Abstract
Emerging evidence supports an important role for T cells in the genesis of hypertension. Because this work has predominantly been performed in experimental animals, we sought to determine whether human T cells are activated in hypertension. We employed a humanized mouse model in which the murine immune system is replaced by the human immune system. Angiotensin II increased systolic pressure to 162 mm Hg vs. 116 mm Hg for sham treated animals. Flow cytometry of thoracic lymph nodes, thoracic aorta and kidney revealed increased infiltration of human leukocytes (CD45+) and T lymphocytes (CD3+ and CD4+) in response to angiotensin II infusion. Interestingly, there was also an increase in the memory T cells (CD3+/CD45RO+) in the aortas and lymph nodes. Prevention of hypertension using hydralazine and hydrochlorothiazide prevented the accumulation of T cells in these tissues. Studies of isolated human T cells and monocytes indicated that angiotensin II had no direct effect on cytokine production by T cells or the ability of dendritic cells to drive T cell proliferation. We also observed an increase in circulating IL-17A producing CD4+ T cells and both CD4+ and CD8+ T cells that produce IFN-γ in hypertensive compared to normotensive humans. Thus, human T cells become activated and invade critical end-organ tissues in response to hypertension in a humanized mouse model. This response likely reflects the hypertensive milieu encountered in vivo and is not a direct effect of the hormone angiotensin II.
Keywords: Inflammation, Memory T cells, Myeloid cells, CD14, CD4+ T cells, CD8+ T cells
INTRODUCTION
In the past several years it has become evident that inflammation contributes to the elevation of blood pressure and end organ damage in numerous experimental models of hypertension. In mice lacking the recombinase-activating gene (RAG-1−/− mice), which lack all lymphocytes, the hypertensive responses to angiotensin II (ang II), deoxycorticosterone acetate-salt challenge, norepinephrine and emotional stress are blunted.1–3 Moreover, these mice are protected against endothelial dysfunction, vascular hypertrophy and do not develop an increase in vascular superoxide as observed in normal mice. Adoptive transfer of T cells, but not B cells, restores the hypertensive response and vascular dysfunction in RAG-1−/− mice.1 Mice with severe combined immunodeficiency are also protected against hypertension.4 Likewise, deletion of the RAG-1 gene in Dahl salt-sensitive rats blunts their hypertension and renal damage upon salt feeding.5 T cells with an effector phenotype infiltrate the kidney and periadventitial tissues, where they release inflammatory cytokines, including interleukin 17A, interferon-γ (IFN-γ) and tumor necrosis factor α(TNF-α) that in turn alter renal and vascular function and participate in end-organ damage.6–8 Genetic deletion of these cytokines or treatment with their specific antagonists blunts hypertension and its attendant end-organ dysfunction.
Despite the overwhelming evidence that inflammation and immunity contribute to hypertension in these experimental models, there is a paucity of information to suggest that hypertension or the factors present in the hypertensive milieu lead to T cell activation in humans. In the present study, we addressed this issue using several approaches. First we used a unique animal model, the bone/liver/thymus (BLT) humanized mouse to determine whether Ang II-induced hypertension is associated with T cell activation and infiltration into key target tissues. Second, we examined the direct effects of ang II on human T cell activation and human dendritic cell (DC) function in culture. Finally, we analyzed the phenotype of circulating T cells in humans with and without hypertension.
METHODS
BLT mice
The Vanderbilt University Institutional Animal Care approved all experiments. Mice were cared for in accordance with the Guide for the Care and Use of Laboratory Animals. Bone marrow, liver, thymus (BLT) mice were produced on a NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) background as previously described.9, 10 Twelve to fourteen weeks after immune reconstitution, osmotic minipumps (Alzet, model 2002) were implanted subcutaneously for infusion of either ang II (490 ng/kg/min) or an isotonic vehicle (sodium chloride/acetic acid solution) for sham mice. In some experiments, hypertension was prevented by co-administration of hydralazine and hydrochlorothiazide as previously described.11
Response of human T cells and dendritic cells to ang II
Two experimental approaches were employed to examine the direct effects of ang II on human immune cells. In the first, human T cells were isolated from the blood of normotensive volunteers using magnetic sorting and 106 cells were exposed to anti-CD3+ coated culture plates with anti-CD28 (2 µg/ml) added to the media for 3 days in the presence or absence of 0.1 µMol/L of ang II. Intracellular staining was employed to identify cells containing IFN-γ, IL-17A and forkhead box P3 (FoxP3) as previously described.12 As a second approach, human monocytes were differentiated to dendritic cells in the presence of GM-CSF and IL-4 with and without addition of 0.1 µMol/L ang II. These cells were then co-cultured with CFSE-labeled T cells from the same volunteer at a ratio of 1 dendritic cell to 10 T cells.
