Abstract
Diet-induced obesity and associated metabolic effects can lead to neurological dysfunction and increase the risk of developing Alzheimer's disease (AD) and Parkinson's disease (PD). Despite these risks, the effects of a high-fat diet on the central nervous system are not well understood. To better understand the mechanisms underlying the effects of high fat consumption on brain regions affected by AD and PD, we used proton magnetic resonance spectroscopy (1H-MRS) to measure neurochemicals in the hippocampus and striatum of rats fed a high fat diet vs. normal low fat chow. We detected lower concentrations of total creatine (tCr) and a lower glutamate-to-glutamine ratio in the hippocampus of high fat rats. Additional effects observed in the hippocampus of high fat rats included higher N-acetylaspartylglutamic acid (NAAG), and lower myo-inositol (mIns) and serine (Ser) concentrations. Post-mortem tissue analyses revealed lower phosphorylated AMP-activated protein kinase (pAMPK) in the striatum but not in the hippocampus of high fat rats. Hippocampal pAMPK levels correlated significantly with tCr, aspartate (Asp), phosphoethanolamine (PE), and taurine (Tau), indicating beneficial effects of AMPK activation on brain metabolic and energetic function, membrane turnover, and edema. A negative correlation between pAMPK and glucose (Glc) indicates a detrimental effect of brain Glc on cellular energy response. Overall, these changes indicate alterations in neurotransmission and in metabolic and bioenergetic function in the hippocampus and in the striatum of rats fed a high fat diet.
Keywords: brain metabolism, diet-induced obesity, high-fat, magnetic resonance spectroscopy, imaging
Introduction
A high fat diet contributes to obesity, insulin resistance, and type 2 diabetes [1]. A high fat diet is also associated with oxidative stress, chronic neuroinflammation, altered mitochondrial function, and decreased hippocampal neurogenesis and plasticity [2,3] in the central nervous system (CNS). Thus, diet-induced obesity may accelerate age-related neural pathology and disease, and increase the brain's vulnerability to insults that contribute to cognitive decline and dementia [4,5]. In fact, greater caloric and fat intake in late middle age increases the risk of dementia and Alzheimer's disease (AD) [6,7]. Being obese is associated with a greater chance of developing dementia [8] and metabolic syndrome, and type 2 diabetes increases an individual's relative risk for developing AD [9].
A high fat diet can also affect neural pathways associated with Parkinson's disease (PD). In studies examining the effects of a high-fat diet on nigrostriatal function and vulnerability, we found that a high fat diet in young adult rats increases dopamine depletion in the 6-hydroxydopamine (6-OHDA)-lesioned model of PD [10, 11]. We also reported that rats fed a high fat diet exhibit attenuated dopamine release in the striatum and increased iron levels and markers of oxidative stress in the substantia nigra [12]. These effects parallel findings reported for normal aging [13-17], which is the greatest contributor to PD and AD in humans. Overall, increasing evidence supports the hypothesis that a high fat diet may accelerate mechanisms related to neural aging.
The goal of the current study was to determine the extent to which markers of metabolic and bioenergetic function are altered in brain regions affected by AD and PD in high fat-fed rats. We used high-field proton magnetic resonance spectroscopy (1H-MRS) to measure a neurochemical profile. 1H-MRS is a non-invasive chemical assay technique that affords quantification of multiple neurochemicals within a given region of interest in the brain. Although we did not conduct repeated measures in our animals, this technique has the added advantage of identifying individual differences in neural responses to a high fat diet in the absence of group differences in translational studies. Our previous 1H-MRS studies have reported altered neurochemical markers of bioenergetics and metabolic function in rodent models of aging, diabetes, and brain injury [18-21]. In the current study, we compared rats fed a high fat diet or standard chow. We measured neurochemical markers in the hippocampus and striatum in living animals in situ as well as proteins related to bioenergetic function (peroxisome proliferator-activated receptor gamma coactivator 1a (PGC-1a), mitochondrial transcription factor A (TFAM), nuclear respiratory factor 1 (NRF-1), and phosphorylation of AMP-activated protein kinase (AMPK)) and astrocyte function (glial fibrillary acidic protein (GFAP)) in hippocampal and striatal tissue collected from the same animals post mortem.
Materials & Methods
Animals and diet
Two-month-old male Fischer 344 (F344; Harlan) rats were given access ad libitum to a high fat diet (D12492 from Research Diets, New Brunswick, NJ; 60% calories from fat, 20% calories from carbohydrate, 20% calories from protein; n=6) or standard rat chow (Harlan Teklad rodent diet 8604 from Teklad Diets, Madison, WI; 14% calories from fat, 54% calories from carbohydrate, 32% calories from protein; n=6) for five months prior to MRI/MRS measurements. Rats were randomly assigned to diet groups. Body weights for the control and high fat groups prior to diet implementation were 265 ± 3 and 266 ± 4 grams, respectively. We did not collect blood for glucose or insulin measures in these rats because we wanted to avoid multiple anesthesia episodes and because our previous studies have documented insulin resistance and glucose intolerance following 60% high fat diets lasting from 5-weeks to 6-months [10-12]. Procedures conformed to the National Research Council's Guide for the Care and Use of Laboratory Animals and were approved by the University of Kansas Institutional Animal Care and Use Committee. Experiments were in compliance with the ARRIVE guidelines.
