Abstract
Few studies have investigated developmental strengths and weaknesses within the cognitive profile of children and adolescents with fragile X syndrome (FXS), a single-gene cause of inherited intellectual impairment. With a prospective longitudinal design and using normalized raw scores (Z scores) to circumvent floor effects, we measured cognitive functioning of 184 children and adolescents with FXS (ages 6 to 16) using the Wechsler Scale of Intelligence for Children on one to three occasions for each participant. Participants with FXS received lower raw scores relative to the Wechsler Scale of Intelligence for Children normative sample across the developmental period. Verbal comprehension, perceptual organization, and processing speed Z scores were marked by a widening gap from the normative sample, while freedom from distractibility Z scores showed a narrowing gap. Key findings include a relative strength for verbal skills in comparison with visuospatial–constructive skills arising in adolescence and a discrepancy between working memory (weakness) and processing speed (strength) in childhood that diminishes in adolescence. Results suggest that the cognitive profile associated with FXS develops dynamically from childhood to adolescence. Findings are discussed within the context of aberrant brain morphology in childhood and maturation in adolescence. We argue that assessing disorder-specific cognitive developmental profiles will benefit future disorder-specific treatment research.
Fragile X syndrome (FXS) is a leading single-gene cause of inherited intellectual impairment. Individuals with FXS exhibit a mutation of the fragile X mental retardation 1 gene (FMR1) on the X chromosome that leads to a decrease in FMR1 protein (FMRP) levels (Verkerk et al., 1991). FMRP levels are generally higher in females than in males with FXS because of the presence of a second X chromosome, which does not carry the FMR1 mutation. Approximately two-thirds of males and one-third of females with FXS have intellectual disability (Hall, Burns, Lightbody, & Reiss, 2008; Loesch et al., 2002; Rousseau et al., 1994). Intellectual disability is characterized by “intellectual and adaptive functioning deficits in conceptual, social, and practical domains” with onset in childhood (American Psychiatric Association, 2013).
Although many studies have investigated the cognitive development of children with FXS, interpretation of findings is limited due to the samples, experimental designs, and test scores used. Methodological limitations have also prevented a comparison of development across cognitive domains that could reveal strengths and weaknesses within the cognitive profile associated with FXS. Thus, the aims of the present study are to (a) assess cognitive trajectories associated with FXS during childhood and adolescence with a robust longitudinal sample and novel methodology and (b) compare trajectories of different cognitive domains in childhood and adolescence.
For early childhood, Bailey, Hatton, and Skinner (1998) and Bailey, Hatton, Skinner, and Mesibov (2001) found progress in overall cognitive and language development before age 7, although the rate of growth was about half of that of typically developing children. Studies focused on broader age ranges reveal less gain in later childhood and adolescence. Over time, children and adolescents with FXS show slower gains in raw scores than do typically developing peers on cognitive assessments (Hall et al., 2008), which in turn is associated with declines in standardized IQ scores (Fisch et al., 1996, 1999, 2010; Fisch, Simensen, & Schroer, 2002; Kover, Pierpont, Kim, Brown, & Abbeduto, 2013; Skinner et al., 2005). Specifically, steep declines in standardized IQ scores have been observed in children with FXS before ages 8 (Skinner et al., 2005), 10 (Dykens et al., 1989), and 11 (Fisch et al., 1996), followed by potential stabilization in adolescence until at least age 14 (Skinner et al., 2005). However, Skinner et al. (2005) did not include females with FXS, Fisch et al. (1996) obtained repeated measures on separate groups of children with nonoverlapping ages, and Dykens et al. (1989) used a small sample size. Prospective longitudinal studies with large samples covering broad age ranges are thus required to track cognitive trajectories through time with greater precision in this condition. Such experimental designs allow modeling of nonlinear growth that could reveal potential patterns associated with declines in IQ scores before age 11 (e.g., sudden or progressive) and whether there is a potential stabilization from age 11 onward.