Studies of circulating T cells in humans
Peripheral blood samples were collected in ethylenediaminetetraacetic acid (EDTA) from 20 hypertensive patients and 20 healthy volunteers, matched for age, sex and BMI. A detailed history of major cardiovascular risk factors for atherosclerosis and characteristics of hypertension were obtained (Table 1). Patient groups did not differ in major cardiovascular risk factors apart from hypertension. Exclusion criteria are listed in the supplementary materials and methods. Office blood pressure was determined in all subjects and 4 resting measurements were averaged. Twenty-four hour ambulatory blood pressures were used to confirm hypertension. The Local Research Ethics Committee of Institute of Cardiology at Jagiellonian University approved collection of tissue specimens and all patients gave written informed consent.
Table 1.
Clinical characteristics of patients studied for circulating T cell phenotypes.
| Clinical features | Control (n=20) |
Hypertension (n=20) |
p value |
|---|---|---|---|
| Female sex (n; %) | 11 (55%) | 11 (55 %) | NS |
| Age (years ± SD) | 52.6 ± 12 | 52.6 ± 11 | NS |
| BMI (kg/m2 ± SD) | 28.2 ± 5.1 | 28.9 ± 5.1 | NS |
| Blood pressure characteristics | |||
| Hypertension (%) | 0% | 100% | <0.05 |
| Office blood pressure | 126±13/83±8 | 150±19/90±11 | <0.05 |
| 24h ABPM (systolic mmHg ± SD) | 120±13 | 143±18 | <0.05 |
| 24h ABPM (diastolic mmHg ± SD) | 75±5 | 85±9 | <0.05 |
| Renin (after supine position) | 10.8±6 | 18±9 | <0.05 |
| Aldosterone (after supine position) | 126±30 | 230±150 | <0.05 |
| Target organ characteristics | |||
| GFR | 74±14 | 78±12 | NS |
| Serum creatinine (µmol/L) | 81±17 | 75 ± 24 | NS |
| LVEDV | 103±26 | 110 ± 28 | NS |
| LVESV | 41±15 | 34 ±11 | NS |
| EF | 65±7 | 65±7 | NS |
| Cardiovascular risk characteristics | |||
| Current Smoking (%) | 2 (10%) | 3 (15%) | NS |
| Ever smoking status (%) | 8 (40%) | 8 (40%) | NS |
| Glucose intolerance/DM | 1 (5%) | 5 (25%) | NS |
| Current blood glucose (mmol/L) | 5.5±0.6 | 6.1±1.5 | NS |
| Total cholesterol (mmol/L) | 5.7±1.0 | 4.9±1.7 | NS |
| Inflammatory/Autoimmune disease | 0 (0%) | 0 (0%) | NS |
| hsCRP level | 0.20±0.18 | 0.16±0.09 | NS |
| WBC (cells/mm3) | 6.3±1.2 | 6.3±1.6 | NS |
| Atherosclerosis and CAD | |||
| Ischemic heart disease (%) | 2 (10%) | 4 (20%) | NS |
| Prior myocardial infarction (%) | 0 (0%) | 3 (15%) | NS |
| Peripheral Arterial Disease (%) | 0 (0%) | 0 (0%) | NS |
| Stroke/TIA | 0 (0%) | 0 (0%) | NS |
| PAD | 0 (0%) | 0 (0%) | NS |
| Medications | |||
| ACE inhibitors (%) | 0 (0%) | 9 (45%) | |
| Angiotensin Receptor Blockers (%) | 0 (0%) | 6 (30%) | |
| Beta – blockers (%) | 0 (0%) | 12 (60%) | |
| Calcium channel blockers (%) | 0 (0%) | 14 (70%) | |
| Alpha – blockers (%) | 0 (0%) | 6 (30%) | |
| Diuretics (%) | 0 (0%) | 9 (45%) | |
| Statins (%) | 1 (5%) | 9 (45%) |
Flow cytometry
Flow cytometry was performed on single cell suspensions of thoracic lymph nodes, the right kidney and the descending thoracic aorta as previously described.12 Human cells were initially identified by anti-human CD45 and subsets were identified using antibodies that specifically react with human cells. Preliminary studies showed that these antibodies did not identify cells in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice without immune reconstitution. Flow cytometry gates were established using flow minus one as previously described and employed in our laboratory (Figure S1).13, 14
Statistics
Data in the manuscript are presented as mean ± standard error of the mean. To compare blood pressure measurements in mice over time, two-way ANOVA with multiple comparisons was employed. To compare numbers of human T cells in various tissues we used either an unpaired T test to analyze or the non-parametric Mann Whitney test when variances between groups were not equal. To show the effect of hypertension on myeloid differentiation, we used one-way ANOVA to analyze the flow cytometry data. For comparison of the frequency of T cell subtypes in humans, unpaired T tests were employed with a Bonferroni correction. Chi square analysis with Bonferonni correction was employed to compare the frequency of risk factors between the normotensive and hypertensive populations. P values reported in the figures and tables represent the adjusted values after multiple testing.
Please see the on-line supplement for complete description of the methods employed.
