Abstract
Previous studies have shown that the basal forebrain (BF) modulates cortical activation via its projections to the entire cortical mantle. However, the organization of these projections is only partially understood or, for certain areas, unknown. In this study, we examined the topographic organization of cholinergic and non-cholinergic projections from the BF to the perirhinal, postrhinal, and entorhinal cortex using retrograde tracing combined with ChAT (choline acetyltransferase) immunohistochemistry in rats. The perirhinal and postrhinal cortex receives major cholinergic and non-cholinergic input from the caudal BF, including the caudal globus pallidus and substantia innominata and moderate input from the horizontal limb of diagonal band, whereas the entorhinal cortex receives major input from the rostral BF, including the medial septum and the vertical and horizontal limbs of diagonal band. In the perirhinal cases, cholinergic projection neurons are distributed more caudally in the caudal globus pallidus than non-cholinergic projection neurons. Compared to the perirhinal cases, the distribution of cholinergic and non-cholinergic neurons projecting to the postrhinal cortex shifts slightly caudally in the caudal globus pallidus. The distribution of cholinergic and non-cholinergic neurons projecting to the lateral entorhinal cortex extends more caudally in the BF than to the medial entorhinal cortex. The ratio of ChAT positive projection neurons to the total projection neurons is higher in the perirhinal/postrhinal cases (26-48%) than in the entorhinal cases (13-30%). These results indicate that the organization of cholinergic and non-cholinergic projections from the BF to the parahippocampal cortex is more complex than previously described.
Keywords: cholinergic and non-cholinergic neurons, parahippocampal region, medial septum, vertical and horizontal limbs of diagonal band, substantia innominata, globus pallidus, retrograde tracing
Introduction
The basal forebrain (BF) is composed of heterogeneous structures, including the medial septum (MS), vertical and horizontal limbs of the diagonal band (VDB, HDB), substantia innominata (SI), and globus pallidus (GP), and provides topographically organized cholinergic projections to the entire cortical mantle (Bigl et al., 1982; Lamour et al., 1982; McKinney et al., 1983; Mesulam et al., 1983a, 1983b; Price and Stern, 1983; Rye et al., 1984; Saper 1984; Woolf et al., 1984; Amaral and Kurz et al., 1985; Luiten et al., 1987; Gaykema et al., 1990; Ghashghaei and Barbas, 2001; Bloem et al., 2014; Zaborszky et al., 2015a). Additionally, the BF sends non-cholinergic projections, including GABAergic, glutamatergic and peptidergic axons to the cortex (Gritti et al., 1997; Hur and Zaborszky, 2005; Zaborszky et al., 2015b). It has been shown that the BF projection, including cholinergic projection, to the cortex plays a critical role for cortical activation and is also implicated in attention, sensory processing, and learning (Buzsaki et al., 1988; Metherate et al., 1992; Muir et al., 1993; Everitt and Robbins, 1997; Dringenberg and Vanderwolf, 1998; Detari et al., 1999; Duque et al., 2000: Jones, 2004; Lin and Nicolelis, 2008; Fuller et al., 2011; Letzkus et al., 2011; Pinto et al., 2013).
Despite several previous anatomical studies, a systematic study of the topographic organization of the BF projections to the perirhinal (PER), postrhinal (POR), and entorhinal cortex (EC) has not been done. The perirhinal and postrhinal cortex receives convergent inputs from several sensory and non-sensory association areas and provides major cortical input to the entorhinal cortex, which in turn sends major cortical input to the hippocampus (Suzuki and Amaral, 1994; Burwell and Amaral, 1998a, 1998b; Witter et al., 2000; Furtak et al., 2007). In the present study, we examined the topographic organization of the BF projections to the PER, POR and EC using retrograde tracers in combination with ChAT (choline acetyltransferase) immunostaining in rats.
Materials and Methods
Forty-one male Sprague Dawley rats (Harlan Laboratories Inc, Indianapolis, IN, weight 167-208g) were used in this study. Of these animals, we used 16 successful cases for further analysis after checking for proper uptake and transport of the tracer, and that injections were restricted to the target cortical region. All experiments were performed in accordance with the US Public Health Service Policy on Human Care and Use of Laboratory Animals, the National Institutes of Health Guidelines for the Care and Use of Animals in Research, and were approved by Rutgers University Institutional Review Board.
Tracer injections
Unilateral injections of retrograde tracers, Fast Blue (FB; Polysciences, Inc., Warrington, PA) and Fluoro-Gold (FG; Fluorochrome, LLC, Denver, CO), both 2% solution in purified water, were made in the perirhinal, postrhinal, and entorhinal cortices. Animals were anesthetized with isoflurane. FB was injected by pressure injection (PV820 device; World Precision Instrument) using glass pipettes (tip diameter, 40-80 μm) and FG was iontophoretically injected by applying a negative, pulsed DC current (7 μA; 7 s on-off cycles; 20 min). In order to avoid leakage along the pipette track we used a modified stereotaxic instrument (SR-50, Narishige) allowing up to 90 degree rotation of the head position. After survival period of 6-10 days, the animals were deeply anesthetized with isoflurane supplemented with urethane (1.5 mL of 0.35g/mL solution) and transcardially perfused with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer. The brains were removed from the skull, postfixed for 4 hours in the same fixative, and stored in 30% sucrose.
Tissue processing
The brains were cut on a freezing microtome in the coronal plane (50 μm thickness) and collected in 0.1 M phosphate buffer (PB). Four alternating series of sections were made; the first series of sections was mounted for FB and FG without further processing, the second series of sections was stained with Nissl, the third series was processed for ChAT (choline acetyltransferase) immunohistochemistry, the fourth series was stored in a cryoprotectant and stored at −20°C. Following mapping with the epifluorescent microscope, coverslips were removed and sections were re-stained with thionine to identify cytoarchitectonic areas. Images of the Nissl-stained sections were overlaid with the appropriate mapping files using the Neurolucida “virtual slice module”.
ChAT processing is as follows. After washing (3 × 10 minutes) in 0.1 M PB, sections were incubated in goat-anti-ChAT* antibody (1:500, EMD Millipore, Billerica, MA, RRID: AB_2079751) (Table 1) at room temperature (RT) overnight. Sections were subsequently rinsed for 3 × 10 minutes in 0.1 M PB and incubated in Cy3 (Indocarbocyanine) IgG or Cy2 (Cyanine) IgG conjugated to donkey anti-goat IgG (1:200, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, RRID: AB_2307351 and AB_2307341, respectively) for 3 hours at RT. Sections were then rinsed for 3 × 10 minutes in 0.1 M PB. The same primary and linking antibodies were used in two previous publications from this laboratory (Unal et al., 2012, 2015).
Table 1.
Antibodies used in this study
| Antigen | Immunogen | Source | Dilution |
|---|---|---|---|
| Anti-ChAT | Human placental enzyme | EMD Millipore, Goat polyclonal, Cat# AB144P, RRID: AB_2079751 |
1:500 |
| Cy2 Anti-Goat | Jackson ImmunResearch Laboratories, Inc. Donkey polyclonal Cat# 705-225-147 RRID: AB_2307341 |
1:200 | |
| Cy3 Anti-Goat | Jackson ImmunResearch Laboratories, Inc. Donkey polyclonal Cat# 705-165-147 RRID: AB_2307351 |
1:200 |
Sections were mounted on glass slides and, after drying at RT, were coverslipped with DEPEX mounting medium (Electron Microscopy Sciences, Hatfield, PA). In order to better localize the projection neurons, sections after plotting were counterstained with Nissl.