However, our ability to track cognitive trajectories of individuals with FXS is limited by the presence of floor effects when standardized IQ scores are employed. Standardized scores lack sensitivity in the low ranges where scores of children with FXS often fall. For instance, Hessl et al. (2009) noted that 40 is the lowest possible standard IQ score on the Wechsler Intelligence Scale for Children—Third Edition (WISC-III), and a wide range of raw scores (0 to 37 depending on the subtest) are associated with a scaled score of 1, the lowest possible scaled score (Wechsler, 1991). To circumvent this issue, Bailey et al. (1998, 2001) used developmental ages from the Battelle Developmental Inventory instead of standardized scores, but this option is only possible for children younger than 7 years old. Skinner et al. (2005) and Kover et al. (2013) used growth scores of the Leiter IQ scale, but this test does not give a measure of verbal IQ. In a previous study conducted by our group, we tracked the cognitive development of 90 boys and 55 girls with FXS, aged 6 to 16 years, on the WISC-III (Hall et al., 2008). To avoid potential floor effects, we used raw scores in our analyses, and compared the rate of cognitive development in participants with FXS to a group of same-gender unaffected siblings who were also administered the WISC-III during the same visit. Results showed that the rate of cognitive development was 2.2 times slower in individuals with FXS compared to their unaffected siblings (Hall et al., 2008). However, in that study, we did not report whether cognitive development was differentially affected on the different subtests or indexes.
Another approach to avoid the confound of possible floor effects is to use normalized raw scores (i.e., compare the participant’s raw score to the average raw score of the normative sample in a particular age band of the test). This method also allows performance on various subtests to be compared to one another (Hessl et al. 2009). To date, this method has been applied to cross-sectional data in individuals with FXS (Hessl et al. 2009). Here we use the same approach to track cognitive trajectories of children with FXS from 6 to 16 years of age in the context of a longitudinal design. Accordingly, our first overarching aim was to produce more specific information about cognitive development in FXS at particular points in time over a critical period of childhood development.
The second aim of this study was to compare the trajectories of specific cognitive domains to one another to assess potential strengths and weaknesses within the cognitive profile of children and adolescents with FXS. In particular, we sought to evaluate whether the profile of strengths and weaknesses associated with FXS changes during development. Specifically, we investigated the following cognitive domains: verbal skills, visuospatial–constructive processing, short-term working memory, and processing speed. Findings from cross-sectional studies suggest a potential strength for verbal skills in comparison with visuospatial–constructive and quantitative reasoning, and impairments in short-term working memory skills. Lachiewicz, Dawson, Spiridigliozzi, and McConkie-Rosell (2006) found that females with FXS have higher verbal abilities than quantitative skills, but Fisch (2006) failed to replicate this finding with a larger sample. Cornish, Munir, and Cross (1998, 1999) documented deficits in visuoconstructive processing ability but did not provide a direct comparison with verbal skills. Mental arithmetic, a skill often associated with visuospatial–constructive abilities, is problematic for males and females with FXS (Hessl et al., 2009; Mazzocco, 2001; Rivera, Menon, White, Glasser, & Reiss, 2002). Mental arithmetic requires attention and short-term working memory, which are also impaired in individuals with FXS (Cornish, Cole, Longhi, Karmiloff-Smith, & Scerif, 2013; Cornish, Scerif, & Karmiloff-Smith, 2007; Munir, Cornish, & Wilding, 2000; Ornstein et al., 2008; van der Molen et al., 2010). However, others have reported a lack of strengths and weaknesses within the cognitive profile associated with FXS (Curfs, Schreppers-Tijdin, Wiegers, Borghgraef, & Fryns, 1989; Simon, Rappaport, Papka, & Wodruff-Pak, 1995). Given mixed findings concerning the presence or absence of a discrepancy between verbal and visuospatial–constructive skills in prior cross-sectional studies, we compared the trajectories of these two cognitive domains during childhood and adolescence in the context of a longitudinal design. We also investigated whether impairments in mental arithmetic and working memory observed in cross-sectional studies are present throughout development. Specifically, we contrasted mental arithmetic and working memory to processing speed. Given that all three cognitive domains require attention, this comparison would enable us to identify idiosyncratic difficulties with mental arithmetic and working memory associated with FXS that cannot be solely accounted for by attentional impairments.
In a previous study by our group (Hall et al., 2008), we tracked cognitive development in males and females with FXS on the WISC-III over two time points and used raw scores to compare the rate of cognitive development in participants with FXS to their typically developing siblings. In the present study, we increased our sample size, added a third time point, and compared the cognitive development of our group of participants with FXS to the WISC-III normative sample. We also compared cognitive development in FXS between cognitive domains.
Method
Participants
The sample included 184 participants (114 males, 70 females) with FXS enrolled in a prospective longitudinal study. FXS full mutation status was confirmed with DNA testing. Participants were between 6 and 16 years of age (mean ± SD at first assessment; males: 11.38 ± 2.71; females 11.27 ± 3.11). Participants were assessed one to three times, with 1 to 6 years between assessments (mean ± SD = 3.37 ± 1.47). Of the male participants, 72 had only 1 data point, 34 had 2 data points, and 8 had 3 data points (for a total of 114 participants and 164 data points). Of the female participants, 40 had only 1 data point, 19 had 2 data points, and 11 had 3 data points (for a total of 70 participants and 111 data points). Data and analyses are presented separately for males and females because males with FXS typically show much greater cognitive impairment than females with FXS (Hall et al., 2008; Loesch et al., 2002; Rousseau et al., 1994).