RESULTS
Effect of angiotensin II-infusion on blood pressure of humanized mice
The blood pressure of humanized mice increased to 162 mmHg following two weeks of ang II infusion when compared to mice that were exposed to a sham infusion (Figure S2). Co-administration of hydralazine and hydrochlorothiazide reduced blood pressure to 112 ± 0.5 mmHg.
Effect of angiotensin II-induced hypertension on the presence of human T cells in the kidney
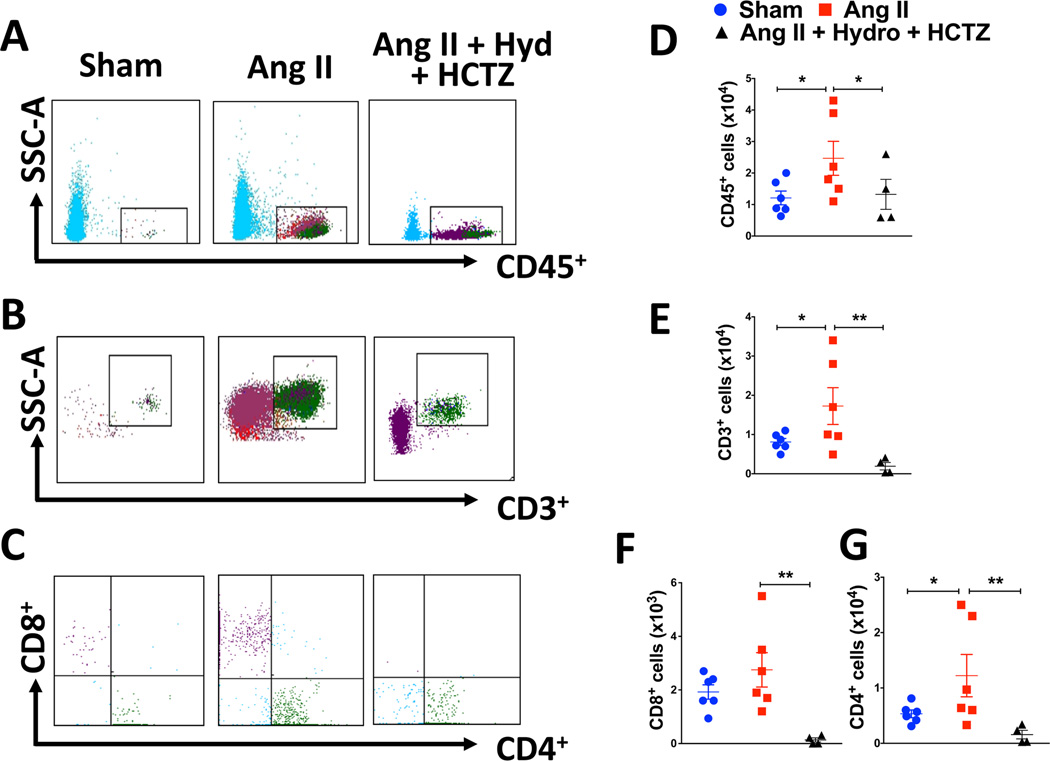
Following 2 weeks of ang II or sham infusion, flow cytometry was performed to quantify the presence of T cell activation in the kidney that had not previously undergone the thymus/liver transplant. As shown in the representative flow cytometry dot plots in figure 1A–C and the mean data in figure 1D–G, there was accumulation of total human leukocytes (CD45+ cells), total T cells (CD3+) and several T cell subpopulations (CD4+ and CD8+) in the kidney. The cells were predominantly composed of CD4+ T cells. There was a non-significant trend for an increase in CD8+ T cells. Among the CD3+ and CD4+ T cells, ang II infusion increased the number of renal CD45RO+ cells indicating an increase in human memory T cells (Table 2). There were relatively few CD69+ T cells in the kidney and these were not changed by ang II infusion (Table S1). Prevention of hypertension by co-administration of hydrochlorothiazide and hydralazine completely abrogated the elevation of both CD4+ and CD8+ T cells and the formation of RO+ cells in the kidney (Figure 1 and table 2).
Figure 1. Effects of hypertension on renal leukocyte and T cell infiltration.
Humanized mice received sham or ang II infusion (490 ng/kg/min) for two weeks with or without co-administration of hydralazine (Hyd) and hydrochlorothiazide (HCTZ). Single cell suspensions were prepared from kidneys of humanized mice and analyzed for total human leukocytes and T cells. Representative dot plots are of human live, singlets gated for (A) total leukocytes (CD45+), (B) total T cells (CD3+), (C) CD8+/CD4+ T cells. The solid black lines in the histogram indicate the gates used for analysis. Summary data are shown for renal accumulation of total leukocytes (D), total T cells (E), CD8+ T cells (F), and CD4+ T cells (G) in response to either a sham or ang II infusion. Data were analyzed using an unpaired T test or the non-parametric Mann Whitney test when variances between groups were not equal, n= 4–6 per group.
Table 2.