Method of analysis
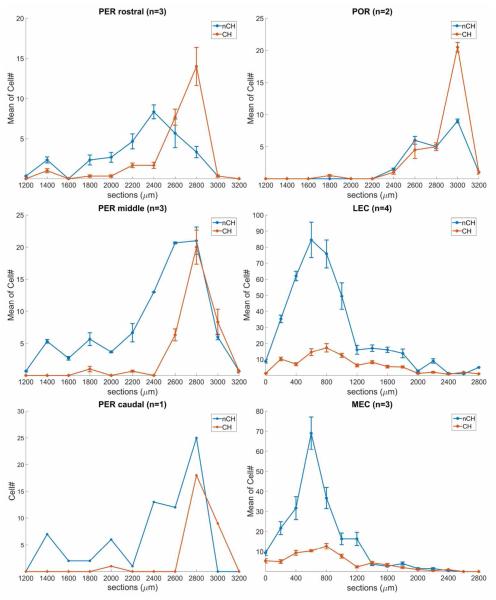
The location and extent of each injection and the distribution of retrogradely (cholinergic and non-cholinergic) labeled cells in the BF were plotted from the histological sections with a Zeiss Axioscop microscope equipped with a Neurolucida (MBF Bioscience, RRID: nif-0000-10294) software and hardware data acquisition system. By using the Zeiss epifluorescent microscope with appropriate filter set (UV G365/LP420; Blue BP450/490-LP520; Green BP546/12-LP590 AXIO), the FB and FG-labeled projection neurons and the CY3 labeled cholinergic neurons could be separately visualized in the same section. Cytoarchitectonically defined borders of BF regions were added to the maps, guided by Nissl-staining of the mapped sections. For quantitative analysis, the number of ChAT negative and ChAT positive projection neurons in the BF was counted using MBF Explorer and the ratio of ChAT positive and negative projection cells to the total projection cells was then calculated. In order to compare the location and number of retrogradely labeled cells in the septum and caudal GP in the individual cases, the mapped series of sections that were 200 μm apart was reorganized and normalized so each section became equidistant to the same fiducial markers. Statistics were done using one way ANOVA with Tukey’s post hoc test. Graphical data in Figure 10 represent mean and standard error of the mean using Excel program.
Figure 10.
Graphical display of the normalized averaged cell numbers in the perirhinal (PER), postrhinal (POR), lateral entorhinal (LEC) and medial entorhinal (MEC) groups. Y axis: mean number of cholinergic (red) and non-cholinergic (blue) neurons in GP for PER and POR groups and in MS/VDB, HDB, SI, and GP for LEC and MEC groups; X axis: distance in μm from the crossing of the anterior commissure (PER, POR cases). In LEC and MEC cases 0 level corresponds to the appearance of the most rostral cholinergic neurons in the medial septum. Bars represent standard deviation of the mean. 166×201mm (300 × 300 DPI)
Injection sites were defined based on the extent of dense fluorescent labeling with the surrounding halo, and the relationship to cytoarchitectonically defined cortical areas in Nissl-stained sections according to Burwell (2001). In this text for BF areas we tried to adhere to the nomenclature adapted by Paxinos and Watson (2005), except for the magnocellular preoptic nucleus that we incorporated into the area of the horizontal limb of the diagonal band (HDB) and we kept the term substantia innominata (SI) for the ‘extended amygdala’ since replacing the classical term would diminish the significance of the corticopetal system (see detailed discussion in Zaborszky et al., 2015c).
Results
BF projections to the perirhinal (PER) cortex
In total, seven injections (FB and FG) were made at various rostrocaudal levels of the perirhinal cortex (Table 2). Retrogradely labeled cells were found in each subregion of the BF; dense labeling was present in the caudal BF, including caudal GP and SI. Labeling was weak in the rostral BF (MS/VDB). Double labeled cells (ChAT positive and retrogradely labeled cells) were densely packed in the caudal GP but were also observed in other BF regions.
TABLE 2.
Retrograde tracer injections in the perirhinal, postrhinal, and entorhinal cortex
| Area injected | Case no. | Tracer | Layers |
|---|---|---|---|
| Perirhinal cortex | |||
| (Rostral) | |||
| Area 35 | R-21 | FG | I, II, III |
| Area 36 | R-25 | FG | II, III, IV, V |
| Area 36 | R-41 | FB | I, II, III, IV, V, VI |
| (Middle) | |||
| Area 36 | R-33 | FG | I, II, III, IV, V |
| Area 36 | R-39 | FB | I, II, III, IV, V |
| Area 36 | R-47 | FB | I, II, III |
| (Caudal) | |||
| Area 36 | R-51 | FB | I, II, III, IV, V |
| Postrhinal cortex | |||
| POR (m-c) | R-29 | FB | I, II, III |
| POR (m-c) | R-49 | FB | I, II, III |
| Entorhinal cortex | |||
| LEC (L) | R-27 | FG | III, V |
| LEC (I) | R-28 | FB | I, II |
| LEC (L) | R-28 | FG | V, VI |
| LEC (L) | R-44 | FG | V, VI |
| MEC (L) | R-14 | FG | II, III, V |
| MEC (M) | R-41 | FG | III, V |
| MEC (I) | R-49 | FG | V, VI |
L, I, M, and m-c in parentheses indicate the lateral, intermediate, and medial part of the entorhinal injections and the mid-caudal part of the postrhinal cortex.
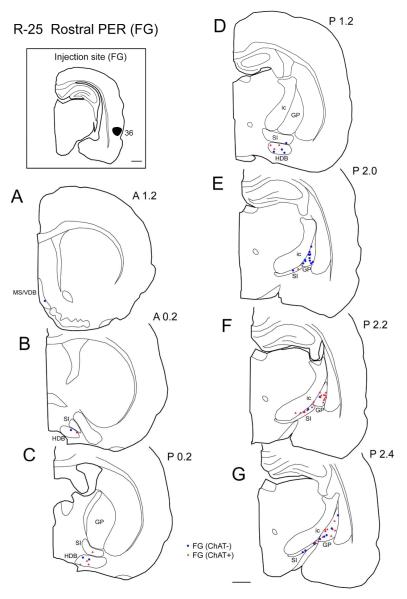
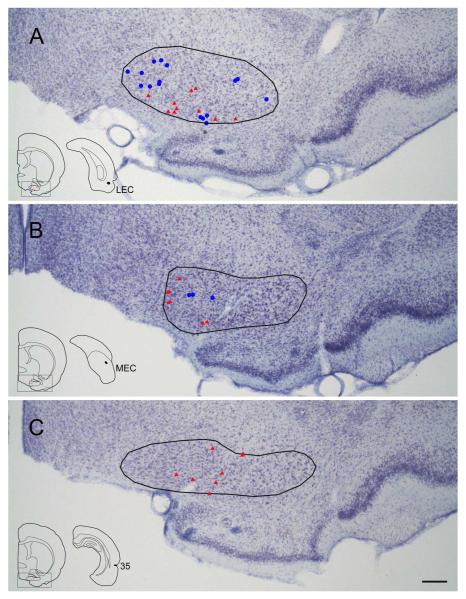
Three injections (FB and FG) were made in the rostral PER (Table 2, Fig. 8A). In a representative case (case R-25), FG was injected in area 36 of the rostral PER (Fig. 1). Retrogradely labeled cells were observed most densely in the caudal GP (Fig. 1E-G) and moderately in the HDB (horizontal limb of the diagonal band) and caudal SI (Fig. 1C-G) and sparsely in the MS/VDB (Fig. 1A). ChAT positive and ChAT negative projection neurons were distributed in each subregion of the BF. Generally, in the GP, between 1.6-2.4 mm caudal to bregma there are more non-cholinergic projection neurons than cholinergic neurons (Fig. 10). However, caudally, between 2.6-2.8 mm there are more cholinergic projection than non-cholinergic neurons (Fig. 10). In the entire BF from 3 cases, 48% of labeled projection neurons were ChAT positive (n = 382; Table 3).
