Abstract
Within the basolateral amygdaloid complex (BLA), Neuropeptide Y (NPY) buffers against protracted anxiety and fear. While the importance of NPY's actions in the BLA is well-documented, little is known about the source(s) of NPY fibers to this region. These current studies identified sources of NPY projections to the BLA using a combination of anatomical and neurochemical approaches. NPY innervation of the BLA was assessed in rats by examining the degree of NPY co-expression within interneurons or catecholaminergic fibers using somatostatin and tyrosine hydroxylase (TH) or dopamine β-hydroxylase (DβH), respectively. Numerous NPY+/somatostatin+ and NPY+/somatostatin- fibers were observed suggesting at least two populations of NPY fibers within the BLA. No co-localization was noted between NPY and TH or DβH immunoreactivities. Additionally, Fluorogold retrograde tracing with immunohistochemistry was used to identify the precise origin of NPY projections to the BLA. FG+/NPY+ cells were identified within the amygdalostriatal transition area (AStr), stria terminalis and scattered throughout the bed nucleus of the stria terminalis (BNST). The subpopulation of NPY neurons in the AStr also co-expressed somatostatin. Subjecting animals to a conditioned fear paradigm increased NPY gene expression within the AStr, whereas no changes were observed within the BLA or stria terminalis. Overall, these studies identified limbic regions associated with stress circuits providing NPY input to the BLA and demonstrated a unique NPY projection from the AStr may participate in the regulation of conditioned fear.
Keywords: amygdalostriatal transition area, Fluorogold, stria terminalis, in situ hybridization, immunohistochemistry, somatostatin, RRID:AB2255365, RRID:AB572229, RRID: AB 572268, RRID: AB 2314408
INTRODUCTION
The generation of a stress response involves the appropriate processing and integration of sensory and emotionally relevant information from a wide variety of brain regions, of which the amygdala plays a critical role. While all amygdala subdivisions influence the expression of anxiety and fear behavior, the basolateral amygdala complex (BLA) is particularly important in the acquisition and expression of fear-related memories and behavior (LeDoux et al., 1990a; Helmstetter and Bellgowan, 1994; Campeau and Davis, 1995; Maren et al., 1996; Muller et al., 1997; Wilensky et al., 1999). Regulation of anxiety and fear by the BLA is complex, consisting of a myriad of neurotransmitters and neuropeptides whose balance ultimately influences BLA output and resultant amygdala-dependent behaviors.
Neuropeptide Y (NPY) is highly expressed in the BLA and other regions associated with the generation of stress responses (Adrian et al., 1983; Allen et al., 1983; de Quidt and Emson, 1986a; 1986b). Changes in NPY gene and peptide expression have been reported in the BLA as well as other limbic regions following exposure to stress (Thorsell et al., 1998; 1999; Krukoff et al., 1999; Conrad and McEwen, 2000; Krysiak et al., 2000; Makino et al., 2000; Sweerts et al., 2001; Sergeyev et al., 2005; Cui et al., 2008), implicating NPY as an important player in these responses. This has been further emphasized by overall increases in stress-related behaviors in NPY knockout mice, or decreases in stress-related behavior in animals overexpressing the peptide (Erickson et al., 1996; Bannon et al., 2000; Thorsell et al., 2000; Redrobe et al., 2003; Tschenett et al., 2003; Primeaux et al., 2005). The precise role of NPY in modulating stress-related behaviors was elucidated by a number of studies showing that injection of NPY, or selective NPY receptor agonists, into the BLA decreases the expression of anxiety-related behaviors (Sajdyk et al., 1999; Sajdyk et al., 2002; Heilig, 2004; Sajdyk et al., 2006; 2008), inhibits the expression of fear-potentiated startle (Gutman et al., 2008) and avoidance training (Flood et al., 1989). While there are three subtypes of NPY receptors expressed within the BLA (Wolak et al., 2003; Kopp et al., 2002; Stanic et al., 2011), the NPY Y1 receptor subtype appears to be the predominant receptor underlying the generation of these anxiolytic-like effects. Of importance, endogenous NPY plays a critical role in the extinction of fear memories. Blockade of NPY Y1 receptors in the BLA leads to a deficit in extinction retention (Gutman et al., 2008) while having no effect on the acquisition of the fear memory. In addition to demonstrating the involvement of NPY Y1 receptors in fear behavior, these findings underscore the importance of endogenous NPY in the expression of anxiety and emotional processing within the BLA.
While the ability of NPY to regulate anxiety and fear is apparent, the origin of projection sources of NPY to the BLA remains unidentified. NPY co-localizes with GABA (McDonald, 1985; McDonald and Pearson, 1989) and somatostatin (SOM) (McDonald, 1989) in a population of class II BLA neurons which are largely local-circuit neurons (McDonald, 1982b; 1992). Projection sources of NPY were postulated to originate from catecholaminergic cell groups although this hypothesis has been discounted (Gustafson et al., 1986; Asan, 1998; Cui et al., 2008). This lack of evidence for projection sources of NPY to the BLA, together with knowledge that NPY is primarily expressed in interneurons throughout the brain (Chronwall et al., 1985; de Quidt and Emson, 1986b), prompted speculation that local-circuit neurons provide the sole source of NPY to the BLA. However, recent studies have challenged this concept and suggest that projection sources containing NPY likely exist and provide substantial innervation to the BLA (Truitt et al., 2009).
The current studies identified local and projection sources of NPY to the BLA using a combination of neuronal tract tracing and immunohistochemical methods. The neurochemical identity of NPY fibers in the BLA was determined using multi-label immunohistochemistry for NPY and SOM, tyrosine hydroxylase (TH), or dopamine β-hydroxylase (DβH). Retrograde tract-tracing [Fluorogold (FG)] was combined with immunohistochemistry for NPY to identify cell bodies of origin. Additionally, NPY gene expression was measured in animals that were subjected to a model of conditioned fear to determine which sources of NPY may be involved in regulating anxiety and fear responses.
MATERIALS AND METHODS
Animals
Male Sprague Dawley (Harlan Laboratories) rats (64-84 days old) were housed 2 or 3 per cage in an AAALAC accredited facility. A 12:12 h light:dark cycle was maintained, and food and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at Rosalind Franklin University of Medicine and Science.
Retrograde tracing of NPY projections to the BLA
Iontophoresis of FG into the BLA
Naïve rats received atropine sulfate (0.4 mg/kg, i.p.) and were anesthetized with sodium pentobarbital (40 mg/kg, i.p.). They were then placed in a stereotaxic apparatus, and the pipette containing FG was lowered into the brain at the level of the BLA [anteroposterior, −3.0; mediolateral, 5.1; dorsoventral, −8.2 mm from bregma] (Paxinos and Watson, 1998). Borosilicate glass pipettes (1.5 mm O.D. × 0.86 I.D.; Harvard Apparatus) were pulled and tips cut to 20 μm diameter under a dissecting microscope before being filled with 4% FG (Fluorochrome, LLC) in 0.1M cacodylic acid, pH 7.0. A bare silver wire was inserted into the pipette and connected to an iontophoresis current source (Stoelting). A retaining current was maintained while lowering the pipette tip to the desired position and for an additional 5 min once the pipette was in position. The tracer was delivered via an alternating current (+ 7 μAmps; 4 s on, 4 s off) for 30 min (in 5 rats designated FG14, FG18, FG24, FG25, and FG29) or 45 min (FG15). A retaining current was maintained for an additional 10 min and during the removal of the pipette tip from the brain. Incisions were closed and rats received topical lidocaine (Sigma-Aldrich) at the incision site and the general analgesic, banamine (2 mg/kg, s.c.).
After allowing 7 days for transport of tracer, rats were anesthetized with sodium pentobarbital (100 mg/kg, i.p.), and transcardially perfused with warm (37°C) phosphate buffered saline (PBS) containing 0.1% procaine and heparin followed by cold paraformaldehyde (PFA; 4%) in PBS containing 0.05% glutaraldehyde. The brains were removed and post-fixed through a graded series of sucrose concentrations (5, 10, 20%) in 4% PFA at 4°C. Tissues were transferred over to phosphate buffer containing 20% sucrose for 1 hr, sectioned into 20 μm slices on a cryostat, mounted on RNase free gelatin-subbed slides, and stored in sealed slide boxes with a desiccant tablet at −80°C until processing for immunohistochemistry.
Dual-label Immunohistochemistry for FG Tracing Study
Tissues were removed from the freezer and allowed to dry at room temperature. A barrier was made around the edge of the slide using a PAP™ pen to assure that tissues remained covered in solution during the incubation steps. Processing of the tissues was performed similarly to that described previously (Urban et al., 2006), except washes were done using slide racks, and longer incubations were carried out in moist chambers to retard evaporation. In brief, sections were rinsed in PBS (pH 7.4; 3 × 5 min) washes. The sections were blocked for 2 hr with 10% normal donkey serum (NDS) containing 0.25% triton X-100 (TX-100) in PBS-gelatin and then incubated for 72 hrs with a rabbit 1° antibody against FG (1:5,000; Chemicon; catalog # AB153: RRID:AB_2314408) in 3% NDS and 0.25% TX-100 at 4°C for 72 hrs. After washes, the sections were incubated with FITC-conjugated donkey anti-rabbit 2° antibody (1:250; Jackson ImmunoResearch Laboratories) in 3% NDS and 0.25% TX-100 for 3 hrs. Tissue was then washed and incubated with a mouse 1° antibody against NPY (NPY02; 1:2500; a generous gift from Dr. Eric Grouzmann) in 3% NDS and 0.25% TX-100 for 72 hrs. After washes, sections were incubated with Cy3-conjugated donkey anti-mouse 2° antibody (1:250; Jackson ImmunoResearch Laboratories) in 3% NDS and 0.25% TX-100 for 3 hrs. Coverslips were applied using PVA-DABCO. Antibody specificity was verified in all immunohistochemistry studies by omitting each 1° antibody in separate wells and assessing specific signal in all appropriate channels.
Conditioned Fear [Conditioned Emotional Response (CER)]
Naïve rats were randomly assigned into one of 3 groups (Shock, No Shock, or Home Cage Control). Rats assigned to the Shock group were placed in a footshock chamber (Med Associates) for a total of 10 min. During the last 10 s of this period, animals received an inescapable scrambled footshock (0.5 mA). This was repeated once a day for a total of 3 days, and on the fourth day animals were placed in the chamber for 10 min but did not receive a shock. An additional group of animals was treated similarly and placed in the chamber for all 4 days, but never received a shock (No Shock group). They served as a control group for the footshock, novel environment (shock chamber), and transfer to and from housing and conditioning rooms. Home Cage Controls were handled for a few minutes each day over 4 days, but were not introduced to the chamber and serve as a control for handling stress. Behavior (time spent exploring, grooming, and freezing as well as number of rearings and defecations) was recorded over the entire 10 min period spent in the shock chamber on day 4, and scored at a later time by an experimenter blind to the assigned treatment group.
