Abstract
Objective
Candida-induced denture stomatitis is a common debilitating problem among denture wearers. Previously, we described the fabrication of a new denture material that released antifungal drugs when immersed in phosphate buffered saline. Here, we use more clinically relevant immersion conditions (human saliva; 37°C) and measure miconazole release and bioactivity.
Materials and Methods
Disks were prepared by grafting PNVP [poly(N-vinyl-2-pyrrolidinone)] onto PMMA [poly(methylmethacrylate)] using plasma initiation (PMMA-g-PNVP) and then loaded with miconazole. Drug-loaded disks were immersed in 10–100% human saliva (1–30 days). Miconazole release was measured and then tested for bioactivity versus miconazole-sensitive and -resistant Candida isolates.
Results
HPLC was used to quantify miconazole levels in saliva. Miconazole-loaded disks released antifungal drug for up to 30 days. Higher drug release was found with higher concentrations of saliva and, interestingly, miconazole solubility was increased with higher saliva concentrations. The released miconazole retained its anticandidal activity. After immersion, the residual miconazole could be quenched and the disks recharged. Freshly recharged disks displayed the same release kinetics and bioactivity as the original disks. Quenched disks could also be charged with chlorhexidine that displayed anticandidal activity.
Conclusions
These results suggest that PMMA-g-PNVP is a promising new denture material for long-term management of denture stomatitis.
Keywords: Candida-associated denture stomatitis (CADS), antifungal treatment, drug delivery, rechargeable biomaterial
Introduction
Removable dentures are a valuable prosthodontic appliance used to replace varying numbers of missing teeth and significantly improve oral function and quality of life (Douglass et al. 2002; Perezous et al. 2005). Denture stomatitis, a non-specific inflammation of the oral mucosa produced by colonizing microorganisms, is a reaction to microbial antigens, toxins, and enzymes. Candida-induced denture stomatitis (CADS) is a common, recurring disease that occurs in up to 67% of denture wearers (Arendorf and Walker 1987; Nikawa et al. 1998; Pereira-Cenci et al. 2008; Ramage et al. 2004; ten Cate et al. 2009; Webb et al. 1998). Frequently, these patients have one or more conditions (e.g. poor oral hygiene, diabetes, reduced salivary flow, etc.) that increase susceptibility to CADS (Dorko et al. 2005; Golecka et al. 2006; Maksymiuk et al. 1984; Musial et al. 1988).
Current management of CADS is heavily dependent on patient compliance. Since many of these patients are elderly and physically and/or mentally compromised (Chandra et al. 2001a; Chandra et al. 2001b; Lamfon et al. 2005; Nikawa et al. 2003; Pesci-Bardon et al. 2006), CADS remains a difficult problem to treat successfully. One new approach is to fabricate denture materials that can be impregnated with anticandidal drugs (Abe et al. 2004; Chow et al. 1999; Kalachandra et al. 2002; Lefebvre et al. 2001; Lin et al. 2003; Matsuura et al. 1997; Quinn 1985; Redding et al. 2009; Schneid 1992; Truhlar et al. 1994). Some of these new materials have shortcomings that limit their usefulness in the clinic. To address these deficiencies, we recently described the fabrication and characterization of a new acrylic-based denture material (PMMA-g-PNVP) that provides sustained antifungal release for 30 or more days and can be charged (“click on”) and discharged (“click off”) with different antifungal drugs so that treatment can be tailored to the clinical needs of the patient (Sun et al. 2013; Villar et al. 2013).
In routine clinical use, dentures are continuously immersed in saliva. To date, this new denture material has not been evaluated for drug-release in human saliva. In the current study, we develop an HPLC-based method for quantitating miconazole in saliva. The new assay is subsequently used to measure miconazole release during immersion of PMMA-g-PNVP disks (loaded with miconazole) in saliva for extended periods of time, followed by assessing the bioactivity of the released anticandidal drug versus clinical Candida isolates.
Materials and Methods
Materials
Unless otherwise indicated, all reagents were analytical grade, purchased from Sigma-Aldrich (St. Louis, MO), and used as received with the exception of azobisisobutyronitrile (AIBN) which was recrystallized from ethanol before use. Miconazole free base (Fisher BioReagents, Pittsburgh, PA) was dissolved in HPLC grade methanol at 0.1 mg/mL and stored at 4°C.
Saliva Collection and Preparation
Whole saliva (oral fluids) was obtained from healthy human subjects (ages 27 – 52yo; 4 females and 4 males) while chewing paraffin wax for 15 minutes. The collected saliva was immediately centrifuged (2000×g) for 10 minutes at 4°C. All samples were pooled, divided into 10 ml aliquots, and then frozen at −20°C. The study was approved by the Institutional Review Board (IRB) at the University of Texas Health Science Center at San Antonio (UTHSCSA) (Protocol: HSC20120105H, renewed 02/10/2015).
Artificial saliva was used to mimic the electrolytes and viscosity of human saliva and the formula described by Cao et al. (Cao et al. 2010) was used. The constituents and their concentrations were combined and adjusted to pH 7.0 [CaCl2-2H2O (2.55 mM), MgCl2-6H2O (0.61 mM), KCl (16.0 mM), NaCl (14.5 mM), NaHPO4-12H2O (6.98 mM), sorbic acid (8.9 mM), sodium carboxymethylcellulose (5 g/L), and sorbitol (236 mM)].
Grafting poly(N-vinyl-2-pyrrolidinone) (PNVP) to acrylic denture resin and subsequent charging with the antifungal drug miconazole
The method of grafting of NVP (N-vinyl-2-pyrrolidinone) to acrylic denture resin (Lucitone 199, poly(methylmethacrylate) [PMMA]; Dentsply International [York, PA]) has been described previously (Sun et al. 2013). Briefly, slabs (10×10×1.35mm) of denture resin (hereafter, referred to as “disks”) were prepared following the manufacturer’s instructions. Subsequently, NVP was grafted onto the disks by immersion for 2 hrs in 20wt% NVP in acetone containing diurethane dimethacrylate (DUMA, 1wt% as a cross-linker) and AIBN (1wt% as an initiator); after 2 hrs, the disks were air dried at 24°C and then plasma treated (Harrick Plasma, Ithaca, NY) for 30 min on each side. The disks were extensively washed with acetone and distilled water, air-dried, and stored in a desiccator.