Magnetic Resonance Imaging and Spectroscopy
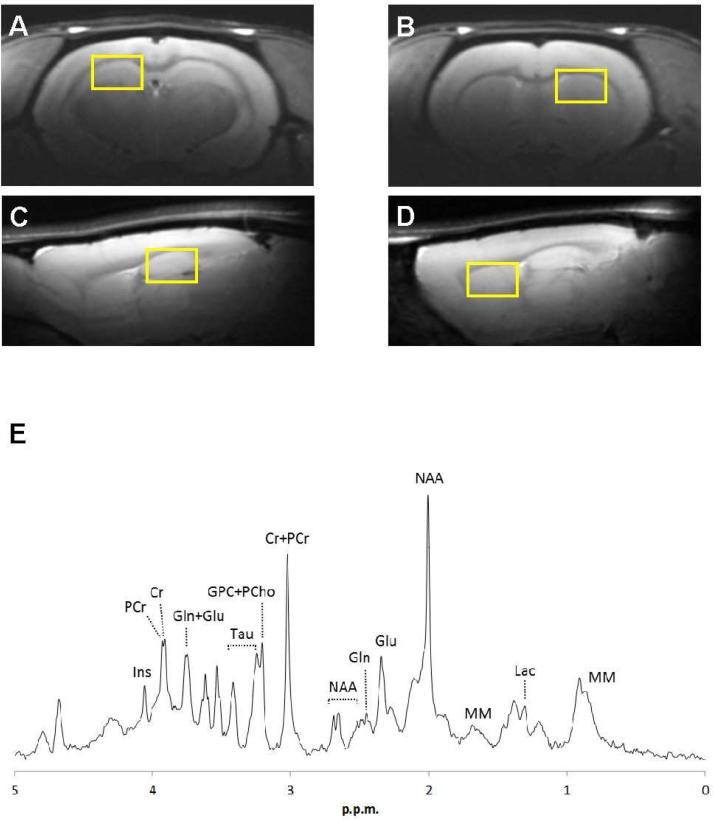
Animals were fasted for 12 hours prior to MR scans to maintain consistency with our previous studies measuring insulin resistance. Isoflurane was administered for 4 minutes at 4% prior to placing the animal in the magnet cradle where anesthesia was maintained at 1.5-3.5% during imaging. Throughout the in vivo experiments, respiration was maintained at 40-80 cycles/min and body temperature was maintained at 37°C via a feedback control system. We collected water-suppressed MR spectra using a STEAM sequence (TE=2ms, TR=4000ms, Varian 9.4T spectrometer) from two regions of interest (ROI) over the hippocampus and striatum (Figure 1A-D). First- and second-order shims were adjusted using FASTMAP [22], and MR spectra (Figure 1E) were analyzed with LCModel software as described previously [20,21]. LCModel uses a basis set of spectra acquired from in vitro samples of pure chemicals to estimate the in vivo neurochemical concentrations, and the unsuppressed water signal from the ROI as a reference for each scan [23]. Peak assignments for individual metabolites in the neurochemical profile have been previously validated [23, 24]. We measured the following neurochemicals: alanine (Ala), ascorbate (Asc), aspartate (Asp), creatine (Cr), γ-aminobutyric acid (GABA); glucose (Glc), glutamine (Gln), glutamate (Glu), glycerophosphocholine (GPC), glutathione (GSH), myo-inositol (mIns), lactate (Lac), macromolecules (Mac), N-acetylaspartate (NAA), N-acetylaspartyl glutamate (NAAG), phosphocholine (PCho), phosphocreatine (PCr), phosphoethanolamine (PE), serine (Ser), and taurine (Tau). In addition, total choline (tCho: GPC + PCho), total creatine (tCr: Cr + PCr), and the ratios of PCr/Cr and Glu/Gln were evaluated. 1H-MRS does not distinguish between intracellular and extracellular compartments. However, since the extracellular volume and the extracellular concentrations of neurochemicals measured by 1H-MRS are each small, the extracellular contribution is generally considered to be negligible.
Figure 1.
Sample voxels chosen for 1H-MRS (A-D) and an MR spectrum (E). Regions of interest for hippocampus (A, C) and striatum (B, D) as identified on anatomical MRI taken from the coronal (top row) and sagittal (bottom row) planes. (E) Sample 1H-MR spectrum from hippocampus of a chow-fed rat. Prominent neurochemical peaks are labeled. Cr = creatine, PCr = phosphocreatine, Gln = glutamine, Glu = glutamate, GPC = Glycerophosphocholine, PC = phosphocholine, mIns = myo-inositol, Lac = lactate, MM = macromolecules, NAA = N-acetylaspartate, Tau = taurine.
The duration of anesthesia did not differ between high fat and chow groups. However, the average concentration of isoflurane that was required to maintain respiration within the target range (40-80/min) was higher in the high fat group (average high fat = 3.1%, average C = 2.7%; p=0.007), likely due to the greater body weights and fat absorption. Although it is possible that this small difference could have influenced brain metabolism, we found no correlations between anesthesia concentrations and levels of Glc, Glu, NAA, or Lac measured in the striatum or hippocampus (R2 ranged from 0 to 0.26). Thus, we think the relatively small group variations in anesthesia are unlikely to have significantly influenced the study conclusions.
Western Blot
We measured GFAP as a marker of astroglial responses in the striatum and hippocampus of rats using western blot. We also measured protein markers of bioenergetic function, including PGC1α, NRF-1, and TFAM, and assessed the activation of AMPK using pAMPK/total AMPK. Antibodies against GFAP, TFAM, phospho-AMPK, and total AMPK were obtained from Cell Signaling Technology (Beverly, MA), and antibodies against PGC1α were obtained from Calbiochem (San Diego, CA). Antibodies against Actin were obtained from Abcam (Cambridge, MA). Goat-anti-rabbit HRP-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced chemiluminescence reagents were purchased from Thermo Scientific (Waltham, MA). All other reagents were obtained from Sigma (St. Louis, MO).
Following MRI/MRS scans, brains were extracted and hippocampus and striatal tissue was dissected freehand using a stainless steel adult rat brain slicer matrix with 1.0 mm coronal slice intervals. Hippocampal and striatal sections corresponded with the ROI analyzed with MRS. Brain tissue was frozen until processed as described previously [11,25]. Specifically, frozen samples were diluted in cell extraction buffer and the tissue was homogenized and centrifuged. A Bradford assay was performed in triplicate to determine sample protein concentrations and then diluted to obtain samples of constant concentration for analysis with SDS-PAGE. All samples were run on 10% gels and then transferred to nitrocellulose membranes for 90 min. After blocking non-specific binding sites with 5% milk for 1 h, membranes were incubated overnight with primary antibody at 4° C. Membranes were then washed with TBST and incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h. Upon exposure, films were scanned at high resolution to obtain digital images. Densitometry analyses were performed using Image J software. Protein content was normalized to the loading control β-actin.
Statistical Analysis
Mean neurochemical concentrations in the high fat vs. control groups were compared using a weighted averages method and corrected for multiple comparisons using Benjamini-Hochberg's procedure.
Protein concentrations obtained with western blot were compared between groups using an unpaired weighted Student's t-test (two high fat condition tissue samples from the hippocampus and one high fat and one control from the striatum were unsuitable for processing for western blots). In order to determine relationships between in vivo 1H-MRS and ex vivo western blot findings, we conducted correlation analyses on results from both hippocampus and striatum. The analyses were conducted across groups. Correlations between 1H-MRS and tissue findings were analyzed by Pearson Correlation. Statistical significance was set at p ≤ 0.05 for both corrected and uncorrected analyses. Data are reported as means ± standard errors of mean (S.E.M.).
Results
Neurochemical differences following a high fat diet
After 5 months of a standard chow or high fat diet, body weights were 374 ± 9 g and 420 ± 8 g, respectively, leading to a significant group X time interaction indicating greater weight gain in the high fat group, F(1,10)=9.674, p=0.05 by the time of MR scans.