Determination of NPY co-expression with markers for interneurons or catecholaminergic fibers
Tissue collection for NPY, SOM, TH, and DβH immunohistochemistry
Rats were removed from the chamber on day 4 of the CER procedure and transferred to their housing room where they were immediately anesthetized with pentobarbital (100 mg/kg, i.p.) and transcardially perfused with warm (37°C) PBS containing 0.1% procaine and heparin, followed by cold 4% PFA in PBS. Naïve and Home Cage Control rats were anesthetized directly from their cages. After perfusion, the brains were post-fixed overnight at 4°C. Tissues were then transferred over to PBS and later sectioned using a vibratome (40μm; Ted Pella).
Multi-label Immunohistochemistry for NPY, SOM, TH, and DβH Fiber Analysis
Immunohistochemistry was performed on free-floating sections from naïve rats or those included in the CER study as that described above. Sections were incubated for 72 hrs with a mouse 1° antibody against NPY (NPY05; 1:10,000; a generous gift from Dr. Eric Grouzmann) in 3% NDS and 0.25% TX-100 with gentle agitation. Sections were rinsed in PBS-gelatin and incubated with biotinylated donkey anti-mouse 2° antibody (1:2,000; Jackson ImmunoResearch Laboratories) for 1 hr. Following washes in PBS-gelatin, the tissues were incubated with avidin-biotin complex (2 μl/ml; ABC reagent, Vector Labs) for 30 min and rinsed in PBS-gelatin. For amplification of the signal, the tissues were incubated with biotinylated tyramide in 0.01% H2O2/PBS for 10 min, rinsed and incubated with FITC-streptavidin (1:250; Jackson ImmunoResearch Laboratories) for 3 hrs. After 4 × 5 min washes in Tris buffered saline (TBS; pH 7.4), sections were incubated with a rabbit 1° antibody against DβH (1:3000; ImmunoStar; RRID:AB_572229) in 3% NDS and 0.25% TX-100 for 72 hrs and rinsed in PBS-gelatin. Tissues were then incubated with Cy5-conjugated donkey anti-rabbit 2° antibody (1:250; Jackson ImmunoResearch Laboratories) in 3% NDS for 3 hrs, rinsed in TBS, and incubated with a rat 1° antibody against SOM (1:200; Millipore; catalog # MAB354; RRID:AB_2255365) or a mouse 1° antibody against TH (1:1000; ImmunoStar; RRID:AB_572268) in 3% NDS and 0.25% TX-100 for 72 hrs. After rinses in PBS-gelatin, sections were incubated with Cy3-conjugated donkey anti-rat or anti-mouse 2° antibody (1:250; Jackson ImmunoResearch Laboratories) in 3% NDS for 3 hrs to visualize SOM or TH labeling, respectively. Lastly, tissue was rinsed in TBS, mounted on gelatin-subbed slides, and air-dried. Coverslips were applied using PVA-DABCO (polyvinyl alcohol-1,4 diazabicyclo[2.2.2.]octane).
Biotinylated tyramide amplification permits the use of lower concentrations of 1° antibody than detectable using standard indirect immunofluorescence methods. This amplification technique is particularly useful when performing multiple-label immunohistochemistry using primary antibodies in the same species. To control for species cross-reactivity of antibodies, the anti-TH antibody was omitted from the multi-label protocol and specificity was verified by observing no signal in the Cy3 channel (excitation laser 543 nm).
Analysis of NPY fiber intensities in the BLA
Every 6th section of tissue throughout the entire extent of the BLA (~12 sections/animal) was used for analysis of each antibody combination. The BLA was defined as including the following subdivisions: dorsolateral and ventromedial subdivision of the lateral amygdaloid nucleus (LA), and the anterior and posterior subdivision of the basolateral amygdaloid nucleus (BL). Immunoreactive staining was visualized using epifluorescent microscopy (Eclipse C600; Nikon) images were captured at 10× magnification.
The contours of the BLA were outlined and average staining intensities were obtained using MetaMorph software (Universal Imaging; RRID:SciRes_000136). The contours were apparent based on differences in intensity between the BLA and adjacent structures. LA and BL subdivisions were not assessed separately because they contain similar staining intensities, which made the borders between the two subdivisions difficult to distinguish. To account for differences in background labeling between sections, staining intensities of the optic tract was subtracted from that of the BLA for each section. The optic tract was devoid of any specific labeling and present in every section, thus making it an appropriate structure for background subtraction. Analysis was performed with the experimenter blind to the assigned group. Data were obtained from 4-10 animals per group.
Modulation of NPY gene expression by Conditioned Fear
Rats were subjected to the conditioned fear paradigm as previously described, removed from the chamber on day 4 and returned to their home cages. They remained there for 1 or 5 hr and were transferred to a separate room where they were killed by decapitation. Brains were removed, snap frozen in chilled 2-methyl butane, and stored at −80°C. Sections through the brain were cut (20 μm) using a cryostat, mounted onto RNase free gelatin-subbed slides, and stored in sealed slide boxes with a desiccant capsule at −80°C until further processing.
In Situ Hybridization Coupled with Emulsion Autoradiography
On the day of processing, slides containing sections of the BLA, were brought to room temperature and sections were allowed to air dry. Tissue slices were postfixed in cold 4% PFA in PBS and rinsed in PBS and 0.1M triethalolamine (TEA). The tissues were then incubated in acetic anhydride (875μl in 400 ml 0.1M TEA) for 10 minutes, rinsed in 2× standard saline citrate (SSC), dehydrated through a graded series of ethanol (70, 95, and 100%) washes, delipidated in chloroform and rehydrated (100, 95% ethanol).
Oligonucleotides complementary to NPY (5’-TGC TGG CGC GTC CTC GCC CGG ATT GTC CGG CTT GGA GGG GTA-3’; Integrated DNA Technologies, Inc.; GenBank accession # AF392061) were end-labeled with 35S-dATP using terminal deoxynucleotidyl transferase. Labeled probes were purified on spin columns, and applied to the tissue (2.5 pmol/ml) in a hybridization buffer consisting of 50% formamide, 10% dextran sulfate, 0.3 M NaCl, 10 mM TRIS, 1 mM EDTA,10 mM DTT, and 1x Denhardts (1% bovine serum albumin, ficoll, and polyvinylpyrollidone). Coverslips were applied over the tissue which was incubated overnight at 37°C in moist chambers. The next day, the coverslips were removed by rinsing the slides in 1X SSC. The tissue underwent a series of 4 washes (15 min) in 1X SSC at 58°C followed by 2 washes (1 hr) in 1X SSC at room temperature. The tissue was dehydrated through a graded series of alcohols (70 and 95% containing 300 mM ammonium acetate to stabilize the formed hybrids). Once dried, the sections were coated with Kodak NTB2 track emulsion diluted 1:1 with 0.6 M ammonium acetate and stored in light tight boxes at 4°C for approximately 2-3 weeks. The slides were developed in Kodak D-19 developer (diluted 1:1 with dH2O) for 4 min followed by a 1 min rinse in dH2O, and fixed (Kodak fixer) for 5 min; all solutions were maintained at 16°C. After a thorough rinsing in dH2O, sections were stained lightly with cresyl violet acetate to define histology, dehydrated through a graded series of alcohols (70, 95, 100%), and cleared in CitriSolv (Fisher Scientific). Coverslips were applied using Permount Mounting media (Fisher Scientific). Signal specificity was verified by treating alternate brain sections with 100-fold excess unlabelled probe in the hybridization mix.
Analysis of NPY mRNA
Sections processed for in situ hybridization were atlas-matched [Paxinos and Watson (1997); RRID:nlx_152120] to correspond with atlas levels spaced through the AStr (2.2-3.3 mm caudal to bregma) or BLA (1.8-3.8 mm caudal to bregma) and analyzed. As NPY cells in the stria terminalis are less abundant, every 6th section of tissue through this region was analyzed. Darkfield images were captured at 20× magnification with a digital camera (Eclipse C600; Nikon) and analyzed using MetaMorph software. Average cell intensity (relative mRNA expression per cell) and cell counts were measured, unilaterally, in 4 or 5 sections for the AStr or BLA, respectively. Background was subtracted using intensities adjacent to each NPY labeled cell. Viewing tissue with bright-field microscopy verified that grain clusters were localized over cells as assessed by cresyl violet staining. Analysis was performed with the experimenter blind to the assigned group. Data were obtained from 4-10 animals per group.
Antibody Characterization
NPY02 (mature NPY) and NPY05 (amidated NPY) antibodies were characterized by demonstrating affinity for NPY with little to no cross-reactivity for the related peripheral peptides, pancreatic polypeptide (PP) or peptide YY (PYY), respectively (Grouzmann et al., 1992). We have also characterized the antibodies by immunohistochemistry, with or without pre-adsorption with the immunizing peptide (1:10 antiserum:peptide; unpublished observations). The NPY02 antibody is directed against the mature form of NPY and thus labels more cell bodies than the NPY05 antibody, which specifically labels the final, amidated, releasable peptide and is more specific to axonal terminals. Therefore, NPY02 was used in the retrograde tracing study and NPY05 was used in fiber analysis studies. Specificity of somatostatin and DβH antisera was also determined by pre-adsorption of the antibody with the immunogenic peptide, which eliminated all detectable labeling as assessed by immunohistochemistry (Sockman and Salvante, 2008; Rostkowski et al., 2009). Likewise, the somatostatin antibody did not cross-react with various other related neuropeptides (manufacturer's technical information), and the DβH antibody produced characteristic bands at the expected molecular weight (72-74 kD), as determined by Western blot of purified DβH (Clarke et al., 2010). The staining pattern of DβH was typical for that of catecholamine neurons and also overlapped in many areas with TH immunoreactivity. The specificity of the TH antibody was verified by Western blot according to the manufacturer. A single 60 kDa band was observed in protein lysates from rat and mouse brain. . Furthermore, the TH antibody did not cross-react with DβH, phenylethanolamine-N-methyltransferase (PNMT), phenylalanine hydroxylase, tryptophan hydroxylase, or dihydropterdine reductase. Specificity of the Fluorogold antiserum was determined by the hypothalamic-specific labeling observed in brains from rats that received peripheral injections of Fluorogold, and the lack of immunostaining in tissue from rats that did not receive injections (Dimitrov and Usdin, 2010).
