Abstract
Background
The nature of the relationship between depressive vulnerability (DV) and acute adversity in the etiology of major depression (MD) remains poorly understood.
Method
Stressful life events (SLEs) and MD onsets in the last year were assessed at four waves in cohort 1 (females) and at two waves in cohort 2 (males and females) from the Virginia Adult Twin Study. Structural equation modeling was conducted in Mplus.
Results
In cohort 1, DV was strongly indexed by depressive episodes over the four waves (paths from +0.72 to 0.79) and predicted by SLEs in the month of their occurrence (+0.31 to 0.36). Wave-specific DV was associated both with stable DV (+0.29 to 0.33) and by forward transmission of DV from the preceding wave (+0.33 to 0.36). SLEs were predicted by stable DV (+0.29) and from SLEs in the preceding month (+0.06). As the cohort aged, MD onsets were better indexed by DV and more poorly predicted by SLEs. Parameter estimates were similar in males and females from cohort 2. In individuals with prior depressive episodes, the association between MD onset and SLEs was weakened while the prediction of SLEs from DV was substantially strengthened. We found no evidence for ‘reverse causation’ from MD episodes to SLEs.
Conclusion
The interrelationship between DV and acute adversity in the etiology of MD is complex and temporally dynamic. DV impacts on MD risk both directly and indirectly through selection into high stress environments. Over time, depressive episodes become more autonomous. Both DV and SLEs transmit forward over time and therefore form clear targets for intervention.
Keywords: Adversity, major depression, stressful life events
Introduction
What is critical in understanding an integrated system is the willingness to go beyond what can be learned by studying component reactions in isolation. One must attempt to develop comprehensive models that integrate different processes in accounting for systemic behavior (Bechtel & Richardson, 1993, p. 171)
Episodes of major depression (MD) commonly arise as a result both of long-term vulnerability and experiences of acute adversity. This vulnerability derives in part from genetic risk factors (Sullivan et al. 2000) and early traumatic experiences (Kessler et al. 1997; Kendler et al. 2000a), and is reflected in personality traits such as neuroticism (Hirschfeld et al. 1989; Boyce et al. 1991; Kendler et al. 1993b). Acute depressogenic adversity is most commonly operationalized as stressful life events (SLEs) which powerfully predict risk for depressive onsets (Brown & Harris, 1978; Kessler, 1997; Kendler et al. 1998).
This simple picture, however, does not adequately capture the complexity of the causal pathways to depressive episodes. First, exposure to SLEs is modestly heritable (Kendler et al. 1993a; Bemmels et al. 2008; Boardman et al. 2011), and the same genetic risk factors and personality traits that increase risk for MD also predispose to SLE exposure (Kendler & Karkowski-Shuman, 1997; van Os et al. 2001; Boardman et al. 2011). Therefore, the SLE–MD association does not likely arise solely because SLEs cause MD but also because they both share common antecedents. Second, as Prince Hamlet says, ‘When sorrows come, they come not single spies, but in battalions.’ One SLE (such as financial difficulties) can predispose to subsequent SLEs (such as marital discord) (Conger et al. 1990). Third, depressive episodes can produce scars manifest by changes in personality, cognitive style and relationship quality (Lewinsohn et al. 1981; Kendler et al. 1993b, 2011). These changes can then increase risk for future episodes so that the liability to depression can perpetuate itself over time. Fourth, the time period over which SLEs have a direct effect on MD onset has been debated with temporal windows ranging from 1 month to 1 year or more (Bebbington et al. 1981, 1984; Solomon & Bromet, 1982; Brugha et al. 1990; Kendler et al. 1998). Fifth, while SLEs have a relatively clear causal association with depressive onsets (Kendler et al. 1999; van Praag, 2004; Kendler & Gardner, 2010), a reverse causal path needs to be considered. That is, depressive episodes can increase the risk for future SLEs (Hammen, 1991). Finally, the association between SLEs and MD may not be stable over the course of a depressive illness. After repeated episodes, as suggested by the kindling hypothesis, depressive onsets may become more autonomous and less closely related to adversity (Post, 1992; Kendler et al. 2000b; Monroe & Harkness, 2005).
While each of these effects has been individually examined, we are unaware of the development and application to longitudinal data of a more comprehensive and integrative etiologic model for depressive vulnerability, SLEs and MD. That is the goal of this report. We seek, by this effort, to estimate jointly the strength of these postulated relationships simultaneously, thereby obtaining a more complete picture of etiologic pathways to depressive episodes.
More explicitly, we develop a model from records of the occurrence of SLEs and MD onsets in each of 12 months prior to a structured interview. We then apply this model to four 1-year periods in cohort 1 – a population-based sample of female twins assessed over a decade. We can thereby examine the temporal stability of these key relationships. We then apply this model to cohort 2 consisting of two waves of data on male and female twins from a population-based registry. This permits us to examine the cross-cohort stability of our estimates and explore the stability of our parameter estimates across sexes. Finally, in cohort 1, we model kindling effects by examining interactions between SLE occurrence and the history of prior depressive episodes.
