Summary
E4402 (RESORT) was a phase 3 randomized prospective trial comparing maintenance rituximab (MR) versus a retreatment (RR) dosing strategy in asymptomatic, low tumor burden (GELF criteria) indolent lymphoma. A planned exploratory sub-study compared the two strategies for small lymphocytic (SLL) and marginal zone lymphomas (MZL). Patients responding to R weekly × 4 were randomized to MR (single dose R every 3 months until treatment failure) or RR (R weekly × 4) at the time of each progression until treatment failure. The primary endpoint was time to treatment failure (TTTF). Patients with SLL (n = 57), MZL (n = 71) and unclassifiable small B-cell lymphoma (n = 3) received induction R. The overall response rate was 40% (95% CI 31-49%; SLL ORR 22.8%; MZL ORR 52.1%); all 52 responders were randomized. At a median of 4.3 years from randomization, treatment failure occurred in 18/23 RR and 15/29 MR. The median TTTF was 1.4 years for RR and 4.8 years for MR (p=.012); median time to first cytotoxic therapy was 6.3 years for RR, not reached for MR (p=.0002). Survival did not differ (p=0.72). In low tumor burden SLL and MZL patients responding to R induction, MR significantly improved TTTF as compared with RR.
Keywords: B-cell lymphoma, indolent lymphoma, rituximab, maintenance
Introduction
The indolent B-cell non-Hodgkin lymphomas (NHL) are clinically and biologically heterogeneous, both among and within individual entities. Standard treatment approaches have yet to be established for the non-follicular subtypes of small lymphocytic lymphoma (SLL) and the marginal zone lymphomas (MZL), including splenic, nodal and extranodal (MALT [mucosa-associated lymphoid tissue]). However, given the typically slow pace of progression and the incurability of patients with advanced-stage disease, a watch-and-wait strategy is often pursued wherein treatment is deferred until progression or development of disease-related symptoms. Whether this strategy remains the most appropriate in the rituximab era is unknown.
Small phase II studies have shown clinical response and a well-tolerated safety profile for the anti-CD20 monoclonal antibody rituximab when administered as front-line, single-agent treatment of SLL and MZL (Hainsworth et al, 2005; Conconi et al, 2003; Kalpadakis et al, 2013). The use of extended schedule (maintenance) rituximab has also shown improved progression-free survival (PFS), although it remains uncertain if the clinical benefit and safety of maintenance therapy merit its routine use versus retreatment with rituximab at the time of disease progression.
To test the hypothesis that patients with low tumor burden indolent NHL who respond to front-line rituximab treatment would have prolonged disease control with extended schedule dosing (Maintenance Rituximab) as compared to rituximab retreatment at the time of disease progression, the Eastern Cooperative Oncology Group (ECOG) designed the RESORT trial (Rituximab Extended Schedule or Retreatment Trial; NCT01406782). The study was powered to definitively test this hypothesis in FL; an exploratory analysis was also conducted in RESORT for SLL and MZL and is the focus of this report.
Methods
Patient eligibility
Patients with previously untreated SLL, nodal MZL, splenic MZL, extranodal MZL (MALT) and FL grade 1 or 2 were eligible as part of a pre-specified exploratory analysis; the FL cohort is not included here and is reported separately (Kahl et al, 2014). Patients were eligible if they had stage III or IV disease, at least one measurable lesion of 2 cm or larger, and low tumor burden by GELF criteria (Brice et al, 1997): no single tumor mass ≥ 7 cm, fewer than 3 nodal masses > 3 cm, no systemic or B symptoms, no splenomegaly > 16 cm by CT scan, no risk of organ compression, no leukemic phase > 5000/mcL, and no cytopenias (neutrophil count > 1500/mcL, hemoglobin > 10 gm/dL, platelets > 100,000/mcL). Patients were ineligible if they were HIV positive, had active infection requiring antibiotics or tested positive for hepatitis B surface antigen, or were pregnant or breast feeding.
Pathology review
Central pathology review by an ECOG hematopathologist (RDG) was utilized to confirm that diagnostic biopsies were classified according to WHO criteria (Swerdlow et al, 2008). Repeat tissue biopsy was mandated if the interval from the initial biopsy to study entry was > 1 year.
Baseline studies
Baseline history, physical examination, height, weight, and performance status were recorded at the time of study entry. Laboratory studies within 2 weeks of study entry included complete blood counts and chemistry panel, lactate dehydrogenase, β2 microglobulin, quantitative immunoglobulin levels and hepatitis B antigen test, as well as pregnancy test if applicable. Baseline CT imaging of the neck, chest, abdomen and pelvis were obtained within 6 weeks prior to study entry. Bone marrow biopsy was obtained at study entry unless a previous sample positive for lymphoma was documented within the preceding 12 months.