Image Preparation and Statistical Analysis
To ensure the highest quality images for publication, all images were imported into Photoshop (Adobe Systems, Inc.) where brightness and contrast were modified and superimposition of the images was performed. All data are reported as mean ± standard error of the mean (S.E.M.). Graphs as well as statistical analysis were performed using GraphPad Prism. Data were analyzed using Student's t-test, one-, or two-way ANOVA where appropriate and statistical significance was set at p < 0.05.
RESULTS
Assessment of the Distribution and Co-localization of NPY- and SOM-ir Fibers in the BLA
To characterize the NPYergic innervation in the BLA we utilized a series of double-label immunohistochemistry studies to identify the extent of NPY fibers from interneuronal versus projection fibers. Previous studies have demonstrated that virtually all of the NPY/GABAergic interneurons in the BLA also express SOM (Levita et al., 2003). Therefore, NPY and SOM co-expression would indicate the presence of NPY in interneuronal populations, and the lack of co-expression would suggest additional sources. In unstressed male rats, NPY-ir fibers (Figure 1A,D,G) were more prevalent than SOM-ir fibers (Figure 1B,E,H) throughout the BLA although higher densities of SOM-ir fibers were found in the LA. Dual-labeled fibers for both NPY and SOM were present throughout the LA and BL division (arrows in Figure 1F and 1I, respectively). In general, these fibers often appeared thicker (arrows in Figure 1D-F) than those single-labeled for NPY (arrowheads in Figure 1G-I). Numerous NPY single-labeled fibers were present throughout the BLA (arrowheads in Figure1F,I); fibers were consistently observed in greater abundance in the BL (Figure 1I) than the LA (Figure 1F).
Figure 1.
Distribution of Neuropeptide Y- (NPY05, green) and somatostatin-(SOM, red) immunoreactive (ir) fibers within the lateral (LA; A-C, D-F) and basolateral amygdaloid nuclei (BL; G-I). The borders of the LA and BL are indicated by dotted lines (A-C). Merged confocal images of each respective row demonstrating co-localization (white) of NPY and SOM immunoreactivity (C,F,I). Single- (arrowheads) and double- labeled (arrows) fibers are indicated. Note the higher degree of single-labeled, NPY-ir fibers within the BL (I) compared with the LA (F). CeA, central amygdaloid complex. Scale bars = 200 μm (A-C) and 20 μm (D-I).
Assessment of the Distribution and Co-expression of NPY, TH, and DβH immunoreactivity in the BLA
The BLA receives catecholaminergic innervation from the brainstem and ventral tegmental area. These are likely sources of NPY to the BLA since the neuropeptide is co-expressed within catecholaminergic cell groups in these regions (Everitt et al., 1984). The distribution and morphology of NPY-ir fibers in the BLA (Figure 2A-C) is consistent and similar to that described above. Both TH- (Figure 2D) and DβH-ir fibers (Figure 2G) were prevalent throughout the rostral-caudal extent of the BLA with a higher distribution in the BL than the LA. Numerous single-labeled TH-ir fibers (right most set of arrowheads in Figure 2C,F,I,L) and dual-labeled TH/DβH-ir fibers (arrows in Figure 2C,F,I,L; co-localization is seen as magenta) were present in both the LA and BL of naïve rats. No co-expression of NPY-ir and TH- or DβH-ir was found within the BLA of naïve rats (far left set of arrowheads in Figure 2C,F,I,L). The same immunohistochemical staining was performed in tissue from stressed rats, with the rationale being that conditioned fear may increase the expression of either marker thereby providing a different profile of co-expression. Exposure to conditioned fear did not alter the co-expression of NPY and TH or DβH immunoreactivities within the BLA.
Figure 2.
Comparative distribution of NPY (green), tyrosine hydroxylase (TH; red) and dopamine β-hydroxylase (DβH; blue) immunoreactivities in the amygdala. Representative confocal photomicrographs of NPY (A-C), TH- (D-F), and DβH-ir (G-I) fibers in the lateral (LA; A,D,G,J) and basolateral (BL; A,D,G,J and C,F,I,L) amygdaloid nucleus of a naïve rat. The border of the BLA is indicated by dotted lines. Merged images from each respective column (C,F,I,L) demonstrating overlap but no co-localization of NPY immunoreactivity with either TH- or DβH-ir fibers. Single-labeled fibers are indicated by arrowheads and double-labeled TH- and DβH-ir fibers (magenta) by arrows. CeA, central amygdaloid nucleus. Scale bars = 500 μm (A,D,G,J) and 10 μm (B,C,E,F,H,I,K,L).
Retrograde Tracing Studies
Pipette tip placements and the degree of FG injection spread are presented in Figure 3A. Placement locations ranged from 2.56 to 3.80 mm caudal to bregma (Paxinos and Watson,1997). FG injections in FG24 and FG14 were confined to the BL division of the BLA, while injections in FG15 were almost completely restricted to the LA component of the BLA. FG25 and FG29 contained spread into both the LA and BL. The injection in FG18 was outside and lateral to the BLA (Den, VEn, and cortex). Therefore, this injection was as a control for FG labeling in FG25 and FG15, which contained tracer that had spread to these adjacent regions to the BLA, at the perimeter of the injection site. FG was not observed in the pipette tracks in all cases (Figure 3C).
Figure 3.
Schematic (A) and representative photomicrographs (B, C) displaying placement of Fluorogold (FG) injections in coronal slices from individual rats. Numbers to the right of each diagram in A represent distance in mm posterior to bregma as indicated by Paxinos and Watson (1997). B. Low magnification, phase-contrast photomicrograph of the BLA identifying the borders of the BLA (indicated by arrows). C. Photomicrograph showing FG deposition within the BLA captured with ultraviolet illumination; identical field of view as B. BL, basolateral amygdaloid nucleus; BSTIA, bed nucleus of the stria terminalis intra-amygdaloid division; CeA, central amygdaloid nucleus; DEn, dorsal endopiriform nucleus; LA, lateral amygdaloid nucleus; Pir, piriform cortex; VEn, ventral endopiriform nucleus. Scale bar = 1mm.
NPY projections to the BLA
In general, retrogradely labeled (FG-ir) cells were observed throughout the brain in patterns consistent with regions previously reported to project to the BLA (Figure 4; Pitkanen, 2000).
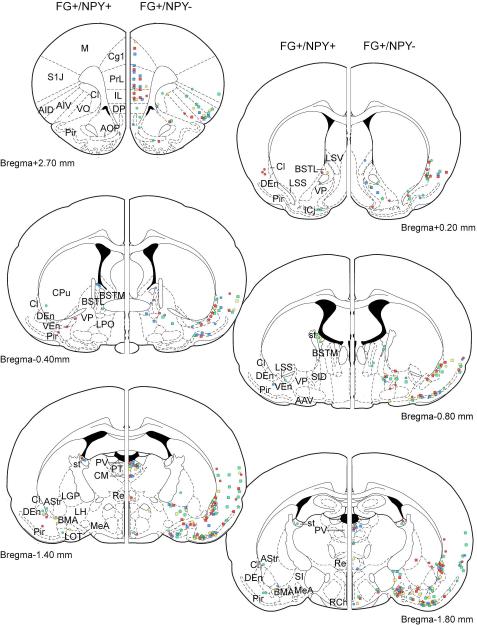
Figure 4.
Schematic indicating location of FG-filled cells immunopositive for NPY at various coronal atlas levels from individual rats. Left hemispheres depict FG+/NPY+ cells observed over multiple sections near each atlas level while right hemispheres depict relative number of FG+/NPY− cells in each section.
Amygdalostriatal transition area (AStr) and stria terminalis
FG-filled cell bodies containing NPY immunoreactivity were observed in numerous regions (Figure 4). The amygdalostriatal transition area (AStr) and stria terminalis contained the largest number of dual-labeled cells. Double-labeled (NPY+/FG+) cells were present in these regions in every rat except for FG14 (injection confined to BL) and FG18 (injection outside the BLA). No FG labeling was observed in the contralateral AStr and stria terminalis.
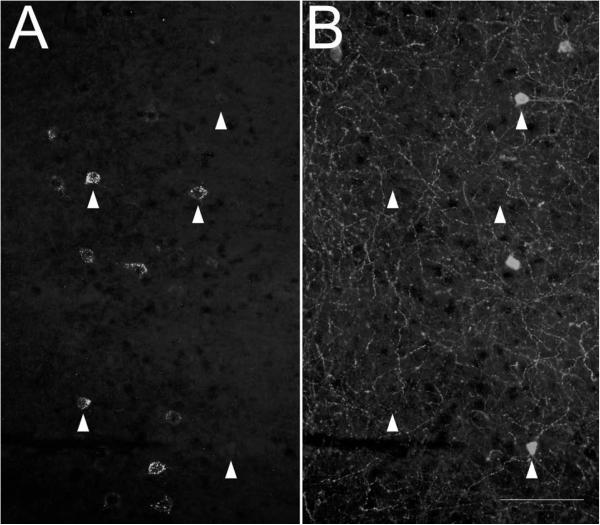
In general, for all tissues analyzed, FG-ir cells were present in each section of the AStr (Figure 4; 5D-J). In general, dual-labeled cells in the AStr contained small- to medium-sized (12-17 μm diameter) round or ovoid somata that were scattered throughout the rostral-caudal extent of the nucleus. However, not all FG-filled cells in the AStr displayed NPY immunoreactivity (Figure 5A-C). The largest proportion (7/8 or 88%) of FG+/NPY+ cells per section occurred in FG24, which had the most anterior injection placement and in which FG spread was restricted to the BL. More posterior injections yielded a smaller percentage of dual-labeled cells (FG14,0/4 or 0%; FG29, 3/8 or 38%; FG25, 3/9 or 33%; FG15, 9/25 or 36%) in the AStr. The larger number of FG-filled cells was observed in FG15, which contained the most posterior injection and in which FG spread was largely restricted the LA division.
Figure 5.