Method
Sample
Participants were derived from two inter-related cohorts of Caucasian same-sex twin pairs who participated in the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (Kendler & Prescott, 2006). All subjects were ascertained from the population-based Virginia Twin Registry formed from a systematic review of birth certificates in the Commonwealth of Virginia. Cohort 1 consisted of female–female twin pairs, born during 1934–1974, who were eligible if both members responded to a mailed questionnaire in 1987–1988. This cohort (n = 2163 at the first wave) was interviewed four times in person or by telephone from 1987 to 1997, with cooperation rates ranging from 85% to 93%. At the fourth wave, an additional younger set of 217 twins interviewed only once before were included. The mean (s.d.) ages of female twins at the four interview waves were, respectively, 30.1 (7.6), 31.6 (7.5), 35.1 (7.5), and 36.3 (8.2). At the first wave, the mean (s.d.) of years of education of this cohort was 14.1 (2.3).
Cohort 2 consisted of male–male/male–female pairs (birth years 1940–1974) ascertained, with a 72% cooperation rate, directly from registry records containing all twin births. Cohort 2 was studied after cohort 1 and we did not include the earlier birth years so as to ascertain samples of similar age. The first interview was completed largely by phone in 1993–1996 and was followed by a second wave of interviews conducted largely face to face in 1994–1998, with a response rate of 83%. For the two waves of the male–female twin sample, the mean ages were 35.5 (9.1) and 37.0 (9.1), respectively. Sample sizes available for analysis in cohorts 1 and 2 are seen in Table 1. At the first wave, the mean (s.d.) of years of education of this cohort was 13.6 (2.6).
Table 1.
Onsets of major depression in the last year across the four waves of assessment in the female-female twin sample and two waves of assessment in the male-female twin sample
| Number of onsets of MD (% of subjects)b | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cohort | Sex | Wave | Total N | Total eligible, Na | Total | Prior episode | No prior episode | |
| Cohort 1 | F | 1 | 2163 | 2137 | 204 (9.5%) | 126 (19.0%) | 78 (5.3%) | |
| Cohort 1 | F | 2 | 2003 | 1979 | 185 (9.3%) | 109 (16.0%) | 76 (5.9%) | |
| Cohort 1 | F | 3 | 1898 | 1871 | 155 (8.3%) | 132 (16.3%) | 23 (2.2%) | |
| Cohort 1 | F | 4 | 1943 | 1911 | 176 (9.2%) | 141 (16.9%) | 35 (3.2%) | |
| Cohort 2 | F | 1 | 1729 | 1650 | 146 (8.8%) | 92 (16.8%) | 55 (4.9%) | |
| Cohort 2 | F | 2 | 1402 | 1335 | 169 (12.7%) | 129 (19.5%) | 40 (6.0%) | |
| Cohort 2 | M | 1 | 5114 | 4904 | 282 (5.8%) | 143 (12.3%) | 139 (3.7%) | |
| Cohort 2 | M | 2 | 4240 | 4079 | 287 (7.0%) | 219 (14.3%) | 68 (2.7%) | |
F, Female; M, male; MD, major depression.
Were not eligible for MD onset either because they were in an episode of MD the entire year or because of missing data.
For simplicity, we do not present the number of subjects in each cohort and wave who have υ. have not had a prior depressive episode which represent the denominator used for the calculation of the percentages of MD onsets in these two groups presented in the last two columns of the table.
Interviews were separated by a minimum of 1 year and were conducted by a carefully trained mental health professional blind to knowledge about the co-twin. Informed consent was obtained prior to all personal interviews and assent prior to all phone interviews. Zygosity was determined by discriminate function analyses using standard twin questions validated against DNA genotyping in 496 pairs (Kendler & Prescott, 1999).
Measures
In all interviews, twins were asked about the occurrence in the last year of 15 symptoms reflecting all DSM-III-R A criteria for MD. As detailed elsewhere (Kendler & Prescott, 2006), questions for this section were adapted from the SCID interview (Spitzer & Williams, 1985). They then aggregated these symptoms in time, reported total number of episodes and dated, to the month, the onset and offset of each episode. Test–retest reliability for last year MD was good: κ = 0.74 (s.e. = 0.08) tetrachoric r = 0.96 ± 0.03. At each interview, each twin was systematically asked about the occurrence, at any time in the preceding 12 months, of 11 ‘personal’ events (such as major marital problems, job loss and assault) and four classes of ‘network’ SLEs (such as death or difficulties getting along with key individuals in their family or social network), each event being dated to the nearest month with high inter-rater reliability (Kendler et al. 1998). Our criteria for the conclusion of one depressive episode and the start of a second was the report that the respondent felt they were ‘back to their usual self’ for a period of at least 2 weeks. While this is a relatively short time period, the respondent had to be free of depressive symptoms so that even if they met a few criteria, they would still be considered in episode.
We examined potential predictors of depressive vulnerability in our female–female twins using a range of variables detailed elsewhere (Kendler et al. 2002; Kendler & Prescott, 2006). Neuroticism was assessed with a measurement model utilizing the mean of scores for the short (12-item) version of the Revised Eysenck Personality Questionnaire (Eysenck & Eysenck, 1975) available at four assessment waves. Genetic risk was assessed from the lifetime history of MD and generalized anxiety disorder in co-twins and parents. Disturbed family environment was measured by 14 items from the Family Environment (Moos & Moos, 1986) using reports for the twin, co-twin and parents. Parental warmth, assessed using the Parental Bonding Instrument (Parker et al. 1979), was calculated for each twin pair as the mean of the self-report and co-twin report of maternal and paternal warmth assessed at our second wave interview.