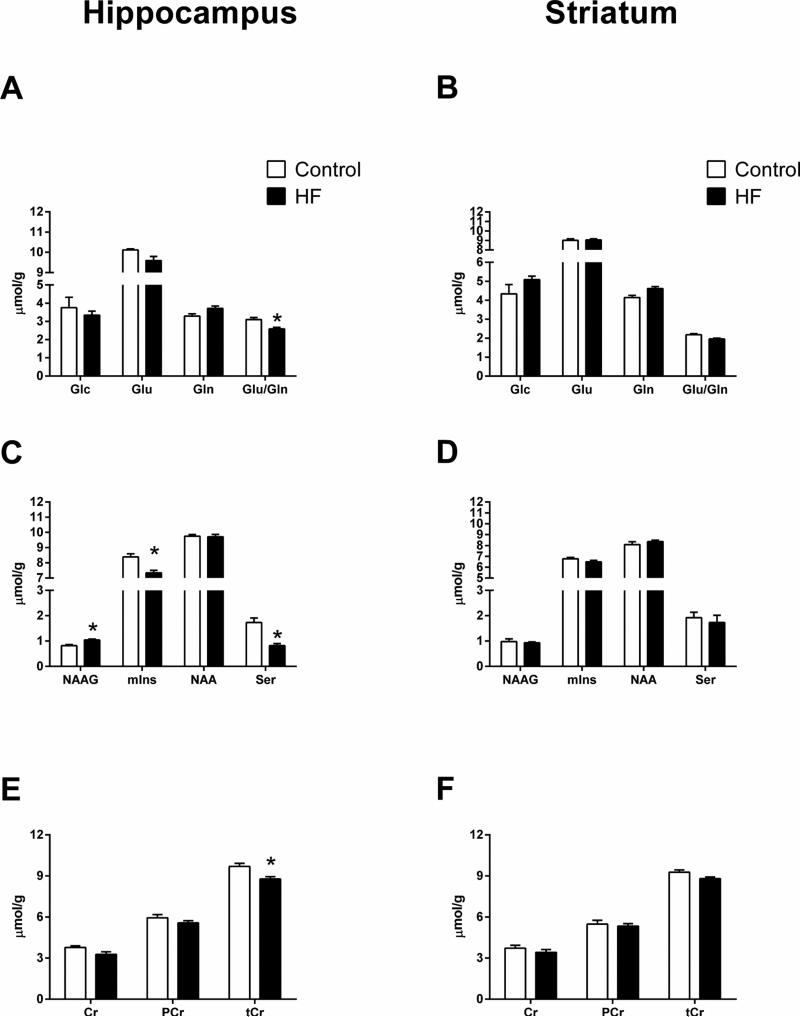
The groups did not differ with regard to Glc concentrations in either the hippocampus or the striatum (Fig. 2a,b). Although Glu and Gln concentrations did not differ significantly between the two groups in either brain region, the high fat group had significantly lower Glu/Gln ratios in the hippocampus (p=0.005), but not in the striatum. In addition, the high fat group exhibited significantly greater NAAG (p=0.003), and lower mIns (p=0.003) and Ser (p=0.001) concentrations in the hippocampus, but not in the striatum (Fig. 2c,d). Although Cr and PCr concentrations did not differ between the two groups in either brain region, tCr concentrations were significantly lower in the hippocampus of the high fat group (p=0.007; Fig. 2e,f). The apparently lower striatal tCr concentrations in the high fat group did not reach statistical significance after adjusting for multiple comparisons. Means, standard errors of means, and unadjusted p values for the other neurochemicals are provided in Table 1.
Figure 2.
Between-groups differences in selected neurochemicals measured with 1H-MRS. Glc, Glu, and Gln in the hippocampus (A) and the striatum (B). The ratio of Glu to Gln was significantly decreased in the hippocampus of the high fat group. NAAG, mIns, NAA, and Ser in the hippocampus (C) and the striatum (D). NAAG was significantly greater in the hippocampus of the high fat group, while mIns and Ser were significantly lower. Cr, PCr and tCr in the hippocampus (E) and the striatum (F). tCr was significantly lower in the hippocampus of the high fat group. Control = Chow; HF = High-fat; Data are expressed as means + S.E.M. See Table 1 for p values.
Table 1.
Neurochemical changes in the high fat vs. control animals.
| Hippocampus |
Striatum |
|||||
|---|---|---|---|---|---|---|
| Control | High Fat | p | Control | High Fat | p | |
| Ala | 0.39 ± 0.04 | 0.38 ± 0.04 | 0.804 | 0.58 ± 0.05 | 0.45 ± 0.07 | 0.159 |
| Asc | 2.84 ± 0.26 | 2.33 ± 0.09 | 0.078 | 2.28 ± 0.16 | 2.13 ± 0.10 | 0.423 |
| Asp | 1.54 ± 0.23 | 1.31 ± 0.16 | 0.415 | 0.81 ± 0.15 | 0.83 ± 0.14 | 0.929 |
| Cr | 3.79 ± 0.11 | 3.28 ± 0.18 | 0.050 | 3.72 ± 0.21 | 3.42 ± 0.20 | 0.319 |
| tCr | 9.70 ± 0.22 | 8.77 ± 0.17 | 0.007 | 9.28 ± 0.17 | 8.80 ± 0.12 | 0.038 |
| PCr | 5.95 ± 0.24 | 5.58 ± 0.15 | 0.221 | 5.48 ± 0.28 | 5.34 ± 0.17 | 0.686 |
| GABA | 1.38 ± 0.06 | 1.20 ± 0.05 | 0.046 | 1.43 ± 0.10 | 1.33 ± 0.05 | 0.393 |
| Glc | 3.75 ± 0.57 | 3.34 ± 0.22 | 0.497 | 4.34 ± 0.49 | 5.09 ± 0.18 | 0.177 |
| Gln | 3.29 ± 0.12 | 3.71 ± 0.13 | 0.048 | 4.14 ± 0.12 | 4.62 ± 0.10 | 0.010 |
| Glu | 10.12 ± 0.05 | 9.59 ± 0.21 | 0.055 | 9.02 ± 0.15 | 9.06 ± 0.11 | 0.821 |
| GPC | 0.94 ± 0.07 | 0.81 ± 0.07 | 0.216 | 0.90 ± 0.04 | 0.81 ± 0.09 | 0.396 |
| tCho | 1.19 ± 0.08 | 1.04 ± 0.07 | 0.169 | 1.46 ± 0.03 | 1.37 ± 0.07 | 0.300 |
| PCho | 0.24 ± 0.04 | 0.22 ± 0.03 | 0.797 | 0.57 ± 0.04 | 0.55 ± 0.05 | 0.746 |
| GSH | 0.90 ± 0.07 | 0.85 ± 0.05 | 0.604 | 0.99 ± 0.03 | 0.98 ± 0.02 | 0.801 |
| mIns | 8.39 ± 0.21 | 7.35 ± 0.16 | 0.003 | 6.77 ± 0.13 | 6.49 ± 0.14 | 0.187 |
| Lac | 1.53 ± 0.07 | 1.63 ± 0.12 | 0.499 | 2.42 ± 0.35 | 1.78 ± 0.13 | 0.111 |
| Mac | 1.78 ± 0.05 | 1.85 ± 0.03 | 0.267 | 1.63 ± 0.04 | 1.70 ± 0.03 | 0.203 |
| NAA | 9.75 ± 0.11 | 9.72 ± 0.15 | 0.884 | 8.09 ± 0.26 | 8.36 ± 0.13 | 0.377 |
| NAAG | 0.82 ± 0.04 | 1.04 ± 0.04 | 0.003 | 0.98 ± 0.11 | 0.93 ± 0.04 | 0.656 |
| PE | 1.54 ± 0.41 | 1.58 ± 0.05 | 0.928 | 1.93 ± 0.19 | 1.85 ± 0.17 | 0.785 |
| Ser | 1.73 ± 0.18 | 0.82 ± 0.08 | 0.001 | 1.92 ± 0.22 | 1.73 ± 0.29 | 0.614 |
| Tau | 6.58 ± 0.19 | 6.38 ± 0.12 | 0.382 | 7.60 ± 0.21 | 7.52 ± 0.17 | 0.772 |
| PCr/Cr | 1.58 ± 0.14 | 1.72 ± 0.15 | 0.505 | 1.50 ± 0.14 | 1.56 ± 0.09 | 0.734 |
| Glu/Gln | 3.10 ± 0.11 | 2.59 ± 0.09 | 0.005 | 2.18 ± 0.06 | 1.96 ± 0.04 | 0.019 |
Values are group means with standard error of mean. p values are uncorrected. Significant p values based on Benjamini-Hochberg's correction procedure are denoted in bold. Abbreviations: Ala, alanine; Asc, ascorbate, Asp, aspartate; Cr, creatine; GABA, gamma aminobutyric acid; Glc, glucose; Gln, glutamine; Glu, glutamate; GPC, glycerophosphocholine; GSH, glutathione; mIns, myo-inositol; Lac, lactate; Mac, macromolecules; NAA, N-acetylaspartate; NAAG, N-acetylaspartyl glutamate; PCho, phosphocholine; PCr, phosphocreatine; PE, phosphoethanolamine; Ser, serine; Tau, taurine; tCho, total choline (GPC & PCho); tCr, total creatine (Cr & PCr).
Protein differences following a high fat diet