Figure 8.
Photomicrographs showing representative injection sites of Fast Blue in the rostral perirhinal cortex (A, R-41), caudal perirhinal cortex (B, R-51), and postrhinal cortex (C, R-29) on Nissl sections. Scale bar = 0.5 mm.
Figure 1.
Distribution of retrogradely labeled cells in the basal forebrain (BF) following an injection of FG into the rostral perirhinal cortex (area 36; case R-25 FG). A black-filled area on the boxed coronal section shows the location of the FG injection site (top left). Line drawings were arranged from rostral to caudal (A-G). Retrogradely labeled cells positive for ChAT are shown by red triangle (ChAT+); non-cholinergic retrogradely labeled cells are indicated by blue filled circle (ChAT−). Note that labeling is denser in the caudal part of the BF, including the caudal globus pallidus (GP) and substantia innominata (SI). Within the caudal GP, it is apparent that non-cholinergic projection neurons outnumber cholinergic projection cells rostrally (panel E), while cholinergic cells are more numerous caudally (F-G). Scale bar = 1 mm.
TABLE 3.
The distribution of cholinergic and non-cholinergic neurons in the basal forebrain projecting to the perirhinal, postrhinal, and entorhinal cortex
| Case | MS/VDB | HDB | SI | SI (c) | GP | total | % | |
|---|---|---|---|---|---|---|---|---|
| Perirhinal cortex | ||||||||
| (Rostral) | ||||||||
| R-21 FG | ChAT− | 0 | 8 | 11 | 31 | 50 | 100 | 52 |
| ChAT+ | 3 | 25 | 9 | 21 | 35 | 93 | 48 | |
| R-25 FG | ChAT− | 3 | 7 | 5 | 7 | 28 | 50 | 52 |
| ChAT+ | 0 | 10 | 4 | 8 | 25 | 47 | 48 | |
| R-41 FB | ChAT− | 1 | 22 | 10 | 7 | 12 | 52 | 57 |
| ChAT+ | 1 | 4 | 8 | 6 | 21 | 40 | 43 | |
| (mid) | ||||||||
| R-33 FG | ChAT− | 4 | 21 | 9 | 28 | 89 | 151 | 60 |
| ChAT+ | 5 | 13 | 4 | 23 | 54 | 99 | 40 | |
| R-39 FB | ChAT− | 2 | 3 | 9 | 33 | 54 | 101 | 60 |
| ChAT+ | 3 | 15 | 2 | 19 | 27 | 66 | 40 | |
| R-47 FB | ChAT− | 3 | 7 | 6 | 30 | 80 | 126 | 66 |
| ChAT+ | 3 | 15 | 3 | 14 | 30 | 65 | 34 | |
| (Caudal) | ||||||||
| R-51 FB | ChAT− | 2 | 6 | 5 | 66 | 68 | 147 | 74 |
| ChAT+ | 4 | 7 | 2 | 12 | 28 | 53 | 26 | |
| Postrhinal cortex | ||||||||
| R-29 FB | ChAT− | 8 | 6 | 8 | 24 | 27 | 73 | 54 |
| ChAT+ | 1 | 1 | 0 | 19 | 40 | 61 | 46 | |
| R-49 FB | ChAT− | 11 | 4 | 4 | 63 | 18 | 100 | 60 |
| ChAT+ | 13 | 6 | 2 | 22 | 25 | 68 | 40 | |
| Entorhinal cortex | ||||||||
| LEC | ||||||||
| R-27 FG | ChAT− | 301 | 134 | 22 | 4 | 4 | 465 | 77 |
| ChAT+ | 59 | 67 | 9 | 3 | 0 | 138 | 23 | |
| R-28 FB | ChAT− | 295 | 93 | 18 | 2 | 5 | 413 | 77 |
| ChAT+ | 91 | 28 | 1 | 0 | 0 | 120 | 23 | |
| R-28 FG | ChAT− | 471 | 107 | 11 | 1 | 4 | 594 | 87 |
| ChAT+ | 49 | 33 | 4 | 0 | 1 | 87 | 13 | |
| R-44 FG | ChAT− | 152 | 19 | 3 | 0 | 2 | 176 | 79 |
| ChAT+ | 14 | 30 | 3 | 0 | 0 | 47 | 21 | |
| MEC | ||||||||
| R-14 FG | ChAT− | 172 | 16 | 0 | 1 | 0 | 189 | 70 |
| ChAT+ | 61 | 21 | 0 | 0 | 0 | 82 | 30 | |
| R-41 FG | ChAT− | 117 | 9 | 1 | 0 | 0 | 127 | 77 |
| ChAT+ | 27 | 11 | 0 | 0 | 0 | 38 | 23 | |
| R-49 FG | ChAT− | 276 | 51 | 0 | 1 | 0 | 328 | 82 |
| ChAT+ | 50 | 22 | 0 | 0 | 0 | 72 | 18 | |
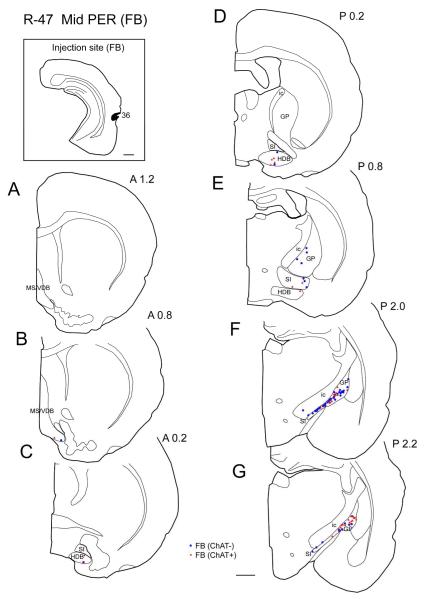
Three injections (FB and FG) were made in the mid-perirhinal cortex (Table 2). In a representative case (case R-47; Fig. 2), FB was injected in area 36 at the mid rostrocaudal level of the PER. Retrogradely labeled cells were observed most densely in the caudal GP/SI (Fig. 2F, G) and labeling decreased rostrally. Similar to case R-25, ChAT positive projection neurons were present most densely in the caudal GP (Fig. 2G). In the GP, approximately between 1.6-2.6 mm there were more non-cholinergic projection neurons than cholinergic ones, but this difference leveled off at about 2.8 mm caudal to bregma (Fig. 10). In the entire BF, from three animals, 38% of labeled projection neurons were ChAT positive (n = 608; Table 3).