To charge (“load”) the PNVP-grafted denture material with miconazole, disks were incubated overnight at 24°C) in an ethanolic solution of 5wt% miconazole at a weight ratio of 1:50. After extensive washing with ethanol and distilled water, the disks were air dried and stored desiccated.
Release of miconazole from PMMA-g-PNVP disks into human saliva
Miconazole-loaded PMMA-g-PNVP disks were placed in Falcon 50mL polypropylene centrifuge tubes and then immersed in 5 mL of varying concentrations (10%, 50% or 100%) of human saliva. The disks were incubated at 37°C with constant shaking (60 rpm). PBS was used as a control and diluent. Saliva was replaced every 1–3 days.
The rate of drug release, after various times of immersion, was determined by replacing the saliva with fresh saliva of the same concentration and then continuing the incubation for an additional 24 hrs. The amount of miconazole released during this 24 hr period was determined using the HPLC method described below and provided an assessment of the relative depletion of the drug loaded denture material.
Assay of miconazole in human saliva
The amount of miconazole released from the charged PMMA-g-PNVP disks was determined using high performance liquid chromatography (HPLC) (BRODIE et al. 1978; Turner and Warnock 1982). An extended C-18 column (ZORBAX 300, 4.6 × 250mm, 5µm particle size; Agilent Technologies, Santa Clara, CA) was used in combination with a Beckman Coulter HPLC system (System Gold with model 125 solvent delivery module, Beckman Coulter, Inc., Atlanta, GA) and a variable wavelength detector to monitor the chromatography at 215 nm. The mobile phase consisted of a 7:3 (v/v) ratio of acetonitrile to 0.01M potassium dihydrogen phosphate buffer (pH 4.5); chromatography was performed at 1 mL/min. Immediately prior to assay, saliva samples were thawed on ice and centrifuged at 2000 ×g for 10 mins. The concentration of miconazole in each saliva sample was calculated using a standard curve of miconazole peak area versus concentration (0.1 to 1.6µg/mL).
Solubility of miconazole in human saliva
Miconazole powder (500µg/ml) was added to 5 mL of 10%, 50%, or 100% human saliva and PBS. After addition, the samples were shaken at 60 rpm for 24 and 48 hrs at 37°C. At the end of incubation, samples were centrifuged at 5000 ×g for 5 mins. The amount of miconazole dissolved in each of the solvents was determined using HPLC.
Assay of chlorhexidine digluconate in human saliva
The amount of chlorhexidine released from the PMMA-g-PNVP disks was measured using a previously described procedure (Cavrini et al. 1981). Briefly, saliva (1 mL) containing chlorhexidine digluconate (CD) was mixed with 1 mL of a 1:1 (v/v) solution of 2M sodium hydroxide and chloroform. The mixture was shaken for 10 min, followed by the addition of 2 mL of 0.1M glycine-HCl (pH 2.0) buffer, and shaken for additional 5 min. The water phase was collected and the absorption at 255nm determined. The concentration of CD was calculated from a standard curve for CD. Saliva alone was used as a negative control.
Bioactivity of miconazole and chlorhexidine released from the PMMA-g-PNVP disks
To assess the bioactivity of miconazole and CD released from the PMMA-g-PNVP disks, Candida isolates, with varying sensitivity to these two drugs, were obtained from the Fungal Testing Laboratory at UTHSCSA. Miconazole-sensitive Candida isolates were: X5314 (MIC: 0.125 µg/ml), X1215 (MIC: 0.062 µg/ml), and 88–111 (MIC: 0.25 µg/ml). Isolate 13–2559 is fluconazole resistant and is also miconazole resistant (MIC >2µg/ml). The anticandida assays were performed in 96 well plates using 100 µL saliva, 100 µL RPMI media, and 1000 CFU Candida / per well. Plates were incubated for 24 hrs at 37°C and turbidity read at 530 nm using a plate reader.
Determination of protein adsorption on disks incubated in human saliva
Miconazole-charged PMMA-g-PNVP disks, immersed in different concentrations of human saliva for 20 days at 37°C, were analyzed for protein adsorption. For analysis, the disks were rinsed 3 times with PBS and washed 3 more times with distilled water. Adsorbed protein was released by shaking the disks in 2.0 mL 1% sodium dodecyl sulfate (SDS) for 6 hrs at room temperature and then quantified using the Micro BCA protein assay (ThermoScientific, Rockford, IL). Bovine serum albumin was used as a standard. Adsorbed proteins were visualized using confocal microscopy and fluorescein isothiocyanate (FITC) staining (excitation 488 nm, emission 505–525 nm).
Quenching and recharging PMMA-g-PNVP disks with miconazole or CD
The drug quenching and recharging procedures were as previously described (Sun et al. 2013). Briefly, previously charged PMMA-g-PNVP disks were immersed in 5% PNVP (1:50 [wt/wt], disks to PNVP) for 8 hrs at 24°C. Disks were subsequently rinsed with distilled water and air dried. To recharge, the disks were incubated in 5 wt% miconazole or 5 wt% CD (1:50 [wt/wt] ratio of disks to drug) as described above.
Statistical analysis of the data
Standard descriptive statistics were calculated and the presented data are the mean ± SEM. Statistical analyses were performed using GraphPad software (Prism Software, La Jolla, CA) and included 2-way analysis of variance (ANOVA) with Bonferroni correction. All statistical tests were two sided with an experiment-wise significance level of 5%. p < 0.05 was considered statistically significant.
