Abstract
Flavonoids are becoming popular nutraceuticals. Different flavonoids show similar or distinct biological effects on different tissues or cell types, which may limit or define their usefulness in cancer prevention and/or treatment application. This review focuses on a few selected flavonoids and discusses their functions in normal and transformed pigment cells, including cyanidin, apigenin, genistein, fisetin, EGCG, luteolin, baicalein, quercetin and kaempferol. Flavonoids exhibit melanogenic or anti-melanogenic effects mainly via transcriptional factor MiTF and/or the melanogenesis enzymes tyrosinase, DCT2 or TYRP-1. To identify a direct target has been a challenge as most studies were not able to discriminate whether the effect(s) of the flavonoid were from direct targeting or represented indirect effects. Flavonoids exhibit an anti-melanoma effect via inhibiting cell proliferation and invasion and inducing apoptosis. The mechanisms are also multi-fold, via ROS-scavenging, immune-modulation, cell cycle regulation and epigenetic modification including DNA methylation and histone deacetylation. In summary, although many flavonoid compounds are extremely promising nutraceuticals, their detailed molecular mechanism and their multi-target (simultaneously targeting multiple molecules) nature warrant further investigation before advancement to translation studies or clinical trials.
Keywords: Flavonoids, Luteolin, melanoma, melanogenesis, prevention
1. Introduction
Flavonoids are a large group of polyphenolic compounds found in a wide range of vegetables and medicinal herbs; so far more than 5000 compounds have been identified [1]. These compounds exhibit a broad range of anti-tumor, anti-allergic, anti-inflammation, anti-fungal and anti-viral functions and have attracted much attention in the chemoprevention and cancer treatment fields. Because they are easily available nutrients in regular diets and as they have exhibited high pharmaceutic potential in preclinical studies, many of these compounds have become popular as nutraceuticals. There have been many publications on flavonoids and their potential roles in the management of cancer; however, information about these compounds vis-a-vis the pathogenesis of melanoma have been limited [2]. As flavonoids show dramatic cell line and tissue specificities [3, 4], it is incumbent that we examine what has been done to date both mechanistically and preclinically before proceeding to translational studies of melanoma.
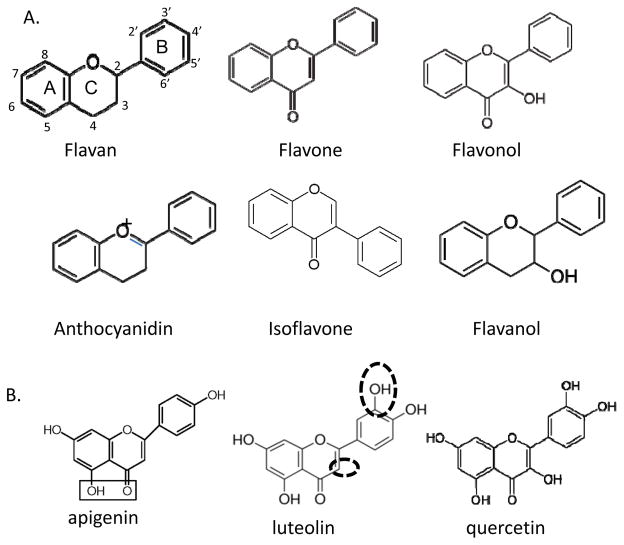
Flavonoids include several major groups of compounds with the shared core backbone structure of flavan: flavones, flavonols (3-hydroxyflavone), flavanols, isoflavones and anthocyanidins (Fig. 1A), all with different side group modifications [5, 6] . The main structure of flavan is comprised of three rings: A, B, and C rings (Fig 1A). The above major groups of flavonoids differ on their modification of side groups on these rings. These side groups play crucial roles in the function of these compounds as the side modification can produce very different activities. Table 1 lists the most commonly used flavonoids that have been tested in melanoma models and their major dietary sources. The most informative data have been derived from these compounds therefore this review will focus on these listed compounds and their possible molecular mechanisms of action in the pathogenesis of melanoma. However this is a limited list as there are many more flavonoids that have been studied in melanocytic cell lineage, including rutin, robinetin, rhamnetin, naringin, chrysin, ipriflavone, tangeritin and more, and some derivatives of these compounds [7–9].
Figure 1. Skeleton structures of major flavonoids and luteolin, apigenin and quercetin.
A: Six main classes of flavonoids are listed, which are the focus of this review. Locations of three rings (A, B and C) are labelled on flavan which are the same for other compounds. B: Structure comparison of luteolin, apigenin and quercetin. The different side groups are circled in luteolin structure. The boxed side groups in apigenin show a typical structure that is able to bind metal ions. Comparing these three popular compounds which sometimes show opposite effects on melanogenesis may provide some hints on how each side group functions biologically.
Table 1.
An Overview of Flavonoids: Sources and Functions
| Category | Major Dietary Sources | Compounds | Pigment Synthesis | Reference | Reference cell line | Function in melanoma prevention/treatment | Reference |
|---|---|---|---|---|---|---|---|
| Anthocyanidin | Berries, grapes | Cyanidin | Increase | [103] | Human | Anti-proliferation | [123] |
| Flavanol | Chocolate, cherry, green tea | EGCG/Epicatechin | Inhibition | [19, 20] | Anti-metastasis, anti- proliferation, apoptosis, immunomodulation | [95, 124, 125] | |
| Flavanone | Citrus fruits | Hesperetin | Increase | [21, 126] | Human, Mouse | No known effect | [8] |
| Hesperidin | Inhibition | [18] | Human, Mouse | No report | |||
| Flavone | Celery, parsley | Apigenin | Increase | [27, 127] | Mouse | Anti-proliferation, anti-metastasis | [98] |
| Luteolin | Inhibition | [23] | Mouse | Anti-proliferation, anti-metastasis | [128, 129] | ||
| Baikal skullcap (medicinal herb) | Baicalein | Inhibition | [22] | Mouse | Anti-proliferation | [48, 130] | |
| Flavonol | Onions, apples, Kale, Leek, broccoli Strawberry |
Quercetin | Increase | [25] | Human | [98] | |
| Increase | [131] | Mouse | Anti-proliferation, anti-metastasis | ||||
| Inhibition | [132] | Mouse | |||||
| Fisetin | Increase | [7, 133] | Human Mouse | Anti-proliferation, anti-metastasis | [107, 108,134] | ||
| Kaempferol | Inhibition | [26] | Mouse | Anti-proliferation (G2/M arrest) | [102] | ||
| Isoflavone | Soybean | Daidzein | No effect | [24] | Mouse, Human | Anti-proliferation, anti-metastasis | [24, 135] |
| Genistein | Increase | [24] | Mouse, Human | Anti-proliferation | [135, 136] |
Cutaneous melanomas arise from skin melanocytes, a cell type that is specialized in synthesizing melanin which contributes to skin color and protection against solar UV (ultra-violet) radiation. Skin color is an important part of beautification [10]; for example, a tan color has become desirable for white skinned individuals (Caucasians) while a lighter color has become more desirable for darker-skinned Asian individuals, especially women. Therefore the skin care industry has been seeking various methods to safely manipulate skin color. As a consequence, there are many studies using flavonoids as skin-whitening agents [11], as listed in Table 1. This review attempts to summarize the known effect of flavonoids in melanogenesis and melanomagenesis and their potential molecular mechanisms. As is revealed in this short review, it is apparent that majority of the pigmentation and anti-melanoma studies have been performed in vitro and/or in B16 mouse melanoma cell lines, indicating that in vivo studies and studies with human cells are still needed to enable clinic use of these compounds.