Figure 2.
Distribution of cholinergic and non-cholinergic retrogradely labeled cells following an injection of FB into the mid-rostrocaudal portion of the perirhinal cortex (case R-47 FB). Conventions as in Figure 1. Note that, as in Figure 1, labeling is present most densely in the caudal BF (caudal GP and SI) (F, G) and decreases rostrally. Cholinergic projection neurons are present densely in the caudal part of caudal GP (G). Scale bar = 1 mm.
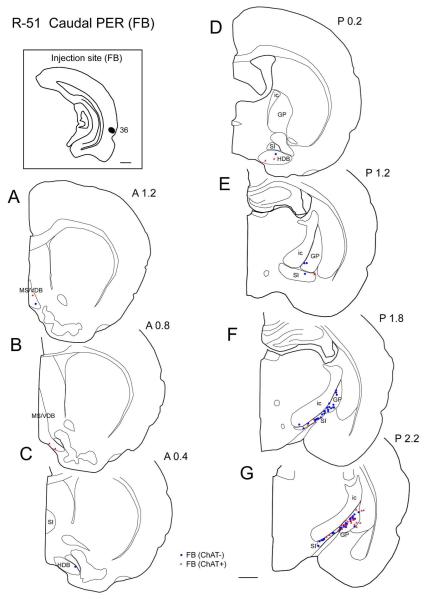
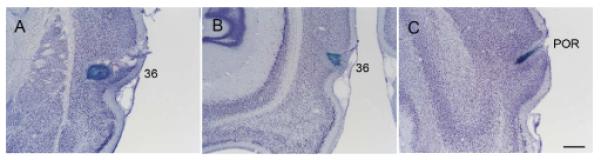
One injection was made in the caudal PER (Table 2, Fig. 8B). In case R-51, FB was injected in area 36 at the caudal level of the perirhinal cortex. As in the rostral and mid-perirhinal cases, labeled cells were found most densely in the caudal part of GP and SI (Figs. 3F-G, 9B) and were present very sparsely in the rostral BF (Figs. 3A-C, 9A). Similarly to rostral and mid-perirhinal injections, non-cholinergic cells between 2.2-2.8 mm were more numerous than cholinergic projection neurons (Fig. 10). In the entire BF, only 26% of labeled projection neurons were ChAT positive (n = 200; Table 3). Comparing with the rostral and mid rostrocaudal PER cases, the ratio of cholinergic projection neurons becomes lower in the caudal perirhinal cortex (Table 3).
Figure 3.
Distribution of cholinergic and non-cholinergic retrogradely labeled cells following an injection of FB into the caudal portion of the perirhinal cortex (case R-51 FB). Conventions as in Figure 1. Note that, as in Figures 1 and 2, labeling is present densely in the caudal BF (caudal GP and SI) (F, G) and decreases rostrally. Cholinergic projection neurons are present densely in the caudal part of caudal GP (G). Scale bar = 1 mm.
Figure 9.
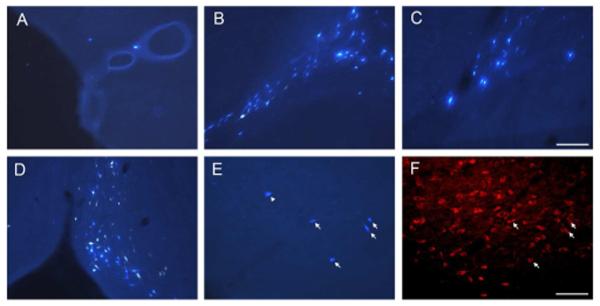
Photomicrographs of retrogradely labeled cells in the HDB (A) and caudal GP (B) following FB injection in the caudal perirhinal cortex (case R-51 FB), in the caudal GP (C) following FB injection in the postrhinal cortex (case R-29 FB), in the MS/VDB (D) following FB injection in the entorhinal cortex (case R-28 FB). Photomicrographs in E and F show the retrogradely labeled cells in the HDB (E) following FB injection in the caudal perirhinal cortex (case R-50 FB) and immunoreactive cells for ChAT (F) for the same location as in E. Arrows indicate double labeled cells for FB and ChAT (E, F) and an arrowhead indicates a FB labeled cell that is ChAT negative (E). Scale bar = 0.1 mm (A-D) and 0.2 mm (E, F).
BF projections to the postrhinal (POR) cortex
In total, two injections (FB) were made in the postrhinal cortex. As in the perirhinal cases, retrogradely labeled cells were found densely in the caudal BF (caudal GP/SI) with density decreasing rostrally.
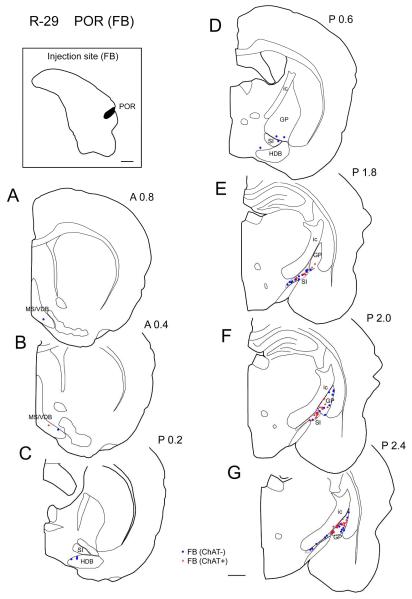
In the representative case (case R-29), FB was injected in the mid-caudal POR (Fig. 8C) and labeled cells were present most densely in the caudal GP/SI (Figs. 4E-G, 9C) and sparsely in HDB, VDB, and SI (Fig. 4A-D). Cholinergic projection neurons were intermingled with non-cholinergic projection neurons and were found most densely in the caudal GP/SI. Within the caudal GP/SI, the density of cholinergic neurons becomes higher in the caudal than in the rostral part (Fig. 4E, F, G). Compared to the perirhinal cases, the distribution of cholinergic and non-cholinergic projection neurons shifts slightly caudally in the caudal GP (Fig. 10). In the entire BF, from two animals, 43% of labeled projection neurons were ChAT positive (n = 302; Table 3).
Figure 4.
Distribution of cholinergic and non-cholinergic retrogradely labeled cells following an injection of FB into the postrhinal cortex (case R-29 FB). Conventions as in Figure 1. Note that, as in perirhinal cases (Figs. 1-3), labeling is dense in the caudal BF (caudal GP and SI) (E-G) and decreases rostrally (A-D). Cholinergic projection neurons are labeled densely in the caudal part of caudal GP (F, G). Scale bar = 1 mm.
BF projections to the entorhinal (EC) cortex
Four injections (FG and FB) in the lateral EC (LEC) and three injections (FG) in medial EC (MEC) were made. Unlike in the perirhinal and postrhinal cases, retrogradely labeled cells were densely present in the rostral BF (MS/VDB) (Figs. 5, 6, 9D) and were absent or very few in the caudal BF. The ratio of non-cholinergic to cholinergic projection neurons in EC cases (3.9) was higher than that in the perirhinal (1.5) and postrhinal (1.3) cases.
Figure 5.