Results
The modified HPLC method for measuring miconazole in human saliva was effective and reliable. Retention times in both PBS and 10% saliva were nearly identical and peak heights were equivalent for the same concentration of miconazole in both solvents (Figures 1A and 1B). In addition, elution of miconazole from the column did not overlap with any of the other components present in the samples (Figures 1A & 1B), including when higher concentrations of saliva were used (data not shown). Further, there was a linear relationship between miconazole concentration (0.1–1.6 µg/mL) and peak height over the range of saliva concentrations tested and PBS. Representative data for miconazole in 10% saliva is shown in Figure 1C.
Figure 1. Chromatographic separation of miconazole dissolved in saliva or PBS.
Miconazole, dissolved in 10% human saliva or PBS, was isolated on a C-18 HPLC column as described in the Methods section. Panel A: Chromatogram of 0.2 µg/mL miconazole dissolved in 10% saliva. Miconazole elutes at 6.7 mins and the area under the curve (proportional to concentration) is shown in the box and calculated by the HPLC. Panel B: Chromatogram of 0.2 µg/ml miconazole dissolved in PBS. Miconazole elutes in an equivalent manner as found in Panel A. Panel C: A representative standard curve for miconazole dissolved in 10% saliva. Each point represents the mean±SEM for 5 determinations. Irrespective of the solvent used, dose-dependency and linearity were equivalent to that shown.
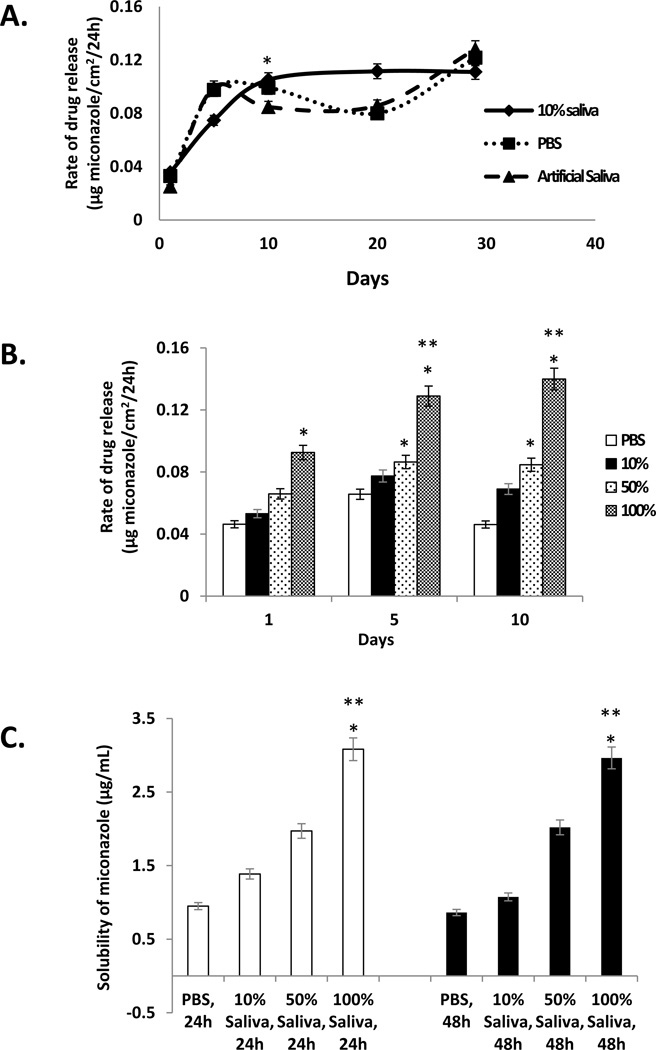
Miconazole release from the PMMA-g-PNVP disks was examined in PBS, artificial saliva, and 10% human saliva (Figure 2A). Twenty four hour release rates were determined over the course of 30 days incubation at 37°C. At early times (day 2), the release rate was relatively slow, but between day 5 and day 10, the 24 hour release rate increased rapidly (and significantly) and then achieved a steady state that was maintained for the remainder of the incubation and independent of the solvent (Figure 2A). In addition, release rate in 10% saliva was slower at the beginning, compared to PBS or artificial saliva, but at later times was more constant.
Figure 2. Twenty four hour release rate of miconazole from PMMA-g-PNVP disks and solubility of miconazole in saliva.
Panel A: Miconazole loaded PMMA-g-PNVP disks, containing 59.8 ± 2.5 µg miconazole/cm2, were immersed in PBS, artificial saliva, or 10% human saliva for up to 30 days at 37°C. At select times, solvents were refreshed, the incubation continued for an additional 24 hrs, and miconazole in the solvent measured using the HPLC method. Each point represents the 24 hr release rate (mean±SEM; n=5). *p ≤ 0.001, day 10 versus the corresponding day 1. Panel B: Miconazole loaded PMMA-g-PNVP disks were immersed in PBS and varying concentrations of human saliva for 1, 5, and 10 days. The miconazole level in each solvent was determined as in Panel A. Each bar represents the 24 hr release rate (mean±SEM, n = 3). *p ≤ 0.02, versus PBS; **p ≤ 0.04, versus Day 1 (100% saliva). Panel C: Solubility of miconazole in PBS and varying concentrations of saliva. Each bar represents the solubility of miconazole in µg/mL (mean±SEM, n = 3). *p ≤ 0.003, versus PBS; **p ≤ 0.005, versus 10% saliva.
Miconazole release studies were also performed using different concentrations of saliva (i.e., 10%, 50% and 100%) and compared to PBS (Figure 2B). Twenty four hour release rates were highly dependent on saliva concentration with the highest release rate found with 100% saliva, followed by 50% saliva and then 10% saliva. As observed in Figure 2A, release rate reached a steady state between day 5 and 10. Since the data suggested that release rate might be dictated by solubility of the drug in saliva, a study was performed to evaluate this possibility (Figure 2C). An excess of miconazole powder was suspended in PBS and various concentrations of human saliva and shaken on an orbital shaker for 24 or 48 hrs. at 37°C. At the end of incubation, the undissolved drug was pelleted and the supernatant assayed for miconazole content. The results show that miconazole reached saturation levels within 24hrs in both control (PBS) and saliva solutions and displayed a dose-dependent increase in solubilized drug with increasing saliva concentrations (Figure 2C).