2. Flavonoids function in melanogenesis
As listed in Table 1, cyanidin, hesperetin, apigenin, genistein and fisetin all exhibited melanogenic effect, i.e., stimulated melanin synthesis. On the other hand, EGCG or other catechins, hesperidin, luteolin, baicalein and kaempferol all inhibited melanin synthesis. For quercetin, two studies showed stimulatory effect and one showed an inhibitory effect. We have listed cell lines (mouse or human) used in each study because the regulation of melanin syntheses may be different in human and mouse normal and malignant cells by these compounds. Indeed, caution when evaluating these compounds across species types needs to be the order of the day.
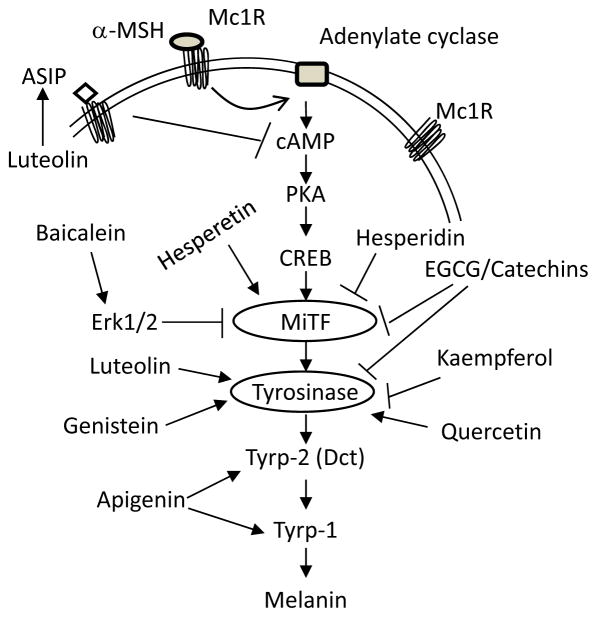
Pigmentation is a very complex biochemical process involving more than 300 loci in mice, according to International Federation of Pigment Research Society website (http://www.espcr.org/micemut/). Most of these loci have corresponding orthologues with human genes. Figure 2 lists the major pathway showing a few key genes in this process. Mainly, upon stimulation by the α-melanocytes stimulating hormone (α-MSH), MC1R (Melanocortin Receptor 1) transmits a signal to cAMP-PKA (cyclic AMP and Protein Kinase A) [12] , whose activation leads to enhanced expression of the melanocytes master transcriptional factor MITF (Microphthalmia Transcription Factor) which in turn activates expression of the major melanogenic enzymes tyrosinase, dopachrome tautomerase (DTC, also known as tyrosine-related protein 2, TYRP-2) and tyrosine-related protein 1 (TYRP-1) via binding to E boxes on their promoters [13–16]. Agouti signaling protein (ASIP) antagonizes the functions of α-MSH and inhibits the melanin synthesis pathway (Fig. 2) [17]. This schema is oversimplified as melanins exist as two classes: eumelanin and pheomelanin and their synthesis share some regulatory features but differ in others. Most of melanogenesis effects of flavonoids have been targeted to this simplified scheme. As shown in Fig. 2, hesperidin and catechins (including EGCG) inhibited MITF protein accumulation [18, 19]; EGCG in addition inhibited tyrosinase accumulation [19, 20]. Hesperetin which stimulated melanogensis, on the other hand, enhanced MITF accumulation; the upstream signal was not investigated [21]. Baicalein, a depigmenting agent, inhibited MITF accumulation via ERK1/2- phosphorylation mediated degradation [22]. Luteolin, genistein, kaempferol and quercetin all targeted tyrosinase directly or indirectly [23–26]. Apigenin did not target tyrosinase, rather it targeted TYRP-2/DCT and TYRP-1, perhaps via p38 mitogen activating protein kinase [27].
Figure 2. Molecular mechanisms of flavonoids on melanin synthesis.
The current understanding of melanin synthesis follows the cAMP-PKA-MITF-tyrosinase scheme, in which MITF serves as the master transcriptional factor activating tyrosinase, DCT and TYRP-1, and receives signals from MC1R. Inhibitory or stimulatory effects of each compound are listed in the scheme with references discussed in the text.
Note that even though luteolin increased tyrosinase protein accumulation in B16 melanoma cells, this compound in the end inhibited melanin synthesis and several studies suggest that it is a skin-whitening agent [23, 28]. This may be because luteolin actually inhibited tyrosinase activity and the upstream α-MSH mediated cAMP signaling [28]. Results from our lab have revealed that luteolin dramatically up-regulated ASIP (Agouti-signaling protein) at mRNA level (17.0 fold of increase as compared to untreated control cells) in human A375 melanoma cells (data not shown). This action may reflect a novel layer of regulation by luteolin for the melanogenesis pathway, but will require extensive studies to validate. As shown in Figure 2, ASIP binds to MC1R and inhibits α-MSH-mediated cAMP/PKA activation and hence inhibits downstream melanin synthesis [17] , ASIP polymorphisms are associated with human pigmentation phenotypes and melanoma risk [29, 30]. To date, ASIP is not known to regulate pigmentation via an autocrine route; a previous study showed ASIP expression at the mRNA and protein level in melanoma cell lines [31], which is consistent with our unpublished results, suggesting this protein may have the potential to exhibit autocrine function.
Of importance is that although some compounds have similar structures, they show drastic differences on melanogenesis regulation. For example, in comparing apigenin and luteolin, there is only one extra hydroxyl group in luteolin (Fig. 1B), yet, apigenin stimulated, while luteolin inhibited, melanin synthesis. It is speculated that the extra hydroxyl group in luteolin played a crucial role in determining some specificities of this compound. Also, compared to luteolin, quercetin has an extra hydroxyl group on the C ring, which apparently also results in different cellular functions (Fig. 1B and Table 1). This differential function of structurally similar flavonoids is not only observed in the melanogensis pathway, it has also been observed in cardiovascular and cancer-related pathways as well [32].
3. Flavonoids function in melanoma prevention. treatment and metastasis prevention
Flavonoids have been widely used as experimental chemoprevention and chemotherapy agents in many different cancer types including breast, prostate, pancreas, bladder, lung and colon cancer [33–35]. Epidemiological studies show that estimated dietary intake of total flavonoids (most of the time it is not specified) is usually (but not always) inversely correlated with cancer risk [36–38]. Carefully designed cancer prevention trials (including melanoma) are currently lacking. Below we summarize the potential molecular mechanisms of flavonoids and their activities in anti-oxidant, anti-inflammation and immune modulation, anti-proliferation, anti-angiogenesis, apoptosis induction and potential epigenetic modification, with most studies executed in vitro, a few in mouse; and some were epidemiological observations.
3.1 Flavonoids as reactive oxygen species (ROS) scavenger for melanoma
Numerous studies have showed that many flavonoids are potent antioxidants, therefore they may serve as effective scavengers of reactive oxygen species [39, 40]. Because excessive ROS cause many problems including DNA, lipid and protein damage and aberrant cellular signaling, flavonoids are apparently protective agents in such conditions.
A number of in vitro assays have been developed to measure the radical scavenger activities in vitro, including 2,2-diphenyl-1-picrylhydrazyl radical (DPPH assay), ferric reducing antioxidant power (FRAP assay), 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulphonate) radical cation (TEAC assay), 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulphonate) radical (ABTS(·+) assay) (ABTS assay), Folin-Ciocalteu reducing capacity (FC assay),electrochemical total reducing capacity, and hypoxanthine/xanthine oxidase system coupled with nitroblue tetrazolium (NBT) reduction (NBT/XO) [28, 41, 42]. All flavonoids listed in Table 1 showed some degrees of free radical scavenger activities, with luteolin and quercetin among the most potent antioxidants in the category [4, 43]. For example, luteolin showed dose-dependent antioxidant activity in DPPH and NBT/XO assays in a cell-free system, as well as in B16 cells by H2DCF-DA (dihydrodichlorofluorescein diacetyl)-based intracellular ROS assays [28].