Identification of FG-filled cells immunopositive for NPY in the amygdalostriatal transition area (AStr) from rats that received iontophoretic delivery of FG into the BLA. Representative photomicrographs displaying A.) FG, and B.) NPY immunoreactivity. C.) Merged image of A and B demonstrating co-localization as indicated by arrowheads. Inset displays high magnification images of a dual-labeled cell. Note: the AStr is distinct from the adjacent capsular part of the central amygdaloid nucleus (CeC), which is devoid of NPY fiber staining, and LA, which encompasses the injection site. Dashed lines indicate region borders. D-J. Schematic indicating location of FG-filled cells immunopositive for NPY through the rostral-caudal extent of the AStr. Each dot represents one dual-labeled cell and is color coded to indicate individual rats (refer to figure 3). ACo, anterior cortical amygdaloid nucleus; BMA, basomedial amygdaloid nucleus, anterior part; BSTIA, bed nucleus of the stria terminalis, intra-amygdaloid division; CeA, central amygdaloid nucleus; Den, dorsal endopiriform nucleus; IPAC, interstitial nucleus of the posterior limb of the anterior commissure; LSS, lateral stripe of the striatum; Pir, piriform cortex; st, stria terminalis; VEn, ventral endopiriform nucleus. Scale bar = 100 μm.
In the stria terminalis, very few FG-ir cells (0-2 per section) were observed (Figure 6A). However, in contrast to the AStr, NPY immunoreactivity was present in the majority of FG filled cells in the stria terminalis (FG14, 6/8 or 75%; FG24, 15/18 or 83%; FG29, 6/6 or 100%; FG25, 4/4 or 100%; Figure 6A-C) but not in FG15 where the placement was in the more posterior BLA (2/7 or 29%). In general, dual-labeled somata were elongated or fusiform (20-25 μm diameter) in appearance and were observed in the more rostral stria terminalis, but were also seen scattered throughout the rostrocaudal extent of the pathway (FG24 and FG29).
Figure 6.
Identification of FG (green)-filled cells immunopositive for NPY (NPY02; magenta) in the stria terminalis (st) of rats that received iontophoretic delivery of FG into the BLA. Representative photomicrographs displaying A.) FG, and B.) NPY immunoreactivity. C.) Merged image of A and B demonstrating co-localization (arrowheads). The presence of NPY fibers in the stria terminalis were used to delineate it from the adjacent internal capsule (ic). D-K. Schematic indicating number and location of FG-filled cells immunopositive for NPY over multiple sections near each coronal atlas level indicated. Each colored dot represents one dual-labeled cell which is coded to an individual rat (see figure 3). AStr, amygdalostriatal transition area; BSTIA, bed nucleus of the stria terminalis, intra-amygdaloid division; CeA, central amygdaloid nucleus; fi, fimbria of the hippocampus. Scale bar = 50 μm.
Other brain regions
Cells co-expressing NPY and FG immunoreactivities were also observed in other brain regions, although less frequently and consistently than the AStr and stria terminalis. These regions included numerous subregions of the bed nucleus of the stria terminalis (intra-amygdaloid division, lateral division, and medial division, anterior part) and the piriform cortex. Of all the regions mentioned above, only the intraamygdaloid division of the bed nucleus of the stria terminalis contained dual-labeled cells in every rat.
A number of brain regions with known projections to the BLA that modulate stress responses were also examined for the presence of FG and NPY co-expression. However, no FG+/NPY+ co-expressing cells were found in the medial prefrontal cortex, locus coeruleus, entorhinal cortex, subiculum or the ventral CA1 region of the hippocampal formation. Although numerous NPY-ir cells were adjacent to, or interspersed among, FG-ir cells within regions (infralimbic, prelimbic, and cingulate cortices; Figure 7) analogous to the human prefrontal cortex, no dual-labeled cells were observed. This was also true for the locus coeruleus, entorhinal cortex, and subiculum. While the CA1 did contain NPY-ir somata, they were restricted to more dorsal aspects of this region than the FG-ir cells.
Figure 7.
Photomicrograph of A.) FG and B.) NPY immunoreactivities in the prefrontal (infralimbic) cortex from rats that received iontophoretic delivery of FG into the BLA. Arrows demonstrate cells immunopositive for FG or NPY; note the lack of co-expression within this area. Scale bar = 100 μm.
Assessment of NPY and SOM Co-localization in Cell Somata within the AStr and Stria Terminalis
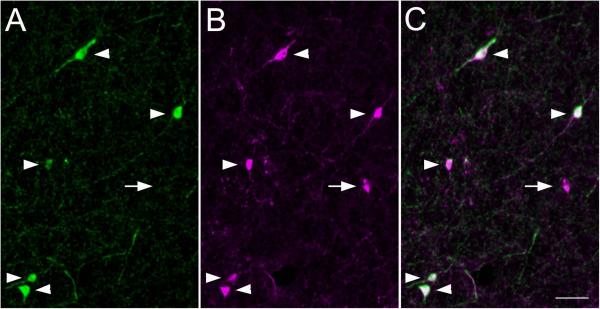
To determine if projections from the AStr or stria terminalis contribute to the dual-labeled NPY/SOM fibers in the BLA, NPY-ir cell bodies within the AStr and stria terminalis were assessed for SOM immunoreactivity. All NPY-ir cells observed in the AStr displayed SOM immunoreactivity (Figure 8) while numerous SOM single-labeled cells were also observed. In contrast, none of the NPY-ir cells assessed in the stria terminalis displayed SOM immunoreactivity.
Figure 8.
Co-expression of A.) NPY (green) and B.) SOM immunoreactivities in cell bodies within the AStr . Representative NPY- and SOM-ir staining patterns in the AStr are shown in panel A and B, respectively. C.) Merged image of A and B demonstrating NPY-ir cells displaying SOM immunoreactivity as indicated by arrowheads. Arrow indicates a single-labeled SOM cell. Scale bar = 50 μm.
Modulation of NPY expression by Conditioned Fear
Rats subjected to the conditioned fear paradigm (Shock rats) displayed typical fear-related behavior when placed in the shock chamber on day 4 (Figure 9). This included spending most of the time motionless (freezing). In contrast, ‘No Shock’ controls spent most of their time actively exploring or rearing. Overall, statistical analysis revealed that ‘Shock’ rats spent significantly less time exploring and grooming (P < 0.0001, Student's t-test), and more time freezing (P < 0.0001) in comparison to ‘No Shock’ controls. Shock-exposed rats also reared significantly less (P = 0.0056) and produced more fecal boli (P = 0.0011) than No Shock rats.
Figure 9.
Behavioral responses on day 4 of the conditioned contextual fear paradigm. Data are expressed as mean + S.E.M. n=4-6 animals per group. Significantly different from corresponding ‘No Shock’ group, p<0.01* or 0.0001**; Student t-test.
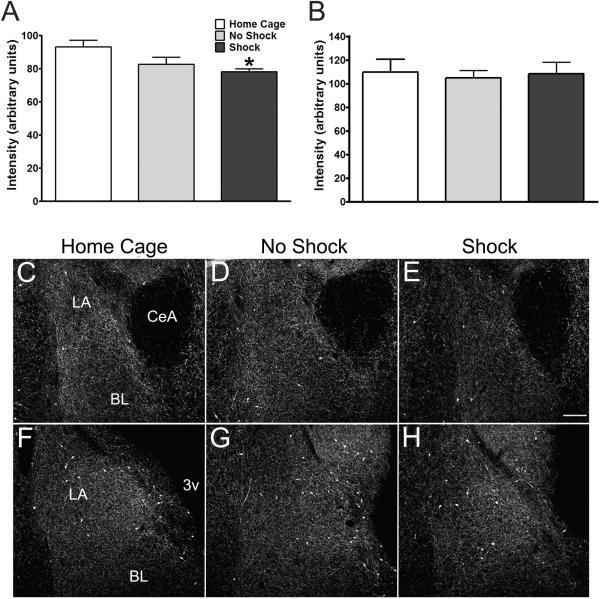
Analysis of BLA sections (2.0 to 3.2 mm caudal to bregma; Paxinos and Watson, 1998) revealed that NPY05-ir fiber intensities were significantly lower in Shock rats when compared with home cage control groups (F2,8 = 4.657; P = 0.0456; one-way ANOVA with Newman Keuls post-hoc test; Figure 10A, C-E). While changes in NPY-ir fiber intensities appeared to encompass a large extent of the anterior BLA, decreases were particularly apparent in fibers bordering the central amygdaloid nucleus and AStr medially and the cortex laterally (Figure 10C-E). Fiber intensities in No Shock controls were not significantly different from Home Cage controls or Shock rats, indicating that exposure to the footshock was critical for the diminution in fiber intensity observed in Shock rats. No changes in NPY-ir fiber intensity were observed in the posterior BLA (3.3 to 4.2 mm caudal to bregma; F2,8 = 0.07804; P = 0.9256; Figure 10B, F-H).
Figure 10.
Amidated NPY-ir (NPY05) fiber intensities in the A.) anterior (2.0 to 3.2 mm caudal to bregma as indicated by Paxinos and Watson, 1997) or B.) posterior (−3.3 to −4.2 mm bregma) BLA of rats exposed to conditioned fear. C-H. Representative photomicrographs of NPY-ir fiber intensities in the anterior (C-E) and posterior (F-H) BLA depicting average intensities shown in A and B, respectively. Note the less intense labeling within the BLA of Shock rats in anterior coronal sections, but not posterior sections. Data are expressed as mean + S.E.M. n=4-6. *statistically significant from home cage group, p < 0.05, One way ANOVA with Newman-Keuls post-hoc test. CeA, central amygdaloid nucleus; BL, basolateral amygdaloid nucleus; LA, lateral amygdaloid nucleus; 3v, 3rd ventricle. Scale bar = 200 μm.
NPY mRNA expression was examined in regions with known NPY projections to the BLA to determine which cell groups might be activated by fear and anxiety. To test this, NPY mRNA-containing cell numbers and expression were compared between the home cage and ‘No shock’ control groups. No differences were observed in cell intensities between No Shock and Home Cage controls in the BLA (F2,24 = 0.2339; P = 0.7932; one-way ANOVA; Figure 11A,C), stria terminalis (F2,9 = 0.4209; P = 0.6688; Figure 12), or AStr (F2,24 = 1.561; P = 0.2306; Figure 13A,C). Similarly, there were no differences in the number of cells expressing NPY mRNA in the BLA (F2,24 = 0.4029; P = 0.6728; one-way ANOVA; Figure 10B) or AStr (F2,24 = 2.849; P = 0.0776; Figure 12B) between Home Cage and No Shock controls. This was important to determine so that any changes, or lack thereof, in NPY mRNA expression after fear conditioning was not due to confounds from exposure to the process of transfer to the conditioning room, or the conditioning chamber.
Figure 11.