Statistical methods
Our dependent variable was onset of a depressive episode meeting DSM-III-R criteria in any of the 12 months prior to interview. Months when individuals were in an episode were censored. SLEs were a binary variable indicating the occurrence of at least one event in that month. The history of prior MD episodes came either from a separate section of the interview that assessed personal history prior to the past year or from previous months in the current year.
For each year, we created a latent variable for depressive vulnerability (DV) using the 12 months of binary depression onsets as measured indicators. To obtain stable estimates with sparse data, the loadings were constrained to equality across months. The latent variables were assigned a mean of zero and residual variance of 1. In our female sample, the latent variables for DV for each of the four 1-year intervals were then treated as indicators for a higher level latent variable representing temporally stable DV. That is, our temporally stable DV is a latent variable indexed by the occurrence of depressive episodes across the waves of interviews. The magnitude of the paths from this DV variable to the wave-specific DV (which are loadings on a common factor) reflects the degree of temporal stability of this long-term vulnerability. Autoregressive paths were added between each adjacent 1-year latent variable to represent forward transmission of DV. The male–male, male–female twin-pair data were modeled similarly except that only two 1-year periods were available, and males and females were treated as separate groups.
Since binary variables served as both dependent and independent variables, model fitting was carried out using the theta parameterization in Mplus 6.11 and mean and variance adjusted weighted least squares (WLSMV) as the estimator (Muthen & Muthen, 2010). The comparative fit of nested models was carried out with corrected χ2 statistics using the difftest utility. Tests involved examining which parameters could be dropped from the model and which could be constrained to equality across waves or across genders.
To examine the effects of a prior history of MD, structural models were set up similarly to the best-fitting model for the overall data, but each model only applied to 1 year of person-month data. The first model fit treated all binary SLE data normally (1 = occurrence, 0 = non-occurrence). The second model was similar except that SLE data were treated as the product of life events and prior history (yes = 1, no = 0), so that the event variable is equal to 1 only if there is an event and the individual had prior depressive episodes. A stronger path in models 2 υ. 1 indicates a positive interaction with prior MD history while weaker path indicates a negative interaction. Classic interactions could not be identified as prior history was confounded with our latent measure of DV.
Ethical standards
Informed consent was obtained prior to all personal interviews and assent prior to all phone interviews.
Results
Female–female sample
Information about the number of onsets of MD in the year prior to the four interview waves for cohort 1 is provided in Table 1. The proportion of subjects with a depressive onset in the last year tended to decline modestly over the four waves. As expected, the rates of last year episodes were much higher in waves with υ. waves without prior MD episodes.
The number of first episodes of MD declined substantially over waves.
Parameter estimates and 95% confidence intervals for our best-fit model, which had an excellent fit [root mean square error of approximation (RMSEA) = 0.002, Comparative fit index (CFI) = 0.99, Tucker–Lewis index (TLI) = 0.99], are seen in Fig. 1. This model had nine key features. First, as expected, stable DV was robustly related to DV at each individual wave. However, the association declined modestly over waves. Second, the wave-specific DV was itself transmitted forward over time gaining modestly in strength as the cohort aged. Third, within waves, as predicted the wave-specific DV was strongly related to risk for onset for all 12 months of the year (but only the first two are depicted in the figure for simplicity). The association between wave-specific DV and risk for onset became modestly stronger as the cohort aged.
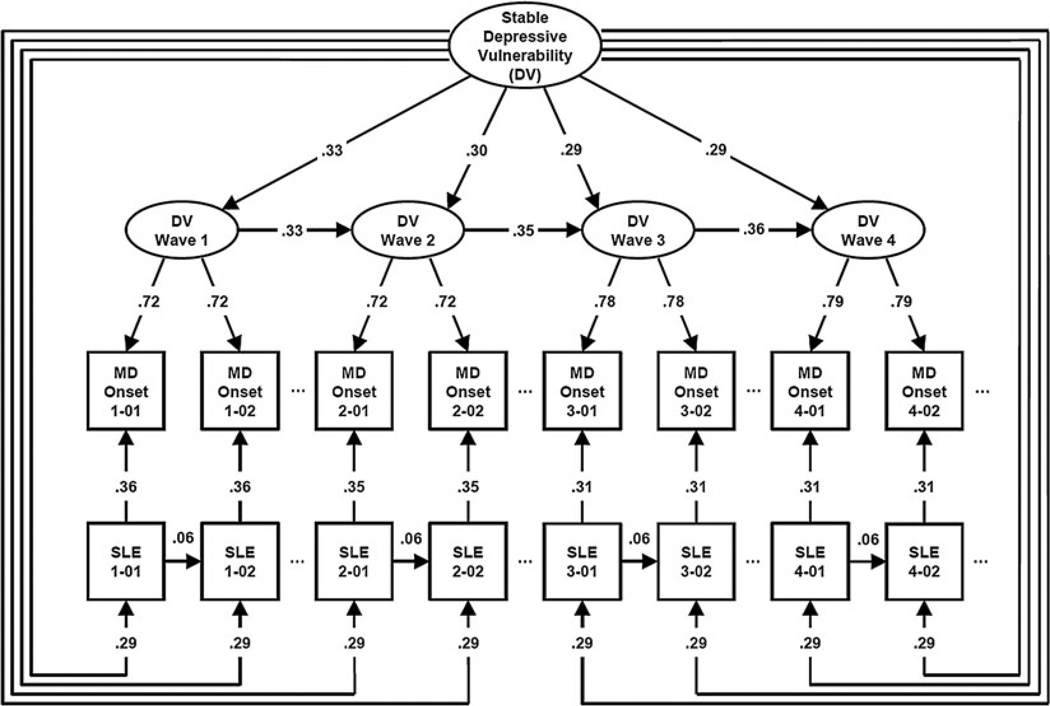
Fig. 1.