Distribution of cholinergic and non-cholinergic retrogradely labeled cells following an injection of FG into the lateral entorhinal cortex (LEC; case R-27 FG). Conventions as in Figure 1. Note that in contrast with the topographic pattern of labeling in the perirhinal and postrhinal cases (Figs. 1-4), labeling is present densely in the rostral BF (MS/VDB) (A, B). Scale bar = 1 mm.
Figure 6.
Distribution of cholinergic and non-cholinergic retrogradely labeled cells following an injection of FG into the medial entorhinal cortex (case R-49 FG). Conventions as in Figure 1. Note that as in the LEC case (Fig. 5), labeling is present densely in the rostral BF (MS/VDB) (A, B). Compared to LEC case (Fig. 5), labeling is confined to more rostral regions in the BF. Scale bar = 1 mm.
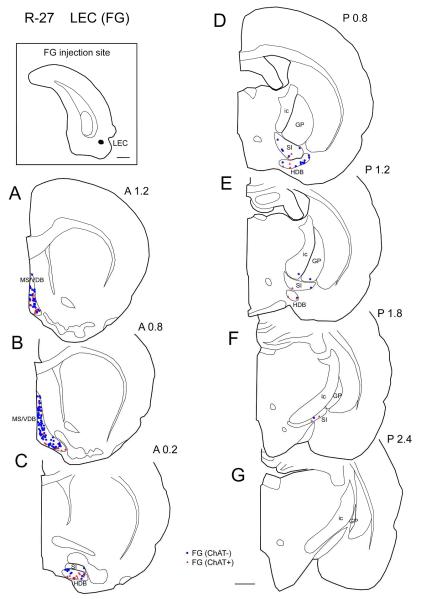
In the representative case of LEC injection (case R-27), FG was injected in the dorsolateral part of LEC and labeled cells were observed very densely in MS/VDB (Fig. 5A, B) and the labeling extends to the HDB and SI (Fig. 5C-F). In the caudal MS/VDB, cholinergic projection neurons were found more densely in the ventral than in dorsal part (Fig. 5B). In the entire BF, from four cases only 19% of labeled projection neurons were colocalized with ChAT (n = 2040; Table 3).
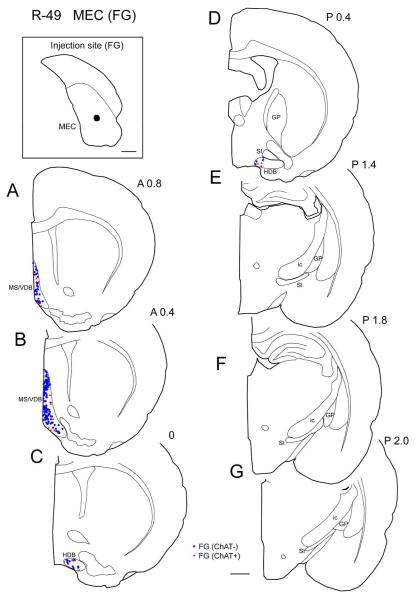
In the representative case of MEC injection (case R-49 FG), FG was injected in the caudal MEC (Fig. 6). As in case R-27, labeled cells were densely present in the MS/VDB (Fig. 6A, B). Labeling in LEC cases extends more caudally (1.8-2.2 mm) than in cases with MEC injections (1.2 mm; Fig. 10), from 3 cases 23% of labeled projection neurons were ChAT positive (n =836; Table 3).
Figure 7 shows the topographical specificity of the HDB projections to the perirhinal and entorhinal cortex, as this BF subdivision contains cells projecting to all cortical areas investigated in this study. In the case of MEC (Fig. 7B), cells were located medially, whereas they were present more diffusely in the case of LEC (Fig. 7A). Finally, labeling in the HDB in MEC cases seem to be different from the pattern of labeling in a perirhinal case, where labeled cells occupy the mid portion of HDB (Fig. 7C).
Figure 7.
Distribution of retrogradely labeled cells in HDB following FG injections in LEC (A, case R-27 FG), MEC (B, case R-14 FG), and perirhinal cortex (C, case R-21 FG). Labeled cells are superimposed on the Nissl-stained images of the mapped sections. Blue circles and red rectangles indicate non-cholinergic and cholinergic retrogradely labeled cells, respectively. The injection site and the actual mapped coronal section are shown in the lower left inset. The boxed area on the coronal map shows the position of the picture, and the outline on the Nissl section shows the border of HDB. Notice that the labeled cells are located medially in HDB in the case of MEC injection (B) whereas labeling is present in both medial and ventral portions of HDB in the case of LEC injection (A). In the perirhinal case, labeling is shifted to the mid portion of HDB (C). Scale bar = 0.25 mm.
Comparing the total number of projection (cholinergic+non-cholinergic) neurons normalized for the size of injection surface (106 μm2) limited to cases where FG was injected in the PER (n=3), MEC (n=3), and LEC (n=3), there is a trend of an increasing number of total projection neurons from PER (45.4±9.2) to LEC (203.2±43.4), with values of MEC (131.3±40.5) in between and the difference between PER and LEC is significant (P<0.5).
Discussion
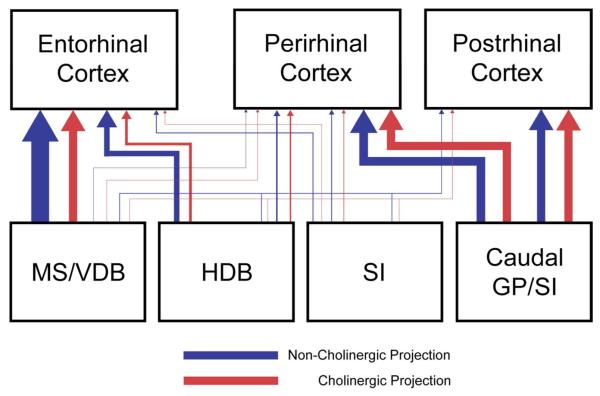
In the present study, we examined the topographic organization of the BF projections to the perirhinal, postrhinal, and entorhinal cortex. We found that these cortical areas receive complementary inputs from the BF in that the perirhinal and postrhinal cortex receives cholinergic and non-cholinergic projections mainly from the caudal part of the BF, including GP/SI as well as from the HDB, whereas the entorhinal cortex (LEC and MEC) receives cholinergic and non-cholinergic projections mainly from the rostral part of the BF (MS/VDB) as well as from the HDB (Fig. 11). The overall number of retrogradely labeled cells projecting to EC is much higher than to PER. The ratio of cholinergic projection neurons to the total projection neurons is higher in PER/POR cases (26-48%) than in EC cases (13-30%). We also revealed that comparing to PER cases, the distribution of cholinergic and non-cholinergic GP neurons projecting to POR shifts slightly caudally within the caudal GP. Furthermore, the distribution of cholinergic and non-cholinergic neurons projecting to LEC extends more caudally in the BF than to MEC. A differential distribution of BF projection neurons according to their cholinergic and/or non-cholinergic transmitter (GP: Fig. 10) or their target (HDB: Fig. 7) suggests organization that is more specific than previously described. The comparison of the fine topography of neurons projecting to PER, POR, and EC with pools of BF neurons that project to other cortical targets suggests interactions within the BF that support specific cognitive operations.
Figure 11.