Effect of ang II and ang II + anti-hypertensive treatment on accumulation of human memory (CD45RO+) cells.
| CD3 | CD4 | CD8 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sham | Ang II | Ang II Hyd + HCTZ |
Sham | Ang II | Ang II Hyd + HCTZ |
Sham | Ang II | Ang II Hyd + HCTZ |
|
| Kidney | 1443±295 | 6600±2923* | 1227±661† | 1266±227 | 6057±2791* | 1161±633 | 337±57 | 674±246 | 4±3† |
| Aorta | 113±19 | 333±116 * | 194±63† | 93±16 | 294±105* | 181±58 | 29±7 | 44±12 | 15±4† |
| Lymph Nodes | 443±233 | 2642±712* | 17736±10333† | 350±181 | 2258±603* | 16638±9470† | 71±34 | 276±79 | 145±90 |
N = 4–6 per group,
p < 0.05 vs sham.
p < 0.05 vs ang II.
Hyd = hydralazine. HCTZ = hydrochlorothiazide
Effect of angiotensin II-induced hypertension on the presence of human T cells in the aorta
Representative flow cytometry for human T cells in the aorta is shown in Figure 2A–C. In contrast to the kidney, relatively few human cells accumulated in the aorta; however, ang II infusion increased the small numbers of human CD45+ and CD3+, and CD4+ and CD8+ T cells (Figure 2D–G) in the aorta compared to sham infusion. Total CD45RO+ memory T cells and CD4+ memory T cells in the aorta were likewise increased by ang II infusion (table 2). Prevention of hypertension with hydralazine and hydrochlorothiazide significantly reduced accumulation of CD3+ and CD8+ T cells in the aorta and reduced the presence of total memory T cells and CD4+ memory T cells. (Figure 2 and table 2). Ang II did not change the few CD69+ T cells in the aorta (Table S1).
Figure 2. Effects of hypertension on leukocyte and T cell infiltration into the descending, thoracic aorta.
Mice underwent sham or Ang II infusion with or without co-administration of hydralazine (Hyd) and hydrochlorothiazide (HCTZ) as in figure 1. Representative dot plots are shown of live, human, singlet cells gated for (A) total leukocytes (CD45+), (B) total T cells (CD3+), (C) CD8+/CD4+ T cells. Summary data are shown for aortic accumulation of total leukocytes (D), total T cells (E), CD8+ T cells (F) and CD4+ T cells (G) in response to either a sham or ang II infusion. Data were analyzed using an unpaired T test or the non-parametric Mann Whitney test when variances between groups were not equal, n= 4–6 per group.
Effect of angiotensin II induced hypertension on the presence of human T cells in lymph nodes
The greatest accumulation of human cells observed in the humanized mice was noted in lymph nodes. Representative flow cytometry dot plots are shown in Figure 3A–C. Ang II-induced hypertension caused a 3 to 5-fold increase in human CD3+, CD4+ and CD8+ T cells in the murine lymph nodes of this model (Figure 3D–G). Ang II also caused a striking increase in the memory CD3+, CD4+ and CD8+/CD45RO+ T cells in the lymph nodes (Table 2). As in the cases of the kidney and aorta, prevention of hypertension abolished the accumulation of total CD3+ cells and both CD4+ and CD8+ T cells (Figure 3 and table 2). Unlike the case of the kidney and aorta, treatment with hydralazine and hydrochlorothiazide paradoxically increased the number of total CD45RO+ memory T cells and CD4+ memory T cells in lymph nodes.
Figure 3. Effect of hypertension on leukocyte and T cell infiltration into thoracic lymph nodes.
Mice underwent sham or Ang II infusion with or without co-administration of hydralazine (Hyd) and hydrochlorothiazide (HCTZ) as in figure 1 and thoracic lymph nodes were harvested from the periaortic region. Representative images are of human live, singlets gated for (A) total leukocytes (CD45+), (B) total T cells (CD3+), (C) CD8+ / CD4+ T cells. The solid black lines in the histogram indicate the gates that used for all of our analysis. Summary data are shown for the presence of total leukocytes (D), total T cells (E), CD8+ T cells (F), and CD4+ T cells (G). Data were analyzed using an unpaired T test or the non-parametric Mann Whitney test when variances between groups were not equal, n= 4–6 per group.
Myeloid differentiation in angiotensin II treated humanized mice
Because of limitations of fluorochromes in our flow cytometry, we were unable to stain for monocyte/macrophages or dendritic cells in the same animals in which we examined T cell populations; however, one can approximate non-T cell populations by subtracting the CD3+ population from CD45+ cells. There were non-significant trends for increases in these cells in the kidney (Figure 4A) and the aorta (Figure 4B). In the lymph nodes, ang II infusion caused a significant increase in this population of human cells (Figure 4C). In one animal, there was a striking increase in the number of CD45+/CD3− cells (Figure 4C); however, a significant difference remained between sham and ang II infused mice even after exclusion of this animal. In selected mice, we examined staining for CD14, a marker of human monocytes and CD83, an activation marker that appears on monocyte-derived dendritic cells (Figure 4D–E). There were relatively few of these human myeloid cells in the kidney of ang II-infused humanized mice, and these were equally distributed between CD14+, CD14+/CD83+ and CD14−/CD83+ cells. In contrast, in the aorta and lymph nodes, the predominant population of human myeloid cells was CD83+/CD14−.