Protein levels of GFAP did not differ between the high fat fed and standard chow-fed rats (Figure 3). Phosphorylated AMPK was significantly lower in the striatum of high fat group (p=0.005; Figure 3), but not in the hippocampus. Although not reaching statistical significance (p=0.065), PGC1α levels appeared lower in the striatum of high fat group (p=0.065). PGC1α did not differ in the hippocampus. There were no significant diet effects on NRF-1 or TFAM, in either striatum or hippocampus.
Figure 3.
Western blot quantification of proteins in hippocampal (A) and striatal (B) tissue. pAMPK/AMPK was significantly lower in the high fat rats than the control rats in striatum, but not in hippocampus; PGC1α levels revealed a non-significant trend to be lower in the high fat group in striatum, but not in hippocampus; there was no significant difference of GFAP, NRF-1, TFAM between high fat rats and control rats in either striatum or hippocampus. Control = Chow; HF = High-fat; Data are expressed as means + S.E.M. *p=0.005.
Correlations between 1H-MRS and tissue protein measures
We detected significant positive correlations between AMPK activation (pAMPK/AMPK) and tCr (r =0.78; p=0.008), Asp (r = 0.68, p=0.02), PE (r = 0.73, p=0.01), Tau (r = 0.82, p=0.002), and a significant negative correlation between pAMPK and Glc (r = −0.75, p=0.008) in the hippocampus. Although there was a positive relationship between tCr concentration and AMPK activation in striatum, this correlation did not reach statistical significance (r=0.60; p=0.068). No other significant correlations were observed between 1H-MRS neurochemicals and proteins measured in tissue in the hippocampus or striatum.
Discussion
In the current study, non-invasive 1H-MRS and western blot analyses were used to compare neurochemical differences between rats fed a high fat diet or standard chow. Our results revealed evidence of altered neurotransmission and bioenergetic function, primarily in the hippocampus, but also in the striatum. We did not find evidence for increased glial activation or decreased neural integrity in either location.
After Glu is released into the synapse, it is rapidly taken up by astrocytes and converted to Gln by glutamine synthase to allow for rapid clearance as part of the Glu-Gln cycle. Although a high fat diet, metabolic syndrome, and obesity have been linked to altered Glu-Gln cycling, the direction of alterations is inconsistent across studies [26-29]. We previously reported that the Glu/Gln ratio was significantly lower in the hippocampus and cortex of aged rats [20]. The lower Glu/Gln ratio that we measured in the hippocampus of high fat rats is consistent with previous work using high fat diet models [28]. This suggests a shift in the balance of the Glu-Gln cycle that may reflect increased Glu turnover. Decreases in Glu degrading enzymes and increased Glu uptake via increased glial Glu transporters GLT-1 and GLAST have been reported following a high fat diet [28]. It is possible that increased glial uptake is a compensatory response to increased synaptic Glu. Because 1H-MRS cannot differentiate between intracellular and extracellular Glu, or between glial cells and neurons, further studies are needed to test this hypothesis. However, increased Glu activity should activate GSH synthesis and enhance the glycolytic pathway, leading to increased Lac formation by astrocytes [30]. Our findings of no differences in GSH, Lac, or Ala between the high fat and control groups, suggest that glycolysis was not affected by our diet regimen. It is likely that differences between high fat diet models and genetic models of obesity (e.g., Zucker obese and Zucker diabetic fatty rats [27, 29]) account for disparate findings in Glu-Gln cycling across studies of metabolic dysfunction.
Higher GFAP expression, suggesting glial activation, as measured by immunohistochemistry has been reported in both the hypothalamus [31] and hippocampus [32] of high fat-fed rodents. We did not detect significant group differences in GFAP protein levels in either the hippocampus or the striatum, however. Moreover, we did not detect significant differences in mIns, a glial cell marker, or NAA, a marker of neural health [20,33]. It is tempting to attribute lack of glial activation to a potential compensatory upregulation of NAAG, since NAAG is neuroprotective against increased glutamate activity [34,35]. By affecting presynaptic metabotropic glutamate receptors, NAAG can inhibit presynaptic glutamate release. We have reported greater NAAG in the hippocampus and cortex of aged rats, both regions that exhibited decreased Glu/Gln ratios [20]. It is possible that NAAG prevented further Glu release that could have been toxic to neurons and resulted in glial proliferation.