The detailed molecular mechanisms of flavonoids remain to be clarified but can be summarized into three major categories:
As chelators for redox-potent transition metal ions, which include Cd2+ Fe2+, Co2+, Ni2+, Cu2+, Cr3+ and Zn2+ [44, 45]. These metals cause an ROS increase via different mechanisms and some are potent carcinogens. The metal binding sites for flavonoids are usually adjacent hydroxyl and/or ketone side groups. For example, the potential metal binding site for apigenin is between the 5-OH group of A ring and the ketone group on C ring (Fig 1B, boxed).
Reacting directly with free radicals via their free hydroxyl group(s) and quench these activities [42]. For example, quercetin scavenges superoxide free radicals mainly function through 3’4’-dihydroxy groups on the B ring [46].
Modulating multiple cellular anti-oxidant systems which re-establish redox balance in cells after oxidative stress.
These functions are not mutually exclusive. In a previous review we summarized the source of ROS in melanoma [47], including mitochondria, NADPH oxidases, nitric oxidases, lipoxygenase, cyclooxygenase 2 (COX-2) and melanosomes. These ROS sources are regulated by major redox transcriptional regulators NRF2 (nuclear factor erythroid 2 [NF-E2]-related factor 2), and the AP-1 family members [48–50], among other factors [51]. NRF2 is an important target for flavonoids as it is also the master transcriptional factor for redox regulation [52]. Luteolin was initially found to be a NRF2 inhibitor in lung carcinoma A549 cells [53]; however, in colorectal and prostate cancers and in neuronal cells luteolin activated NRF2 [54–56]. This compound also enhanced NRF2 translocation into the nucleus where it functions as a transcriptional activator in neuronal cells [57]. Furthermore, luteolin inhibited Cr(VI)-induced malignant cell transformation of human lung epithelial cells by targeting multiple ROS mediated cell signaling pathways [58]. Luteolin inhibited NRF2 target glutathione S-transferase in SK-Mel-28 human melanoma cells [59]; we found that luteolin inhibited NRF2 protein accumulation at 30 μM but stimulated NRF2 accumulation at 8 μM in SK-Mel-28 cells (Liu-Smith et al., unpublished data). These studies suggest that luteolin exhibits different effects on the same target gene in different cell lines, or even opposite effects on the same target at different concentrations. On the other hand, apigenin showed more consistent effects in different cell lines or in different studies: apigenin stimulates NRF2 activities in prostate cancer, mouse skin epidermal JB6 P+ cells, hepatocellular carcinoma HEPG2-C8 cells and primary hepatocytes [56, 60–63], via MAPK pathway, epigenetic modification of NRF2 promoter, or PI3K pathway. Quercetin shows similar NRF2-enhancing effect as apigenin, with the end results of activating NRF2-regulated antioxidant genes including heme-oxygenase 1, NAD(P)H Dehydrogenase, Quinone 1(NQO1) and genes for glutathione synthesis [64–67]. Genistein and EGCG also induced NRF2 in different cellular background for invoking a protective antioxidant mechanism [68–70]. For other targets, baicalein enhanced Cox-2 expression [71], but luteolin, apigenin, genistein suppressed its expression or function [72–75]. Luteolin, quercetin and apigenin also exhibit AP-1 inhibitory effects [76–78].
Mitochondria and ROS-generating enzymes can also be targets for flavonoids; however, published data show that flavonoids serve either as ROS scavengers or ROS stimulators. In A375 cells apigenin directly targeted and compromised the oxidative phosphorylation system in mitochondria and induced ROS levels which led to cell death [79]. Similarly, baicalein also induced ROS in B16 cells, possibly via12-lipoxygenase [48]; and quercetin increased ROS levels in DB-1 melanoma cells via inhibiting bio-reduction capacity, namely the glutathione-S transferase and NQO1 levels [80]. On the other hand, luteolin directly inhibited xanthine oxidase activity in a dose-dependent manner and reduced cellular ROS levels in B16 cells [28]. As all antioxidants have the potential to be converted into pro-oxidants, it is not surprising to see these conflicting results. Our own experiments with luteolin showed dose-dependent differential stimulating and inhibitory results on NRF2 (described above) accumulation in the same cell line, we speculate that some of the flavonoids may require a specific dose range to act as antioxidants, or else they may stimulate ROS production. Despite much evidence that flavonoids serve as ROS scavengers, the antioxidant property is not the only mechanism for their protective roles for human cells [81]. Next we will discuss their other cellular roles.
3.2 Flavonoids function in anti-inflammation and immune-modulation in melanoma
There is strong evidence that inflammation and immune suppression play important roles in melanoma etiology, progression, and even prognosis: 1) the major environmental risk factor Ultra-Violet (UV) radiation causes skin immune suppression [82] , 2) melanoma tumors contain large amount of infiltrated immune cells [83] and 3) BRAF inhibitor-mediated immunosuppression is a reason for therapeutic failure [84]. Also inflammation exhibits an intrinsic correlation with oxidative stress which is highly elevated in melanoma [85]. For all these aspects, the anti-inflammatory properties of flavonoids in preclinical have shown a potentially important impact on melanoma etiology, prevention, and treatment outcomes. Briefly, flavonoids modulate inflammatory effects through a few key mediators in melanoma and skin tissues: AP-1 family transcriptional factors [86], NFκB [87], STAT3 [88] and nitric oxidases (mainly iNOS and nNOS) [89, 90].. AP-1 and NFκB are able to up-regulate cytokine expression such as IL-8 [91, 92]; as stated above, AP-1 can be inhibited by luteolin, quercetin and apigenin [76–78]. Luteolin was shown to promote proteasome-mediated degradation of STAT3 and thus blocked the inflammatory signals from cytokines such as IL6 and IL10 [93]. Quercetin, on the other hand impaired STAT3 nuclear localization via altering its phosphorylation [94]. EGCG prevents UV-induced immunosuppression via a mechanism that involves production of IL-12. In IL-12 knockout mice or mice injected with anti-IL-12 antibodies, EGCG lost its ability to inhibit UV-induced immune -suppression [95]. Nitric oxide (NO) plays an important role in the melanoma inflammatory microenvironment which promotes tumor growth and metastasis; iNOS-expression in melanoma is negatively correlated with patient survival [96]; and nNOS is up-regulated in melanoma and is a potential target for melanoma therapy [90]. Paracrine NO production led to decreased CXC chemokine ligand 10 (CXCL10) levels which resulted in less inflammatory tumor microenvironment in melanoma patients and WM1727A, A375 and SB2 melanoma cell lines [96]. Flavonoids exhibit complex reactions with NO. In cell free system flavonoids have NO-scavenger activity but may generate superoxide at the same time [97]; under oxidative stress flavonoids may play an anti-inflammation role via inhibiting iNOS or inhibiting the NFκB pathway [97]. Thus it is likely that the impact of flavonoids on NO levels (perhaps also ROS levels) is dependent on the flavonoid type, concentration and cellular conditions such as expression levels of iNOS.
3.3 Flavonoids anti-proliferative, apoptotic induction and anti-metastatic activities
Flavonoids exhibit anti-proliferative and anti-apoptotic effects via HGF/SF-Met signaling, MAPK pathway, cell cycle regulation, differentiation induction and PI3K-AKT pathway. Like their function in melanogenesis, different flavonoids exhibit different effects on their cellular targets -which are quite diverse.