Figure 4. Effect of hypertension on myeloid differentiation in the kidney, aorta and lymph nodes.
Summary data from mice following sham or angiotensin II (ang II) infusion (490 ng/kg/min) of non-CD3+ CD45+ cells in the kidney (A), descending, thoracic aorta (B), and thoracic lymph nodes (C). The flow cytometry gating strategy used to identify myeloid cells is shown (D). The kidney, aorta and lymph nodes of the humanized mice all showed differentiation of the monocytes to dendritic cells (E). Myeloid differentiation was not significantly shifted in any of the tissues when we compared monocyte (CD14+CD83) vs. activated monocyte (CD14+CD83+) vs. dendritic cell (CD14CD83+) populations.
Direct effects of ang II on human T cells and dendritic cell function
Our above data suggest that normalizing blood pressure prevents human T cell activation in vivo, suggesting that ang II does not directly stimulate T cell cytokine production. One criticism of these experiments is that the anti-hypertensive drugs might have off-target effects to inhibit T cell activation. To further study the direct effect of ang II on human immune cells, we performed two additional experiments. In the first, we analyzed the effect of ang II (0.1 µMol/L) on the production of IL-17A, IFN-γ, FoxP3 and TNF-α in human T cells cultured for 7 days on anti-CD3 plates using intracellular staining. As shown in figure 5A – 5D, there was no direct effect of ang II on the production of TH1 cytokines or FoxP3 by CD3+, CD4+ or CD8+ T cells. Because murine T cells can produce ang II,15 we considered the hypothesis that endogenous ang II might affect human T cell production of cytokines. Co-incubation human T cells with ramipril (1 µMol/L), however, had no effect on production of any cytokines measured (data not shown). In another experiment, we cultured human monocytes for 7 days with granulocyte/macrophage colony stimulating factor (GM-CSF) and IL-4 in the presence and absence of ang II (0.1 µMol/L). The resultant differentiated dendritic cells were then cultured with CFSE- labeled T cells from the same donor. As shown in figure 5E and S3, there was no effect of ang II on the ability of human DCs to drive T cell proliferation. Moreover, ang II did not directly stimulate the accumulation of isoketal-protein adducts in human DCs, as evidenced by intracellular staining with the isoketal adduct antibody D11 (Figure 5F).
Figure 5. Direct effect of ang II on T cell phenotype and dendritic cell activation.
Human T cells were cultured on anti-CD3 plates containing anti-DC28 (2 µg/ml) without and with ang II (0.1 µMol/L) for 7 days and intracellular staining was performed to detect the cytokines IFN-γ and IL-17A and the transcription factor FoxP3 (A–D). Human monocytes were differentiated into dendritic cells by exposure to GM-CSF and IL-4 for 7 days without and with ang II (0.1 µMol/L). The differentiated DCs were then co-cultured with CFSE-labeled T cells from the same donor at a ratio of 1:10. Proliferation was measured by CFSE dilution (E). Isoketal-protein adducts in DCs were determined by intracellular staining with isoketal adduct antibody D11 (F).
Inflammatory profile of circulating T cells in humans with and without hypertension
To further characterize the effect of hypertension on T cell phenotype, we studied 20 normotensive and 20 hypertensive humans, matched for sex, age and BMI. Hypertensive patients had significantly higher office blood pressures and both systolic and diastolic blood pressures as measured by ambulatory blood pressure monitoring. Plasma renin and aldosterone levels were elevated in the hypertensive group compared to the normotensive subjects. As shown in figure 6A and 6B, the percent of both CD4+ and CD8+/CD45RO+ circulating T cells was greater in the hypertensive patients than in the normotensive controls. Intracellular staining showed that CD4+ T cells of humans with hypertension produce greater amounts of IL-17A than normotensive controls (Figure 6C and D). Very few circulating CD8+ T cells were positive for IL-17A (data not shown). Intracellular staining for IFN-γ and TNFα revealed that both of these cytokines were increased in CD4+ T cells and CD8+ T cells of humans with hypertension and were commonly co-produced within the same cells (Figure 6E and 6F).
Figure 6.
Characterization of circulating T cells in humans with and without hypertension (n = 20 for each). Blood was obtained from patients with hypertension (HTN) and normotensive controls (CTR) and T cells were stained for CD3, CD4, CD8 and the memory marker CD45RO (panels A and B). Intracellular staining was performed to identify cells producing IL-17A (panels C and D) and for IFN-γ and TNFα (panel E and F). Data were analyzed using an unpaired T test with Bonferroni correction for multiple comparisons.