Parameter estimates from our best-fitting model applied to four waves of interviews in cohort 1. For simplicity, we present only the first 2 months of the 12-month periods for each of the four waves. Each of the arrows represents standardized partial correlations. DV, Depressive vulnerability; MD, major depression; SLE, stressful life event. For MD onset and SLEs, the numbers in the boxes refer to wave and month in wave so that 1-01 reflects the first month in wave 1 and 4-02 the second month of wave 4. Variables in ellipses are latent while variables in rectangles are observed.
Fourth, within each month, SLE occurrence predicted risk for depressive onset. The strength of this relationship declined modestly across waves. From these estimates, we can calculate that at wave 1, the wave-specific DV explained 0.722 = 52% of the variance in liability to depressive onsets compared to 0.362 = 13% for SLE occurrence. Thus, at wave 1, DV accounted for four times as much variance in predicting onsets as did SLE occurrence. This ratio increased over time equaling 4.3, 6.1 and 6.2 at, respectively, waves 2, 3 and 4. Fifth, we modeled a path from SLEs at month n to depressive onsets at month n + 1. This path was not significant, suggesting that the temporal window for the direct impact of SLEs on risk for onset was restricted to a single month.
Sixth, we tried to add a ‘reverse causation’ path from depressive onsets in month n to SLEs in month n + 1. This was also not significant, indicating the depressive onsets did not substantially increase risk for future SLEs. Seventh, the best-fit model did, however, include a path for the forward transmission over time for SLEs. Although some SLEs are not repeatable over short time intervals (e.g. divorce), we still found that events in one month directly but modestly predicted events in the next month. Eighth, SLE occurrence was predicted by the stable DV at the same level across all four waves. We can therefore estimate the within-month sources of correlation between SLEs and depressive onsets. Looking at wave 1, we have a direct path of +0.36 and an indirect path via the stable and wave 1 depressive vulnerabilities which equals +0.07. Finally, we tried to add a path from the wave-specific DV to SLEs. It was not, however, statistically significant.
Male–female sample with two waves
As seen in Table 1, last-year prevalence rates for MD were generally similar in the females from our second cohort and our first cohort, both being higher than those seen in males from our second cohort. However, higher rates of MD were generally seen for both sexes in the second wave assessments in cohort 2, largely done face to face, than in our first wave interview, performed over the telephone.
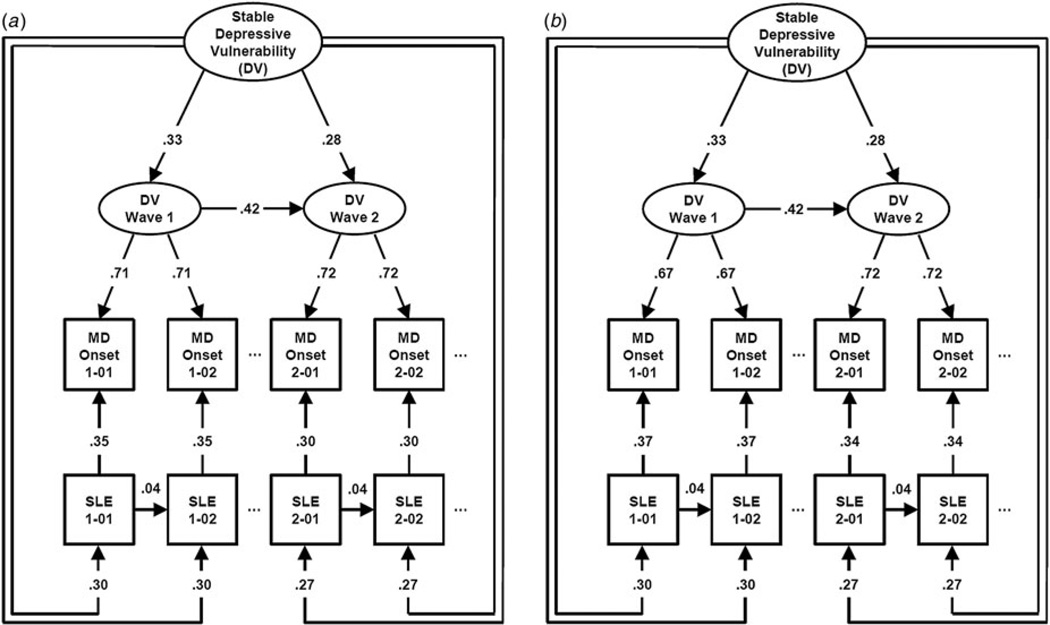
The best fitting joint model for the two waves of interviews for males and females had a nearly perfect fit to the data (RMSEA = 0.00, CFI = 1.00, TLI = 1.00). Parameter estimates are seen in Fig. 2(a,b) for females and males, respectively. Looking first at the females, path estimates are very similar to those seen in our female–female sample in Fig. 1. A few small differences include slightly stronger paths between the first and second wave DV, and slightly weaker paths for transmission of SLEs from 1 month to the next. Wave-specific DV indexes depressive episodes onsets slightly more strongly in wave 2 than wave 1 with the opposite effect seen for the causal effects of SLEs on onsets. The association of wave-specific and stable DV was modestly stronger in wave 1 than wave 2.