The decreased Ser we measured in this hippocampus of our high fat group has significant functional implications. In the brain, D-serine is a co-agonist with glutamate at NMDA receptors (especially the NR2b subunit), playing an essential role in learning and memory [36,37]. D-serine levels are regulated by insulin levels. A recent study of the streptozotocin-treated rat model of Type 1 diabetes reported elevated hippocampal D-serine in these hypoinsulinemic rats [38]. Interestingly, insulin treatment decreased D-serine insulin levels and improved cognitive function in these animals. Conversely, hippocampal cognitive deficits were associated with lower serine racemase (the enzyme that synthesizes D-serine from L-serine) and D-serine concentrations in aged rats [39]. Increased serum insulin levels have been measured in aged rats [40] and in rats fed a high fat diet [10, 11], and likely accounted for the decreased Ser in the current study. The mechanism underlying this effect is unclear, however, because a high fat diet decreases insulin transport across the blood-brain barrier [41]. We recently measured similar cerebral spinal fluid insulin levels in chow and high fat rats despite elevated serum insulin in the latter group [Ma D et al unpublished findings]. Given these findings, it is possible that the lack of elevated Glc concentrations in the brains of our high fat group resulted from a similar unknown mechanism. Alternatively, Glc levels could have been maximal in both groups, given the hyperglycemic effects of isoflurane anesthesia [42]. Further studies are required to determine the mechanism underlying insulin's effects of Ser and Glc in the brain, and to determine the functional consequences of hyperinsulinemia during the pre-diabetic phase.
Creatine (Cr) and phosphocreatine (PCr) play a central role in energy metabolism, buffering rapid changes in ADP/ATP ratios [43]. At times of high energy demand, PCr can be used to fuel metabolic needs, resulting in a decreased PCr/Cr ratio. Conversely, PCr can be reserved during times of low energy demand, providing an energy supply buffer [44]. Neuroprotective effects of Cr have been reported both in vitro and in vivo [45]. In the current study, neither Cr nor PCr/Cr ratios differed significantly between groups. Total creatine (tCr = Cr+PCr) was significantly lower in the hippocampus of HIGH FAT rats, consistent with earlier work in a high fat ketogenic diet [46]. Because our high fat diet was not ketogenic (carbohydrates were 20% of calories), it is tempting to attribute fat content as the critical factor for lower tCr. However, the relative contributions of increased fat content vs decreased protein (20% in high fat diet vs 32% in chow) to decreased tCr are unclear. It is possible that lower levels of anabolic precursors and/or synthesis-related enzymes such as arginine:glycine amidinotransferase (AGAT) accounted for the lower tCr levels in our high fat group. AGAT activity is regulated by growth hormone and thyroxine, both of which are affected by a high fat diet, obesity, and diabetes [47,48]. Another possible mechanism is reduced Cr kinase activity, which has been reported in serum of high fat rats [49] and in the brains of streptozotocin-induced diabetic rats [50]. It is also possible that increased reactive oxygen species production under these conditions led to decreased Cr kinase activity [51] and a less efficient Cr-PCr system, ultimately affecting tCr levels [45]. The absence of differences in GSH and Asc, however, suggest that oxidative stress was not increased by the high fat diet.
Alternatively, decreased creatine uptake in the brain of high fat-fed rats could have contributed to decreased Cr and tCr concentrations. Creatine is transported into the brain via the SLC6A8 transporter [52]. To our knowledge, the effects of a high fat diet on SLC6A8 levels or function have not been described. Recent studies, however, report that STE-20/SPS1-related proline-alanine-rich protein kinase (SPAK) and oxidative stress responsive 1 kinase (OSR1) are increased in kidneys of high fat-fed mice [53]. Both SPAK and OSR1 negatively regulate SLC6A8 creatine transport [54]. Further studies are necessary to identify the mechanism underlying decreased brain Cr and tCr following a high fat diet.
As a major regulator of energy metabolism, AMPK is sensitive to cellular AMP/ATP ratios, becoming activated via AMP binding and phosphorylation at its Thr172 residue by upstream kinases at times of metabolic stress [55]. Increasing evidence indicates that AMPK is a neuroprotective factor [56,57], and a high fat diet likely reduces AMPK activity in brain. Hippocampal levels of both total and phosphorylated AMPK were reduced after high fat consumption [57], and our previous study found that a high fat diet greatly reduced the phosphorylation of AMPK in rat striatum [11]. We also found lower activation of AMPK (measured by pAMPK/total AMPK) in the striatum of high fat rats, suggesting either lower cellular energy demand or suboptimal responses to cellular energy demands.
Given the decrease in pAMPK, it is surprising that PGC-1α and its downstream targets NRF-1 and TFAM were not significantly decreased. AMPK activity upregulates PGC-1α [58], a master regulator of mitochondrial function. By increasing transcription of NRF-1 and TFAM, PGC-1α mediates AMPK-induced gene expression [59] and plays a major role in neuroprotection [60]. However, a previous study reporting that a high fat diet decreased PGC-1α in human skeletal muscle without affecting NRF-1 and TFAM [61] suggests that upstream effects do not always affect downstream targets.
Analyses correlating individual 1H-MRS neurochemicals to tissue proteins in the hippocampus and striatum found that pAMPK/AMPK correlated positively with measures indicating maintenance of brain energy metabolism and bioenergetics (tCr, Asp, PE), membrane phospholipid integrity (PE), and decreased edema (Tau) in the hippocampus. In the cell, tCr reflects the metabolically-linked Cr and PCr, which provides an essential energy reserve for normal cellular function [62], while Asp is an important product of the mitochondrial citric acid cycle [63]. In the brain, PE has been reported to reflect membrane synthesis and mitochondrial respiration [64, 65]. Decreased Tau is believed to reflect edema [21]. Despite a lack of difference in Glc concentration between the high fat and chow groups, the significant negative correlation between pAMPK/AMPK and Glc in the hippocampus suggests a relationship between brain Glc levels and brain energy maintenance. None of the other tissue proteins correlated significantly with 1H-MRS measures. Together, these data suggest that lower levels of tCr impair the activation of brain AMPK, even when PCr/Cr ratios are unchanged. This would be consistent with a previous study reporting that creatine supplementation activates AMPK in rat skeletal muscle [66]. They also point to beneficial effects of increased AMPK activation. Finally, these findings also suggest that despite similar gains in body weight within the high fat group, the effects of the high fat diet on brain Glc concentration and energy metabolism are variable, even in inbred F344 rats. The mechanisms that underlie this variable neural response are unclear. Future studies taking advantage of 1H-MRS prior to high fat diet implementation, or during earlier time points under the diet, might offer clues to differential neural vulnerability to metabolic challenge.