Flavonoids inhibited xenografted B16-BL6 mouse melanoma growth in the decreasing order of effectiveness: EGCG, apigenin, quercetin, with the latter two compounds showing similar effects as tamoxifen [98]. EGCG inhibited colony formation in soft-agar [99], possibly by inhibiting cyclin D1, CDK2 and PCNA (Proliferating Cell Nuclear Antigen) while inducing p21waf1/cip1 and p27kip1, and promoting apoptosis [100]. EGCG reduced MITF protein accumulation via ERK1/2-independent mechanism [19], which may also contribute to its anti-proliferation effect because MITF is generally a melanoma survival gene [101]. Similarly, apigenin induced G2/M cell cycle arrest via inhibiting CDK1 activity [102], which may contribute to its anti-melanoma activity in vivo on xenografted B16 cells [98]. Cyanidin glucopyranoside induced B16 differentiation via up-regulating cAMP, tyrosinase expression, and the differentiation marker MART-1 [103]. Both EGCG and quercetin inhibited HGF/SF-Met signaling, a key regulator of melanoma migration and invasion [104, 105]. Fisetin inhibited 451Lu cell proliferation via disrupting the β-catenin/MITF signaling pathway [106]; also inhibited melanoma cell invasion and metastasis through inhibiting the epithelial to mesenchymal transition in a three-dimensional skin model and in a xenografted mice model [107, 108]. Combination treatment of xenografted A375 and SK-Mel-28 tumors with fisetin and RAF inhibitor Sorafenib showed greater reduction in tumor growth than single compounds or control mice due to multiple mechanisms, including induction of apoptosis, inhibition of proliferation and angiogenesis, and inhibition of the MAPK and PI3K pathways [109].
Overall, whether flavonoids directly target the affected genes is not clear; what is clear is that all these compounds, more or less, show an anti-proliferation and/or anti-metastatic effect against melanoma (Table 1). Recent development in nanotechnology have made flavonoids much more effective in targeting melanoma cells both in vitro and in vivo [110–112]; therefore in the near future we may witness clinical use of these promising natural compounds. However, whether individual compounds delivered by nanoparticles needs to be assessed as drugs first is an issue that has not yet been addressed by regulatory bodies.
3.4 Flavonoids in epigenetic modification: histone acetylation and DNA methylation
The diverse targets of flavonoids may be directly related to their diverse structures [113]. However, there may be a substantial contribution for the epigenetic modification function of flavonoids. Increasing evidence suggests that many flavonoids are able to regulate gene expression via epigenetic approaches including histone modification, DNA methylation and miRNA/lncRNA (microRNA and long non-coding RNA) [114]. These epigenetic modifications may affect much diversified target genes. Histones can be modified by acetylation and methylation via histone acetyl transferase (HAT), histone deacetylase (HDAC), histone methyl transferase (HMT) and histone demethylase (HDM) [115]. DNA can be modified by methylation via DNA methyl transferases [114]; how DNA is de-methylated is still not clear and is under intensive investigation [116]. The epigenetic modification functions of most flavonoids from Table 1 are listed in Table 2, with most data obtained from cell types other than melanoma. Only limited studies were performed in melanoma cell lines. DNA methyl transferases (DNMTs), HDACs and HAT are common targets of flavonoids in melanoma, and these enzymes affect the expression of tumor suppressors such as p21CIP1 and p16INK4A [117, 118]. In several human melanoma cell lines, green tea polyphenols (mixture of epicatechin monomers) showed significant inhibitory effect on HDAC activities and class I HDAC proteins, and promoted HAT activity, resulting in proliferation inhibition and cell killing [117, 119]. This mechanism may explain a previous observation that EGCG up-regulated p16INK4A, p27KIP1 and p21CIP1 protein levels in A375 and Hs294t melanoma cells [100], as it was well known that these tumor suppressor genes were subjected to epigenetic silencing in melanoma cells [120–122].
Table 2.
Known functions of flavonoids in epigenetics
| Flavonoids | Targets | Reference |
|---|---|---|
| EGCG/Epicatechin | DNMT, HDAC3, HAT, | [137–139] |
| Hesperetin | DNMT | [140] |
| Apigenin | DNMT, HDAC | [140, 141] |
| Luteolin | DNMT | [140] |
| Baicalein | DNMT | [142] |
| Quercetin | DNMT1, HDAC, HAT | [143, 144] |
| Kaempferol | HDAC | [145] |
| Daidzein | DNMT | [140, 146] |
| Genistein | DNMT | [118, 140,146, 147] |
DNMT: DNA methyl Transferase; HDAC: Histone Deacetylase; HAT: Histone acetyl transferase.
4. Conclusions
Flavonoids are widely available from food and herbs, and have the potential to become therapeutic agents with minimum toxicity. However, not many (if any) clinical trials have been done to establish the profile of flavonoids and the toxicity curve at the doses required to prevent or treat cancer in humans, more in vivo studies and human trials are needed to explore their clinical activities. Although it is difficult to pinpoint each compound’s intracellular target, their overall effectiveness should be noted. Lack of specificity may be because they are able to simultaneously target many different genes, but that may be the exact reason for their functional versatility. Also, like other chemical compounds, flavonoids are subjected to metabolism and the metabolites may also be active components in vivo, rendering it even more difficult to identify a single target. Investigators and the public should respect this diversity of action and not to be limited by the “targeted therapy” mantra in the exploration of clinical usefulness of nutraceuticals.
Acknowledgments
FLS is supported by NIH/NCI K07 award (CA160756) and FLM by the Waltmar Foundation.(to FLS and FLM)
Abbreviations
- cAMP-PKA
cyclic AMP-protein kinase A
- DTC2
dopachrome tautomerase
- TYRP-1
tyrosine-related protein 1
- EGCG
epigallocatechin gallate
- MiTF
microphthalmia transcription factor
- NRF2
nuclear factor erythroid 2 [NF-E2]-related factor 2
- ROS
reactive oxygen species
- DNMT
DNA methyl transferases
- NO
nitric oxide
Footnotes
Conflicts of interests
These authors state no conflict of interests.