Fig. 2.
Parameter estimates from our best-fitting model applied to (a) two waves of interviews in females from cohort 2, and (b) two waves of interviews in males from cohort 2. For abbreviations, see legend to Fig. 1.
We could constrain to equality across sexes all but one set of paths – the loadings from the wave-specific DV to MD onset which were slightly higher in females at wave one. Other differences in the parameter estimates between males and females emerge only because of differences in the underlying variance.
Kindling in the female–female sample
We fitted separate models to all four waves of our female–female sample examining all SLEs and then examining the interaction between SLEs and a history of one or more prior depressive episode. In all four waves, in the interactive υ. standard model, paths from SLEs to MD onset were considerably smaller and the path from DV to SLEs much higher (Table 2). We also see a more modest decline in the interactive υ. standard model for the path from DV to MD episodes but this effect gets smaller across waves and becomes quite modest in the last two waves.
Table 2.
The impact of a history of prior depressive episodes on key path estimates of the modela
| Wave | All/prior MD |
Path estimates: Wave DV→MD onset |
Path estimates: Wave DV→SLE |
Path estimates: SLE→SLE |
Path estimates: SLE→MD onset |
|---|---|---|---|---|---|
| 1 | All | 0.70 | 0.16 | 0.12 | 0.30 |
| Prior | 0.59 | 0.49 | 0.18 | 0.17 | |
| 2 | All | 0.70 | 0.20 | 0.13 | 0.33 |
| Prior | 0.65 | 0.38 | 0.23 | 0.22 | |
| 3 | All | 0.78 | 0.22 | 0.09 | 0.20 |
| Prior | 0.73 | 0.50 | 0.06 | 0.12 | |
| 4 | All | 0.78 | 0.20 | 0.10 | 0.21 |
| Prior | 0.75 | 0.44 | 0.14 | 0.09 |
DV, Depressive vulnerability; MD, major depression; SLE, stressful life event.
For all the models, value of RMSEA was ⩽0.02, CFI ⩾0.91 and TLE ⩾0.90.
Predictors of depressive vulnerability
Stable DV was defined in our model as a common factor indexed by occurrence of MD episodes across waves. However, because it was of interest to examine the predictors of the key variable in our model, we conducted exploratory analyses using the rich information available in our data set (Kendler & Prescott, 2006). The four strongest predictors for stable DV in our female–female pairs were (i) the personality trait neuroticism (Eysenck & Eysenck, 1975), (ii) genetic risk (Kendler et al. 2002), (iii) disturbed family environment (Moos & Moos, 1986), and (iv) low parental warmth (Parker et al. 1979). That is, our measure of DV reflects genetic and temperamental risk factors for MD as well as early environmental adversities.
Discussion
We sought, in this paper, to clarify the temporal and causal inter-relationships between DV, SLE occurrence and onset of MD episodes. We here review eight noteworthy findings from our analyses.
First, our model demonstrates two pathways through which a stable DV can impact on risk for MD episodes. The first and stronger pathway is mediated through the wave-specific DV which is directly associated with risk for MD onset. This pathway can be best conceptualized as involving neurobiological, temperamental and cognitive factors, and is largely ‘within the skin.’ The second and weaker pathway involves selection into or the creation of high-risk environments that increase risk for SLEs which in turn heightened risk for MD. This is best understood as operating largely ‘outside the skin’ (Kendler, 2001).
Second, DV was strongly transmitted forward in time from one wave to the next. These findings are congruent with prior evidence that depressive episodes can scar personality, cognitive style and relationship quality (Lewinsohn et al. 1981; Kendler et al. 1993b, 2011). That this can occur independently of genetic risk has been shown in both a clinical-historical (Kendler & Halberstadt, 2013) and a longitudinal-symptomatic (Kendler et al. 2011) study of monozygotic twins demonstrating diverging depressive trajectories despite identical genetic liabilities. These forward transmission pathways for DV form an obvious focus for clinical intervention that could not only relieve current symptoms but break a self-perpetuating cycle of dysfunction.