Several limitations of our study should be mentioned. We did not measure blood glucose or insulin. Although we are confident that our rats were insulin resistant based on our previous studies [10-12], it is possible that additional correlations may have been revealed between measures of insulin resistance and brain chemistry. The invasive techniques required to measure dopamine function were beyond the scope of this study and at nanomolar levels [67] dopamine is below the minimum concentration levels that can be detected by in vivo 1H-MRS, which are in the low millimolar range. Our previous study reported, however, that a high fat diet attenuates striatal dopamine release without affecting dopamine content [12]. Regarding anesthesia, although isoflurane is known to depress CNS metabolism [68], there is little literature on the effects of anesthesia, specifically isoflurane, on metabolites visible by MRS. Unfortunately, it is not possible to image rodents without anesthesia. Inhaled isoflurane is the preferred anesthetic because it can be adjusted quickly, particularly in animals that are inaccessible deep in the MR magnet bore. As stated in the Methods, we do not believe our conclusions are confounded by our anesthesia protocol.
In summary, our findings indicate that a high fat diet in adult rats results in significant neurochemical alterations in the hippocampus and striatum, brain regions associated with AD and PD. These effects implicate altered neurotransmission and bioenergetic dysfunction following a high fat diet that mimic some, but not all, of the effects of neural aging. These effects may underlie links between diet-induced obesity, metabolic syndrome, type 2 diabetes, and AD and PD [9, 69]. With the increased incidence of obesity and metabolic disorders, it is important to understand these diet-induced effects within the CNS in order to determine their relationship to neurological dysfunction. Our study provides evidence of changes that can be detected with a non-invasive neuroimaging technique that could be translated to human studies. This could further the potential for developing therapeutic options for cognitive and motor impairment in individuals with metabolic disorders.
Highlights.
Measures indicating glutamate turnover were increased in the hippocampus but not the striatum of high fat rats as measured by 1H-MRS.
Serine concentrations were decreased in the hippocampus of high fat rats as measured by 1H-MRS.
Total creatine was decreased in the hippocampus of high fat rats as measured by 1H-MRS.
pAMPK protein content was decreased in the striatum of high fat rats as measured by Western blot.
pAMPK protein content was positively correlated with 1H-MRS measures indicating improved neuronal function and negatively correlated with glucose concentration.
ACKNOWLEDGEMENTS
The work was supported by AG035982, HD02528 and RR016745.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE/CONFLICT OF INTEREST
The authors declare that they have no competing interests.
References
- 1.Marshall JA, Bessesen DH. Dietary fat and the development of type 2 diabetes. Diabetes Care. 2002;25(3):620–622. doi: 10.2337/diacare.25.3.620. [DOI] [PubMed] [Google Scholar]
- 2.Lindqvist A, Mohapel P, Bouter B, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13(12):1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- 3.Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. PPARgamma agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology. 2012;153(1):329–338. doi: 10.1210/en.2011-1502. [DOI] [PubMed] [Google Scholar]
- 4.Uranga RM, Bruce-Keller AJ, Morrison CD, Fernandez-Kim SO, Ebenezer PJ, Zhang L, Dasuri K, Keller JN. Intersection between metabolic dysfunction, high fat diet consumption, and brain aging. J Neurochem. 2010;114(2):344–361. doi: 10.1111/j.1471-4159.2010.06803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce-Keller AJ, Keller JN, Morrison CD. Obesity and vulnerability of the CNS. Biochim Biophys Acta. 2009;1792(5):395–400. doi: 10.1016/j.bbadis.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42(5):776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 7.Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258–1263. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- 8.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr., Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O'Brien PC, Palumbo PJ. The risk of dementia among persons with diabetes mellitus: a population-based cohort study. Ann N Y Acad Sci. 1997;826:422–427. doi: 10.1111/j.1749-6632.1997.tb48496.x. [DOI] [PubMed] [Google Scholar]
- 10.Morris JK, Bomhoff GL, Stanford JA, Geiger PC. Neurodegeneration in an animal model of Parkinson's disease is exacerbated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2010;299(4):R1082–1090. doi: 10.1152/ajpregu.00449.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma D, Shuler JM, Raider KD, Rogers RS, Wheatley JL, Geiger PC, Stanford JA. Effects of discontinuing a high-fat diet on mitochondrial proteins and 6-hydroxydopamine-induced dopamine depletion in rats. Brain Res. 2015;1613:49–58. doi: 10.1016/j.brainres.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris JK, Bomhoff GL, Gorres BK, Davis VA, Kim J, Lee PP, Brooks WM, Gerhardt GA, Geiger PC, Stanford JA. Insulin resistance impairs nigrostriatal dopamine function. Exp Neurol. 2011;231(1):171–180. doi: 10.1016/j.expneurol.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drayer B, Burger P, Darwin R, Riederer S, Herfkens R, Johnson GA. MRI of brain iron. AJR Am J Roentgenol. 1986;147(1):103–110. doi: 10.2214/ajr.147.1.103. [DOI] [PubMed] [Google Scholar]
- 14.Hebert MA, Gerhardt GA. Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in fischer 344 rats. Brain Res. 1998;797(1):42–54. doi: 10.1016/s0006-8993(98)00370-9. [DOI] [PubMed] [Google Scholar]
- 15.Ke Y, Chang YZ, Duan XL, Du JR, Zhu L, Wang K, Yang XD, Ho KP, Qian ZM. Age-dependent and iron-independent expression of two mRNA isoforms of divalent metal transporter 1 in rat brain. Neurobiol Aging. 2005;26(5):739–748. doi: 10.1016/j.neurobiolaging.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Venkateshappa C, Harish G, Mythri RB, Mahadevan A, Bharath MM, Shankar SK. Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: implications for Parkinson's disease. Neurochem Res. 2012;37(2):358–369. doi: 10.1007/s11064-011-0619-7. [DOI] [PubMed] [Google Scholar]