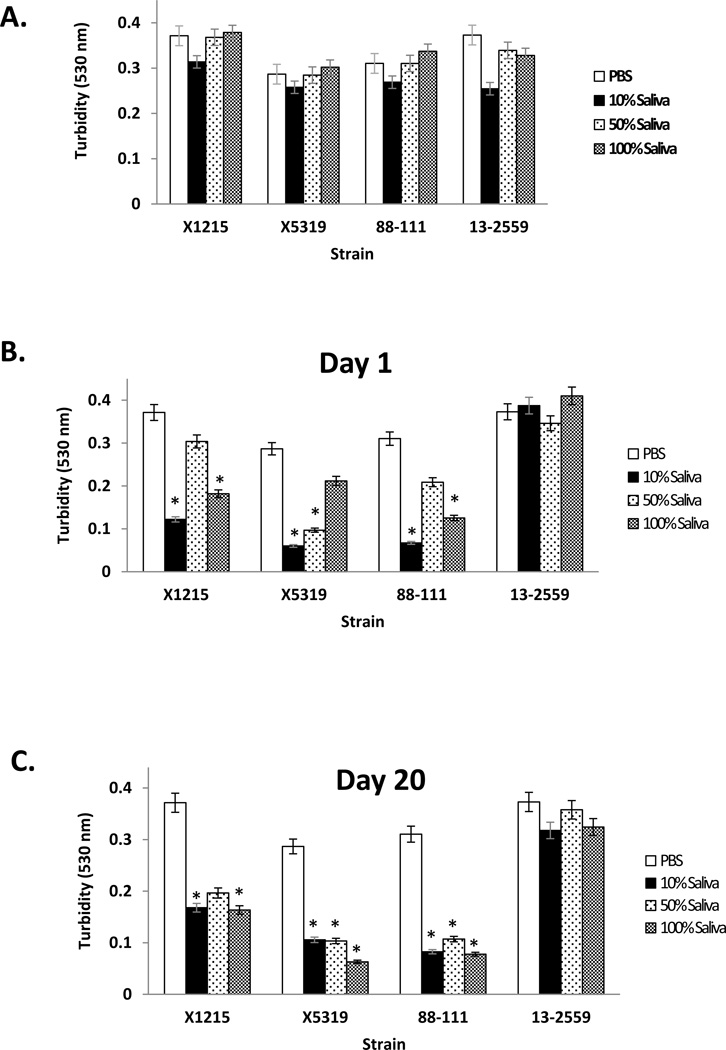
The bioactivity of miconazole released from the PMMA-g-PNVP disks was evaluated by measuring its effect on Candida growth using a turbidity assay. In control experiments, different concentrations of human saliva, compared to PBS alone (not exposed to disks), had little effect on the growth of both miconazole-sensitive and miconazole-resistant isolates (Figure 3A). After one day of immersion in human saliva, but not PBS controls, miconazole released from the PMMA-g-PNVP disks significantly inhibited miconazole-sensitive Candida isolates (i.e., X1215, X5314, 88–111), but not the insensitive 13–2559 isolate (Figure 3B). Interestingly, some variation in the degree of inhibition was noted with each isolate, depending on the saliva concentration. By day 20, miconazole released from the disks continued to show robust anticandidal activity against the sensitive isolates (Figure 3C). These results demonstrate that miconazole released from the PMMA-g-PNVP disks through 20 days of incubation was effective against Candida isolates known to be sensitive to this drug, but not to one known to be resistant, and the bioactivity of the disks was unaffected by prolonged exposure to varying concentrations of human saliva.
Figure 3. Anticandidal activity of miconazole released from PMMA-g-PNVP disks in human saliva.
Bioactivity of the released drug against Candida was evaluated in a standard growth assay using turbidity measurements as described in the Methods. Panel A: The effect of varying concentrations of human saliva (10%, 50%, and 100%) and PBS alone on drug-sensitive and insensitive Candida isolates was evaluated in the assay. Little to no effect on cell growth was noted. Panel B: Miconazole-loaded PMMA-g-PNVP disks were immersed in varying concentrations of human saliva for 1 day, the solvent replaced, and then collected for analysis of bioactivity in the growth assay after 24 hrs. PBS alone (not exposed to drug-loaded disks) was used as a control. Anticandidal activity was observed against miconazole-sensitive isolates, but not the insensitive one. *P ≤ 0.049 versus PBS. Panel C Miconazole-loaded PMMA-g-PNVP disks were immersed in varying concentrations of human saliva for 20 days and the solvent replaced 24 hrs before collection for bioactivity analysis. Anticandidal activity against sensitive isolates continued to be found. The data are the mean±SEM, n = 3. *P ≤ 0.05, vs. PBS.