Correlative studies
Quality of life assessments (QOL) included patient-reported outcomes of illness-related anxiety and health-related QOL, and will be reported separately (Wagner et al, 2015). Quantitative serum immunoglobulin levels were obtained at baseline and serially thereafter, with results summarized below.
Protocol treatment
Institutional Review Board approval was established at each study site, with signed consent obtained for each enrolled patient. All patients received induction rituximab 375 mg/m2 weekly in weeks 1-4 followed by restaging CT scans at week 13. Responses were classified by the NCI 1999 criteria in effect at the time of trial design (Cheson et al, 1999). Patients with partial response (PR), complete response (CR) or CR unconfirmed (CRu) were randomized to maintenance rituximab (MR) or to retreatment rituximab (RR).
MR patients received one dose of rituximab 375 mg/m2 every 3 months until treatment failure. The maintenance dosing interval was based upon pharmacokinetic analyses available at the time of study protocol design (Berinstein et al, 1998; Gordan et al, 2005). Responding patients in the RR arm were observed and treated with rituximab weekly × 4 upon disease progression. Retreatment was repeated until treatment failure. Patients in both the RR and MR arms underwent physical examination and laboratory studies every 13 weeks with restaging CT imaging every 26 weeks.
Statistical considerations
The primary endpoint of the study was time to treatment failure (TTTF). Treatment failure in the RR arm was defined as < PR to retreatment, time to progression (TTP) < 26 weeks, initiation of alternative therapy, or inability to complete planned therapy. Treatment failure in the MR arm included disease progression anytime during maintenance, initiation of alternative therapy, or the inability to complete planned therapy. Secondary endpoints included time to first cytotoxic therapy and treatment-related toxicity. Both were defined as the time from step 2 randomization. Additional analyses included overall survival, risk of transformation, and duration of response, which was defined as the time from first documented response to first progression.
The study was designed among follicular patients, while non-follicular indolent histologies were enrolled as part of a planned exploratory analysis. Interim analysis of TTTF was planned for all semi-annual Data Monitoring Committee (DMC) meetings beginning at approximately 25% of the planned events. At the Fall 2011 meeting, with 78% information available, the DMC concluded on futility among follicular patients for the study and decided to release the results. Patients were given the option to come off study and choose best treatment strategy based on personal choice. Since inability to complete protocol therapy was considered an event for the primary endpoint of TTTF, treatment withdrawals after the DMC letter were not to count as a treatment failure. The data for the TTTF analysis was locked as of November 1, 2011; there was no other censoring event except the administrative locking. For all other endpoints, the data was locked on September 20, 2013.
Descriptive statistics were used to summarize baseline demographic and clinical characteristics for the patient population. Comparison between treatment arms was conducted according to the intent-to-treat principal among all randomized patients with correct histology, regardless of eligibility status. The log-rank test was used for the comparison of all time-to-event endpoints, stratified on age (< 60 years vs. other) and time from diagnosis (< 1 year vs. ≥ 1 year). The Kaplan-Meier method and Cox proportional regression models were used to estimate failure rate, hazard ratios (HRs), and 95% CIs. Fisher's exact test/Chi-square test and t-test were used to compare proportions and means, respectively. Toxicity was evaluated on all patients who received any study treatment, regardless of eligibility. A two-sided P-value of 0.05 or less was used to claim statistical significance.
Results
Patient characteristics
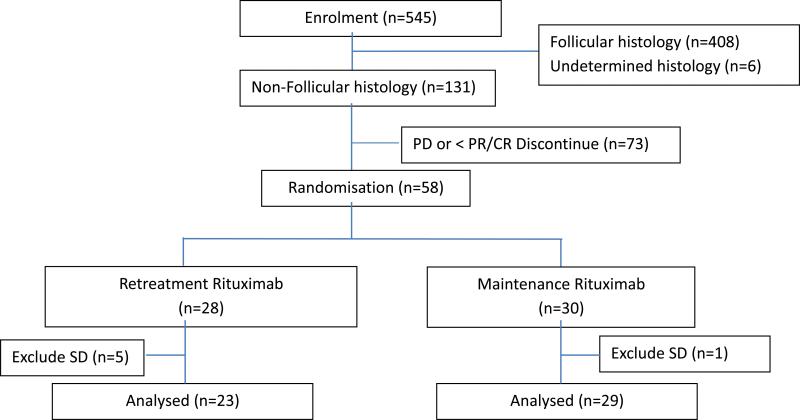
The study was activated in November 2003 and closed to accrual in September 2008. 131 patients with non-follicular lymphoma were enrolled (Figure 1). Follicular lymphoma results (408 patients) are reported separately (Kahl et al, 2014). Overall, 93% of non-follicular cases had tissue submitted for central pathology review.