- 17.Venkateshappa C, Harish G, Mahadevan A, Srinivas Bharath MM, Shankar SK. Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: implications for neurodegeneration in Alzheimer's disease. Neurochem Res. 2012;37(8):1601–1614. doi: 10.1007/s11064-012-0755-8. [DOI] [PubMed] [Google Scholar]
- 18.Choi IY, Lee P, Wang WT, Hui D, Wang X, Brooks WM, Michaelis EK. Metabolism changes during aging in the hippocampus and striatum of glud1 (glutamate dehydrogenase 1) transgenic mice. Neurochem Res. 2014;39(3):446–455. doi: 10.1007/s11064-014-1239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang WT, Lee P, Yeh HW, Smirnova IV, Choi IY. Effects of acute and chronic hyperglycemia on the neurochemical profiles in the rat brain with streptozotocin-induced diabetes detected using in vivo (1)H MR spectroscopy at 9.4 T. J Neurochem. 2012;121(3):407–417. doi: 10.1111/j.1471-4159.2012.07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris JL, Yeh HW, Swerdlow RH, Choi IY, Lee P, Brooks WM. High-field proton magnetic resonance spectroscopy reveals metabolic effects of normal brain aging. Neurobiol Aging. 2014;35(7):1686–1694. doi: 10.1016/j.neurobiolaging.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris JL, Yeh HW, Choi IY, Lee P, Berman NE, Swerdlow RH, Craciunas SC, Brooks WM. Altered neurochemical profile after traumatic brain injury: (1)H-MRS biomarkers of pathological mechanisms. J Cereb Blood Flow Metab. 2012;32(12):2122–2134. doi: 10.1038/jcbfm.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29(6):804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- 23.Pfeuffer J, Tkác I, Provencher SW, Gruetter R. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time (1)H NMR spectra of the rat brain. J Magn Reson. 1999;141(1):104–120. doi: 10.1006/jmre.1999.1895. [DOI] [PubMed] [Google Scholar]
- 24.Tkác I, Rao R, Georgieff MK, Gruetter R. Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1H NMR spectroscopy. Magn Reson Med. 2003;50(1):24–32. doi: 10.1002/mrm.10497. [DOI] [PubMed] [Google Scholar]
- 25.Morris JK, Zhang H, Gupte AA, Bomhoff GL, Stanford JA, Geiger PC. Measures of striatal insulin resistance in a 6-hydroxydopamine model of Parkinson's disease. Brain Res. 2008;1240:185–195. doi: 10.1016/j.brainres.2008.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sookoian S, Pirola CJ. Alanine and aspartate aminotransferase and glutamine-cycling pathway: their roles in pathogenesis of metabolic syndrome. World J Gastroenterol. 2012;18(29):3775–3781. doi: 10.3748/wjg.v18.i29.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sickmann HM, Waagepetersen HS, Schousboe A, Benie AJ, Bouman SD. Obesity and type 2 diabetes in rats are associated with altered brain glycogen and amino-acid homeostasis. J Cereb Blood Flow Metab. 2010;30(8):1527–1537. doi: 10.1038/jcbfm.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valladolid-Acebes I, Merino B, Principato A, Fole A, Barbas C, Lorenzo MP, García A, Del Olmo N, Ruiz-Gayo M, Cano V. High-fat diets induce changes in hippocampal glutamate metabolism and neurotransmission. Am J Physiol Endocrinol Metab. 2012;302(4):E396–402. doi: 10.1152/ajpendo.00343.2011. [DOI] [PubMed] [Google Scholar]
- 29.Langley SC, York DA. Increased type II glucocorticoid-receptor numbers and glucocorticoid-sensitive enzyme activities in the brain of the obese Zucker rat. Brain Res. 1990;533(2):268–274. doi: 10.1016/0006-8993(90)91349-l. [DOI] [PubMed] [Google Scholar]
- 30.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–38. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calvo-Ochoa E, Hernandez-Ortega K, Ferrera P, Morimoto S, Arias C. Short-term high-fat-and fructose feeding produces insulin signaling alterations accompanied by neurite and synaptic reduction and astroglial activation in the rat hippocampus. J Cereb Blood Flow Metab. 2014;34(6):1001–1008. doi: 10.1038/jcbfm.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karczewska-Kupczewska M, Tarasow E, Nikolajuk A, Stefanowicz M, Matulewicz N, Otziomek E, Górska M, Straczkowski M, Kowalska I. The effect of insulin infusion on the metabolites in cerebral tissues assessed with proton magnetic resonance spectroscopy in young healthy subjects with high and low insulin sensitivity. Diabetes Care. 2013;36(9):2787–2793. doi: 10.2337/dc12-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas AG, Olkowski JL, Slusher BS. Neuroprotection afforded by NAAG and NAALADase inhibition requires glial cells and metabotropic glutamate receptor activation. Eur J Pharmacol. 2001;426:35–38. doi: 10.1016/s0014-2999(01)01198-0. [DOI] [PubMed] [Google Scholar]
- 35.Cai Z, Lin S, Rhodes PG. Neuroprotective effects of N-acetylaspartylglutamate in a neonatal rat model of hypoxia-ischemia. Eur J Pharmacol. 2002;437(3):139–45. doi: 10.1016/s0014-2999(02)01289-x. [DOI] [PubMed] [Google Scholar]
- 36.Wolosker H. Serine racemase and the serine shuttle between neurons and astrocytes. Biochim Biophys Acta. 2011;1814:1558–1566. doi: 10.1016/j.bbapap.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Radzishevsky I1, Sason H, Wolosker H. D-serine: physiology and pathology. Curr Opin Clin Nutr Metab Care. 2013;16(1):72–75. doi: 10.1097/MCO.0b013e32835a3466. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Song Y, Wang H, Liu C, Zhongzhe L, Liu Y, Kong Y. Insulin treatment prevents the increase in D-serine in hippocampal CA1 area of diabetic rats. Am J Alzheimers Dis Other Demen. 2015;30(2):201–208. doi: 10.1177/1533317514545379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turpin FR, Potier B, Dulong JR, Sinet PM, Alliot J, Oliet SH, Dutar P, Epelbaum J, Mothet JP, Billard JM. Reduced serine racemase expression contributes to age-related deficits in hippocampal cognitive function. Neurobiol Aging. 2011;32(8):1495–1504. doi: 10.1016/j.neurobiolaging.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Larkin LM, Reynolds TH, Supiano MA, Kahn BB, Halter JB. Effect of aging and obesity on insulin responsiveness and glut-4 glucose transporter content in skeletal muscle of Fischer 344 x Brown Norway rats. J Gerontol A Biol Sci Med Sci. 2001;56(11):B486–492. doi: 10.1093/gerona/56.11.b486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–1533. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- 42.Zuurbier CJ, Keijzers PJ, Koeman A, Van Wezel HB, Hollmann MW. Anesthesia's effects on plasma glucose and insulin and cardiac hexokinase at similar hemodynamics and without major surgical stress in fed rats. Anesth Analg. 2008;106(1):135–142. doi: 10.1213/01.ane.0000297299.91527.74. [DOI] [PubMed] [Google Scholar]