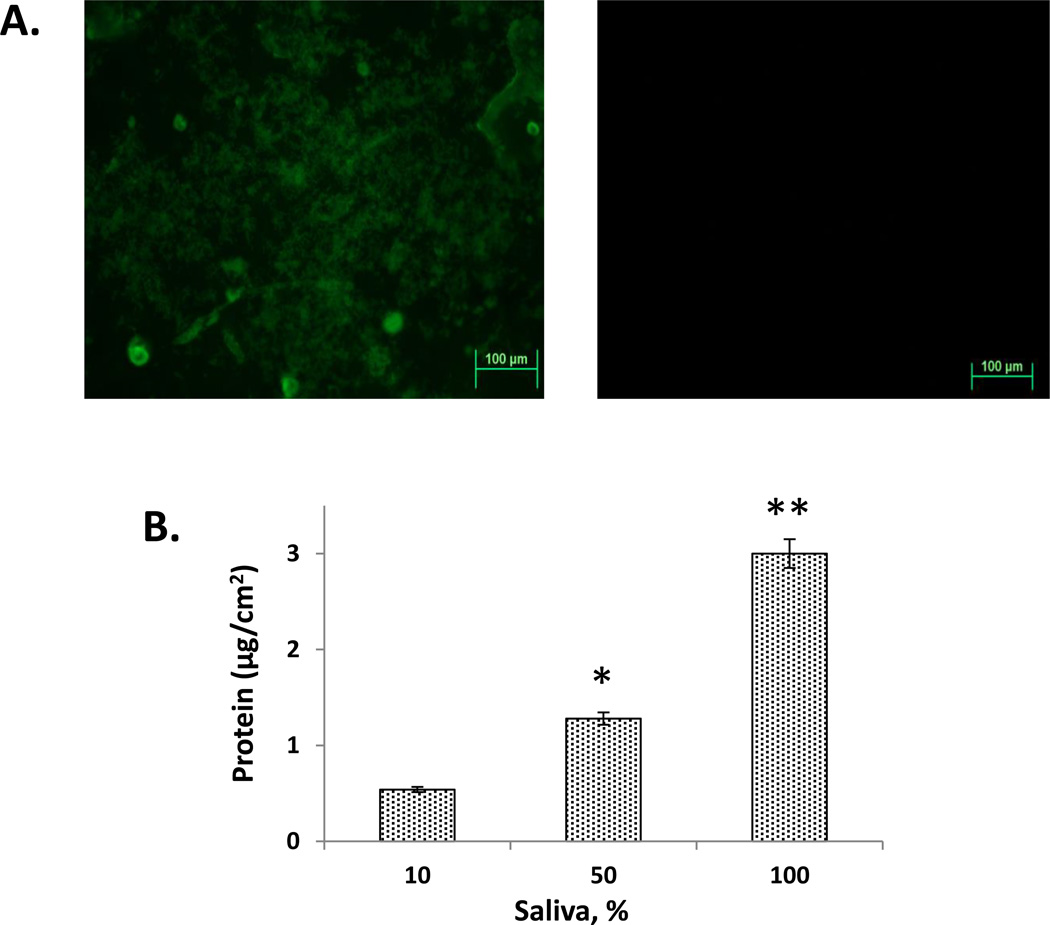
Since saliva had an effect on miconazole release, the adsorption of salivary proteins to the disks was evaluated. Initially, the presence of protein on the disks was confirmed by FITC staining and confocal microscopy (Figure 4A). Strong fluorescence on the surface of the disks immersed in 10% saliva for 20 days was evident, whereas there was no fluorescence found with the PBS control disks. To quantitate the amount of protein adsorbed, the disks were incubated in SDS and the released protein measured. Protein adsorption increased significantly with increasing saliva concentration (Figure 4B). Disks incubated in 100% saliva had the highest level of protein adsorption at 3.00±0.47 µg/cm2.
Figure 4. Adsorption of salivary proteins to PMMA-g-PNVP disks.
Panel A: PMMA-g-PNVP disks immersed in 10% human saliva for 20 days were stained with FITC and viewed in a confocal microscope. Positive staining was observed on disks incubated in saliva (left image) but not PBS (right image). Panel B: Quantitation of protein adsorption using the microBCA assay. Disks were incubated for 20 days in 10%, 50%, and 100% saliva, the adsorbed proteins released with SDS, and then assayed. Protein adsorption significantly increased with increasing saliva concentration. *P ≤ 0.015 versus 10%, ** P ≤ 0.001 versus 10%.
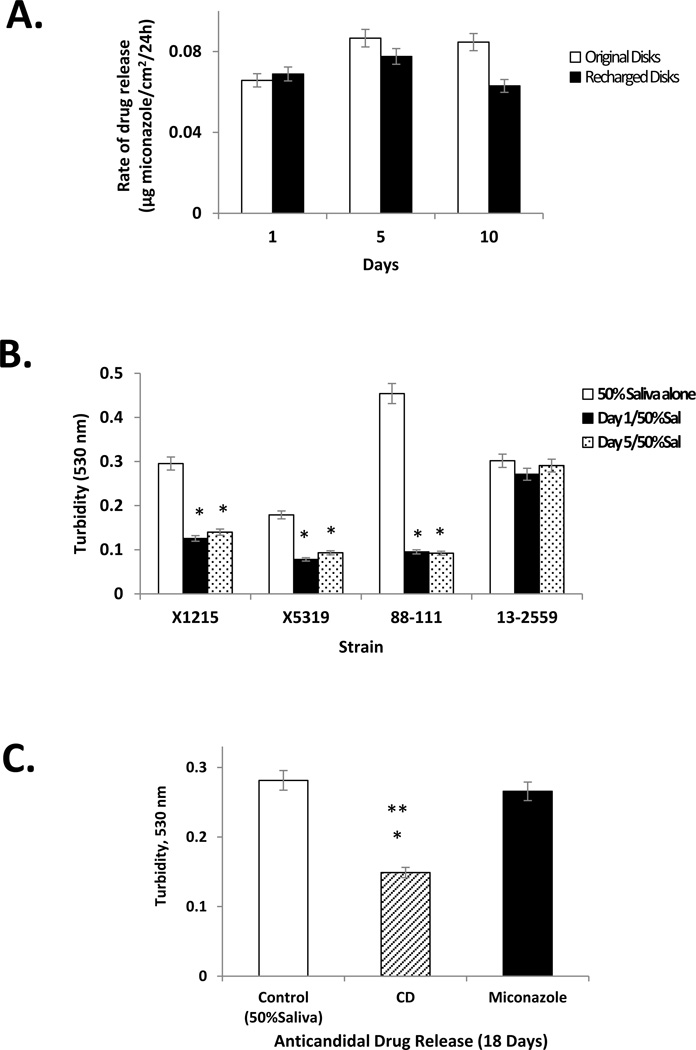
The ability to remove residual antifungal from the PMMA-g-PNVP disks and re-charge them with another round of the same drug or a different drug was explored next. Disks containing residual miconazole after immersion in saliva were quenched in 5% PNVP and then recharged with miconazole or CD. The release kinetics of miconazole from the recharged disks in 50% saliva was nearly equal to that of the originally charged disks (Figure 5A). Further, anticandidal activity of the miconazole released from the recharged disks was similar to that of the original disks (Figure 5B). Recharging the disks, originally charged with miconazole, with CD also proved effective. The rate of release of CD from the recharged disks in 50% saliva after 18 days was 1.13 µg CD/cm2/24hrs. The released CD maintained its anticandidal activity against the miconazole-resistant isolate 13–2559 (Figure 5C). These results demonstrate that PMMA-g-PNVP disks have the ability to effectively bind and release anticandidal drugs over extended periods of time in human saliva. In addition, this novel denture material can be quenched and recharged with the same drug or another one from a different category to effectively manage treatment for an individual patient.