Figure 1.
Consort diagram: Study enrollment and randomization.
The baseline characteristics of the non-follicular cohort are summarized in Table 1. Of the 131 patients, 57 (41.6%) had SLL, 71 MZL (51.8%) and 3 were unclassifiable (2.2%). Eight of the 131 proved to be ineligible for enrollment (no core biopsy to confirm histology, 1; no measurable disease, 1; did not meet GELF criteria, 3; stage I disease, 1; B-symptoms, 1; unclassified B-NHL, 1). 93% of patients were within one year of initial diagnosis at the time of study enrollment.
Table 1.
Characteristics of small lymphocytic (SLL) and marginal zone lymphoma (MZL) patients enrolled on E4402
| Characteristic | All Patients (N = 131) | Randomized Patients | |
|---|---|---|---|
| RR (N = 23) | MR (N =29) | ||
| Median age | 65.5 (29.5-86.3) | 61.3 (47.3-86.3) | 65.5 (38.5-85.4) |
| Male sex (%) | 45.8 | 30.4 | 51.7 |
| White race (%) | 93 | 95.7 | 93.1 |
| PS 0 (%) | 80.2 | 87 | 93 |
| BM involvement (%) | 63.4 | 39.1 | 62.1 |
| Elevated β2M (%) | 71.7 | 60.9 | 69 |
| Elevated LDH (%) | 15.3 | 17.4 | 27.6 |
| Hgb < 12 g/dL (%) | 22 | 8.7 | 24.1 |
| Stage (%) | |||
| I-II | 1.5 | 0 | 3.4 |
| III | 27.4 | 30.3 | 20.6 |
| IV | 80.9 | 69.6 | 75.9 |
| Time from diagnosis < 1 year (%) | 93.1 | 91.3 | 89.7 |
| Histology (%) | |||
| SLL | 43.5 | 26.1 | 24.1 |
| MZL, splenic | 3.8 | 4.3 | 13.8 |
| MZL, nodal | 21.3 | 34.8 | 31 |
| MZL, extranodal | 29 | 30.4 | 27.6 |
| Unclassifiable | 2.3 | 4.3 | 3.4 |
Of 58 patients originally assigned to step 2 randomization, two were ineligible and six achieved <PR to step 1 rituximab therapy. Thus, 52 patients were correctly randomized to RR or MR. Treatment arms were balanced. Four of the five patients with splenic MZL were randomized to RR.
Clinical responses
The overall response rate (ORR) to induction rituximab weekly × 4 (Step 1) was 39.7% (95% CI 31.2-48.6%), significantly lower than the ORR of 71.8% (95% CI 67-76%) observed in the follicular lymphoma cohort (p<.0001). The ORR for SLL was only 22.8%, all PR, with a higher ORR for MZL of 52.1%. CR/CRu was achieved in 9 of the 71 MZL patients (12.7%). All patients with splenic MZL (n=5) responded with 2 CR, as did 15/38 MALT lymphomas with 6 CR and 17/28 nodal MZL with 1 CR (Table 2). Of patients with initial PR following Step 1, 6/18 on RR and 16/24 on MR converted to CR/CRu. Two of the three patients with unclassifiable indolent B-cell lymphoma responded, including one with CR.
Table 2.