- 43.Choe CU, Nabuurs C, Stockebrand MC, Neu A, Nunes P, Morellini F, Sauter K, Schillemeit S, Hermans-Borgmeyer I, Marescau B, Heerschap A, Isbrandt D. L-arginine:glycine amidinotransferase deficiency protects from metabolic syndrome. Hum Mol Genet. 2013;22(1):110–123. doi: 10.1093/hmg/dds407. [DOI] [PubMed] [Google Scholar]
- 44.Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beal MF. Neuroprotective effects of creatine. Amino Acids. 2011;40(5):1305–1313. doi: 10.1007/s00726-011-0851-0. [DOI] [PubMed] [Google Scholar]
- 46.DeVivo DC, Leckie MP, Ferrendelli JS, McDougal DB., Jr Chronic ketosis and cerebral metabolism. Ann Neurol. 1978;3(4):331–337. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- 47.Lu C, Kumar PA, Sun J, Aggarwal A, Fan Y, Sperling MA, Lumeng CN, Menon RK. Targeted deletion of growth hormone (GH) receptor in macrophage reveals novel osteopontin-mediated effects of GH on glucose homeostasis and insulin sensitivity in diet-induced obesity. J Biol Chem. 2013;288(22):15725–15735. doi: 10.1074/jbc.M113.460212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.List EO, Palmer AJ, Berryman DE, Bower B, Kelder B, Kopchick JJ. Growth hormone improves body composition, fasting blood glucose, glucose tolerance and liver triacylglycerol in a mouse model of diet-induced obesity and type 2 diabetes. Diabetologia. 2009;52(8):1647–1655. doi: 10.1007/s00125-009-1402-z. [DOI] [PubMed] [Google Scholar]
- 49.Amin KA, Kamel HH, Abd Eltawab MA. The relation of high fat diet, metabolic disturbances and brain oxidative dysfunction: modulation by hydroxy citric acid. Lipids Health Dis. 2011;10:74. doi: 10.1186/1476-511X-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao X, Bassirat M, Zeinab K, Helme RD. Effects of diabetes on creatine kinase activity in streptozotocin-diabetic rats. Chin Med J (Engl) 1999;112(11):1028–1031. [PubMed] [Google Scholar]
- 51.Genet S, Kale RK, Baquer NZ. Effects of free radicals on cytosolic creatine kinase activities and protection by antioxidant enzymes and sulfhydryl compounds. Mol Cell Biochem. 2000;210(1-2):23–28. doi: 10.1023/a:1007071617480. [DOI] [PubMed] [Google Scholar]
- 52.Tachikawa M, Hosoya K-i. Transport characteristics of guanidino compounds at the blood-brain barrier and blood-cerebrospinal fluid barrier: relevance to neural disorders. Fluids Barriers CNS. 2011;8:13. doi: 10.1186/2045-8118-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davies M, Fraser SA, Galic S, Choy SW, Katerelos M, Gleich K, Kemp BE, Mount PF, Power DA. Novel mechanisms of Na+ retention in obesity: phosphorylation of NKCC2 and regulation of SPAK/OSR1 by AMPK. Am J Physiol Renal Physiol. 2014;307(1):F96–F106. doi: 10.1152/ajprenal.00524.2013. [DOI] [PubMed] [Google Scholar]
- 54.Fezai M, Borras EB, Ben-Attia M, Hoseinzadeh Z, Lang F. Negative regulation of the creatine transporter SLC6A8 by SPAK and OSR1. Kidney Blood Press Res. 2014;39(6):546–554. doi: 10.1159/000368465. [DOI] [PubMed] [Google Scholar]
- 55.Amato S, Man HY. Bioenergy sensing in the brain: the role of AMP-activated protein kinase in neuronal metabolism, development and neurological diseases. Cell Cycle. 2011;10(20):3452–3460. doi: 10.4161/cc.10.20.17953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M. AMP-activated protein kinase: a potential player in Alzheimer's disease. J Neurochem;2011;118(4):460–474. doi: 10.1111/j.1471-4159.2011.07331.x. [DOI] [PubMed] [Google Scholar]
- 57.Wu A, Ying Z, Gomez-Pinilla F. Oxidative stress modulates Sir2alpha in rat hippocampus and cerebral cortex. Eur J Neurosci. 2006;23(10):2573–2580. doi: 10.1111/j.1460-9568.2006.04807.x. [DOI] [PubMed] [Google Scholar]
- 58.Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:1340–1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- 59.Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20(2):98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 61.Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54(7):1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 62.Haga KK, Khor YP, Farrall A, Wardlaw JM. A systematic review of brain metabolite changes, measured with 1H magnetic resonance spectroscopy, in healthy aging. Neurobiol Aging. 2009;30:353–363. doi: 10.1016/j.neurobiolaging.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Moffett JR, Tieman SB, Winberger DR, Coyle JT, Namboodiri AM. N-Acetylaspartate: a unique neuronal molecule in the central nervous system. In: Back N, Cohen IR, Kritchevsky D, Lajtha A, Paoletti R, editors. Adv Exp Med Biol. Springer; New York, NY.: 2006. [Google Scholar]
- 64.Wijnen JP, Scheenen TW, Klomp DW, Heerschap A. 31P magnetic resonance spectroscopic imaging with polarisation transfer of phosphomono- and diesters at 3T in the human brain: relation with age and spatial differences. NMR Biomed. 2010;23:968–976. doi: 10.1002/nbm.1523. [DOI] [PubMed] [Google Scholar]
- 65.Modi HR, Katyare SS, Patel MA. Ageing-induced alterations in lipid/phospholipid profiles of rat brain and liver mitochondria: implications for mitochondrial energy-linked functions. J Membr Biol. 2008;221:51–60. doi: 10.1007/s00232-007-9086-0. [DOI] [PubMed] [Google Scholar]
- 66.Ceddia RB, Sweeney G. Creatine supplementation increases glucose oxidation and AMPK phosphorylation and reduces lactate production in L6 rat skeletal muscle cells. J Physiol. 2004;555(Pt 2):409–421. doi: 10.1113/jphysiol.2003.056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanford JA, Currier TD, Purdom MS, Gerhardt GA. Nomifensine reveals age-related changes in K+-evoked striatal DA overflow in F344 rats. Neurobiol Aging. 2001;22:495–502. doi: 10.1016/s0197-4580(00)00243-8. [DOI] [PubMed] [Google Scholar]
- 68.Maekawa T, Tommasino C, Shapiro HM, Keifer-Goodman J, Kohlenberger RW. Local cerebral blood flow and glucose utilization during isoflurane anesthesia in the rat. Anesthesiology. 1986;65(2):144–151. doi: 10.1097/00000542-198608000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Hu G, Housilahti P, Bidel S, Antikainen R, Tuomilehto J. Type 2 diabetes and the risk of Parkinson's disease. Neurology. 2007;67:1955–1959. doi: 10.2337/dc06-2011. [DOI] [PubMed] [Google Scholar]