Data presented here were collected as part of a prospective longitudinal study of FXS. A subset of these data (145/184 participants with one data point and all participants with two data points) have been presented elsewhere (Hall et al., 2008). In comparison with Hall et al.’s study, the current study includes an additional third data point for 19 participants and 39 participants with one data point. Mean full scale IQ scores were 47 for males with FXS (SD =10, range = 40–101) and 76 for females with FXS (SD = 20, range = 40–123). Given that a large proportion of participants obtained full scale IQ scores at or close to the floor score (40) of the test (males: 33% with a score of 40 and 79% ≤ 50; females: 1.4% with a score of 40 and 13% ≤ 50), we used normalized raw scores (Z scores), calculated from the WISC-III normative data in the current study. Standard (scaled) scores for each index are presented in Table 1. (Note that the floor of each index is 50 and the floor for the full scale IQ is 40.) Assessments took place in the participants’ homes or at our research center. Participants were recruited throughout the United States and Canada via advertisements through the National Fragile X Foundation and genetic clinics. Median and mean household income based on 2006–2010 US Census data for the participants’ zip codes (Population Studies Center, 2015) were greater than the nationwide average (all ps < .05; mean ± SD household income per zip code for our sample: median income = $67,125 ± $23,921, mean income = $81,752 ± $30,994). Participants and their parents gave written informed assent and consent to participate in the study at each assessment. Stanford University’s Research Ethics Board approved the study.
Table 1.
WISC-III index standard scores and full scale IQ for males and females with FXS
| WISC-III Index | Males With FXS (N = 114)a
|
Females With FXS (N = 70)
|
||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Verbal comprehension | 56 | 10 | 50–103 | 83 | 20 | 50–120 |
| Perceptual organization | 55 | 9 | 50–111 | 78 | 19 | 50–124 |
| Processing speed | 55 | 10 | 50–96 | 83 | 18 | 50–134 |
| Freedom from distractibility | 51 | 5 | 50–81 | 71 | 17 | 50–112 |
| Full scale IQ | 47 | 10 | 40–101 | 76 | 20 | 40–123 |
Note: WISC-III, Wechsler Intelligence Scale for Children—Third Edition; FXS, fragile X syndrome.
N =113 males for processing speed.
Measurement and data transformation into normalized scores
Participants completed the WISC-III (Wechsler, 1991) one to three times between the ages of 6 and 16 years old. The study started in 1997 before publication of the WISC-IV or WISC-V, hence the use of the WISC-III. The WISC-III is divided into four indexes that correspond to the four cognitive domains under investigation. The verbal comprehension index is a measure of verbal skills that taps into a range of knowledge base including word and practical knowledge retrieval from long-term verbal memory, concept formation, and verbal abstract reasoning. The Perceptual Organization Index examines visual perception, attention to detail, visual abstract reasoning, and visuospatial–constructive processing. The Freedom From Distractibility Index evaluates attention, short-term auditory working memory, numerical reasoning, and mental arithmetic. The Processing Speed Index taps attention, visual-motor coordination, and processing speed. The last two indexes both assess attention but the Processing Speed Index is less taxing on working memory than is the Freedom From Distractibility Index.
To reach our first aim, we modeled quadratic growth to assess the trajectory of performance on each index from 6 to 16 years for this longitudinal study sample. To address our second aim, we compared the trajectories of quadratic growth of the following WISC-III indexes: Verbal Comprehension Index versus Perceptual Organization Index; and Freedom From Distractibility Index versus Processing Speed Index, respectively.
For each index, we calculated normalized scores (Z scores) based on procedures described by Hessl et al. (2009). We obtained raw score means and standard deviations from the WISC-III normative sample to calculate Z scores for each datum of each participant.1 After consultation with the test developers, we used raw score means and standard deviations for each 1-year age band because they include an equal distribution of males and females and a proportional representation of ethnicity based on US Census data, which is not the case for all 3-month age bands used by Hessl et al. We calculated a normalized Z score for each subtest for each participant as such:
where zij is the z score for ith individual in the jth age band (we used 1-year age bands from 6 to 16, i.e., 11 age bands), rij is the raw score for the ith individual in the jth age band, and μj and σj are the raw score mean and standard deviation of the normalization sample within the jth age band.