Response to rituximab induction therapy by WHO lymphoma subtype (Step 1)
| Response3 | CLL/SLL1 n (%) | Splenic MZL2 n (%) | Extranodal MZL n (%) | Nodal MZL n (%) | Unclassifiable B-cell n (%) | Total n (%) |
|---|---|---|---|---|---|---|
| CR | 0 | 2 (40) | 6 (15.8) | 0 | 1 (33.3) | 9 (6.9) |
| CRu | 0 | 0 | 0 | 1 (3.6) | 0 | 1 (0.8) |
| PR | 13 (22.8) | 3 (60) | 9 (23.7) | 16 (57.1) | 1 (33.3) | 42 (32.1) |
| SD | 41 (71.9) | 0 | 19 (50) | 11 (39.3) | 1 (33.3) | 72 (55) |
| PD | 2 (3.5) | 0 | 1 (2.6) | 0 | 0 | 3 (2.3) |
| Unevaluable | 1 (1.8) | 0 | 3 (7.9) | 0 | 0 | 4 (3.1) |
| Total | 57 | 5 | 38 | 28 | 3 | 131 |
Chronic lymphocytic leukemia/Small lymphocytic lymphoma
Marginal zone lymphoma
CR = Complete remission, CRu = CR unconfirmed, PR = Partial remission, SD = Stable disease, PD = Progressive disease
Time to treatment failure, response duration and time to first cytotoxic therapy
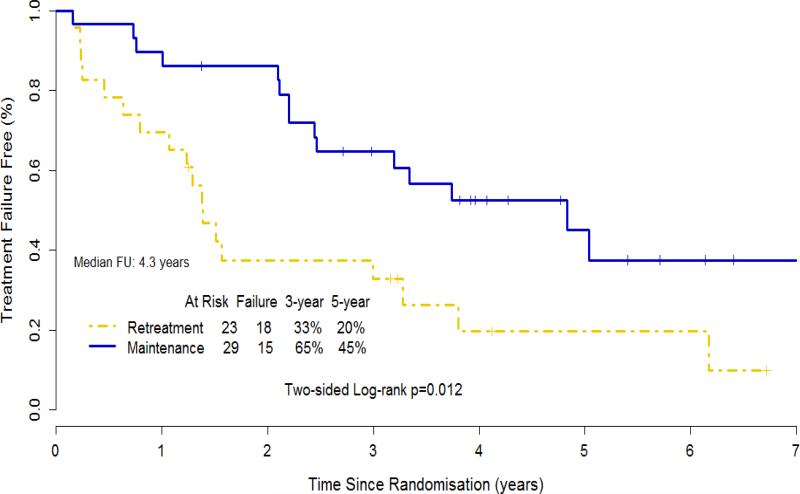
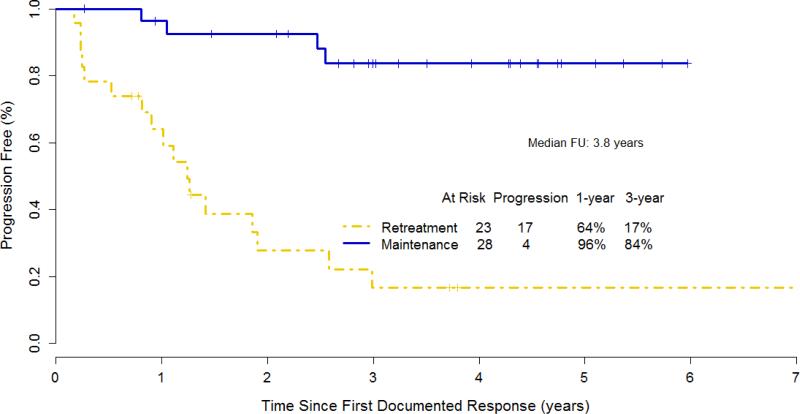
With a median follow-up of 4.3 years from step 2 randomization, a total of 33 treatment failures were observed: 18/23 for RR and 15/29 for MR patients (Table 3). Time to progression < 6 months and initiation of alternative therapy were the most frequent failure types in RR, while patient withdrawal or treatment refusal was more frequent in MR patients. The median time to rituximab failure was 1.39 years for RR and 4.83 years for MR (p=.012) (Figure 2). The median duration of response was 1.24 years for RR (considers only the first rituximab course) and not reached for MR (Figure 3).
Table 3.
Reasons for treatment failure after randomization to retreatment rituximab (RR) or maintenance rituximab (MR).
| ASSIGNED TREATMENT ARM | ||||
|---|---|---|---|---|
| RR (n) | MR (n) | TOTAL | ||
| (n) | % | |||
| Failure Type | ||||
| Adverse Event/side effect/complications | - | 2 | 2 | 6.1 |
| Patient withdraw/refuse | 3 | 5 | 8 | 24.2 |
| Alternative Therapy | 5 | - | 5 | 15.2 |
| Other Disease | 1 | 3 | 4 | 12.1 |
| No response (Arm A) | 2 | - | 2 | 6.1 |
| Time to Progression <=6 mo | 7 | 3 | 10 | 30.3 |
| Other | - | 2 | 2 | 6.1 |
| Total | 18 | 15 | 33 | 100.00 |
Figure 2.
Kaplan-Meier estimate for time to rituximab failure for 52 patients with small lymphocytic or marginal zone lymphoma, including 2 with unclassifiable indolent B-cell lymphoma.
Figure 3.
Kaplan- Meier Estimate for duration of response for 51 patients (data missing for 1 case) with small lymphocytic or marginal zone lymphoma, including 2 patients with unclassifiable indolent B-cell lymphoma.