Figure 5. PMMA-g-PNVP disks recharged with miconazole or CD maintain release characteristics and antifungal activity.
Panel A: Previously used PMMA-g-PNVP disks were quenched to remove residual miconazole, recharged with fresh miconazole, and then immersed in 50% human saliva for 1, 5, or 10 days to follow drug release. Twenty-four hour drug release was calculated for the recharged disks and compared to release kinetics of the original disks. Panel B: Anticandidal activity of miconazole released from PMMA-g-PNVP disks that had been recharged with fresh miconazole. Data are mean±SEM, n = 3. *P ≤ 0.05, vs. 50% saliva control. Panel C: Previously used miconazole-loaded PMMA-g-PNVP disks were quenched, recharged with CD, and then immersed in 50% human saliva for 18 days. A release sample was obtained on day 18 and then tested for bioactivity against the miconazole-resistant Candida isolate 13–2559. A significant inhibition of Candida growth was observed for CD-containing saliva, but not for similarly prepared saliva samples containing miconazole. Data are mean±SEM, n = 3. *P ≤ 0.05, versus miconazole (18 days); **P ≤ 0.001, versus 50% saliva.
Discussion
In the current study, we evaluated the characteristics of our recently developed denture material (PMMA-g-PNVP) in human saliva to replicate conditions found in vivo. The results showed that when disks, charged with miconazole, were immersed in either saliva or PBS, the release kinetics were similar. In addition, drug release continued for extended periods of time (> 1 month) in both solvents and bioactivity of the anticandidal drug was unaffected. The PMMA-g-PNVP disks could also be recharged with either the same or a different drug after immersion in saliva.
Human whole saliva is a complex biological secretion that contains various fluids, electrolytes, proteins, and cells that often interfere with successful detection/quantitation of salivary analytes. In order to identify the best method for quantitating miconazole in saliva, we needed to evaluate the performance of several previously reported methods. Colorimetric and spectrophotometric (UV absorbance) methods were effective at measuring miconazole in serum (Cavrini, Di Pietra, & Raggi 1981), but were not sensitive enough for our application (sensitivity >3µg/mL in saliva). Another previously published method used HPLC to quantitate miconazole in human saliva (Turner & Warnock 1982) and econazole in plasma. With recent improvements in sample handling and column technology, we adapted the HPLC method without special sample preparation (e.g. deproteination) and were able to directly quantitate miconazole levels in PBS and varying saliva concentrations without interference.
Here we confirm that the anticandidal drug, miconazole, binds to PMMA-g-PNVP under conditions that preserve the physicochemical properties of the denture material and the bioactivity of the drug. In addition, when miconazole-loaded PMMA-g-PNVP disks were immersed in PBS or human saliva (to replicate the clinical condition) at 37°C, drug release was sustained for at least one month. Interestingly, we observed that the amount of miconazole released was proportional to the concentration of saliva (Figure 2B). Subsequently, we found that the solubility of miconazole was increased in higher concentrations of saliva (Figure 2C), providing at least a partial explanation for the increased drug release observed with higher saliva concentrations. The pH of saliva did not appear to play a role in drug release since miconazole is a weak base and saliva has a pH of 8.34 versus pH 7.0 for PBS. However, protein adsorption was greater on disks immersed in higher saliva concentrations (Figure 4). In this case, it’s possible that drug re-adsorption may be hindered due to a higher amount of protein coating.
Saliva is known to contain a number of proteins (e.g. proteases, DNAases and RNAases) and microorganisms that may inactivate drugs used to treat oral diseases. Our current results demonstrate that prolonged exposure to saliva has no adverse effect on the activity of miconazole against sensitive isolates (Figure 3).
In the current study we show, as before (Sun et al. 2013), that PMMA-g-PNVP disks can be charged with miconazole, the drug released over time (>1 month), quenched to remove residual drug, and then re-charged with fresh miconazole or other drugs. Here, we demonstrate that prolonged immersion in varying concentrations of human saliva do not affect the performance of the disks. The slight reduction in miconazole release found with recharged disks at 10 days is currently under investigation and may be due to protein adsorption of on the surface of the quenched disks (Figure 5A). We also noted in the current study that disks previously charged with miconazole can be quenched and recharged with CD and that released CD can be quantitated in saliva using spectrophotometry (Cavrini, Di Pietra, & Raggi 1981). Further, we demonstrated that CD released from newly recharged PMMA-PNVP disks suppressed growth of miconazole resistant isolate 13–2259 (Figure 5C). These results indicate that the PMMA-g-PNVP technology is versatile and able deliver different drugs based on the needs of the patient and address other challenges such as drug resistance.