Summary of the topographic organization of BF projections to the perirhinal, postrhinal, and entorhinal cortex in rats. Red and blue lines indicate cholinergic and non-cholinergic projections. Thickness of lines indicates the relative strength of projections. The perirhinal and postrhinal cortices receive cholinergic and non-cholinergic projections mainly from the caudal basal forebrain (caudal GP and SI), whereas the entorhinal cortex receives projections mainly from the rostral basal forebrain (MS/VDB). Cholinergic neurons projecting to the perirhinal and postrhinal cortex comprise 26-48% of the total projection neurons (cholinergic and non-cholinergic projection neurons) and 13-30% in the entorhinal cases.
Basal forebrain projections to the perirhinal, postrhinal, and entorhinal cortex
To our knowledge, the present study is the first to systematically examine the topographic organization of the cholinergic and non-cholinergic projections to the perirhinal, postrhinal, and entorhinal cortex, although some previous findings are consistent with our present results. Saper (1984) found that following a HRP-WGA injection in the rat perirhinal cortex, retrogradely labeled cells were present in the ventrolateral part of the caudal GP. Woolf et al. (1984) reported cholinergic projections to the perirhinal cortex from the ‘subpallidal SI’ and ‘nucleus basalis’ in rats. However, inadequate documentation in those studies prevents detailed comparison of findings with the present study. In the present study, we mapped the distribution of cholinergic and non-cholinergic projection neurons to the PER in the entire BF and found that the BF projection neurons to the perirhinal cortex are present most densely in the caudal GP/SI and that the density of cholinergic projection neurons increases from rostral to caudal within the GP/SI. A few projection neurons to the perirhinal cortex were also observed in more rostral parts of the BF, including SI, HDB, and VDB. The present study also revealed that about 26-48% of neurons that project to the PER are cholinergic, and there is a trend toward a higher cholinergic ratio in the rostral PER. The findings of our retrograde experiments are consistent with anterograde studies indicating that GP, SI, and HDB give rise to labeling in the perirhinal cortex (Lamour et al., 1984; Saper, 1984; Luiten et al., 1985, 1987; Grove, 1988).
In previous studies that investigated BF projections to cortex, the postrhinal cortex (POR) was not identified. The present study clarified that the topography of the BF projection (cholinergic and non-cholinergic) to POR is similar to that of perirhinal cortex in that the caudal part of GP/SI is the site that gives rise to the densest cholinergic and non-cholinergic projections to the POR. However, the pattern of cholinergic and non-cholinergic projection neurons is slightly different from that of the perirhinal cases (Fig. 10). It remains to be investigated whether the same or different cholinergic cells project to these two cortical regions.
The present results for EC are consistent with previous findings that the EC receives projections from MS/VDB and HDB (Alonso and Kohler, 1984; Saper, 1984; Woolf et al., 1984; Manns et al., 2001). Furthermore, it provides new findings that projections to LEC, as compared to MEC, extend further caudally in the BF. Our study also revealed that neurons that project to the MEC and LEC show distinct topography in the HDB (Fig. 7).
Comparison with the basal forebrain projections to other cortical regions
Although the BF as a whole gives rise to projections to the entire cortical mantle, each cortical region receives topographically organized input from the BF (Bigl et al., 1982; Lamour et al., 1982; McKinney et al., 1983; Mesulam et al., 1983a, 1983b; Price and Stern, 1983; Rye et al., 1984; Saper, 1984; Woolf et al., 1984; Amaral and Kurz, 1985; Luiten et al., 1987; Gaykema et al., 1990; Ghashghaei and Barbas, 2001; Bloem et al., 2014; Zaborszky et al., 2015a). For example, previous studies have shown that the temporal cortex, including auditory, visual and insular cortex, receives projections mainly from the caudal GP (Lamour et al., 1982; Price and Stern, 1983; Rye et al., 1984; Saper, 1984), which is similar to the pattern of BF projections to the perirhinal and postrhinal cortex we observed in the present study. In addition to a gross topography, it has been shown that interconnected cortical regions tend to receive projections from spatially overlapping pools of neurons in the BF (Zaborszky et al., 2015a). It is possible, although it remains to be examined, that the overlapping pool of neurons in the BF receives the same inputs and/or is interconnected via local collaterals and thus capable to modulate the associated cortical areas in a coordinated fashion.
Supporting this idea, it has been shown that PER and POR are reciprocally connected (Burwell and Amaral, 1998a; Agster and Burwell, 2009) and our study disclosed that PER and POR receive projections from the same BF area. Interestingly, the lateral visual association cortex (area 18a) and PER are interconnected (Shi and Cassel, 1997; Burwell and Amaral, 1998a) and BF projections to occipital area 18a (Carey and Rieck, 1987) and PER (present study) seem to originate from a partially common BF area. Furthermore, the ventral auditory association cortex and PER are interconnected (Shi and Cassel, 1997; Burwell and Amaral, 1998a; Paperna and Malach, 1991) and projections to these auditory areas (Price and Stern, 1983; Rye et al., 1984; Saper, 1984) and PER (present study) originate from the partially common BF area. In addition, projections to S1/S2 cortical areas were shown to originate from the GP/internal capsule area (Baskerville et al., 1993), partially comparable to the location of BF cells projecting to the insular cortex (Zaborszky et al., 2015a) and PER (present study) and the S2 cortex projects via insular cortex to the PER (Burwell and Amaral, 1998a; Shi and Cassel, 1998). It remains to be tested in future experiments whether indeed these pairs of cortical areas are jointly modulated by their BF input in specific cognitive operations.
The entorhinal cortex receives its cholinergic projection from the MS/VDB and HDB areas. Interestingly, the medial visual association cortex (18b) and MEC receive BF projections from a partially common BF region (Rieck and Carey, 1984) and the MEC projects to the medial visual area (Agster and Burwell, 2009). The retrosplenial cortex (RS) also receives its BF projection from the MS/VDB/HDB area (Saper, 1984; Gyengesi et al., 2013) and the MEC projects to the retrosplenial cortex (Insausti et al., 1997; Agster and Burwell, 2009). Furthermore, the medial visual cortex and the RS are reciprocally connected (Vogt and Miller, 1983).
The hippocampus receives BF projections mainly from the MS/VDB (McKinney et al., 1983; Rye et al., 1984; Saper, 1984; Woolf et al., 1984; Amaral and Kurz, 1985; Gaykema et al., 1990). The topographic pattern of the BF projection to the hippocampus is similar to that to the entorhinal cortex, as we observed in the present study, and these structures are interconnected (Witter et al., 2000). Although PER/POR and entorhinal cortex are interconnected, the BF projections to these areas originate from very different BF regions. This indicates that the notion that interconnected cortical areas receive projections from overlapping pool of BF neurons may not apply for every cortical region.
The perirhinal and postrhinal cortices receive convergent inputs from several association cortices (Suzuki and Amaral, 1994; Burwell and Amaral, 1998a) and provide cortical input to the entorhinal cortex, which in turn sends major cortical input to the hippocampus (Naber et al., 1997; Burwell and Amaral, 1998a, 1998b; Witter et al., 2000). The cortico-hippocampal pathway consists of two parallel but interacting pathways (Witter et al., 2000) so that the perirhinal cortex sends projection mainly to LEC, whereas the postrhinal cortex projects mainly to MEC (Naber et al., 1997; Burwell and Amaral, 1998b). The perirhinal cortex and LEC are important for processing object or nonspatial information (Hargreaves et al., 2005; Winters and Bussey, 2005a, 2005b; Deshmukh and Knierim, 2011), while the postrhinal cortex and MEC are important for processing object-place, contextual information (Furtak et al., 2012) and spatial information (Hargreaves et al., 2005; Moser et al., 2008), respectively. Although PER and POR projection neurons are located in the same BF area (i.e., GP), there are subtle differences in their projection pattern (Figure 10).