DISCUSSION
The major finding of the current study is that the hypertensive stimulus of ang II promotes accumulation of human T cells in the kidney, aorta and lymph nodes of humanized mice. These cells exhibit an increase in the memory cell marker CD45RO and to a lesser extent the activation marker CD69. In addition, CD3− CD45+ cells, which represent other myeloid cells, are increased in lymph nodes by ang II infusion. There also appears to be differentiation of human monocytes to activated DCs in hypertensive mice. Finally, we demonstrate that circulating T cells of humans with hypertension exhibit evidence of activation, as indicated by an increased percent of memory T cells and an increase in production of IL-17A and IFN-γ.
While substantial data support the role of T cells in the genesis of experimental hypertension, there is a paucity of data showing that these cells are activated as a result of human hypertension. Many years ago, Olsen noted periarteriolar infiltration of immune cells in humans with hypertension, and pointed out that these appeared to be lymphocytes and monocytes.16 We found that blood levels of IL-17A are three-times higher in diabetic humans with hypertension as compared to those without hypertension.6 Herrera et al showed that the T cell suppressing agent mycophenolate mofetil lowers blood pressure in humans with rheumatoid arthritis or psoriasis.17 Recently, Yoshida et al demonstrated that the TNF-α antagonist infliximab lowers blood pressure in patients with rheumatoid arthritis.18 Of note, the incidence of hypertension is increased in humans with such autoimmune diseases.19, 20 Youn et al showed that humans with hypertension have an increased number of circulating immunosenescent pro-inflammatory CD8+ T cells compared to age-matched non-hypertensive subjects.21 These cells produced increased amounts of IFN-γ, TNF-α and the cytotoxic molecules granzyme B and perforin than CD8+ T cells from normal subjects. Immunohistochemical studies of renal biopsies revealed an increase in both CD4+ and CD8+ T cells in humans with hypertensive nephrosclerosis compared to controls. These authors also observed an increase of CXCR3 cytokines in the sera of hypertensive patients.
Our current studies of circulating T cells from humans with hypertension confirm the findings of Youn et al that CD8+ T cells produce large amounts of TNFα and IFN-γ. We also found that CD4+ T cells of hypertensive humans produce increased amounts of IL-17A and IFN-γ. The increased number of TH17 cells is compatible with our prior finding that humans with hypertension and diabetes have higher circulating levels of IL-17A than patients with diabetes alone.6 In the present study, we also found an increase in the percent of circulation memory CD4+ and CD8+ T cells. We recently showed that memory T cells are major sources of IFN-γ and IL-17A in mice with hypertension, and that these cells seem to prime hypertensive responses to repeated challenges with high salt or ang II.22 Thus, the finding of increased circulating memory cells might have pathophysiological significance for sustaining hypertension in humans.
The accumulation of human T cells in the kidney of humanized mice is similar to the accumulation of renal T cells observed by Youn et al and parallels similar increases that we and others have observed in mice with experimental hypertension.21, 23, 24 It is likely that cytokines released from T cells in the kidney promote sodium and volume retention and lead to renal damage. We recently found that IFN-γ modulates phosphorylation and activation of the Na-K-2Cl cotransporter, Na-Cl cotransporter, and Ste20/SPS-1-related proline-alanine-rich kinase in response to long-term ang II infusion.25 In this study, we established an important role of IL-17A and IFN-γ in modulating expression of Na/H-exchanger isoform 3 and the motor myosin VI in the proximal tubule and in the anti-natriuretic and anti-diuretic effects of ang II. The propensity of human T cells to accumulate in the kidney supports the notion that these cells might contribute to human hypertension in a similar fashion.
It is also of interest that we observed a striking increase in human T cells in the thoracic lymph nodes of the humanized mice in response to ang II infusion. This was paralleled by an increase in cells bearing the memory cell marker CD45RO. Memory cells persist after an initial expansion of a naïve pool, and it is therefore logical to conclude that the increase in total T cells and memory T cells in the lymph nodes was due to proliferation and expansion in response to ang II infusion not observed in the sham infused mice. We cannot exclude the role of chemokines and adhesion molecules that might also have been increased by ang II in the lymph nodes and kidneys of the humanized mice that could have attracted the human cells to these organs. It is notable that while prevention of hypertension using hydralazine and hydrochlorothiazide reduced the total number of T cells in the lymph nodes, the number of memory T cells in the lymph nodes increased. This might reflect differences in the effect of hypertension as well as direct effects of angiotensin II on trafficking of memory cells in secondary lymphoid organs.