Third, our results clarify the temporal relationship between SLEs and depressive onsets. The window of directly increased risk for depressive onsets after a SLE in our findings is short, lasting only a single month. While consistent with some prior work (Brown & Harris, 1978), these results suggest that windows of risk of 6 months (Brugha et al. 1990), 10 months (Bebbington et al. 1984), 1 year (Solomon & Bromet, 1982), or 6 years (Caspi et al. 2003) used in prior research are likely to be too long. While SLEs did not, in our model, directly predict onsets outside the month of occurrence, they did so indirectly by causing future events which in turn predicted onsets. That is, the causal pathway from one SLE to depressive onset in the next month is SLE1→SLE2→MD2 where subscripts 1 and 2 represent months 1 and 2.
Fourth, contrary to expectations, depressive onsets do not in and of themselves predispose to future SLEs. While we know that being depressed can impair functional ability in multiple realms (Barnett & Gotlib, 1988; Klerman & Weissman, 1992; Lagerveld et al. 2010), once we control for the causal pathway from SLE to MD and from SLE to SLE across time, we find no evidence for MD episodes to cause subsequent SLEs.
Fifth, in our first cohort, we examined changes in the etiologic pathways to MD episodes over four waves as the cohort aged. Wave-specific DV in our model is caused both by stable DV and DV from the last wave. As our cohort ages, the influence of the stable DV declines and the influence of the DV transmitted from the last wave increases. That is, as our sample moves from early to mid-adulthood, the control of DV is shaped increasingly by prior experiences in adulthood and less from the enduring effects of genes, personality and childhood adversity. We also see a change in influences on depressive episodes themselves. As our cohort grows older, depressive onsets are more strongly indexed by the wave-specific DV and more weakly caused by SLEs. Over adulthood, depressive episodes may become more autonomous in nature and less sensitive to environmental precipitants. We cannot rule out the possibility that this effect arises from a decline in the average severity of SLEs although this is unlikely as health-related problems in the subject and their social network increase with age and are often among the more depressogenic events (Kendler et al. 1998). These results were all also seen in the two waves of our second cohort. This pattern of findings is also open to a further alternative explanation. Individuals with high DV are surely more prone to first and subsequent onsets of MD and environmental adversity may play less of a role in depressive onsets in them compared to those with low DV. The proportion of such high DV individuals in 1-year prevalence samples of depressed cases would then likely increase as any cohort ages. This hypothesized explanation of our results, however, is inconsistent with prior studies of kindling in this sample where we showed robust within-individual reductions in the SLE-onset association as individuals experienced additional depressive episodes (Kendler et al. 2000b).
Sixth, in the four waves of our first cohort, we examined differences in key parameter estimates from our model in those without υ. with a prior depressive episode. Congruent with prior results from the ‘kindling’ literature (Post, 1992; Kendler et al. 2000b; Monroe & Harkness, 2005), our modeling showed that the causal pathway from SLEs to MD was substantially weaker in those with υ. without prior depressive episodes. These results are consistent with the changes seen in our cohorts over time because as our cohort ages, the proportion of depressive episodes that are first onsets υ. recurrences steadily decline. Our kindling analysis also produced one unanticipated finding. SLE occurrence was more strongly predicted by the level of DV in individuals with υ. without a history of MD. For those with high DV, this vulnerability has at most a modest impact on selection into stressful situations prior to their first depressive episode. Once they manifest their first full depressive syndrome, this ‘stress-selection’ process becomes more robust, perhaps through increasingly strained inter-personal relationships (Petty et al. 2004).
Seventh, consistent with a recent developmental twin study (Gillespie et al. 2015), our model contained two different kinds of environmental contributants to MD: (i) early adversities such as a disturbed family environment and poor parental warmth which, by influencing DV, produced a long-lasting effect and (ii) concurrent SLEs which had a substantial but quite short-lived impact on risk.
Eighth, the main features of our model were similar in men and women. We have previously examined sex difference in this sample for a broad array of developmental risk factors for MD and found some noteworthy differences (Kendler & Gardner, 2014). In this model, which focuses on the temporal structure of risk for MD, we found, by contrast, very modest differences. For all intents and purposes, the relationship between DV, SLEs and onset risk for MD appears to be the same in men and women.
Limitations
These results should be interpreted in the context of six potentially significant methodological limitations. First, our sample includes only adult white twins born in Virginia and our results may not extrapolate to other samples. With respect to the rates of psychopathology, these twins are broadly representative of the general US population (Kendler et al. 1996; Kendler & Prescott, 2006). For example, our 1-year prevalence rates for MD in females and males are quite similar to those reported in the National Comorbidity Survey (Kessler et al. 1994). Second, our modeling treated each twin as an individual and did not formally correct for the modest correlations between twin pairs for the variables in our model. However, the correlation of the key dependent variable, onset of MD in a particular month, was actually too low in our twin pairs (+0.014) to produce any meaningful bias in our findings. Third, the assessments of SLEs were similar but not identical across the waves of our interviews. This could have contributed to some of the differences in parameter estimates across waves. Fourth, all the data analyzed in this report comes from self-reports albeit only over the past 12 months. SLEs were assessed in a different section of the interview from depressive symptoms. However, we cannot rule out that some of these results arise from recall errors or bias. Fifth, it might have been desirable to examine the effects of specific classes of SLEs or to use ratings available in our data set on long-term contextual threat (Kendler et al. 1998). However, attempts to do so in our sample proved unworkable due to the rarity of more specific subtypes of life events. Obviously, the parameter estimates in our model for SLEs are dependent on our methods of measurement. If our sample was large enough to model only severe SLEs, the relevant path estimates might have been substantially larger. Finally, although it is tempting to interpret our model as saying that stable DV ‘causes’ wave-specific DV that is not an accurate statement. Rather, stable DV is a latent factor indexed by the wave-specific measures of DV.