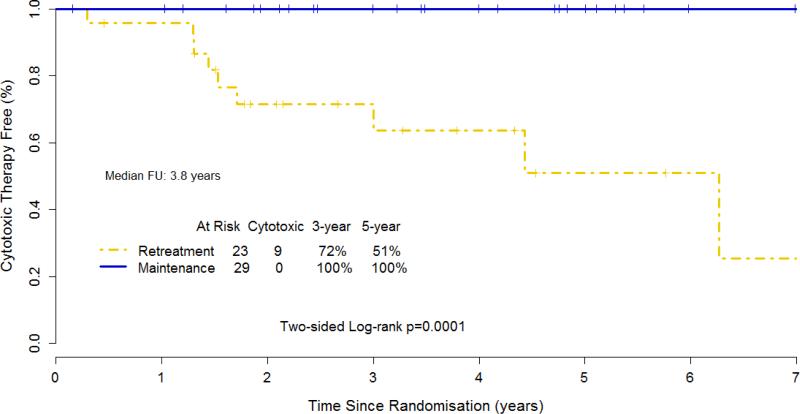
The time to first cytotoxic therapy (chemotherapy or radiation therapy; median follow-up 3.8 years) was 6.3 years for RR and not reached for MR (p = .0002; Figure 4). No patient randomized to MR had received cytotoxic treatment at the time of last analysis.
Figure 4.
Kaplan-Meier estimate for time to first cytotoxic therapy for 52 patients with small lymphocytic or marginal zone lymphoma, including 2 with unclassifiable indolent B-cell lymphoma, who responded to induction therapy with rituximab.
Disease transformation and overall survival
Of the 131 non-follicular patients, four randomized to RR developed transformed lymphoma. These included two extranodal MZL (MALT) and a nodal MZL patient who developed diffuse large B-cell lymphoma, and a patient with nodal MZL who developed follicular lymphoma. A patient with SLL randomized to MR developed Burkitt-like lymphoma.
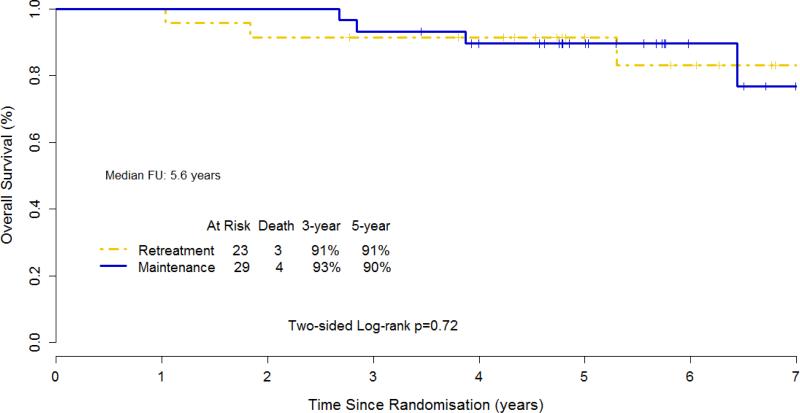
Overall survival (OS) among the 52 randomized patients did not differ between the two study arms, with 5-year OS 91% for RR and 90% for MR (p=0.72) (Figure 5).
Figure 5.
Kaplan- Meier Estimate for overall survival for 52 patients with small lymphocytic or marginal zone lymphoma, including 2 patients with unclassifiable indolent B-cell lymphoma.
Toxicity
Grade 3 toxicities were infrequent and included allergic reaction or rash (3 events), ventricular arrhythmia, fatigue, infection, syncope, neuropathic pain and dyspnea (1 event each), and cytokine release syndrome (2 events) (Table 4). One episode of grade 4 back pain was documented; grade 4 neutropenia occurred in one patient on MR.
Table 4.
Toxicity incidence, grade 3-5, with induction rituximab (N=119)
| Grade | |||
|---|---|---|---|
| Toxicity Type | 3 (n) | 4 (n) | 5 (n) |
| Allergic reaction | 1 | - | |
| Ventricular arrhythmia NOS | 1 | - | - |
| Fatigue | 1 | - | - |
| Rash/desquamation | 1 | - | - |
| Urticaria | 1 | - | - |
| Infection, Grade 0-2 neutropenia, nerve-peripheral | 1 | - | - |
| Syncope | 1 | - | - |
| Back, pain | - | 1 | - |
| Neuropathic, pain | 1 | - | - |
| Dyspnea | 1 | - | - |
| Cytokine release syndrome | 2 | - | - |
| WORST DEGREE | 7 | 1 | - |
Second primary cancers were observed in nine patients receiving only induction rituximab, with another five identified in RR and four in MR patients. Aside from one patient who developed CLL and another with acute non-lymphocytic leukemia, all second primaries were non-hematopoietic malignancies. Health-related quality of life was comparable between the treatment arms, indicating MR was well tolerated. HRQL results are based on an aggregated sample (follicular and non-follicular) due to the sample size and are presented separately (Wagner et al, 2015).