NPY mRNA expression in the BLA of rats subjected to conditioned fear. Data are expressed as mean cell intensities (A) or average number of labeled cells (B) + S.E.M. X-axes indicates time after removal from the shock chamber on day 4. n=4-6 per group; Two way ANOVA. C.) Representative dark-field photomicrographs of emulsion-coated sections depicting NPY mRNA expression in cells within the BLA of the different groups. Scale bar = 50 μm.
Figure 12.
NPY mRNA expression within the stria terminalis of rats subjected to conditioned fear. X-axis indicates time after removal from the shock chamber on day 4. Data are expressed as mean cell intensities + S.E.M. Data are expressed as mean cell intensities + S.E.M. n=4-6 per group; Two way ANOVA.
Figure 13.
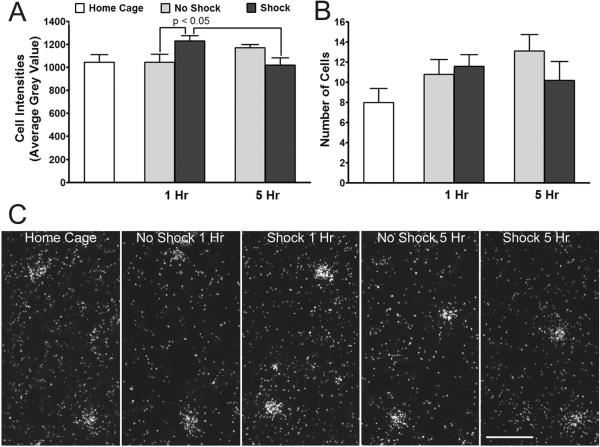
NPY mRNA expression in the AStr of rats subjected to conditioned fear. Data are expressed as mean cell intensities (A) or average number of labeled cells (B) + S.E.M. X-axes indicates time after removal from the shock chamber on day 4. n=4-6 per group; Two way ANOVA followed by Bonferroni post-hoc test. C.) Representative dark-field photomicrographs of emulsion-coated sections showing NPY mRNA-expressing cells within the AStr among groups at different time points. Note the more intense labeling of cells in shocked rats at the 1 hr time point. Scale bar = 50 μm.
Within the BLA, NPY mRNA-expressing cells (Figure 11C) were scattered throughout the rostral-caudal aspect of the nucleus in a manner similar to the distribution of NPY-ir cells. No main effect of conditioning (Shock; F1,34 = 0.0589; P = 0.8097; or F1,34 = 0.0888; P = 0.7676; two-way ANOVA) or conditioning time post-exposure interaction (F1,34 = 0.7080; P = 0.4060; or F1,34 = 0.6963; P = 0.4099) on average cell intensity or cell number, respectively, was observed between No Shock and Shock rats (Figure 11).
Due to the low cell number and widespread distribution of NPY mRNA-expressing cell in the stria terminalis, only cell intensity was assessed. No main effect of conditioning (F1,14 = 0.2575; P = 0.6198) or interaction (F1,14 = 0.7475; P = 0.4018) on average cell intensity was observed in the stria terminalis (Figure 12).
Within the AStr, while there was no effect of fear conditioning on the overall number of NPY mRNA-expressing cells, there was a significant increase in cell intensity that was manifest 1 hour, but not 5 hours, after completion of the conditioned fear. No main effect of conditioning alone on average cell intensity was observed (F1,34 = 0.1128; P = 0.7391; two-way ANOVA comparing No Shock and Shock rats), however, conditioning was dependent on time post-exposure to chamber (interaction; F1,34 = 9.831; P = 0.0035; Figure 13A,C). Shocked rats had significantly higher intensities of NPY mRNA expression at 1 hr post-removal from the shock chamber in comparison to No Shock controls at the same time point and to Shock rats at 5hr (P < 0.05; Bonferroni post-hoc analysis). No main effect of conditioning (F1,34 = 0.4412; P = 0.5110) or interaction (F1,34 = 1.409; P = 0.2434) on cell number occurred among Shock and No Shock rats (Figure 13B).
DISCUSSION
These studies demonstrate the heterogeneity of NPY input to the BLA. While interneurons provide one source of NPY innervation in the BLA, there are other sites, AStr, BNST and stria terminalis, that also send NPYergic projections to this brain region. FG injections confined to the BL or LA subdivision labeled NPY-ir somata within various regions of the forebrain suggesting that both of these BLA subdivisions receive NPY innervation from extra-BLA sources. The predominant novel NPYergic projection was identified from the AStr to the BLA. This projection also expressed SOM immunoreactivity. Additionally, NPY mRNA levels in the AStr were increased in response to a fear-related stress. Since NPY plays an important role in mediating fear-related behaviors (Gutman et al., 2008) and stress resilience (Sajdyk et al., 2008), identification of additional NPY inputs to the BLA contribute significantly to further our understanding of the NPY circuits involved in the regulation of these responses and behaviors.
While activation of NPY receptors in the BLA participates in the modulation of anxiety and stress resilience, little is known about the source or regulation of NPY neural circuits within, or to, this brain region. The dense plexus of NPY fibers in the BLA, especially in comparison to the low to moderate number of cell bodies, led us to consider the existence of additional sources of NPY fibers to the BLA. Within the BLA, NPY is expressed in a subpopulation of interneurons (McDonald and Pearson, 1989) and non-pyramidal projection neurons (McDonald et al., 2012) that also expresses SOM (McDonald, 1989). Initially, we took advantage of the high degree of co-expression between NPY and SOM and reasoned that double-labeled fibers would predominantly be from BLA interneurons (Levita et al.,2003) whereas those single-labeled for NPY would suggest a source of NPY from outside the BLA. Our results demonstrated extensive co-expression of NPY and SOM in fibers within the BLA, but there were also populations that expressed either one peptide or the other in both the BL and LA. Based on earlier evidence presented by McDonald (1989), single-labeled SOM fibers were not unexpected since not all SOM-ir interneurons express NPY. As the vast majority of NPY-expressing BLA interneurons also express SOM, these populations of single-labeled NPY fibers suggested additional sources of NPY to the BLA. As it turns out from recent evidence, the coexistence of SOM and NPY does not necessarily indicate interneurons but now labels a novel population of forebrain projection neurons (McDonald et al., 2012).
NPY Projections from the AStr to the BLA
The specificity of the AStr-BLA projections is demonstrated by the lack of FG-labeled cells in the AStr when the FG injection placement was outside the BLA [FG18; such as in the DEn, VEn, PRh, LEnt, and Pir; (Insausti et al., 1987; Jolkkonen et al., 2001; Majak and Morys, 2007)]. We consistently observed FG-filled cells containing NPY immunoreactivity throughout the rostral-caudal extent of the AStr regardless of whether the FG injections were placed within the rostral-caudal extent of the BL or LA [using the anatomical borders defined by Paxinos and Watson, (1997)]. These findings indicated that NPY projections from the AStr likely provide innervation to a large extent of the BLA. In a related study using an anterograde tracer injected in the AStr, Jolkkonen et al., (2001) demonstrated patchy terminal labeling in the LA, but not the BL. While we observed more wide-ranging AStr to BLA projections, the differences between these studies may lie in the differences of techniques used as well as the relative placement of the injections. Some of the representative injection sites displayed in Jolkonnen et al., (2001) are medial to the location of the AStr illustrated by others (Alheid et al., 1995; Paxinos and Watson, 1997), and in the vicinity of the interstitial nucleus of the posterior limb of the anterior commissure. The distribution of FG-labeled cells observed in the present studies was located lateral to these injection sites. A similar NPY projection from the AStr has recently been reported (McDonald and Zaric, 2015) in addition to BLA-projecting NPY neurons from the entorhinal cortex and amygdalopiriform transition area. The visualization of these other cell groups could be due to the larger FG injections which would label more neurons, thus identifying these additional projections (McDonald and Zaric, 2015).
Widespread co-localization of NPY-ir cell bodies with SOM immunoreactivity was observed in the AStr. This suggests that NPY projections from the AStr to the BLA contain SOM, and thus provide a portion of the SOM fibers in the BLA. The extensive co-localization of NPY and SOM immunoreactivity in the AStr is in agreement with a study by McDonald (1989) describing virtually complete overlap in NPY and SOM immunoreactivity in cell somata in the lateral capsular subdivision of the central amygdalar nucleus (CLC), the dorsal part of which corresponds to the amygdalostriatal transition area described by Paxinos and Watson (1997). Within this study, it was proposed that NPY and SOM dual-labeled cells in the CLC correspond to aspiny neurons observed in Golgi preparations. Interestingly, a projection (presumably an axon) from a Golgi-impregnated, spine-sparse neuron in the dorsolateral part of the CLC is shown extending into the BLA (McDonald, 1982a). Our data extends this proposal, suggesting that the spine-sparse neurons in the dorsal CLC/AStr area could represent the NPY-containing AStr projections to the BLA reported here.
Axonal terminals from AStr projections to the BLA have been described as having a similar appearance to cholinergic terminals (Jolkkonen et al., 2001). The postulation that NPY-containing, AStr projections to the BLA are cholinergic is unlikely. While NPY and SOM are expressed in regions of the basal forebrain that provide the major source of cholinergic input to the amygdala, these peptides are not known to be co-expressed with choline-acetyl-transferase (ChAT), a marker for cholinergic neurons (Walker et al., 1989), and are restricted to local-circuit neurons (Zaborszky and Duque, 2000). Basal forebrain NPY neurons also display different electrophysiological properties than cholinergic neurons (Duque et al., 2000) and different dendritic profiles (Duque et al., 2007). As the neurochemical makeup of the observed NPY-negative, AStr projections to the BLA remain unknown, it's possible that these could represent cholinergic neurons.