References
- 1.Testai L. Flavonoids and mitochondrial pharmacology: A new paradigm for cardioprotection. Life Sci. 2015;135:68–76. doi: 10.1016/j.lfs.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Tong LX, Young LC. Nutrition: the future of melanoma prevention? J Am Acad Dermatol. 2014;71(1):151–60. doi: 10.1016/j.jaad.2014.01.910. [DOI] [PubMed] [Google Scholar]
- 3.Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev. 2014;8(16):122–46. doi: 10.4103/0973-7847.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sak K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr Cancer. 2014;66(2):177–93. doi: 10.1080/01635581.2014.864418. [DOI] [PubMed] [Google Scholar]
- 5.Ververidis F, et al. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol J. 2007;2(10):1214–34. doi: 10.1002/biot.200700084. [DOI] [PubMed] [Google Scholar]
- 6.Egert S, Rimbach G. Which sources of flavonoids: complex diets or dietary supplements? Adv Nutr. 2011;2(1):8–14. doi: 10.3945/an.110.000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takekoshi S, Nagata H, Kitatani K. Flavonoids enhance melanogenesis in human melanoma cells. Tokai J Exp Clin Med. 2014;39(3):116–21. [PubMed] [Google Scholar]
- 8.Rodriguez J, et al. Effects of several flavonoids on the growth of B16F10 and SK-MEL-1 melanoma cell lines: relationship between structure and activity. Melanoma Res. 2002;12(2):99–107. doi: 10.1097/00008390-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Martinez Conesa C, et al. Treatment of metastatic melanoma B16F10 by the flavonoids tangeretin, rutin, and diosmin. J Agric Food Chem. 2005;53(17):6791–7. doi: 10.1021/jf058050g. [DOI] [PubMed] [Google Scholar]
- 10.Korac RR, Khambholja KM. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn Rev. 2011;5(10):164–73. doi: 10.4103/0973-7847.91114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan MT. Novel tyrosinase inhibitors from natural resources - their computational studies. Curr Med Chem. 2012;19(14):2262–72. doi: 10.2174/092986712800229041. [DOI] [PubMed] [Google Scholar]
- 12.Swope VB, et al. Defining MC1R regulation in human melanocytes by its agonist alpha-melanocortin and antagonists agouti signaling protein and beta-defensin 3. J Invest Dermatol. 2012;132(9):2255–62. doi: 10.1038/jid.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasumoto K, et al. Microphthalmia-associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase gene. Mol Cell Biol. 1994;14(12):8058–70. doi: 10.1128/mcb.14.12.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bentley NJ, Eisen T, Goding CR. Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator. Mol Cell Biol. 1994;14(12):7996–8006. doi: 10.1128/mcb.14.12.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang D, Tsuji Y, Setaluri V. Selective down-regulation of tyrosinase family gene TYRP1 by inhibition of the activity of melanocyte transcription factor, MITF. Nucleic Acids Res. 2002;30(14):3096–106. doi: 10.1093/nar/gkf424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou L, Panthier JJ, Arnheiter H. Signaling and transcriptional regulation in the neural crest-derived melanocyte lineage: interactions between KIT and MITF. Development. 2000;127(24):5379–89. doi: 10.1242/dev.127.24.5379. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki I, et al. Agouti signaling protein inhibits melanogenesis and the response of human melanocytes to alpha-melanotropin. J Invest Dermatol. 1997;108(6):838–42. doi: 10.1111/1523-1747.ep12292572. [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ, et al. Hesperidin, A Popular Antioxidant Inhibits Melanogenesis via Erk1/2 Mediated MITF Degradation. Int J Mol Sci. 2015;16(8):18384–95. doi: 10.3390/ijms160818384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DS, et al. (-)-Epigallocatechin-3-gallate and hinokitiol reduce melanin synthesis via decreased MITF production. Arch Pharm Res. 2004;27(3):334–9. doi: 10.1007/BF02980069. [DOI] [PubMed] [Google Scholar]
- 20.Sato K, Toriyama M. Depigmenting effect of catechins. Molecules. 2009;14(11):4425–32. doi: 10.3390/molecules14114425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YC, Liu KC, Chiou YL. Melanogenesis of murine melanoma cells induced by hesperetin, a Citrus hydrolysate-derived flavonoid. Food Chem Toxicol. 2012;50(3–4):653–9. doi: 10.1016/j.fct.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Li X, et al. Baicalein inhibits melanogenesis through activation of the ERK signaling pathway. Int J Mol Med. 2010;25(6):923–7. doi: 10.3892/ijmm_00000423. [DOI] [PubMed] [Google Scholar]
- 23.An SM, et al. Flavonoids, taxifolin and luteolin attenuate cellular melanogenesis despite increasing tyrosinase protein levels. Phytother Res. 2008;22(9):1200–7. doi: 10.1002/ptr.2435. [DOI] [PubMed] [Google Scholar]
- 24.Wang HZ, et al. Effects of genistein and daidzein on the cell growth, cell cycle, and differentiation of human and murine melanoma cells(1) J Nutr Biochem. 2002;13(7):421–426. doi: 10.1016/s0955-2863(02)00184-5. [DOI] [PubMed] [Google Scholar]
- 25.Nagata H, et al. Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and in normal human melanocytes. Pigment Cell Res. 2004;17(1):66–73. doi: 10.1046/j.1600-0749.2003.00113.x. [DOI] [PubMed] [Google Scholar]
- 26.Rho HS, et al. Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules. 2011;16(4):3338–44. doi: 10.3390/molecules16043338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye Y, et al. Flavonoids, apigenin and icariin exert potent melanogenic activities in murine B16 melanoma cells. Phytomedicine. 2010;18(1):32–5. doi: 10.1016/j.phymed.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Choi MY, et al. Whitening activity of luteolin related to the inhibition of cAMP pathway in alpha-MSH-stimulated B16 melanoma cells. Arch Pharm Res. 2008;31(9):1166–71. doi: 10.1007/s12272-001-1284-4. [DOI] [PubMed] [Google Scholar]
- 29.Kanetsky PA, et al. A polymorphism in the agouti signaling protein gene is associated with human pigmentation. Am J Hum Genet. 2002;70(3):770–5. doi: 10.1086/339076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gudbjartsson DF, et al. ASIP and TYR pigmentation variants associate with cutaneous melanoma and basal cell carcinoma. Nat Genet. 2008;40(7):886–91. doi: 10.1038/ng.161. [DOI] [PubMed] [Google Scholar]
- 31.Voisey J, Kelly G, Van Daal A. Agouti signal protein regulation in human melanoma cells. Pigment Cell Res. 2003;16(1):65–71. doi: 10.1034/j.1600-0749.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 32.Yi L, et al. Differential suppression of intracellular reactive oxygen species-mediated signaling pathway in vascular endothelial cells by several subclasses of flavonoids. Biochimie. 2012;94(9):2035–44. doi: 10.1016/j.biochi.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 33.Romagnolo DF, Selmin OI. Flavonoids and cancer prevention: a review of the evidence. J Nutr Gerontol Geriatr. 2012;31(3):206–38. doi: 10.1080/21551197.2012.702534. [DOI] [PubMed] [Google Scholar]
- 34.Yao H, et al. Dietary flavonoids as cancer prevention agents. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2011;29(1):1–31. doi: 10.1080/10590501.2011.551317. [DOI] [PubMed] [Google Scholar]
- 35.Sharma K, Batra P. Anti-cancer potential of flavonoids: recent trends and future perspectives. Biotechnol J. 2013;3:439–459. doi: 10.1007/s13205-013-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi M, et al. Flavonoids, proanthocyanidins, and cancer risk: a network of case-control studies from Italy. Nutr Cancer. 2010;62(7):871–7. doi: 10.1080/01635581.2010.509534. [DOI] [PubMed] [Google Scholar]
- 37.Rossi M, et al. Flavonoids, proanthocyanidins, and the risk of stomach cancer. Cancer Causes Control. 2010;21(10):1597–604. doi: 10.1007/s10552-010-9588-4. [DOI] [PubMed] [Google Scholar]
- 38.Neuhouser ML. Dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer. 2004;50(1):1–7. doi: 10.1207/s15327914nc5001_1. [DOI] [PubMed] [Google Scholar]