Previous studies have shown that disruption of cholinergic transmission in the perirhinal cortex impairs object recognition (Tang et al., 1997; Winters and Bussey, 2005b). The observation that perirhinal and lateral visual association cortex receive their input from partially common BF space (caudal GP/SI) is compatible with the suggestion that the visual and perirhinal cortex is jointly modulated through BF cholinergic input to support specific cognitive function. It remains for future functional studies to establish the significance of the observation that the medial visual association area, the retrosplenial cortex and the entorhinal cortex receive their cholinergic input from the partially common BF region in the septum.
Cholinergic, GABAergic and glutamatergic projections
BF projection neurons comprise cholinergic, GABAergic, and glutamatergic neurons (Gritti et al., 1997; Manns et al., 2001; Hur and Zaborszky, 2005; Henny and Jones, 2008). In the present study, cholinergic projection neurons to the perirhinal/postrhinal and entorhinal cortex were found to make up approximately 26-48% and 13-30% of the total number of projection neurons, respectively.
GABAergic BF neurons project to the entorhinal cortex (Manns et al., 2001) and the small proportion of cholinergic projection neurons to the entorhinal cortex suggests that non-cholinergic, possibly GABAergic modulation becomes important. It was recently shown that cortically projecting GABAergic neurons containing parvalbumin, which send projection to cortical parvalbumin interneurons, are important for enhancing cortical gamma band oscillations (Kim et al., 2015). Cholinergic neurons excite cortically projecting GABAergic neurons via their collaterals in the BF (Yang et al., 2014) and the differential contribution of cholinergic projections to the perirhinal and entorhinal cortex suggests that GABAergic and cholinergic modulation of these cortical areas is under complex regulation.
In a study investigating the BF projections to the entorhinal cortex (Manns et al., 2001), it was suggested that a substantial fraction of the projection to this cortical area is glutamatergic, based on the presence of phosphate activated glutaminase (PAG). However, PAG is not a specific marker for glutamatergic neurons. Using a specific marker for glutamatergic neurons, it was shown that only 5% of BF projections are directed to the prefrontal or somatosensory cortex (Hur and Zaborszky, 2005). It remains for future studies to investigate the contribution of BF glutamatergic neurons projecting to the entorhinal cortex.
Using retrograde tracing combined with ChAT immunohistochemistry, we show that the perirhinal and postrhinal cortices receive cholinergic and non-cholinergic projections mainly from the caudal part of the basal forebrain whereas the entorhinal cortex receives projections mainly from the rostral part of the basal forebrain in rats.
Acknowledgements for support
NIH/NINDS 23945 to LZ
Funding
This work was supported by the National Institutes of Health (NS023945 to L.Z.) Acknowledgements The authors thank Peter Gombkoto, Ph.D. for preparing Figure 10.
Abbreviations
- BF
basal forebrain
- ChAT
choline acetyltransferase
- EC
entorhinal cortex
- FB
Fast Blue
- FG
Fluoro-Gold
- GP
globus pallidus
- HDB
horizontal limb of diagonal band
- ic
internal capsule
- LEC
lateral entorhinal cortex
- MEC
medial entorhinal cortex
- MS
medial septum
- PER
perirhinal cortex
- POR
postrhinal cortex
- RS
retrosplenial cortex
- SI
substantia inominata
- VDB
vertical limb of diagonal band
Footnotes
The authors declare no conflict of interest
References
- Agster KL, Burwell RD. Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Hippocampus. 2009;19:1159–1186. doi: 10.1002/hipo.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Kohler C. A study of the reciprocal connections between the septum and the entorhinal area using anterograde and retrograde axonal transport methods in the rat brain. J Comp Neurol. 1984;225:327–343. doi: 10.1002/cne.902250303. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol. 1985;240:37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- Baskerville KA, Chang HT, Herron P. Topography of cholinergic afferents from the nucleus basalis of Meynert to representational areas of sensorimotor cortices in the rat. J Comp Neurol. 1993;335:552–562. doi: 10.1002/cne.903350407. [DOI] [PubMed] [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull. 1982;8:727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- Bloem B, Schoppink L, Rotaru DC, Faiz A, Hendriks P, Mansvelder HD, van de Berg WD, Wouterlood FG. Topographic mapping between basal forebrain cholinergic neurons and the medial prefrontal cortex in mice. J Neurosci. 2014;34:16234–16246. doi: 10.1523/JNEUROSCI.3011-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998a;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol. 1998b;391:293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Burwell RD. Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. J Comp Neurol. 2001;437:17–41. doi: 10.1002/cne.1267. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RG, Rieck RW. Topographic projections to the visual cortex from the basal forebrain in the rat. Brain Res. 1987;424:205–215. doi: 10.1016/0006-8993(87)91463-6. [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, Knierim JJ. Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front Behav Neurosci. 2011;5:69. doi: 10.3389/fnbeh.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detari L, Rasmusson DD, Semba K. The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Prog Neurobiol. 1999;58:249–277. doi: 10.1016/s0301-0082(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Involvement of direct and indirect pathways in electrocorticographic activation. Neurosci Biobehav Rev. 1998;22:243–257. doi: 10.1016/s0149-7634(97)00012-2. [DOI] [PubMed] [Google Scholar]
- Duque A, Balatoni B, Detari L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. J Neurophysiol. 2000;84:1627–1635. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annual review of psychology. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Ahmed OJ, Burwell RD. Single neuron activity and theta modulation in postrhinal cortex during visual object discrimination. Neuron. 2012;76:976–988. doi: 10.1016/j.neuron.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus. 2007;17:709–722. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Luiten PG, Nyakas C, Traber J. Cortical projection patterns of the medial septum-diagonal band complex. J Comp Neurol. 1990;293:103–124. doi: 10.1002/cne.902930109. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Neural interaction between the basal forebrain and functionally distinct prefrontal cortices in the rhesus monkey. Neuroscience. 2001;103:593–614. doi: 10.1016/s0306-4522(00)00585-6. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383:163–177. [PubMed] [Google Scholar]
- Grove EA. Efferent connections of the substantia innominata in the rat. J Comp Neurol. 1988;277:347–364. doi: 10.1002/cne.902770303. [DOI] [PubMed] [Google Scholar]
- Gyengesi E, Andrews ZB, Paxinos G, Zaborszky L. Distribution of secretagogin-containing neurons in the basal forebrain of mice, with special reference to the cholinergic corticopetal system. Brain Res Bull. 2013;94:1–8. doi: 10.1016/j.brainresbull.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neuorsci. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study. J Comp Neurol. 2005;483:351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- Insausti R, Herrero MT, Witter MP. Entorhinal cortex of the rat: cytoarchitectonic subdivisions and the origin and distribution of cortical efferents. Hippocampus. 1997;7:146–183. doi: 10.1002/(SICI)1098-1063(1997)7:2<146::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Jones BE. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog Brain Res. 2004;145:157–169. doi: 10.1016/S0079-6123(03)45011-5. [DOI] [PubMed] [Google Scholar]