We then obtained a Z score for each WISC-III index by calculating the mean of the Z scores for the subtests included in each index. The Verbal Comprehension Index includes the information, similarities, vocabulary, and comprehension subtests; the Perceptual Organization Index includes the picture completion, picture arrangement, block design, and object assembly subtests; the Freedom From Distractibility Index includes the arithmetic and digit span subtests; and the Processing Speed Index includes the coding and symbol search subtests. WISC-III subtests were divided into four indexes based on results of a factor analysis (Wechsler, 1991). The subtests included in the Verbal Comprehension Index include orally presented questions and answers that test knowledge of commonly known facts (information) and community and social rules (comprehension), as well as the ability to find a concept unifying two given words (similarities) and to define words (vocabulary). Subtests in the Perceptual Organization Index include illustrations of everyday scenes where the participants must identify missing items (picture completion) or order the scenes in a logical sequence (picture arrangement); this index also requires participants to reproduce visual designs with blocks (block design) and solve puzzles representing common images (object assembly). The Freedom From Distractibility Index includes one subtest where participants perform mental calculations (arithmetic) and another where they repeat sequences of numbers in forward and backward order (digit span). For the Processing Speed Index, participants fill out a grid of symbols according to a legend (coding) and identify the presence or absence of target symbols (symbol search) as quickly as possible.
According to WISC-III standardized administration procedures (Wechsler, 1991), a raw score of 0 was given for subtests that were attempted but could not be completed due to behaviors preventing test administration (e.g., minimal expressive language or limited attention span) or the participant’s inability to provide an accurate response or to understand the task. Trained research assistants with experience working with participants with FXS administered the WISC-III and used behavior reinforcement techniques when appropriate to encourage compliance. Despite these efforts, a variable number of participants received raw scores of 0 across the 10 subtests (mean ± SD participants per subtest: males: 25 ± 15; females: 1.8 ± 1.8) as is often the case with participants with FXS. For males, the object assembly subtest had the smallest number of raw scores of 0 (7 participants) and the symbol search subtest had the largest (55 participants). None of the female participants obtained a raw score of 0 on the information, arithmetic, and digit span subtests, and 1 to 6 female participants obtained a raw score of 0 on the other subtests. Subtests with a raw score of 0 were included in the calculation of Z scores.
If a subtest was not administered for reasons other than the participant’s inability to complete the subtest (e.g., lack of time), the mean of the Z scores for the remaining subtests was calculated to obtain the index Z score. For example, if scores were missing for the comprehension subtest, the Z score for verbal comprehension was calculated based on the mean of the remaining verbal subtests (information, similarities, and vocabulary). Data were missing for five subtests: comprehension (1 participant), digit span (14 participants), coding (4 participants), symbol search (19 participants), and object assembly (1 participant). The data for the current study were obtained from several research protocols including one in which digit span and symbol search were not administered (these subtests are optional in the WISC-III); hence, a higher number of missing data occurred for these subtests.
Statistical analysis procedures
Longitudinal analyses were performed with MPlus 7.2 (Muthén & Muthén, 1998–2012). We used longitudinal mixed effects modeling with maximum likelihood estimation to model trajectories of WISC-III normalized index scores for males and females with FXS as a function of age. We treated missing data as missing at random conditional on observed information (Little & Rubin, 2002). Mixed effects modeling using maximum likelihood holds the advantage that we can include participants with both single and multiple time points in our analyses and with individually varying time elapsed between time points.
To accomplish our first aim, we obtained estimated trajectories for each of the four WISC-III index Z scores for males and females with FXS, for a total of 8 trajectories. A quadratic growth model was chosen to properly capture nonlinear developments over time. WISC-III indexes were entered as within-subjects random-effects variables and age and age2 (age squared) were entered as fixed-effect variables to allow modeling of quadratic growth. The estimated trajectories of quadratic growth are shown in Figure 1, overlaid onto the observed data. Model equations for each trajectory are as follows: estimated Z score = intercept + S1(age) + S2(age2) + error, where x is the age used for estimation and the error term includes both random effect and residual error. The parameter estimates of each trajectory are shown in Appendix A. For each index, we estimated Z scores at ages 6, 11, and 16 years and then calculated the change from 6 to 11 years and 11 to 16 years by subtracting the estimated Z score at 11 years from the estimated Z score at 6 years, and at 16 years from 11 years (see change scores from 6 to 11 and 11 to 16 in Table 2). We assessed whether the change over time in Z score (from 6 to 11 years and 11 to 16 years) for each index was statistically significant to identify potential critical cognitive developmental periods for each WISC-III index. We chose 6 and 16 years because they are the minimum and maximum ages for administration of the WISC-III, and 11 years given previous studies reporting declines in IQ scores in childhood before age 11 years followed by potential stabilization in adolescence until at least 14 years (Dykens et al., 1989; Fisch et al., 1996; Skinner et al., 2005). For brevity, we henceforth refer to the developmental period before age 11 years and childhood and from 11 years onward as adolescence.