Resource utilization
Accounting for all administered rituximab doses for the 52 patients randomized to step 2, including the 4 induction doses, patients assigned to RR received an average of 5.74 doses (range 4-12 , median 4) while those in the MR arm received on average 18.5 doses (range 5-31 ; median 19).
Discussion
Achieving and maintaining durable remission in patients with advanced-stage SLL and MZL remains an unmet challenge in lymphoma patient care. Historically, patients with symptomatic disease received chemotherapy or immuno-chemotherapy, while asymptomatic patients were followed and treated at the time of disease progression. Immunotherapy with rituximab has shown single-agent activity and an acceptable safety profile in previously untreated patients and in those with relapsed disease (Hainsworth et al, 2005; Conconi et al, 2003; Kalpadakis et al, 2013). The present study sought to extend this experience in a multicenter, cooperative group setting with the rationale that asymptomatic patients with low tumor burden would be optimal for standard single-agent rituximab induction therapy. The experimental phase of this exploratory analysis in SLL and MZL tested the hypothesis that MR would prolong disease control and delay the time to traditional cytotoxic treatment, either chemotherapy or radiation therapy.
SLL has low level CD20 expression and showed lower ORR and CR compared to FL in the original pivotal rituximab trial (McLaughlin et al, 1998). This has been confirmed in the present study, with 22.8% PR and no CR among 57 patients. However, SLL responders benefitted from MR versus RR, with no MR patient progressing to cytotoxic therapy during the time of follow-up despite the fact that no patient achieved CR with maintenance therapy.
Treatment in both the MR and RR arms was well tolerated, with infrequent grade >/= 3 toxicities. The occurrence of second primary cancers in both this non-follicular patient cohort and in the follicular lymphoma patients treated on this study (Kahl et al, 2014) was relatively high. It is unclear whether the explanation for this observation relates to the underlying indolent lymphoma, age of the study population, or an association with anti-CD20 therapy warrants further investigation.
Obinutuzumab (GA101), the first type 2 anti-CD20 therapeutic monoclonal antibody, was recently shown to provide higher ORR when given in combination with chlorambucil as compared with rituximab-chlorambucil for the initial therapy of CLL (Goede et al, 2014). Potential mechanisms for obinutuzumab efficacy include enhanced antibody-dependent cytotoxicity (ADCC) and direct tumor cell killing, or improved synergy with chlorambucil versus rituximab.
The marginal zone lymphomas include the clinically heterogeneous subtypes of nodal, extranodal (MALT) and splenic MZL. CD20 expression is typically higher in MZL than in SLL, but responsiveness to single-agent rituximab, while higher, is still below that seen in follicular lymphomas. The ORR for MZL in the present study was 52.1%, with 17/28 nodal, 15/38 extranodal and 5/5 splenic MZL patients responding to induction rituximab. Responses improved following randomization, with one-third of MZL patients with PR converting to CR/CRu on both the MR (16/24) and the RR arms (6/18). Importantly, no SLL or MZL patient who responded to induction rituximab and received MR proceeded to cytotoxic therapy during study follow-up.
Given the very different response rates and time to rituximab failure observed for SLL compared to MZL, and for each of these subtypes as compared with FL (Kahl et al, 2014), future studies using single agent anti-CD20 therapy should avoid combining these distinct patient populations. Limitations of this analysis of SLL and MZL include its exploratory nature and the resulting limited patient numbers. There were a greater number of study withdrawals due to patient or physician preference in the MR arm, although a sensitivity analysis excluding these patients did not change the study outcomes or conclusions. As all E4402 patients received induction rituximab, it is unknown whether SLL and MZL patients who are observed until overt disease progression or symptoms would have a shorter time to cytotoxic therapy.