NPY Projections from the Stria Terminalis to the BLA
NPY-containing projections from the stria terminalis to the BLA described here represent the first report of BLA projections from this region. Other amygdala subdivisions have been shown to receive projections from the stria terminalis and supracapsular division of the bed nucleus of the stria terminalis [BSTS; (Shammah-Lagnado et al., 2000)], the latter of which forms two columns of cells that are found within and extend along the length of the stria terminalis (for review see Alheid et al., 1998). We cannot conclusively state whether the FG and NPY dual-labeled cells observed were interspersed between fibers of passage within the stria terminalis or located within the BSTS. The majority of dual-labeled cells were seen in more lateral aspects of the stria terminalis, in proximity to where the lateral subdivision of the BSTS would be located. Based on this observation, along with knowledge that the BLA projects to the BSTS (Shammah-Lagnado et al., 2000) and is reciprocally connected to various other subdivisions of the lateral bed nucleus of the stria terminalis (BNST) (Woolf and Butcher, 1982; Rao et al., 1987; Grove, 1988; Dong et al., 2000; Pitkanen, 2000), it would not be surprising if the dual-labeled cells we observed were within the BSTS. However, it is important to note that many of these FG+/NPY+ cells had an elongated or fusiform appearance (Figure 5A-C), consistent with solitary neurons that are tightly packed between fiber bundles coursing through the stria terminalis (Alheid et al., 1998). FG+/NPY+ cells were seen throughout the rostral-caudal extent of the stria terminalis, although double-labeled cells were consistently observed in the anterior stria terminalis when FG injections were in the more posterior BLA. Similar to the AStr, dual-labeling was present in the stria terminalis regardless of location of the injection site, suggesting these projections innervate a large area of the BLA, though posterior FG injections tended to label cells within the anterior stria terminalis. No FG-ir cell bodies were observed in the stria terminalis when the injection was placed completely outside the BLA (FG18), demonstrating that regions adjacent and lateral to the BLA, such as the DEn, VEn, PRh, LEnt, and Pir, do not receive input from the stria terminalis as previously reported (Insausti et al., 1987; Jolkkonen et al., 2001; Majak and Morys, 2007).
Lack of NPY input to the BLA from the Medial Prefrontal Cortex (mPFC) and Locus Coeruleus (LC)
Known to be key regions involved in the expression of stress responses, the mPFC and LC were originally postulated to be sources of NPY innervation to the BLA. Both sites have known projections to the BLA, provide various inputs to modulate stress responses, and express NPY (de Quidt and Emson, 1986a; 1986b). Connections between the mPFC and BLA form the neuronal basis for the extinction of conditioned fearful memories (for review see Sotres-Bayon et al., 2004). Since endogenous NPY in the BLA modulates extinction to a conditioned auditory stimulus (Gutman et al., 2008), it was anticipated that NPY-containing mPFC projections to the BLA would be identified. However, while plentiful NPY-ir somata were seen scattered among FG-ir cells in the mPFC, no co-labeled cells were observed.
As seen with the mPFC, there were no BLA-projecting NPYergic neurons in the locus coeruleus. Attempts at identifying the possible co-expression of NPY with markers for catecholaminergic fibers, TH and DβH, indicated that these NPY fibers were not likely coming from brainstem sources, and this was supported by lack of FG+/NPY+ cells in the brainstem. While studies typically visualize NPY cell bodies using colchicine, in most brain regions we observed a similar number of NPY-ir somata as reported in colchicine-treated rats (de Quidt and Emson, 1986b), although we consistently saw fewer NPY-ir somata in the locus coeruleus. Therefore, as an additional means to assess whether noradrenergic cells provide NPY input to the BLA, we assessed NPY- and DβH-ir fiber co-localization within the BLA of rats subjected to conditioned fear, as well as naïve rats. NPY-ir fibers did not co-localize with DβH in any condition, reinforcing the concept that adrenergic and noradrenergic cell groups do not provide an appreciable source of NPY to the BLA (Gustafson et al., 1986; Asan, 1998; Cui et al., 2008).
Functional Considerations with Regard to Stress Responses
Identification of a novel NPY projection from the AStr to the BLA and demonstration of increases in NPY gene expression in the AStr after conditioned fear suggests that this pathway is important in regulating fear responses. Using an antibody that recognizes the amidated, or releasable, form of NPY (Grouzmann et al.,1992) we demonstrated that immunoreactivity for amidated NPY was decreased in the BLA on day 4 after exposure to the conditioning box, suggesting release of the peptide in response to conditioned fear. These changes were strongest within anterior BLA sections. In a series of control experiments, NPY immunoreactivity was not altered in animals that were conditioned (shocked) for three days and were taken from their home cage (not placed in the chamber on day 4). Furthermore, these effects were only observed after the three day conditioning paradigm; single exposure to the shock did not alter the response of NPY immunoreactivity. This postulation of NPY release also corroborates the findings of Gutman et al., (2008) who demonstrated that extinction of fear responses is dependent upon NPY release in the BLA. While we cannot definitively say that the specific AStr NPY pathway mediates these actions, the correlation of changes in peptide immunoreactivity and gene expression support this as an important pathway and other gene-based technologies will be useful in further elucidating the regulation of these NPY inputs to the BLA.
As the results from the fiber analysis do not consider which subset of NPY neurons are responding to the fear paradigm, we evaluated changes in NPY mRNA levels in individual cell soma of the AStr and stria terminalis, known projections to the BLA, as well as cell soma of the BLA itself. NPY gene expression was elevated in the AStr 1hr following removal of the conditioned stimulus and returned to baseline levels within 5hr. These changes were specific to the AStr. NPY mRNA levels remained stable in the BLA and stria terminalis at all timepoints examined. While these findings cannot identify whether changes in NPY-ir and gene expression are occurring in the same cell, they do support the postulation that NPY-containing AStr projections to the BLA respond to the fear paradigm.
The NPY Y1r and Y5 receptors that mediate the anxiolytic effects of NPY are located throughout the extent of the BLA (Wolak et al., 2003; Rostkowski et al., 2009) and reports of anxiolysis following exogenous delivery of NPY into the BLA do not appear to be dependent on the anterior-posterior location of the injection location (Sajdyk et al., 2008). Thus, the region-specific effects in NPY immunoreactivity observed likely represent a subset of NPY-containing circuits within the BLA activated in response to the fear paradigm. The importance of the AStr in emotionality is demonstrated by a number of neuroanatomical and electrophysiological studies. The AStr receives much of the same sensory inputs from the thalamus and cortex as the BLA (LeDoux et al., 1990b; Turner and Herkenham, 1991; Bordi and LeDoux, 1992; Mascagni et al., 1993; Romanski and LeDoux, 1993; Bordi et al., 1993; Uwano et al., 1995; Shi and Cassell, 1997; 1998; Doron and LeDoux, 1999; Toyomitsu et al., 2002), and the AStr and BLA receive reciprocal connections (Jolkkonen et al., 2001; Wang et al., 2002; Fudge et al., 2002; 2004; Novejarque et al., 2004; Fudge and Tucker, 2009). Interestingly, when animals are presented with an auditory stimulus the AStr exhibits shorter response latencies than amygdala nuclei (Bordi et al., 1993; Uwano et al., 1995). Furthermore, signal transduction from the LA to the AStr propagates with higher velocity and less attenuation than LA-BL connections (Wang et al., 2002), suggesting that the AStr is involved in reflexive responses. Speculation that the AStr is involved in triggering an orienting response (i.e. head direction and saccadic eye movements) to environmental stimuli (Doron and LeDoux, 1999; Shammah-Lagnado et al., 1999) has been supported by the identification of efferent projections of the AStr to the substantia nigra pars lateralis (Jolkkonen et al., 2001), the latter of which innervates the superior and inferior colliculi. The orienting response would be important for proper assessment and identification of a threat and/or the removal of a threat.
One interpretation of our current findings is that NPY-containing AStr projections to the BLA relay emotionally relevant information to the BLA in regards to learned and/or unlearned aspects of the environmental context. Interestingly, differential activation of AStr neurons has been reported in response to a novel context and immediately following testing for conditioned contextual fear responses (Trogrlic et al., 2011). The well-reported anxiolytic properties of NPY in the BLA, would suggest that the AStr-BLA connections may act to buffer amygdala-mediated downstream responses, such as activation of fear-associated behaviors to stress, and/or termination of the behavior when the stressor is removed. As further justification for the AStr in regulating conditioned fear-induced behaviors, lesions of the central amygdala were more effective at preventing fear-associated responses to conditioned auditory stimuli, such as freezing behavior, when they included the AStr (Iwata et al., 1986; LeDoux et al., 1986).
In summary, our data confirmed that local-circuit neurons are not the only source of NPY to the BLA and represent the first study to identify projection sources, which include the AStr and stria terminalis. Shortly after exposure to a fearful context, immunoreactivity for the amidated, releasable form of NPY was reduced within the BLA, and NPY gene expression was increased within the AStr hours after presentation of the stimulus. These data support endogenous regulation of NPY release and neural activity within BLA-related brain circuits in response to a conditioned fear paradigm. Furthermore, NPY projections from the AStr to the BLA represent a novel anxiolytic circuit, perhaps serving as an adaptive response to conditioned fear. Future studies will be aimed at identifying inputs to these AStr cells as well as the downstream cells in the BLA that receive AStr innervation. This will be important in further determining the role of NPY in modulating fear and stress responses.
Table 1.
Primary antibodies used in immunohistochemical studies
| Antigen | Immunogen | Description | Dilution |
|---|---|---|---|
| NPY02 | amino acids 11-24 of human NPY | Mouse monoclonal (Grouzmann et al., 1992) | 1:2,500 |
| NPY05 | amino acids 32-36 of human NPY | Mouse monoclonal (Grouzmann et al., 1992) | 1:10,000 |
| SOM | amino acids 1-14 of cyclic somatostatin | Rat monoclonal; Millipore clone YC7 AB354 RRID:AB2255365 | 1:200 |
| DβH | purified from bovine adrenal medulla | Rabbit polyclonal Immunostar #22806 RRID:AB572229 | 1:3,000 |
| TH | 34kDa catalytic core of TH purified from PC12 cells | Mouse monoclonal Immunostar #22941 RRID: AB 572268 | 1:1,000 |
| FG | Fluorogold | Rabbit polyclonal Chemicon #AB153 RRID: AB 2314408 | 1:5,000 |
Table 2.