Using strict flow minus one gating criteria, we also observed an increase in CD3+ cells lacking either CD4 or CD8 surface markers, particularly in the lymph nodes. We have observed a similar increase in double negative murine T cells in hypertensive mice. It is conceivable that these double negative T cells are actually CD4+ cells that have lost surface CD4, as has been observed during intense T cell stimulation with phorbol esters or myxoma virus.26, 27
We have previously proposed that mild elevations in blood pressure, such as observed in pre-hypertension, lead to formation of neoantigens that activate T cells which exacerbate vascular remodeling and renal dysfunction, leading to further elevations of blood pressure.28 Our current finding that preventing the initial elevation in blood pressure prevents human T cell activation is in keeping with this concept. Our results also indicate that ang II does not directly activate human T cells. We and others have previously shown that T cells contribute to salt-sensitive hypertension in rats and mice.1, 29 These conditions are associated with suppression of ang II production, indicating that this octapeptide is not needed for this action of immune cells in hypertension. Our results with pharmacological prevention of hypertension should be viewed with some caution however because pharmacologic agents can have off target effects that might directly inhibit T cell function. As an example, we previously showed that hydralazine potently inhibits the NADPH oxidase,30 and thus might prevent formation of immunogenic isoketal-protein adducts independent of its blood pressure lowering effects. To avoid the confounding effects of such agents, we examined the direct effect of ang II on both human T cells and human DCs however failed to observe a change in the production of inflammatory cytokines or the T regulatory cell transcription factor FoxP3. In preliminary experiments, we also found that norepinephrine did not enhance the ability of DCs to drive T cell proliferation.
Taken together, we view these findings as suggesting that human immune cells likely take cues from other tissues in vivo to become activated in hypertension. In keeping with this, we recently found chronic vascular oxidative stress induces activation of both DCs and T cells in mice, likely by generating isoketals within the vessel that are taken up by DCs.31 It is possible therefore that vascular oxidative stress associated with hypertension promotes human T cell activation.
We encountered several limitations in the present study that precluded in-depth study of these mice. We found that these mice did not tolerate the two surgeries needed for implantation of telemetry units for blood pressure measurement and subsequent osmotic minipump placement, and therefore relied upon tail cuff measurement of blood pressure. Our studies should also be interpreted with caution because it is unclear how effectively human T cell receptors interact with mouse adhesion molecules and chemokines. It is possible, for example, that vascular adhesion molecules in humans might more avidly attract activated T cells to vessels than we observed in the humanized mouse. The humanized mouse also presents a limited window of time, ranging from 4 to 12 weeks after reconstitution with human tissues before graft vs. host disease develops, and therefore longer-term hypertensive challenges are problematic. In our studies, none of the mice exhibited signs of graft vs. host disease, such as weight loss, diarrhea or hair loss. Because human T cell expansion did not occur in the sham infused animals, we do not believe that the T cell activation observed in the ang II-treated group was due to graft vs. host disease.
Supplementary Material
PERSPECTIVES.
This is the first study to demonstrate that human T cells respond to a hypertensive stimulus such as ang II by expanding and infiltrating the kidney and vasculature. Our results might also help explain the striking benefit of blood pressure correction observed in the recent SPRINT trial.32 It is conceivable that intensive blood pressure lowering by any means can prevent activation of human immune cells and therefore reduce cardiovascular inflammation.
NOVELTY AND SIGNIFICANCE.
1. What Is New?
Ang II-induced hypertension leads to T cell activation in humanized mice.
Humans with hypertension have an increase in the frequency of circulating memory T cells and an increase in the CD4+ T cells producing IL-17A and CD8+ T cells producing IFN-γ.
2. What Is Relevant?
Animal models have been used as surrogates to study hypertension in humans.
Inflammation is a key component in experimental hypertension, and the current study shows that human cells are activated in the setting of Ang II-induced hypertension and in humans with hypertension.
Correction of blood pressure prevented T cell accumulation in the kidney, aorta and lymph nodes of humanized mice.
3. Summary>
Inflammation has been shown to play a key role in the promotion and maintenance of hypertension in experimental animals and has been indirectly implicated in human hypertension. We provide the first evidence that human T cells are activated by a hypertensive stimulus like angiotensin II.
Phenotypic characterization of circulating T cells in humans might provide insight into immune activation in hypertension.
Acknowledgments
SOURCES OF FUNDING
This work was supported by a postdoctoral fellowship from the American Heart Association (13POST14440041), AHA Strategically Focused Research Network Grants 14SFRN20420046 and 14SFRN20770001 and NIH grants R01HL039006, P01HL058000, P01HL095070, P01GM015431, R01HL110353, R01HL108701, K08HL121671, K08DK090146, R01HL105294, T32 HL69765, F32 HL124972 and the Foundation for Polish Science Welcome (FNP/2009/Welcome02; TM, TJG) and Polish Ministry of Science (Diamentowy Grant; to AK, AJ and MobilnoscPlus to TM), Polish National Science Centre (Agreement 2011/03/B/NZ4/02454) and International Senior Research Fellowship from the Wellcome Trust (TJG).
Footnotes
Conflict (s) of interest/disclosure (s) statement: The authors have no conflict of interests.