- 39.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63(7):1035–42. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 40.Majewska M, et al. Evaluation of antioxidant potential of flavonoids: an in vitro study. Acta Pol Pharm. 2011;68(4):611–5. [PubMed] [Google Scholar]
- 41.Magalhaes LM, et al. Methodological aspects about in vitro evaluation of antioxidant properties. Anal Chim Acta. 2008;613(1):1–19. doi: 10.1016/j.aca.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 42.Gulcin I. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012;86(3):345–91. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- 43.Cai Q, Rahn RO, Zhang R. Dietary flavonoids, quercetin, luteolin and genistein, reduce oxidative DNA damage and lipid peroxidation and quench free radicals. Cancer Lett. 1997;119(1):99–107. doi: 10.1016/s0304-3835(97)00261-9. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Guo M. Studies on transition metal-quercetin complexes using electrospray ionization tandem mass spectrometry. Molecules. 2015;20(5):8583–94. doi: 10.3390/molecules20058583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olennikov DN, Kashchenko NI, Chirikova NK. A novel HPLC-assisted method for investigation of the Fe2+-chelating activity of flavonoids and plant extracts. Molecules. 2014;19(11):18296–316. doi: 10.3390/molecules191118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jovanovic SV, et al. Flavonoids as Antioxidants. Journal of the American Chemical Society. 1994;116(11):4846–4851. [Google Scholar]
- 47.Liu-Smith F, Dellinger R, Meyskens FL., Jr Updates of reactive oxygen species in melanoma etiology and progression. Arch Biochem Biophys. 2014;563:51–5. doi: 10.1016/j.abb.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chou DS, et al. Baicalein induces proliferation inhibition in B16F10 melanoma cells by generating reactive oxygen species via 12-lipoxygenase. Free Radic Biol Med. 2009;46(8):1197–203. doi: 10.1016/j.freeradbiomed.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 49.Denkert C, et al. Expression of cyclooxygenase 2 in human malignant melanoma. Cancer Res. 2001;61(1):303–8. [PubMed] [Google Scholar]
- 50.Kappelmann M, Bosserhoff A, Kuphal S. AP-1/c-Jun transcription factors: regulation and function in malignant melanoma. Eur J Cell Biol. 2014;93(1–2):76–81. doi: 10.1016/j.ejcb.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Denat L, et al. Melanocytes as instigators and victims of oxidative stress. J Invest Dermatol. 2014;134(6):1512–8. doi: 10.1038/jid.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12(12):931–47. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 53.Tang X, et al. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic Biol Med. 2011;50(11):1599–609. doi: 10.1016/j.freeradbiomed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Pandurangan AK, et al. Luteolin, a bioflavonoid inhibits Azoxymethane-induced colorectal cancer through activation of Nrf2 signaling. Toxicol Mech Methods. 2014;24(1):13–20. doi: 10.3109/15376516.2013.843111. [DOI] [PubMed] [Google Scholar]
- 55.Zhang YC, et al. Antioxidant and Nrf2 inducing activities of luteolin, a flavonoid constituent in Ixeris sonchifolia Hance, provide neuroprotective effects against ischemia-induced cellular injury. Food Chem Toxicol. 2013;59:272–80. doi: 10.1016/j.fct.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 56.Wu PS, et al. Luteolin and Apigenin Attenuate 4-Hydroxy-2-Nonenal-Mediated Cell Death through Modulation of UPR, Nrf2-ARE and MAPK Pathways in PC12 Cells. PLoS One. 2015;10(6):e0130599. doi: 10.1371/journal.pone.0130599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu J, et al. Luteolin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE pathway. Free Radic Biol Med. 2014;71:186–95. doi: 10.1016/j.freeradbiomed.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 58.Pratheeshkumar P, et al. Luteolin inhibits Cr(VI)-induced malignant cell transformation of human lung epithelial cells by targeting ROS mediated multiple cell signaling pathways. Toxicol Appl Pharmacol. 2014;281(2):230–41. doi: 10.1016/j.taap.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balyan R, et al. Bioactivation of luteolin by tyrosinase selectively inhibits glutathione S-transferase. Chem Biol Interact. 2015;240:208–18. doi: 10.1016/j.cbi.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Paredes-Gonzalez X, et al. Apigenin reactivates Nrf2 anti-oxidative stress signaling in mouse skin epidermal JB6 P + cells through epigenetics modifications. AAPS J. 2014;16(4):727–35. doi: 10.1208/s12248-014-9613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang CS, et al. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch Toxicol. 2013;87(1):167–78. doi: 10.1007/s00204-012-0913-4. [DOI] [PubMed] [Google Scholar]
- 62.Paredes-Gonzalez X, et al. Induction of NRF2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm Drug Dispos. 2015 doi: 10.1002/bdd.1956. [DOI] [PubMed] [Google Scholar]
- 63.Gao AM, et al. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis. 2013;34(8):1806–14. doi: 10.1093/carcin/bgt108. [DOI] [PubMed] [Google Scholar]
- 64.Kimura S, et al. Essential role of Nrf2 in keratinocyte protection from UVA by quercetin. Biochem Biophys Res Commun. 2009;387(1):109–14. doi: 10.1016/j.bbrc.2009.06.136. [DOI] [PubMed] [Google Scholar]
- 65.Tanigawa S, Fujii M, Hou DX. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic Biol Med. 2007;42(11):1690–703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 66.Yao P, et al. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J Hepatol. 2007;47(2):253–61. doi: 10.1016/j.jhep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 67.Granado-Serrano AB, et al. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: Involvement of p38. Chem Biol Interact. 2012;195(2):154–64. doi: 10.1016/j.cbi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Wang R, et al. Genistein attenuates ischemic oxidative damage and behavioral deficits via eNOS/Nrf2/HO-1 signaling. Hippocampus. 2013;23(7):634–47. doi: 10.1002/hipo.22126. [DOI] [PubMed] [Google Scholar]
- 69.Zhai X, et al. Dietary flavonoid genistein induces Nrf2 and phase II detoxification gene expression via ERKs and PKC pathways and protects against oxidative stress in Caco-2 cells. Mol Nutr Food Res. 2013;57(2):249–59. doi: 10.1002/mnfr.201200536. [DOI] [PubMed] [Google Scholar]
- 70.Na HK, Surh YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol. 2008;46(4):1271–8. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 71.Cha MH, et al. Baicalein inhibits adipocyte differentiation by enhancing COX-2 expression. J Med Food. 2006;9(2):145–53. doi: 10.1089/jmf.2006.9.145. [DOI] [PubMed] [Google Scholar]
- 72.Hu C, Kitts DD. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol Cell Biochem. 2004;265(1–2):107–13. doi: 10.1023/b:mcbi.0000044364.73144.fe. [DOI] [PubMed] [Google Scholar]
- 73.Pandurangan AK, et al. Luteolin, a bioflavonoid inhibits azoxymethane-induced colon carcinogenesis: Involvement of iNOS and COX-2. Pharmacogn Mag. 2014;10(Suppl 2):S306–10. doi: 10.4103/0973-1296.133285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chung MH, et al. Genistein inhibits phorbol ester-induced NF-kappaB transcriptional activity and COX-2 expression by blocking the phosphorylation of p65/RelA in human mammary epithelial cells. Mutat Res. 2014;768:74–83. doi: 10.1016/j.mrfmmm.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 75.Van Dross RT, Hong X, Pelling JC. Inhibition of TPA-induced cyclooxygenase-2 (COX-2) expression by apigenin through downregulation of Akt signal transduction in human keratinocytes. Mol Carcinog. 2005;44(2):83–91. doi: 10.1002/mc.20123. [DOI] [PubMed] [Google Scholar]
- 76.Hirano T, et al. Luteolin, a flavonoid, inhibits AP-1 activation by basophils. Biochem Biophys Res Commun. 2006;340(1):1–7. doi: 10.1016/j.bbrc.2005.11.157. [DOI] [PubMed] [Google Scholar]