Previously, we reported that the common denture material Lucitone 199 with grafted PNVP was able to reversibly bind anticandidal drugs through hydrogen bonding and dipole-dipole interactions (Sun et al. 2013). The physical/mechanical properties of the acrylic material were not affected by PNVP grafting, although resin solubility was compromised if the grafted PNVP was >8%. Since dentures are in constant contact with saliva, the current study specifically examined and demonstrated that the previously measured characteristics in PBS are also found in human saliva (e.g quenching/recharging of drugs; release drug bioactivity). However, miconazole release in saliva was greater than in PBS. The current preclinical results strengthen our earlier results and indicate that plasma-grafting of PNVP onto conventional dental resin is an approach with great potential for long-term management of patients with CADS. The effectiveness of this rechargeable anticandidal material in the clinic, however, will require additional studies.
Conclusions
A new HPLC procedure has been developed for accurately measuring miconazole in human saliva. The rate of miconazole release was sustained for up to 30 days and release was greater in more concentrated saliva. Further, it was demonstrated that saliva does not interfere with miconazole release from the rechargeable denture material or its anticandidal activity. Bioactivity of miconazole released in saliva was retained and showed anticandidal activity against clinical isolates of miconazole-sensitive Candida strains.
Acknowledgments
This study was supported by NIH and NIDCR T-32 grants DE021084, DE014318, the COSTAR Program, and a VA Merit Review (1I01BX001103). We are grateful to Dr. Nathan Wiederhold, Director of the Fungal Testing Laboratory (UTHSCSA), for kindly providing the Candida isolates used in this study and to Dr. David Dean (Professor, Comprehensive Dentistry, UTHSCSA) for his scientific input and careful editing of the manuscript.
Footnotes
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: A. Malakhov, J. Wen contributed to major data acquisition, analysis and interpretation, and the first draft of the manuscript. B-X. Zhang, H. Wang and H. Geng contributed to data acquisition, analysis, and interpretation. X-D. Chen contributed to critically reviewing the data, its interpretation, and revision of the manuscript. Y. Sun and C-K. Yeh conceived the design of the study, provided data analysis and interpretation, and prepared and approved the final version of the manuscript. All authors have carefully read and approved the final version of the manuscript and agree to be accountable for all aspects of the work.
References
- Abe Y, Ishii M, Takeuchi M, Ueshige M, Tanaka S, Akagawa Y. Effect of saliva on an antimicrobial tissue conditioner containing silver-zeolite. J Oral Rehabil. 2004;31:568–573. doi: 10.1111/j.1365-2842.2004.01267.x. available from: PM: 15189314. [DOI] [PubMed] [Google Scholar]
- Arendorf TM, Walker DM. Denture stomatitis: a review. J Oral Rehabil. 1987;14:217–227. doi: 10.1111/j.1365-2842.1987.tb00713.x. available from: PM: 3298586. [DOI] [PubMed] [Google Scholar]
- Brodie RR, Chasseaud LF, Walmsley LM. High-performance liquid chromatographic determination of the antimycotic agent, econazole in plasma. J Chromatogr. 1978;155:209–213. doi: 10.1016/s0021-9673(00)83955-2. available from: PM: 681489. [DOI] [PubMed] [Google Scholar]
- Cao Z, Sun X, Yeh CK, Sun Y. Rechargeable infection-responsive antifungal denture materials. J Dent Res. 2010;89:1517–1521. doi: 10.1177/0022034510379604. available from: PM: 20940361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrini V, Di Pietra AM, Raggi MA. Colorimetric determination of miconazole nitrate in pharmaceutical preparations. Pharm Acta Helv. 1981;56:163–165. available from: PM: 7255504. [PubMed] [Google Scholar]
- Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001a;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. available from: PM: 11514524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, Douglas LJ, Ghannoum MA. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res. 2001b;80:903–908. doi: 10.1177/00220345010800031101. available from: PM: 11379893. [DOI] [PubMed] [Google Scholar]
- Chow CK, Matear DW, Lawrence HP. Efficacy of antifungal agents in tissue conditioners in treating candidiasis. Gerodontology. 1999;16:110–118. doi: 10.1111/j.1741-2358.1999.00110.x. available from: PM: 10825850. [DOI] [PubMed] [Google Scholar]
- Dorko E, Baranova Z, Jenca A, Kizek P, Pilipcinec E, Tkacikova L. Diabetes mellitus and candidiases. Folia Microbiol (Praha) 2005;50:255–261. doi: 10.1007/BF02931574. available from: PM: 16295665. [DOI] [PubMed] [Google Scholar]
- Douglass CW, Shih A, Ostry L. Will there be a need for complete dentures in the United States in 2020? J Prosthet Dent. 2002;87:5–8. doi: 10.1067/mpr.2002.121203. available from: PM: 11807476. [DOI] [PubMed] [Google Scholar]
- Golecka M, Oldakowska-Jedynak U, Mierzwinska-Nastalska E, Adamczyk-Sosinska E. Candida-associated denture stomatitis in patients after immunosuppression therapy. Transplant Proc. 2006;38:155–156. doi: 10.1016/j.transproceed.2005.12.078. available from: PM: 16504690. [DOI] [PubMed] [Google Scholar]
- Kalachandra S, Dongming L, Offenbacher S. Controlled drug release for oral condition by a novel device based on ethylene vinyl acetate (EVA) copolymer. J Mater Sci Mater Med. 2002;13:53–58. doi: 10.1023/a:1013634518797. available from: PM: 15348205. [DOI] [PubMed] [Google Scholar]