LITERATURE CITED
- 1.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the t cell in the genesis of angiotensin ii induced hypertension and vascular dysfunction. J Exp Med. 2007:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of t-lymphocyte activation and vascular inflammation produced by angiotensin ii-induced hypertension. Circ Res. 2010:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marvar PJ, Vinh A, Thabet S, Lob HE, Geem D, Ressler KJ, Harrison DG. T lymphocytes and vascular inflammation contribute to stress-dependent hypertension. Biol Psychiatry. 2012:774–782. doi: 10.1016/j.biopsych.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin ii-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010:R1089–R1097. doi: 10.1152/ajpregu.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the dahl salt-sensitive rat. Hypertension. 2006:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 6.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin ii-induced hypertension and vascular dysfunction. Hypertension. 2010:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marko L, Kvakan H, Park JK, Qadri F, Spallek B, Binger KJ, Bowman EP, Kleinewietfeld M, Fokuhl V, Dechend R, Muller DN. Interferon-gamma signaling inhibition ameliorates angiotensin ii-induced cardiac damage. Hypertension. 2012:1430–1436. doi: 10.1161/HYPERTENSIONAHA.112.199265. [DOI] [PubMed] [Google Scholar]
- 8.Singh P, Bahrami L, Castillo A, Majid DS. Tnf-alpha type 2 receptor mediates renal inflammatory response to chronic angiotensin ii administration with high salt intake in mice. Am J Physiol Renal Physiol. 2013:F991–F999. doi: 10.1152/ajprenal.00525.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and cd34+ cell transplantation. Blood. 2006:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in nod/scid/il2 receptor {gamma} chain(null) mice. Blood. 2005:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, Harrison DG. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res. 2014:616–625. doi: 10.1161/CIRCRESAHA.114.302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saleh MA, McMaster WG, Wu J, et al. Lymphocyte adaptor protein lnk deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest. 2015:1189–1202. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgarth N, Roederer M. A practical approach to multicolor flow cytometry for immunophenotyping. Journal of immunological methods. 2000:77–97. doi: 10.1016/s0022-1759(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 14.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the b7/cd28 t-cell costimulation axis prevents experimental hypertension. Circulation. 2010:2529–2537. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of t-cell function by endogenously produced angiotensin ii. Am J Physiol Regul Integr Comp Physiol. 2009:R208–R216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen F. Inflammatory cellular reaction in hypertensive vascular disease in man. Acta Pathol Microbiol Scand A. 1972:253–256. [PubMed] [Google Scholar]
- 17.Herrera J, Ferrebuz A, Macgregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006:S218–S225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida S, Takeuchi T, Kotani T, Yamamoto N, Hata K, Nagai K, Shoda T, Takai S, Makino S, Hanafusa T. Infliximab, a tnf-alpha inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J Hum Hypertens. 2014:165–169. doi: 10.1038/jhh.2013.80. [DOI] [PubMed] [Google Scholar]
- 19.Miller IM, Ellervik C, Yazdanyar S, Jemec GB. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. Journal of the American Academy of Dermatology. 2013:1014–1024. doi: 10.1016/j.jaad.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 20.Panoulas VF, Metsios GS, Pace AV, John H, Treharne GJ, Banks MJ, Kitas GD. Hypertension in rheumatoid arthritis. Rheumatology (Oxford) 2008:1286–1298. doi: 10.1093/rheumatology/ken159. [DOI] [PubMed] [Google Scholar]
- 21.Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, Choi YS, Lee SH, Kang SM, Jang Y, Yoo OJ, Shin EC, Park S. Immunosenescent cd8+ t cells and c-x-c chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013:126–133. doi: 10.1161/HYPERTENSIONAHA.113.00689. [DOI] [PubMed] [Google Scholar]
- 22.Itani HA, Xiao L, Saleh MA, et al. Cd70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ Res. 2016:1233–1243. doi: 10.1161/CIRCRESAHA.115.308111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirabo A, Fontana V, de Faria AP, et al. Dc isoketal-modified proteins activate t cells and promote hypertension. J Clin Invest. 2014:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG. Oligoclonal cd8+ t cells play a critical role in the development of hypertension. Hypertension. 2014:1108–1115. doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA. Renal transporter activation during angiotensin-ii hypertension is blunted in interferon-gamma−/− and interleukin-17a−/− mice. Hypertension. 2015:569–576. doi: 10.1161/HYPERTENSIONAHA.114.04975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruegg CL, Rajasekar S, Stein BS, Engleman EG. Degradation of cd4 following phorbol-induced internalization in human t lymphocytes. Evidence for distinct endocytic routing of cd4 and cd3. J Biol Chem. 1992:18837–18843. [PubMed] [Google Scholar]
- 27.Barry M, Lee SF, Boshkov L, McFadden G. Myxoma virus induces extensive cd4 downregulation and dissociation of p56lck in infected rabbit cd4+ t lymphocytes. J Virol. 1995:5243–5251. doi: 10.1128/jvi.69.9.5243-5251.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol. 2013:R407–R414. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munzel T, Kurz S, Rajagopalan S, Thoenes M, Berrington WR, Thompson JA, Freeman BA, Harrison DG. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound nadh oxidase. A new action for an old drug. J Clin Invest. 1996:1465–1470. doi: 10.1172/JCI118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J, Saleh MA, Kirabo A, et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016:50–67. doi: 10.1172/JCI80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright JT, Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.