- Kim T, Thankachan S, McKenna JT, McNally JM, Yang C, Choi JH, Chen L, Kocsis B, Deisseroth K, Strecker RE, Basheer R, Brown RE, McCarley RW. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci U S A. 2015;112:3535–3540. doi: 10.1073/pnas.1413625112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamour Y, Dutar P, Jobert A. Topographic organization of basal forebrain neurons projecting to the rat cerebral cortex. Neurosci Lett. 1982;34:117–122. doi: 10.1016/0304-3940(82)90162-8. [DOI] [PubMed] [Google Scholar]
- Lamour Y, Dutar P, Jobert A. Cortical projections of the nucleus of the diagonal band of Broca and of the substantia innominata in the rat: an anatomical study using the anterograde transport of a conjugate of wheat germ agglutinin and horseradish peroxidase. Neuroscience. 1984;12:395–408. doi: 10.1016/0306-4522(84)90061-7. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Lin SC, Nicolelis MA. Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron. 2008;59:138–149. doi: 10.1016/j.neuron.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiten PG, Gaykema RP, Traber J, Spencer DG., Jr. Cortical projection patterns of magnocellular basal nucleus subdivisions as revealed by anterogradely transported Phaseolus vulgaris leucoagglutinin. Brain Res. 1987;413:229–250. doi: 10.1016/0006-8993(87)91014-6. [DOI] [PubMed] [Google Scholar]
- Luiten PG, Spencer DG, Jr., Traber J, Gaykema RP. The pattern of cortical projections from the intermediate parts of the magnocellular nucleus basalis in the rat demonstrated by tracing with Phaseolus vulgaris-leucoagglutinin. Neurosci Lett. 1985;57:137–142. doi: 10.1016/0304-3940(85)90052-7. [DOI] [PubMed] [Google Scholar]
- Manns ID, Mainville L, Jones BE. Evidence for glutamate, in addition to acetylcholine and GABA, neurotransmitter synthesis in basal forebrain neurons projecting to the entorhinal cortex. Neuroscience. 2001;107:249–263. doi: 10.1016/s0306-4522(01)00302-5. [DOI] [PubMed] [Google Scholar]
- McKinney M, Coyle JT, Hedreen JC. Topographic analysis of the innervation of the rat neocortex and hippocampus by the basal forebrain cholinergic system. J Comp Neurol. 1983;217:103–121. doi: 10.1002/cne.902170109. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983a;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983b;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Volicer L, Marquis JK, Mufson EJ, Green RC. Systematic regional differences in the cholinergic innervation of the primate cerebral cortex: distribution of enzyme activities and some behavioral implications. Ann Neurol. 1986;19:144–151. doi: 10.1002/ana.410190206. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Muir JL, Page KJ, Sirinathsinghji DJ, Robbins TW, Everitt BJ. Excitotoxic lesions of basal forebrain cholinergic neurons: effects on learning, memory and attention. Behav Brain Res. 1993;57:123–131. doi: 10.1016/0166-4328(93)90128-d. [DOI] [PubMed] [Google Scholar]
- Naber PA, Caballero-Bleda M, Jorritsma-Byham B, Witter MP. Parallel input to the hippocampal memory system through peri- and postrhinal cortices. Neuroreport. 1997;8:2617–2621. doi: 10.1097/00001756-199707280-00039. [DOI] [PubMed] [Google Scholar]
- Paperna T, Malach R. Patterns of sensory intermodality relationships in the cerebral cortex of the rat. J Comp Neurol. 1991;308:432–456. doi: 10.1002/cne.903080310. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed Elsevier Academic Press; 2005. [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci. 2013;16:1857–1863. doi: 10.1038/nn.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Stern R. Individual cells in the nucleus basalis--diagonal band complex have restricted axonal projections to the cerebral cortex in the rat. Brain Res. 1983;269:352–356. doi: 10.1016/0006-8993(83)90145-2. [DOI] [PubMed] [Google Scholar]
- Rieck R, Carey RG. Evidence for a laminar organization of basal forebrain afferents to the visual cortex. Brain Res. 1984;297:374–380. doi: 10.1016/0006-8993(84)90579-1. [DOI] [PubMed] [Google Scholar]
- Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13:627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. J Comp Neurol. 1984;222:313–342. doi: 10.1002/cne.902220302. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid projections of rat temporal cortex. J Comp Neurol. 1997;382:153–175. [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cascade projections from somatosensory cortex to the rat basolateral amygdala via the parietal insular cortex. J Comp Neurol. 1998;399:469–491. doi: 10.1002/(sici)1096-9861(19981005)399:4<469::aid-cne3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Tang Y, Mishkin M, Aigner TG. Effects of muscarinic blockade in perirhinal cortex during visual recognition. Proc Natl Acad Sci U S A. 1997;94:12667–12669. doi: 10.1073/pnas.94.23.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal CT, Golowasch JP, Zaborszky L. Adult mouse basal forebrain harbors two distinct cholinergic populations defined by their electrophysiology. Front Behav Neurosci. 2012;6:1–14. doi: 10.3389/fnbeh.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal CT, Pare D, Zaborszky L. Impact of basal forebrain cholinergic inputs on basolateral amygdala neurons. J Neurosci. 2015;35:853–863. doi: 10.1523/JNEUROSCI.2706-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Miller MW. Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J Comp Neurol. 1983;216:192–210. doi: 10.1002/cne.902160207. [DOI] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J Neurosci. 2005a;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Removal of cholinergic input to perirhinal cortex disrupts object recognition but not spatial working memory in the rat. Eur J Neurosci. 2005b;21:2263–2270. doi: 10.1111/j.1460-9568.2005.04055.x. [DOI] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Eckenstein F, Butcher LL. Cholinergic systems in the rat brain: I. projections to the limbic telencephalon. Brain Res Bull. 1984;13:751–784. doi: 10.1016/0361-9230(84)90236-3. [DOI] [PubMed] [Google Scholar]
- Yang C, McKenna JT, Zant JC, Winston S, Basheer R, Brown RE. Cholinergic neurons excite cortically projecting basal forebrain GABAergic neurons. J Neurosci. 2014;34:2832–2844. doi: 10.1523/JNEUROSCI.3235-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, Nadasdy Z. [Advance Access publication, August 19, 2013];Neurons in the Basal Forebrain Project to the Cortex in a Complex Topographic Organization that Reflects Corticocortical Connectivity Patterns: An Experimental Study Based on Retrograde Tracing and 3D Reconstruction. Cereb Cortex. 2015a 25:118–137. doi: 10.1093/cercor/bht210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Duque A, Gielow M, Gombkoto P, Nadasdy Z, Somogyi J. Organization of the basal forebrain cholinergic projection system: specific or diffuse? In: Paxinos G, editor. The Rat Nervous System. 4th edition Elsevier Inc.; Amsterdam: 2015b. pp. 491–507. [Google Scholar]
- Zaborszky L, Amunts K, Palomero-Gallagher N, Zilles K. Basal forebrain anatomical systems in MRI space. In: Toga A, editor. Brain Mapping: An Encyclopedic Reference. Elsevier Inc.; Amsterdam: 2015c. pp. 395–409. [Google Scholar]