- 77.Wattel A, et al. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NF kappa B and AP-1. J Cell Biochem. 2004;92(2):285–95. doi: 10.1002/jcb.20071. [DOI] [PubMed] [Google Scholar]
- 78.Patil RH, et al. Apigenin inhibits PMA-induced expression of pro-inflammatory cytokines and AP-1 factors in A549 cells. Mol Cell Biochem. 2015;403(1–2):95–106. doi: 10.1007/s11010-015-2340-3. [DOI] [PubMed] [Google Scholar]
- 79.Das S, et al. Apigenin-induced apoptosis in A375 and A549 cells through selective action and dysfunction of mitochondria. Exp Biol Med (Maywood) 2012;237(12):1433–48. doi: 10.1258/ebm.2012.012148. [DOI] [PubMed] [Google Scholar]
- 80.Thangasamy T, et al. Quercetin selectively inhibits bioreduction and enhances apoptosis in melanoma cells that overexpress tyrosinase. Nutr Cancer. 2007;59(2):258–68. doi: 10.1080/01635580701499545. [DOI] [PubMed] [Google Scholar]
- 81.Mladenka P, et al. Cardiovascular effects of flavonoids are not caused only by direct antioxidant activity. Free Radic Biol Med. 2010;49(6):963–75. doi: 10.1016/j.freeradbiomed.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 82.Norval M, Halliday GM. The consequences of UV-induced immunosuppression for human health. Photochem Photobiol. 2011;87(5):965–77. doi: 10.1111/j.1751-1097.2011.00969.x. [DOI] [PubMed] [Google Scholar]
- 83.Toh B, et al. Detection, enumeration, and characterization of immune cells infiltrating melanoma tumors. Methods Mol Biol. 2013;961:261–77. doi: 10.1007/978-1-62703-227-8_17. [DOI] [PubMed] [Google Scholar]
- 84.Steinberg SM, Turk MJ. BRAF-inhibition and tumor immune suppression. Oncoimmunology. 2015;4(2):e988039. doi: 10.4161/2162402X.2014.988039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13(3):789–94. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 86.Schonthaler HB, Guinea-Viniegra J, Wagner EF. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann Rheum Dis. 2011;70(Suppl 1):i109–12. doi: 10.1136/ard.2010.140533. [DOI] [PubMed] [Google Scholar]
- 87.McNulty SE, et al. Comparative expression of NFkappaB proteins in melanocytes of normal skin vs. benign intradermal naevus and human metastatic melanoma biopsies. Pigment Cell Res. 2004;17(2):173–80. doi: 10.1111/j.1600-0749.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- 88.Flashner-Abramson E, et al. Targeting melanoma with NT157 by blocking Stat3 and IGF1R signaling. Oncogene. 2015 doi: 10.1038/onc.2015.229. [DOI] [PubMed] [Google Scholar]
- 89.Grimm EA, Sikora AG, Ekmekcioglu S. Molecular pathways: inflammation-associated nitric-oxide production as a cancer-supporting redox mechanism and a potential therapeutic target. Clin Cancer Res. 2013;19(20):5557–63. doi: 10.1158/1078-0432.CCR-12-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang Z, et al. Targeting nitric oxide signaling with nNOS inhibitors as a novel strategy for the therapy and prevention of human melanoma. Antioxid Redox Signal. 2013;19(5):433–47. doi: 10.1089/ars.2012.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yin J, et al. Tetramethylpyrazine inhibits migration of SKOV3 human ovarian carcinoma cells and decreases the expression of interleukin-8 via the ERK1/2, p38 and AP-1 signaling pathways. Oncol Rep. 2011;26(3):671–9. doi: 10.3892/or.2011.1334. [DOI] [PubMed] [Google Scholar]
- 92.Bar-Eli M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology. 1999;67(1):12–8. doi: 10.1159/000028045. [DOI] [PubMed] [Google Scholar]
- 93.Selvendiran K, et al. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: an implication for the antitumor potential of flavonoids. Cancer Res. 2006;66(9):4826–34. doi: 10.1158/0008-5472.CAN-05-4062. [DOI] [PubMed] [Google Scholar]
- 94.Cao HH, et al. Quercetin exerts anti-melanoma activities and inhibits STAT3 signaling. Biochem Pharmacol. 2014;87(3):424–34. doi: 10.1016/j.bcp.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 95.Meeran SM, Mantena SK, Katiyar SK. Prevention of ultraviolet radiation-induced immunosuppression by (-)-epigallocatechin-3-gallate in mice is mediated through interleukin 12-dependent DNA repair. Clin Cancer Res. 2006;12(7 Pt 1):2272–80. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- 96.Tanese K, Grimm EA, Ekmekcioglu S. The role of melanoma tumor-derived nitric oxide in the tumor inflammatory microenvironment: its impact on the chemokine expression profile, including suppression of CXCL10. Int J Cancer. 2012;131(4):891–901. doi: 10.1002/ijc.26451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duarte J, Francisco V, Perez-Vizcaino F. Modulation of nitric oxide by flavonoids. Food Funct. 2014;5(8):1653–68. doi: 10.1039/c4fo00144c. [DOI] [PubMed] [Google Scholar]
- 98.Caltagirone S, et al. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer. 2000;87(4):595–600. doi: 10.1002/1097-0215(20000815)87:4<595::aid-ijc21>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 99.Liu JD, et al. Inhibition of melanoma growth and metastasis by combination with (-)-epigallocatechin-3-gallate and dacarbazine in mice. J Cell Biochem. 2001;83(4):631–42. doi: 10.1002/jcb.1261. [DOI] [PubMed] [Google Scholar]
- 100.Nihal M, et al. Anti-proliferative and proapoptotic effects of (-)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005;114(4):513–21. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- 101.Hartman ML, Czyz M. Pro-survival role of MITF in melanoma. J Invest Dermatol. 2015;135(2):352–8. doi: 10.1038/jid.2014.319. [DOI] [PubMed] [Google Scholar]
- 102.Casagrande F, Darbon JM. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: regulation of cyclin–dependent kinases CDK2 and CDK1. Biochem Pharmacol. 2001;61(10):1205–15. doi: 10.1016/s0006-2952(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 103.Serafino A, et al. Differentiation of human melanoma cells induced by cyanidin-3-O-beta-glucopyranoside. FASEB J. 2004;18(15):1940–2. doi: 10.1096/fj.04-1925fje. [DOI] [PubMed] [Google Scholar]
- 104.Kwak IH, et al. Epigallocatechin-3-gallate inhibits paracrine and autocrine hepatocyte growth factor/scatter factor-induced tumor cell migration and invasion. Exp Mol Med. 2011;43(2):111–20. doi: 10.3858/emm.2011.43.2.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cao HH, et al. Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol Cancer. 2015;14:103. doi: 10.1186/s12943-015-0367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Syed DN, et al. Fisetin inhibits human melanoma cell growth through direct binding to p70S6K and mTOR: findings from 3-D melanoma skin equivalents and computational modeling. Biochem Pharmacol. 2014;89(3):349–60. doi: 10.1016/j.bcp.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pal HC, et al. Fisetin inhibits human melanoma cell invasion through promotion of mesenchymal to epithelial transition and by targeting MAPK and NFkappaB signaling pathways. PLoS One. 2014;9(1):e86338. doi: 10.1371/journal.pone.0086338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pal HC, et al. Fisetin, a dietary flavonoid, augments the anti-invasive and anti-metastatic potential of sorafenib in melanoma. Oncotarget. 2015 doi: 10.18632/oncotarget.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pal HC, et al. Fisetin, a phytochemical, potentiates sorafenib-induced apoptosis and abrogates tumor growth in athymic nude mice implanted with BRAF-mutated melanoma cells. Oncotarget. 2015;6(29):28296–311. doi: 10.18632/oncotarget.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lemarie F, et al. Antitumor activity of the tea polyphenol epigallocatechin-3-gallate encapsulated in targeted vesicles after intravenous administration. Nanomedicine (Lond) 2013;8(2):181–92. doi: 10.2217/nnm.12.83. [DOI] [PubMed] [Google Scholar]