Since the design of this trial over ten years ago, other strategies have emerged that could be considered for the low tumor burden setting. These include the use of targeted agents such as B-cell receptor (BCR) inhibitors (Byrd et al, 2013), combination induction therapies including lenalidomide plus rituximab (Fowler et al, 2014), or the use of minimal residual disease in peripheral blood and/or bone marrow to guide the type and duration of maintenance treatment (Ladetto et al, 2013). The aim of these approaches for indolent NHL is to provide durable disease and symptom control with deferral of cytotoxic therapy for as long as possible in what, for now, remains an incurable disease for virtually all patients. Given the improvement in response observed for MZL patients receiving maintenance rituximab, it is possible that an extended induction schedule and/or alternative dosing schema may be beneficial. Future trials should explore optimization of rituximab among individual B-cell lymphoproliferative disorders, and take into account variances in response associated with gender and age (Pfreundschuh et al, 2014).
In summary, this clinical trial of previously untreated, low tumor burden, non-follicular indolent B-cell lymphoma patients who achieved CR or PR to four weekly doses of rituximab showed MR to be superior to RR for the primary endpoint of time to treatment failure. MR was also superior for time to first cytotoxic therapy, with no MR patient requiring such treatment during study follow-up. MR patients received three-fold more rituximab doses than did those randomized to RR. While larger studies will be necessary to verify these results, MR is an acceptable strategy for low tumor burden SLL and MZL patients who choose rituximab monotherapy over watchful waiting and who achieve PR or CR to induction treatment.
Acknowledgements
The authors wish to acknowledge the ECOG Coordinating and Statistical Centers for their expert assistance throughout this clinical trial, the CTSU and our many co-investigators. We also offer our sincere thanks to the patients and families who took part in the E4402/RESORT study. This trial was supported in part by Public Health Service Grants CA21115, CA23318, CA66636, CA49957, CA21076, CA17145 and CA13650 from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Presented in part at the American Society of Clinical Oncology 2012 Annual Meeting, Chicago, IL, USA
Authorship
Michael Williams: protocol development, patient enrollment, data analysis, manuscript writing
Fangxin Hong: data analysis, manuscript writing
Randy Gascoyne: pathology review, manuscript editing
Lynne Wagner: protocol development, manuscript editing
John Krauss: protocol development, patient enrollment
Sandra Horning: protocol development, patient enrollment
Thomas Habermann: patient enrollment, manuscript editing
Lode Swinnen: patient enrollment, manuscript editing
Stephen Schuster: patient enrollment, manuscript editing
Christopher Peterson: patient enrollment, manuscript editing
Mark Sborov: patient enrollment, manuscript editing
S. Eric Martin: patient enrollment, manuscript editing
Matthias Weiss: patient enrollment, manuscript editing
W. Christopher Ehmann: patient enrollment, manuscript editing
Brad Kahl: protocol development, patient enrollment, data analysis, manuscript writing
Author disclosures
Michael E. Williams: Research funding from Genentech/Roche.
Fangxin Hong: None
Randy D. Gascoyne: Consulting fees and research funding from Genentech/Roche.
Lynne I. Wagner: None
John C. Krauss: None
Thomas M. Habermann: None
Lode J. Swinnnen: None
Stephen J. Schuster: None
Christopher G. Peterson: None
Mark D. Sborov: None
S. Eric Martin: None
Matthias Weiss: None
W. Christopher Ehmann: None
Sandra J. Horning: Employment Genentech/Roche.
Brad S. Kahl: Consulting fees and research funding from Genentech/Roche.
References
- Berinstein NL, Grillo-Lopez AJ, White CA, Bence-Bruckler I, Maloney D, Czuczman M, Green D, Rosenberg J, McLaughlin P, Shen D. Association of serum rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Annals of Oncology. 1998;9:995–1001. doi: 10.1023/A:1008416911099. [DOI] [PubMed] [Google Scholar]
- Brice P, Bastion Y, Lepage E, Brousse N, Haïoun C, Moreau P, Straetmans N, Tilly H, Tabah I, Solal-Céligny P. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d'Etude des Lymphomes Folliculaires. Journal of Clinical Oncology. 1997;15:1110–7. doi: 10.1200/JCO.1997.15.3.1110. [DOI] [PubMed] [Google Scholar]
- Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF, O'Brien S. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. New England Journal of Medicine. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NR, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI sponsored international working group. Journal of Clinical Oncology. 1999;17:1244–1255. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- Conconi A, Martinelli G, Thieblemont C, Ferreri AJM, Devizzi L, Peccatori F, Ponzoni M, Pedrinis E, Dell'Oro F, Pruneri G, Filipazzi V, Dietrich PY, Gianni AM, Coiffier B, Cavalli F, Zucca E. Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type. Blood. 2003;102:2741–2745. doi: 10.1182/blood-2002-11-3496. [DOI] [PubMed] [Google Scholar]
- Fowler NH, Davis RE, Rawal S, Nastoupil L, Hagemeister FB, McLaughlin P, Kwak L W, Romaguera JE, Fanale MA, Fayad LE, Westin JR, Shah J, Orlowski RZ, Wang M, Turturro F, Oki Y, Claret LC, Feng L, Baladandayuthapani V, Tariq Muzzafar, Tsai KY, Samaniego F, Neelapu SS. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncology. 2014;15:1311–1318. doi: 10.1016/S1470-2045(14)70455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, Chagorova T, de la Serna J, Dilhuydy MS, Illmer T, Opat S, Owen CJ, Samoylova O, Kreuzer KA, Stilgenbauer S, Döhner H, Langerak AW, Ritgen M, Kneba M, Asikanius E, Humphrey K, Wenger M, Hallek M. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. New England Journal of Med 2014. 2014;370:1101–10. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- Gordan LN, Grow WB, Pusateri A, Douglas V, Mendenhall NP, Lynch JW. Phase II trial of indivisualized rituximab dosing for patients with CD20-positive lymphoproliferative disorders. Journal of Clinical Oncology. 2005;23:1096–1102. doi: 10.1200/JCO.2005.12.171. [DOI] [PubMed] [Google Scholar]
- Hainsworth JD, Litchy S, Shaffer DW, Lackey VL, Grimaldi M, Greco FA. Maximizing therapeutic benefit of rituximab: maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin's lymphoma--a randomized phase II trial of the Minnie Pearl Cancer Research Network. Journal of Clinical Oncology. 2005;23:1088–1095. doi: 10.1200/JCO.2005.12.191. [DOI] [PubMed] [Google Scholar]
- Kahl BS, Hong F, Williams ME, Gascoyne RD, Wagner LI, Krauss JC, Habermann TM, Swinnen LJ, Schuster SJ, Peterson CG, Sborov MD, Martin SE, Weiss M, Ehmann WC, Horning SJ. Rituximab extended schedule or retreatment trial (RESORT) for low tumor burden follicular lymphoma: Eastern Cooperative Oncology Group Protocol E4402. Journal of Clinical Oncology. 2014;32:3096–3102. doi: 10.1200/JCO.2014.56.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpadakis C, Pangalis GA, Angelopoulou MK, Sachanas S, Kontopidou FN, Yiakoumis X, Kokoris SI, Dimitriadou EM, Dimopoulou DN, Moschogiannis M, Korkolopoulou P, Kyrtsonis MC, Siakantaris MP, Papadaki T, Tsaftaridis P, Plata E, Papadaki HE, Vassilakopoulo TPs. Treatment of splenic marginal zone lymphoma with rituximab monotherapy: progress report and comparison with splenectomy. The Oncologist. 2013;18:190–197. doi: 10.1634/theoncologist.2012-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladetto M, Lobetti-Bodoni C, Mantoan B, Ceccarelli M, Boccomini C, Genuardi E, Chiappella A, Baldini L, Rossi G, Pulsoni A, Di Raimondo F, Rigacci L, Pinto A, Galimberti S, Bari A, Rota-Scalabrini D, Ferrari A, Zaja F, Gallamini A, Specchia G, Musto P, Gaia Rossi F, Gamba E, Evangelista A, Vitolo U. Persistence of minimal residual disease in bone marrow predicts outcome in follicular lymphoma treated with a rituximab-intensive program. Blood. 2013;122:3759–3766. doi: 10.1182/blood-2013-06-507319. [DOI] [PubMed] [Google Scholar]
- McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. Journal of Clinical Oncology. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- Pfreundschuh M, Muller C, Zeynalova S, Kuhnt E, Wiesen MHJ, Held G, Rixecker T, Poeschel V, Zwick C, Reiser M, Schmitz N, Murawski N. Suboptimal dosing of rituximab in male and female patients with DLBCL. Blood. 2014;123:640–646. doi: 10.1182/blood-2013-07-517037. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. WHO Press; Geneva, Switzerland: 2008. [Google Scholar]
- Wagner LI, Zhao F, Hong F, Williams ME, Gascoyne RD, Krauss JC, Advani RH, Go RS, Habermann TM, Leach JW, O'Connor B, Schuster SJ, Cella D, Horning SJ, Kahl BS. Anxiety and health-related quality of life among patients with indolent non-Hodgkin's lymphoma randomized to two different rituximab dosing regimens: Results from ECOG Trial 4402 (RESORT). Journal of Clinical Oncology. 2015;33:740–748. doi: 10.1200/JCO.2014.57.6801. [DOI] [PMC free article] [PubMed] [Google Scholar]