Abbreviations used in figures
| AAV | anterior amygdaloid area, ventral part |
| ACo | anterior cortical amygdaloid nucleus |
| AHiAL | amygdalohippocampal area |
| AID | agranular insular cortex, dorsal part |
| AIV | agranular insular cortex, ventral part |
| AOP | anterior olfactory nucleus, posterior part |
| AStr | amygdalostriatal transition area |
| BL | basolateral amygdaloid nucleus |
| BLA | basolateral amygdaloid complex |
| BLV | basolateral amygdaloid nucleus, ventral part |
| BMA | basomedial amygdaloid nucleus, anterior part |
| BMP | basomedial amygdaloid nucleus, posterior part |
| BSTIA | bed nucleus of the stria terminalis, intraamygdaloid division |
| BSTL | bed nucleus of the stria terminalis, lateral division |
| BSTM | bed nucleus of the stria terminalis, medial division, anterior part |
| CeA | central amygdaloid nucleus |
| Cg1 | cingulate cortex, area 1 |
| Cl | claustrum |
| CM | central medial thalamic nucleus |
| CPu | caudate putamen (striatum) |
| DβH | dopamine β hydroxylase |
| DEn | dorsal endopiriform nucleus |
| DMD | dorsomedial hypothalamic nucleus, dorsal part |
| DP | dorsal peduncular cortex |
| Fi | fimbria of the hippocampus |
| IAM | interanteromedial thalamic nucleus |
| ic | internal capsule |
| ICj | islands of Calleja |
| IL | infralimbic cortex |
| IMD | intermediodorsal thalamic nucleus |
| IPAC | interstitial nucleus of the posterior limb of the anterior commissure |
| LA | lateral amygdaloid nucleus |
| LGP | lateral globus pallidus |
| LH | lateral hypothalamic area |
| LHbM | lateral habenular nucleus, medial part |
| LOT | nucleus of the lateral olfactory tract |
| LPO | lateral preoptic area |
| LSS | lateral stripe of the striatum |
| LSV | lateral septal nucleus, ventral part |
| M | motor cortex |
| MeA | medial amygdaloid nucleus |
| NPY | neuropeptide Y |
| PaMP | paraventricular hypothalamic nucleus, medial parvicellular part |
| PH | posterior hypothalamic area |
| Pir | piriform cortex |
| PLCo | posterolateral cortical amygdaloid nucleus (C2) |
| PrL | prelimbic cortex |
| PT | paratenial thalamic nucleus |
| PV | paraventricular thalamic nucleus |
| RCh | retrochiasmatic area |
| Re | reuniens thalamic nucleus |
| S1J | primary somatosensory cortex, jaw region |
| SI | substantia innominata |
| SIB | substantia innominata, basal part |
| SID | substantia innominata, dorsal part |
| SL | semilunar nucleus |
| st | stria terminalis |
| TH | tyrosine hydroxylase |
| VEn | ventral endopiriform nucleus |
| VMH | ventromedial hypothalamic nucleus |
| VO | ventral orbital cortex |
| VP | ventral pallidum |
| 3v | 3rd ventricle |
ACKNOWLEDGMENTS
The authors thank Mary R. DeJoseph for invaluable technical assistance. Immunohistochemical analysis of brain tissue was aided, in part, through the use of the Rosalind Franklin University of Medicine and Science Microscope and Imaging Core Facility.
Support: NIH grants MH062621, MH090297, and MH081152.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that this work was not previously published and is not currently considered for publication elsewhere, and that there are no conflicts of interest, nor any other scientific misconduct to report.
ROLE OF AUTHORS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: RJL, ABR, JHU. Acquisition of data: RJL, ABR. Analysis and interpretation of results: RJL, ABR, JHU. Drafting of the manuscript: RJL, ABR, JHU. Critical revision of the manuscript for important intellectual content: RJL, ABR, JHU. Statistical analysis: RJL, JHU. Obtained funding: JHU. Administrative, technical, and material support: RJL, ABR, JHU. Study supervision: RJL, ABR, JHU.
References
- Adrian TE, Allen JM, Bloom SR, Ghatei MA, Rossor MN, Roberts GW, Crow TJ, Tatemoto K, Polak JM. Neuropeptide Y distribution in human brain. Nature. 1983;306(5943):584–6. doi: 10.1038/306584a0. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Beltramino CA, De Olmos JS, Forbes MS, Swanson DJ, Heimer L. The neuronal organization of the supracapsular part of the stria terminalis in the rat: the dorsal component of the extended amygdala. Neuroscience. 1998;84(4):967–96. doi: 10.1016/s0306-4522(97)00560-5. [DOI] [PubMed] [Google Scholar]
- Alheid GF, de Olmos J, Beltramino CA1. Amygdala and Extended Amygdala. In: Paxinos G, editor. The Rat Nervous System. 2nd ed Academic Press, Inc.; San Diego, CA: 1995. pp. 495–578. [Google Scholar]
- Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, Polak JM. Neuropeptide Y distribution in the rat brain. Science. 1983;221(4613):877–9. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Bannon AW, Seda J, Carmouche M, Francis JM, Norman MH, Karbon B, McCaleb ML. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000;868(1):79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- Bordi F, LeDoux J. Sensory tuning beyond the sensory system: an initial analysis of auditory response properties of neurons in the lateral amygdaloid nucleus and overlying areas of the striatum. J Neurosci. 1992;12(7):2493–503. doi: 10.1523/JNEUROSCI.12-07-02493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F, LeDoux J, Clugnet MC, Pavlides C. Single-unit activity in the lateral nucleus of the amygdala and overlying areas of the striatum in freely behaving rats: rates, discharge patterns, and responses to acoustic stimuli. Behav Neurosci. 1993;107(5):757–69. doi: 10.1037/0735-7044.107.5.757. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15(3 Pt 2):2312–27. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero DA, O'Donohue TL. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience. 1985;15(4):1159–81. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- Clarke GL, Bhattacherjee A, Tague SE, Hasan W, Smith PG. ss-adrenoceptor blockers increase cardiac sympathetic innervation by inhibiting autoreceptor suppression of axon growth. J Neurosci. 2010;30(37):12446–54. doi: 10.1523/JNEUROSCI.1667-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, McEwen BS. Acute stress increases neuropeptide Y mRNA within the arcuate nucleus and hilus of the dentate gyrus. Brain Res Mol Brain Res. 2000;79(1-2):102–9. doi: 10.1016/s0169-328x(00)00105-4. [DOI] [PubMed] [Google Scholar]
- Cui H, Sakamoto H, Higashi S, Kawata M. Effects of single-prolonged stress on neurons and their afferent inputs in the amygdala. Neuroscience. 2008;152(3):703–12. doi: 10.1016/j.neuroscience.2007.12.028. [DOI] [PubMed] [Google Scholar]
- de Quidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system--I. Radioimmunoassay and chromatographic characterisation. Neuroscience. 1986a;18(3):527–43. doi: 10.1016/0306-4522(86)90056-4. [DOI] [PubMed] [Google Scholar]
- de Quidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system--II. Immunohistochemical analysis. Neuroscience. 1986b;18(3):545–618. doi: 10.1016/0306-4522(86)90057-6. [DOI] [PubMed] [Google Scholar]
- Dimitrov E, Usdin TB. Tuberoinfundibular peptide of 39 residues modulates the mouse hypothalamic-pituitary-adrenal axis via paraventricular glutamatergic neurons. J Comp Neurol. 2010;518(21):4375–94. doi: 10.1002/cne.22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Petrovich GD, Swanson LW. Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Res. 2000;859(1):1–14. doi: 10.1016/s0006-8993(99)02246-5. [DOI] [PubMed] [Google Scholar]
- Doron NN, LeDoux JE. Organization of projections to the lateral amygdala from auditory and visual areas of the thalamus in the rat. J Comp Neurol. 1999;412(3):383–409. [PubMed] [Google Scholar]
- Duque A, Balatoni B, Detari L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. J Neurophysiol. 2000;84(3):1627–35. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- Duque A, Tepper JM, Detari L, Ascoli GA, Zaborszky L. Morphological characterization of electrophysiologically and immunohistochemically identified basal forebrain cholinergic and neuropeptide Y-containing neurons. Brain Struct Funct. 2007;212(1):55–73. doi: 10.1007/s00429-007-0143-3. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381(6581):415–21. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Hokfelt T, Terenius L, Tatemoto K, Mutt V, Goldstein M. Differential coexistence of neuropeptide Y (NPY)-like immunoreactivity with catecholamines in the central nervous system of the rat. Neuroscience. 1984;11(2):443–62. doi: 10.1016/0306-4522(84)90036-8. [DOI] [PubMed] [Google Scholar]
- Flood JF, Baker ML, Hernandez EN, Morley JE. Modulation of memory processing by neuropeptide Y varies with brain injection site. Brain Res. 1989;503(1):73–82. doi: 10.1016/0006-8993(89)91706-x. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, McClain C. Amygdaloid inputs define a caudal component of the ventral striatum in primates. J Comp Neurol. 2004;476(4):330–47. doi: 10.1002/cne.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110(2):257–75. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Tucker T. Amygdala projections to central amygdaloid nucleus subdivisions and transition zones in the primate. Neuroscience. 2009;159(2):819–41. doi: 10.1016/j.neuroscience.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouzmann E, Comoy E, Walker P, Burnier M, Bohuon C, Waeber B, Brunner H. Production and characterization of four anti-neuropeptide Y monoclonal antibodies. Hybridoma. 1992;11(4):409–24. doi: 10.1089/hyb.1992.11.409. [DOI] [PubMed] [Google Scholar]
- Grove EA. Efferent connections of the substantia innominata in the rat. J Comp Neurol. 1988;277(3):347–64. doi: 10.1002/cne.902770303. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Card JP, Moore RY. Neuropeptide Y localization in the rat amygdaloid complex. J Comp Neurol. 1986;251(3):349–62. doi: 10.1002/cne.902510306. [DOI] [PubMed] [Google Scholar]
- Gutman AR, Yang Y, Ressler KJ, Davis M. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J Neurosci. 2008;28(48):12682–90. doi: 10.1523/JNEUROSCI.2305-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38(4):213–24. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav Neurosci. 1994;108(5):1005–9. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: III. Subcortical afferents. J Comp Neurol. 1987;264(3):396–408. doi: 10.1002/cne.902640307. [DOI] [PubMed] [Google Scholar]
- Iwata J, LeDoux JE, Meeley MP, Arneric S, Reis DJ. Intrinsic neurons in the amygdaloid field projected to by the medial geniculate body mediate emotional responses conditioned to acoustic stimuli. Brain Res. 1986;383(1-2):195–214. doi: 10.1016/0006-8993(86)90020-x. [DOI] [PubMed] [Google Scholar]
- Jolkkonen E, Pikkarainen M, Kemppainen S, Pitkanen A. Interconnectivity between the amygdaloid complex and the amygdalostriatal transition area: a PHA-L study in rat. J Comp Neurol. 2001;431(1):39–58. doi: 10.1002/1096-9861(20010226)431:1<39::aid-cne1054>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26(3):259–83. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, Wong H, Walsh JH, Hökfelt T. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111(3):443–532. doi: 10.1016/s0306-4522(01)00463-8. [DOI] [PubMed] [Google Scholar]