- Lamfon H, Al Karaawi Z, McCullough M, Porter SR, Pratten J. Composition of in vitro denture plaque biofilms and susceptibility to antifungals. FEMS Microbiol Lett. 2005;242:345–351. doi: 10.1016/j.femsle.2004.11.032. available from: PM: 15621458. [DOI] [PubMed] [Google Scholar]
- Lefebvre CA, Wataha JC, Cibirka RM, Schuster GS, Parr GR. Effects of triclosan on the cytotoxicity and fungal growth on a soft denture liner. J Prosthet Dent. 2001;85:352–356. doi: 10.1067/mpr.2001.115249. available from: PM: 11319532. [DOI] [PubMed] [Google Scholar]
- Lin DM, Kalachandra S, Valiyaparambil J, Offenbacher S. A polymeric device for delivery of anti-microbial and anti-fungal drugs in the oral environment: effect of temperature and medium on the rate of drug release. Dent Mater. 2003;19:589–596. doi: 10.1016/s0109-5641(02)00109-4. available from: PM: 12901982. [DOI] [PubMed] [Google Scholar]
- Maksymiuk AW, Thongprasert S, Hopfer R, Luna M, Fainstein V, Bodey GP. Systemic candidiasis in cancer patients. Am J Med. 1984;77:20–27. available from: PM: 6093530. [PubMed] [Google Scholar]
- Matsuura T, Abe Y, Sato Y, Okamoto K, Ueshige M, Akagawa Y. Prolonged antimicrobial effect of tissue conditioners containing silver-zeolite. J Dent. 1997;25:373–377. doi: 10.1016/s0300-5712(96)00050-4. available from: PM: 9241955. [DOI] [PubMed] [Google Scholar]
- Musial CE, Cockerill FR, III, Roberts GD. Fungal infections of the immunocompromised host: clinical and laboratory aspects. Clin Microbiol Rev. 1988;1:349–364. doi: 10.1128/cmr.1.4.349. available from: PM: 3069198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa H, Hamada T, Yamamoto T. Denture plaque--past and recent concerns. J Dent. 1998;26:299–304. doi: 10.1016/s0300-5712(97)00026-2. available from: PM: 9611934. [DOI] [PubMed] [Google Scholar]
- Nikawa H, Jin C, Makihira S, Egusa H, Hamada T, Kumagai H. Biofilm formation of Candida albicans on the surfaces of deteriorated soft denture lining materials caused by denture cleansers in vitro. J Oral Rehabil. 2003;30:243–250. doi: 10.1046/j.1365-2842.2003.01024.x. available from: PM: 12588495. [DOI] [PubMed] [Google Scholar]
- Pereira-Cenci T, Bel Cury AA, Crielaard W, ten Cate JM. Development of Candida-associated denture stomatitis: new insights. J Appl Oral Sci. 2008;16:86–94. doi: 10.1590/S1678-77572008000200002. available from: PM: 19089197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perezous LF, Flaitz CM, Goldschmidt ME, Engelmeier RL. Colonization of Candida species in denture wearers with emphasis on HIV infection: a literature review. J Prosthet Dent. 2005;93:288–293. doi: 10.1016/j.prosdent.2004.11.015. available from: PM: 15775931. [DOI] [PubMed] [Google Scholar]
- Pesci-Bardon C, Fosse T, Serre D, Madinier I. In vitro antiseptic properties of an ammonium compound combined with denture base acrylic resin. Gerodontology. 2006;23:111–116. doi: 10.1111/j.1741-2358.2006.00088.x. available from: PM: 16677185. [DOI] [PubMed] [Google Scholar]
- Quinn DM. The effectiveness, in vitro, of miconazole and ketoconazole combined with tissue conditioners in inhibiting the growth of Candida albicans. J Oral Rehabil. 1985;12:177–182. doi: 10.1111/j.1365-2842.1985.tb00633.x. available from: PM: 3886866. [DOI] [PubMed] [Google Scholar]
- Ramage G, Tomsett K, Wickes BL, Lopez-Ribot JL, Redding SW. Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:53–59. doi: 10.1016/j.tripleo.2003.04.002. available from: PM: 15243471. [DOI] [PubMed] [Google Scholar]
- Redding S, Bhatt B, Rawls HR, Siegel G, Scott K, Lopez-Ribot J. Inhibition of Candida albicans biofilm formation on denture material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:669–672. doi: 10.1016/j.tripleo.2009.01.021. available from: PM: 19426921. [DOI] [PubMed] [Google Scholar]
- Schneid TR. An in vitro analysis of a sustained release system for the treatment of denture stomatitis. Spec Care Dentist. 1992;12:245–250. doi: 10.1111/j.1754-4505.1992.tb00458.x. available from: PM: 1308323. [DOI] [PubMed] [Google Scholar]
- Sun X, Cao Z, Yeh CK, Sun Y. Antifungal activity, biofilm-controlling effect, and biocompatibility of poly(N-vinyl-2-pyrrolidinone)-grafted denture materials. Colloids Surf B Biointerfaces. 2013;110:96–104. doi: 10.1016/j.colsurfb.2013.04.043. available from: PM: 23708753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Cate JM, Klis FM, Pereira-Cenci T, Crielaard W, de Groot PW. Molecular and cellular mechanisms that lead to Candida biofilm formation. J Dent Res. 2009;88:105–115. doi: 10.1177/0022034508329273. available from: PM: 19278980. [DOI] [PubMed] [Google Scholar]
- Truhlar MR, Shay K, Sohnle P. Use of a new assay technique for quantification of antifungal activity of nystatin incorporated in denture liners. J Prosthet Dent. 1994;71:517–524. doi: 10.1016/0022-3913(94)90193-7. available from: PM: 8006850. [DOI] [PubMed] [Google Scholar]
- Turner A, Warnock DW. Determination of miconazole in human saliva using high-performance liquid chromatography. J Chromatogr. 1982;227:229–232. doi: 10.1016/s0378-4347(00)80377-1. available from: PM: 7056815. [DOI] [PubMed] [Google Scholar]
- Villar CC, Lin AL, Cao Z, Zhao XR, Wu LA, Chen S, Sun Y, Yeh CK. Anticandidal activity and biocompatibility of a rechargeable antifungal denture material. Oral Dis. 2013;19:287–295. doi: 10.1111/odi.12000. available from: PM: 22957799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. Candida-associated denture stomatitis. Aetiology and management: a review. Part 1. Factors influencing distribution of Candida species in the oral cavity. Aust Dent J. 1998;43:45–50. doi: 10.1111/j.1834-7819.1998.tb00152.x. available from: PM: 9583226. [DOI] [PubMed] [Google Scholar]