Conclusions
The models presented here capture important features of the complex and temporally dynamic interrelationship between the sustained influences of DV and the short-term impact of acute adversity in the etiology of MD. DV – substantially influenced by personality, genetic risk and early adversities – impacts on risk for MD both directly on episode onset and indirectly through increasing exposures to stressful situations. SLEs are important but temporally discrete risk factors for depressive onsets. Both DV and SLEs perpetuate themselves over time contributing to the self-sustaining aspects of depressive illness. Over a decade of middle adulthood, important developmental changes are seen in the etiology of MD. DV becomes more influenced by prior DV and less by stable long-term risk factors. MD onsets become more strongly related to wave-specific DV and less influenced by SLEs. These results also emerged clearly in our ‘kindling’ analyses.
These results have implications for both our conceptualization and treatment of psychiatric illness. They demonstrate cross-level causal effects and outside the skin pathways – consistent with the need for multilevel models of psychiatric illness (Kendler, 2012; 2014). As suggested by our introductory quote, to develop realistic etiologic models for complex psychiatric disorders such as MD, we need to identify and study individually important risk factors, but then develop comprehensive models that integrate these different components so that we can account for the ‘systemic behavior’ of the overall disease process (Bechtel & Richardson, 1993). Our approach also highlights the forward transmission over time of both DV and SLEs. Using available pharmacologic and psychotherapeutic tools, our model supports the importance of intervening in these processes, thereby breaking the self-perpetuating nature of depressive illness.
Acknowledgments
This work was supported in part by grants MH40828 and MH49492 from the US National Institutes of Health. The Virginia Twin Registry is supported by grant UL1RR031990. Dr Carol Prescott played a central role in the design and implementation of this twin study.
Footnotes
Declaration of Interest
None.
References
- Barnett PA, Gotlib IH. Psychosocial functioning and depression: distinguishing among antecedents, concomitants, and consequences. Psychological Bulletin. 1988;104:97–126. doi: 10.1037/0033-2909.104.1.97. [DOI] [PubMed] [Google Scholar]
- Bebbington PE, Sturt E, Tennant C, Hurry J. Misfortune and resilience: a community study of women. Psychological Medicine. 1984;14:347–363. doi: 10.1017/s0033291700003603. [DOI] [PubMed] [Google Scholar]
- Bebbington PE, Tennant C, Hurry J. Adversity and the nature of psychiatric disorder in the community. Journal of Affective Disorders. 1981;3:345–366. doi: 10.1016/0165-0327(81)90004-5. [DOI] [PubMed] [Google Scholar]
- Bechtel W, Richardson RC. Discovering Complexity: Decomposition and Localization as Strategies in Scientific Research. Princeton, NJ: Princeton University Press; 1993. [Google Scholar]
- Bemmels HR, Burt SA, Legrand LN, Iacono WG, McGue M. The heritability of life events: an adolescent twin and adoption study. Twin Research and Human Genetics. 2008;11:257–265. doi: 10.1375/twin.11.3.257. [DOI] [PubMed] [Google Scholar]
- Boardman JD, Alexander KB, Stallings MC. Stressful life events and depression among adolescent twin pairs. Biodemography and Social Biology. 2011;57:53–66. doi: 10.1080/19485565.2011.574565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce P, Parker G, Barnett B, Cooney M, Smith F. Personality as a vulnerability factor to depression. British Journal of Psychiatry. 1991;159:106–114. doi: 10.1192/bjp.159.1.106. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Social Origins of Depression: A Study of Psychiatric Disorder in Women. London: Tavistock; 1978. [Google Scholar]
- Brugha TS, Bebbington PE, Sturt E, MacCarthy B, Wykes T. The relation between life events and social support networks in a clinically depressed cohort. Social Psychiatry and Psychiatric Epidemiology. 1990;25:308–313. doi: 10.1007/BF00782886. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Conger GH, Elder GH, Jr, Lorenz FO, Conger KJ, Simons RL, Whitbeck LB, Huck S, Melby JN. Linking economic Hardship to marital quality and instability. Journal of Marriage and the Family. 1990;52:643–656. [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire. London: Hodder and Stoughton; 1975. [Google Scholar]
- Gillespie NA, Eaves LJ, Maes H, Silberg JL. Testing models for the contributions of genes and environment to developmental change in adolescent depression. Behavior Genetics. 2015;45:382–393. doi: 10.1007/s10519-015-9715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RMA, Klerman GL, Lavori PW, Keller MB, Griffith P, Coryell W. Premorbid personality assessments of first onset of major depression. Archives of General Psychiatry. 1989;46:345–350. doi: 10.1001/archpsyc.1989.01810040051008. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Twin studies of psychiatric illness: an update. Archives of General Psychiatry. 2001;58:1005–1014. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- Kendler KS. The dappled nature of causes of psychiatric illness: replacing the organic-functional/hardware-software dichotomy with empirically based pluralism. Molecular Psychiatry. 2012;17:377–388. doi: 10.1038/mp.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. The structure of psychiatric science. American Journal of Psychiatry. 2014;171:931–938. doi: 10.1176/appi.ajp.2014.13111539. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Archives of General Psychiatry. 2000a;57:953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Eaves LJ, Loken EK, Pedersen NL, Middeldorp CM, Reynolds C, Boomsma D, Lichtenstein P, Silberg J, Gardner CO. The impact of environmental experiences on symptoms of anxiety and depression across the life span. Psychological Sciences. 2011;22:1343–1352. doi: 10.1177/0956797611417255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Dependent stressful life events and prior depressive episodes in the prediction of major depression: the problem of causal inference in psychiatric epidemiology. Archives of General Psychiatry. 2010;67:1120–1127. doi: 10.1001/archgenpsychiatry.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Sex differences in the pathways to major depression: a study of opposite-sex twin pairs. American Journal of Psychiatry. 2014;171:426–435. doi: 10.1176/appi.ajp.2013.13101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in women. American Journal of Psychiatry. 2002;159:1133–1145. doi: 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Halberstadt LJ. The road not taken: life experiences in monozygotic twin pairs discordant for major depression. Molecular Psychiatry. 2013;18:975–984. doi: 10.1038/mp.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. Journal of Nervous and Mental Disease. 1998;186:661–669. doi: 10.1097/00005053-199811000-00001. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski-Shuman L. Stressful life events and genetic liability to major depression: genetic control of exposure to the environment? Psychological Medicine. 1997;27:539–547. doi: 10.1017/s0033291797004716. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale M, Kessler R, Heath A, Eaves L. A twin study of recent life events and difficulties. Archives of General Psychiatry. 1993a;50:789–796. doi: 10.1001/archpsyc.1993.01820220041005. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of personality and major depression in women. Archives of General Psychiatry. 1993b;50:853–862. doi: 10.1001/archpsyc.1993.01820230023002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Pedersen NL, Farahmand BY, Persson PG. The treated incidence of psychotic and affective illness in twins compared with population expectation: a study in the Swedish Twin and Psychiatric Registries. Psychological Medicine. 1996;26:1135–1144. doi: 10.1017/s0033291700035856. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. A population-based twin study of lifetime major depression in men and women. Archives of General Psychiatry. 1999;56:39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. 1st. New York: Guilford Press; 2006. [July 26, 2006]. [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the ‘kindling’ hypothesis. American Journal of Psychiatry. 2000b;157:1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annual Review of Psychology. 1997:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychological Medicine. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Klerman GL, Weissman MM. The course, morbidity, and costs of depression. Archives of General Psychiatry. 1992;49:831–834. doi: 10.1001/archpsyc.1992.01820100075013. [DOI] [PubMed] [Google Scholar]
- Lagerveld SE, Bultmann U, Franche RL, van Dijk FJ, Vlasveld MC, van der Feltz-Cornelis CM, Bruinvels DJ, Huijs JJ, Blonk RW, van der Klink JJ, Nieuwenhuijsen K. Factors associated with work participation and work functioning in depressed workers: a systematic review. Journal of Occupational Rehabilitation. 2010;20:275–292. doi: 10.1007/s10926-009-9224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Steinmetz JL, Larson DW, Franklin J. Depression-related cognitions: antecedent or consequence? Journal of Abnormal Psychology. 1981;90:213–219. doi: 10.1037//0021-843x.90.3.213. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the ‘kindling’ hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychological Review. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Moos R, Moos B. Family Environment Scale Manual. 2nd. Palo Alto, CA: Consulting Psychologists Press; 1986. [Google Scholar]
- Muthen LK, Muthen BO. version 6.0 ed. 6th. Los Angeles, CA: Muthen & Muthen; 2010. Mplus User’s Guide: 1998–2010. [Google Scholar]
- Parker G, Tupling H, Brown L. A parental bonding instrument. British Journal of Medical Psychology. 1979;52:1–10. [Google Scholar]
- Petty SC, Sachs-Ericsson N, Joiner TE., Jr Interpersonal functioning deficits: temporary or stable characteristics of depressed individuals? Journal of Affective Disorders. 2004;81:115–122. doi: 10.1016/S0165-0327(03)00158-7. [DOI] [PubMed] [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. American Journal of Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Solomon Z, Bromet E. The role of social factors in affective disorder: an assessment of the vulnerability model of Brown and this colleagues. Psychological Medicine. 1982;12:123–130. doi: 10.1017/s0033291700043361. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-III-R (SCID) New York: Biometrics Research Department, New York State Psychiatric Institute; 1985. [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- van Os J, Park SB, Jones PB. Neuroticism, life events and mental health: evidence for person- environment correlation. British Journal of Psychiatry Supplement. 2001;40:s72–s77. doi: 10.1192/bjp.178.40.s72. [DOI] [PubMed] [Google Scholar]
- van Praag HM. Can stress cause depression? Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28:891–907. doi: 10.1016/j.pnpbp.2004.05.031. [DOI] [PubMed] [Google Scholar]