- Krukoff TL, MacTavish D, Jhamandas JH. Effects of restraint stress and spontaneous hypertension on neuropeptide Y neurones in the brainstem and arcuate nucleus. J Neuroendocrinol. 1999;11(9):715–23. doi: 10.1046/j.1365-2826.1999.00391.x. [DOI] [PubMed] [Google Scholar]
- Krysiak R, Obuchowicz E, Herman ZS. Conditioned fear-induced changes in neuropeptide Y-like immunoreactivity in rats: the effect of diazepam and buspirone. Neuropeptides. 2000;34(3-4):148–57. doi: 10.1054/npep.2000.0804. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990a;10(4):1062–9. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci. 1990b;10(4):1043–54. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Iwata J, Reis DJ. Interruption of projections from the medial geniculate body to an archi-neostriatal field disrupts the classical conditioning of emotional responses to acoustic stimuli. Neuroscience. 1986;17(3):615–27. doi: 10.1016/0306-4522(86)90034-5. [DOI] [PubMed] [Google Scholar]
- Levita L, Mania I, Rainnie DG. Subtypes of substance P receptor immunoreactivie interneurons in the rat basolateral amygdala. Brain Res. 2003;981(1-2):41–51. doi: 10.1016/s0006-8993(03)02870-1. [DOI] [PubMed] [Google Scholar]
- Majak K, Morys J. Endopiriform nucleus connectivities: the implications for epileptogenesis and epilepsy. Folia Morphol (Warsz ) 2007;66(4):267–71. [PubMed] [Google Scholar]
- Makino S, Baker RA, Smith MA, Gold PW. Differential regulation of neuropeptide Y mRNA expression in the arcuate nucleus and locus coeruleus by stress and antidepressants. J Neuroendocrinol. 2000;12(5):387–95. doi: 10.1046/j.1365-2826.2000.00451.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996;110(4):718–26. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ, Coleman JR. Corticoamygdaloid and corticocortical projections of the rat temporal cortex: a Phaseolus vulgaris leucoagglutinin study. Neuroscience. 1993;57(3):697–715. doi: 10.1016/0306-4522(93)90016-9. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol. 1982a;208(4):401–18. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982b;212(3):293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Morphology of peptide-containing neurons in the rat basolateral amygdaloid nucleus. Brain Res. 1985;338(1):186–91. doi: 10.1016/0006-8993(85)90266-5. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Coexistence of somatostatin with neuropeptide Y, but not with cholecystokinin or vasoactive intestinal peptide, in neurons of the rat amygdala. Brain Res. 1989;500(1-2):37–45. doi: 10.1016/0006-8993(89)90297-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Projection neurons of the basolateral amygdala: a correlative Golgi and retrograde tract tracing study. Brain Res Bull. 1992;28(2):179–85. doi: 10.1016/0361-9230(92)90177-y. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Zaric V. Subpopulations of somatostatin-immunoreactive non pyramidal neurons in the amygdala and adjacent external capsule project to the basal forebrain: evidence for the existence of GABAergic projection neurons in the cortical nuclei and basolateral nuclear complex. Front Neural Circuits. 2012;6:46. doi: 10.3389/fncir.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non pyramidal neurons of the basolateral amygdala. Neurosci Lett. 1989;100(1-3):53–8. doi: 10.1016/0304-3940(89)90659-9. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Zaric V. Extrinsic origins of the somatostatin and neuropeptide Y innervation of the rat basolateral amygdala. Neuroscience. 2015;294:82–100. doi: 10.1016/j.neuroscience.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111(4):683–91. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Novejarque A, Lanuza E, Martinez-Garcia F. Amygdalostriatal projections in reptiles: a tract-tracing study in the lizard Podarcis hispanica. J Comp Neurol. 2004;479(3):287–308. doi: 10.1002/cne.20309. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd ed. Academic Press; New York: 1997. [Google Scholar]
- Pitkanen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala: A functional analysis. Second ed. Oxford University Press Inc.; New York: 2000. pp. 31–115. [Google Scholar]
- Primeaux SD, Wilson SP, Cusick MC, York DA, Wilson MA. Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology. 2005;30(9):1589–97. doi: 10.1038/sj.npp.1300705. [DOI] [PubMed] [Google Scholar]
- Rao ZR, Shiosaka S, Tohyama M. Origin of cholinergic fibers in the basolateral nucleus of the amygdaloid complex by using sensitive double-labeling technique of retrograde biotinized tracer and immunocytochemistry. J Hirnforsch. 1987;28(5):553–60. [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Herzog H, Quirion R. Neuropeptide Y (NPY) Y2 receptors mediate behaviour in two animal models of anxiety: evidence from Y2 receptor knockout mice. Behav Brain Res. 2003;141(2):251–5. doi: 10.1016/s0166-4328(02)00374-1. [DOI] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex. 1993;3(6):515–32. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- Rostkowski AB, Teppen TL, Peterson DA, Urban JH. Cell-specific expression of neuropeptide Y Y1 receptor immunoreactivity in the rat basolateral amygdala. J Comp Neurol. 2009;517(2):166–76. doi: 10.1002/cne.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Fitz SD, Shekhar A. The role of neuropeptide Y in the amygdala on corticotropin-releasing factor receptor-mediated behavioral stress responses in the rat. Stress. 2006;9(1):21–8. doi: 10.1080/10253890600557315. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, Gehlert DR, Urban JH, Shekhar A. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28(4):893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacology. 2002;43(7):1165–72. doi: 10.1016/s0028-3908(02)00234-4. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999;368(2-3):143–7. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Sergeyev V, Fetissov S, Mathe AA, Jimenez PA, Bartfai T, Mortas P, Gaudet L, Moreau JL, Hokfelt T. Neuropeptide expression in rats exposed to chronic mild stresses. Psychopharmacology (Berl) 2005;178(2-3):115–24. doi: 10.1007/s00213-004-2015-3. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Afferent connections of the interstitial nucleus of the posterior limb of the anterior commissure and adjacent amygdalostriatal transition area in the rat. Neuroscience. 1999;94(4):1097–123. doi: 10.1016/s0306-4522(99)90280-4. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Beltramino CA, McDonald AJ, Miselis RR, Yang M, de Olmos J, Heimer L, Alheid GF. Supracapsular bed nucleus of the stria terminalis contains central and medial extended amygdala elements: evidence from anterograde and retrograde tracing experiments in the rat. J Comp Neurol. 2000;422(4):533–55. doi: 10.1002/1096-9861(20000710)422:4<533::aid-cne5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid projections of rat temporal cortex. J Comp Neurol. 1997;382(2):153–75. [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;399(4):440–68. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Sockman KW, Salvante KG. The integration of song environment by catecholaminergic systems innervating the auditory telencephalon of adult female European starlings. Dev Neurobiol. 2008;68(5):656–68. doi: 10.1002/dneu.20611. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontalamygdala interactions in fear extinction. Learn Mem. 2004;11(5):525–35. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Stanic D, Mulder J, Watanabe M, Hokfelt Characterization of NPY Y2 receptor protein expresion in the mouse brain. II. Coexistence with NPY, the Y1 receptor and other neurotransmitter-related molecules. J Comp Neurol. 2011;519(7):1219–1257. doi: 10.1002/cne.22608. [DOI] [PubMed] [Google Scholar]
- Sweerts BW, Jarrott B, Lawrence AJ. The effect of acute and chronic restraint on the central expression of prepro-neuropeptide Y mRNA in normotensive and hypertensive rats. J Neuroendocrinol. 2001;13(7):608–17. doi: 10.1046/j.1365-2826.2001.00674.x. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Carlsson K, Ekman R, Heilig M. Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport. 1999;10(14):3003–7. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R, Mathe AA, Heilig M. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci U S A. 2000;97(23):12852–7. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Svensson P, Wiklund L, Sommer W, Ekman R, Heilig M. Suppressed neuropeptide Y (NPY) mRNA in rat amygdala following restraint stress. Regul Pept. 1998;75-76:247–54. doi: 10.1016/s0167-0115(98)00075-5. [DOI] [PubMed] [Google Scholar]
- Toyomitsu Y, Nishijo H, Uwano T, Kuratsu J, Ono T. Neuronal responses of the rat amygdala during extinction and reassociation learning in elementary and configural associative tasks. Eur J Neurosci. 2002;15(4):753–68. doi: 10.1046/j.1460-9568.2002.01889.x. [DOI] [PubMed] [Google Scholar]
- Trogrlic L, Wilson YM, Newman AG, Murphy M. Context fear learning specifically activates distinct populations of neurons in amygdala and hypothalamus. Learn Mem. 2011;18(10):678–87. doi: 10.1101/lm.2314311. [DOI] [PubMed] [Google Scholar]
- Truitt WA, Johnson PL, Dietrich AD, Fitz SD, Shekhar A. Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience. 2009;169(2):284–294. doi: 10.1016/j.neuroscience.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschenett A, Singewald N, Carli M, Balducci C, Salchner P, Vezzani A, Herzog H, Sperk G. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur J Neurosci. 2003;18(1):143–8. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: a test of the amygdala's role in sensory processing. J Comp Neurol. 1991;313(2):295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- Urban JH, Leitermann RJ, DeJoseph MR, Somponpun SJ, Wolak ML, Sladek CD. Influence of dehydration on the expression of neuropeptide Y Y1 receptors in hypothalamic magnocellular neurons. Endocrinology. 2006;147(9):4122–31. doi: 10.1210/en.2006-0377. [DOI] [PubMed] [Google Scholar]
- Uwano T, Nishijo H, Ono T, Tamura R. Neuronal responsiveness to various sensory stimuli, and associative learning in the rat amygdala. Neuroscience. 1995;68(2):339–61. doi: 10.1016/0306-4522(95)00125-3. [DOI] [PubMed] [Google Scholar]
- Walker LC, Koliatsos VE, Kitt CA, Richardson RT, Rokaeus A, Price DL. Peptidergic neurons in the basal forebrain magnocellular complex of the rhesus monkey. J Comp Neurol. 1989;280(2):272–82. doi: 10.1002/cne.902800208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Kang-Park MH, Wilson WA, Moore SD. Properties of the pathways from the lateral amygdala nucleus to basolateral nucleus and amygdalostriatal transition area. J Neurophysiol. 2002;87(5):2593–601. doi: 10.1152/jn.2002.87.5.2593. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19(24):RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol. 2003;464(3):285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic projections to the basolateral amygdala: a combined Evans Blue and acetylcholinesterase analysis. Brain Res Bull. 1982;8(6):751–63. doi: 10.1016/0361-9230(82)90102-2. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Duque A. Local synaptic connections of basal forebrain neurons. Behav Brain Res. 2000;115(2):143–58. doi: 10.1016/s0166-4328(00)00255-2. [DOI] [PubMed] [Google Scholar]