- 111.Chen CC, et al. Improving anticancer efficacy of (-)-epigallocatechin-3-gallate gold nanoparticles in murine B16F10 melanoma cells. Drug Des Devel Ther. 2014;8:459–74. doi: 10.2147/DDDT.S58414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Siddiqui IA, et al. Excellent anti-proliferative and pro-apoptotic effects of (-)-epigallocatechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomedicine. 2014;10(8):1619–26. doi: 10.1016/j.nano.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 113.Leopoldini M, et al. Structure, Conformation, and Electronic Properties of Apigenin, Luteolin, and Taxifolin Antioxidants. A First Principle Theoretical Study. J Phys Chem. 2004;108:92–96. [Google Scholar]
- 114.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Busch C, et al. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin Epigenetics. 2015;7(1):64. doi: 10.1186/s13148-015-0095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guengerich FP. Introduction: Metals in Biology: alpha-Ketoglutarate/Iron-Dependent Dioxygenases. J Biol Chem. 2015;290(34):20700–1. doi: 10.1074/jbc.R115.675652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Katiyar SK, et al. Epigenetic alterations in ultraviolet radiation-induced skin carcinogenesis: interaction of bioactive dietary components on epigenetic targets. Photochem Photobiol. 2012;88(5):1066–74. doi: 10.1111/j.1751-1097.2011.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vahid F, et al. The role dietary of bioactive compounds on the regulation of histone acetylases and deacetylases: a review. Gene. 2015;562(1):8–15. doi: 10.1016/j.gene.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 119.Prasad R, Katiyar SK. Polyphenols from green tea inhibit the growth of melanoma cells through inhibition of class I histone deacetylases and induction of DNA damage. Genes Cancer. 2015;6(1–2):49–61. doi: 10.18632/genesandcancer.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fan T, et al. EZH2-dependent suppression of a cellular senescence phenotype in melanoma cells by inhibition of p21/CDKN1A expression. Mol Cancer Res. 2011;9(4):418–29. doi: 10.1158/1541-7786.MCR-10-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Venza M, et al. Epigenetic regulation of p14ARF and p16INK4A expression in cutaneous and uveal melanoma. Biochim Biophys Acta. 2015;1849(3):247–56. doi: 10.1016/j.bbagrm.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 122.Furuta J, et al. Promoter methylation profiling of 30 genes in human malignant melanoma. Cancer Sci. 2004;95(12):962–8. doi: 10.1111/j.1349-7006.2004.tb03184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Diaconeasa Z, et al. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int J Mol Sci. 2015;16(2):2352–65. doi: 10.3390/ijms16022352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taniguchi S, et al. Effect of (-)-epigallocatechin gallate, the main constituent of green tea, on lung metastasis with mouse B16 melanoma cell lines. Cancer Lett. 1992;65(1):51–4. doi: 10.1016/0304-3835(92)90212-e. [DOI] [PubMed] [Google Scholar]
- 125.Katiyar SK. Skin photoprotection by green tea: antioxidant and immunomodulatory effects. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3(3):234–42. doi: 10.2174/1568008033340171. [DOI] [PubMed] [Google Scholar]
- 126.Usach I, et al. Hesperetin induces melanin production in adult human epidermal melanocytes. Food Chem Toxicol. 2015;80:80–4. doi: 10.1016/j.fct.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 127.Ye Y, et al. Activation of p38 MAPK pathway contributes to the melanogenic property of apigenin in B16 cells. Exp Dermatol. 2011;20(9):755–7. doi: 10.1111/j.1600-0625.2011.01297.x. [DOI] [PubMed] [Google Scholar]
- 128.Ruan JS, et al. Luteolin reduces the invasive potential of malignant melanoma cells by targeting beta3 integrin and the epithelial-mesenchymal transition. Acta Pharmacol Sin. 2012;33(10):1325–31. doi: 10.1038/aps.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.George VC, et al. Comparative studies to evaluate relative in vitro potency of luteolin in inducing cell cycle arrest and apoptosis in HaCaT and A375 cells. Asian Pac J Cancer Prev. 2013;14(2):631–7. doi: 10.7314/apjcp.2013.14.2.631. [DOI] [PubMed] [Google Scholar]
- 130.Martinez C, et al. Effects of several polyhydroxylated flavonoids on the growth of B16F10 melanoma and Melan-a melanocyte cell lines: influence of the sequential oxidation state of the flavonoid skeleton. Melanoma Res. 2003;13(1):3–9. doi: 10.1097/00008390-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 131.Takekoshi S, Matsuzaki K, Kitatani K. Quercetin stimulates melanogenesis in hair follicle melanocyte of the mouse. Tokai J Exp Clin Med. 2013;38(4):129–34. [PubMed] [Google Scholar]
- 132.Fujii T, Saito M. Inhibitory effect of quercetin isolated from rose hip (Rosa canina L.) against melanogenesis by mouse melanoma cells. Biosci Biotechnol Biochem. 2009;73(9):1989–93. doi: 10.1271/bbb.90181. [DOI] [PubMed] [Google Scholar]
- 133.Kumagai A, et al. A potent inhibitor of SIK2, 3, 3', 7-trihydroxy-4'-methoxyflavon (4'-O-methylfisetin), promotes melanogenesis in B16F10 melanoma cells. PLoS One. 2011;6(10):e26148. doi: 10.1371/journal.pone.0026148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Syed DN, et al. Involvement of ER stress and activation of apoptotic pathways in fisetin induced cytotoxicity in human melanoma. Arch Biochem Biophys. 2014;563:108–17. doi: 10.1016/j.abb.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Menon LG, et al. Effect of isoflavones genistein and daidzein in the inhibition of lung metastasis in mice induced by B16F-10 melanoma cells. Nutr Cancer. 1998;30(1):74–7. doi: 10.1080/01635589809514644. [DOI] [PubMed] [Google Scholar]
- 136.Russo A, et al. Genistin inhibits UV light-induced plasmid DNA damage and cell growth in human melanoma cells. J Nutr Biochem. 2006;17(2):103–8. doi: 10.1016/j.jnutbio.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 137.Fang MZ, et al. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63(22):7563–70. [PubMed] [Google Scholar]
- 138.Moseley VR, et al. Green tea polyphenol epigallocatechin 3-gallate, contributes to the degradation of DNMT3A and HDAC3 in HCT 116 human colon cancer cells. Anticancer Res. 2013;33(12):5325–33. [PMC free article] [PubMed] [Google Scholar]
- 139.Choi KC, et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009;69(2):583–92. doi: 10.1158/0008-5472.CAN-08-2442. [DOI] [PubMed] [Google Scholar]
- 140.Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137(1 Suppl):223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- 141.Pandey M, et al. Plant flavone apigenin inhibits HDAC and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: in vitro and in vivo study. Mol Carcinog. 2012;51(12):952–62. doi: 10.1002/mc.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gerhauser C. Epigenetics, Plant (Poly) phenolics, and Cancer Prevention. In: Daayf F, Lattanzio V, editors. Recent advances in polyphenol research. Wiley; Chicago: 2009. pp. 143–207. [Google Scholar]
- 143.Lee WJ, Shim JY, Zhu BT. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharmacol. 2005;68(4):1018–30. doi: 10.1124/mol.104.008367. [DOI] [PubMed] [Google Scholar]
- 144.Lee WJ, Chen YR, Tseng TH. Quercetin induces FasL-related apoptosis, in part, through promotion of histone H3 acetylation in human leukemia HL–60 cells. Oncol Rep. 2011;25(2):583–91. doi: 10.3892/or.2010.1097. [DOI] [PubMed] [Google Scholar]
- 145.Berger A, et al. Kaempferol, a new nutrition-derived pan-inhibitor of human histone deacetylases. J Nutr Biochem. 2013;24(6):977–85. doi: 10.1016/j.jnutbio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 146.Gilbert ER, Liu D. Flavonoids influence epigenetic-modifying enzyme activity: structure - function relationships and the therapeutic potential for cancer. Curr Med Chem. 2010;17(17):1756–68. doi: 10.2174/092986710791111161. [DOI] [PubMed] [Google Scholar]
- 147.Fang MZ, et al. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;11(19 Pt 1):7033–41. doi: 10.1158/1078-0432.CCR-05-0406. [DOI] [PubMed] [Google Scholar]