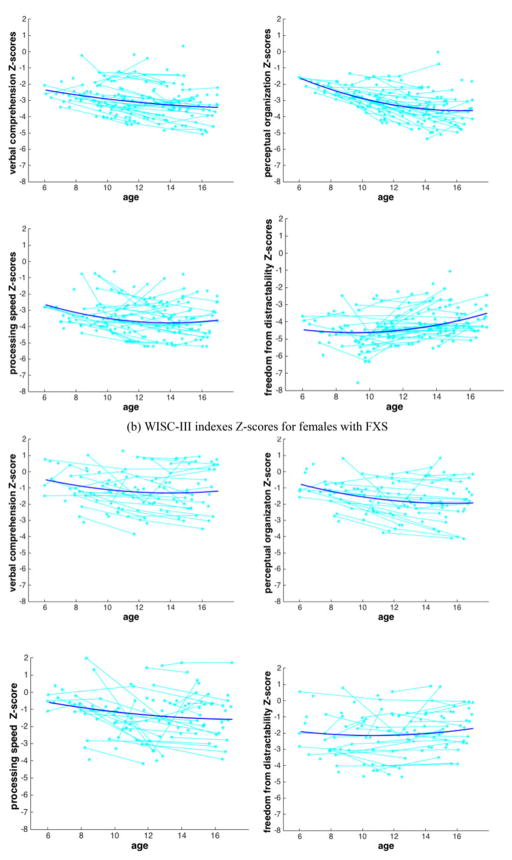
Figure 1.
Observed data and estimated trajectories of the Wechsler Intelligence Scale for Children—Third Edition Z scores based on mixed effects modeling for (a) males and (b) females with fragile X syndrome. The estimated trajectories of quadratic growth for each Wechsler Intelligence Scale for Children index Z score according to age (years) are shown (dark blue lines online only), overlaid onto the observed data (light blue dots and lines online only). Overall, verbal comprehension, perceptual organization, and processing speed Z scores were marked by a widening gap from the normative sample; while freedom from distractibility Z scores showed a narrowing gap.
Table 2.
Estimated longitudinal changes in WISC-III index Z scores based on mixed effects modeling
| WISC-III Index | Males With FXS (N = 114)
|
Females With FXS (N = 70)
|
||
|---|---|---|---|---|
| Estimate | p | Estimate | p | |
| Verbal comprehension | ||||
| Change from 6 to 11 | −0.677 | <.001 | −0.726 | .015 |
| Change from 11 to 16 | −0.397 | .002 | −0.088 | .633 |
| Perceptual organization | ||||
| Change from 6 to 11 | −1.497 | <.001 | −0.918 | <.001 |
| Change from 11 to 16 | −0.567 | <.001 | −0.261 | .077 |
| Processing speed | ||||
| Change from 6 to 11 | −0.977 | <.001 | −0.669 | .008 |
| Change from 11 to 16 | −0.073 | .649 | −0.328 | .034 |
| Freedom from distractibility | ||||
| Change from 6 to 11 | −0.101 | .661 | −0.256 | .399 |
| Change from 11 to 16 | 0.811 | <.001 | 0.314 | .032 |
Note: WISC-III, Wechsler Intelligence Scale for Children—Third Edition; FXS, fragile X syndrome.
To address our second aim, additional analyses were performed to compare trajectories of quadratic growth of different WISC-III indexes. We first compared the trajectories of quadratic growth of verbal comprehension with perceptual organization and freedom from distractibility with processing speed (Figure 2). These two trajectories were simultaneously modeled in a multiple group analysis environment in MPlus (group: males and females). We estimated Z scores at ages 6, 11, and 16 years and assessed whether Z scores differed between indexes at specific ages (6, 11, and 16 years). For example, we subtracted the estimated Z score for verbal comprehension at age 6 years from the estimated Z score for perceptual organization at age 6 years (see all comparisons at ages 6, 11, and 16 in Table 3). We also assessed whether the change over time in Z score differed between indexes (e.g., change from 6 to 11 years for verbal comprehension vs. change from 6 to 11 years for perceptual organization). For example, we obtained a change score A by subtracting the estimated Z scores for verbal comprehension at age 11 years from age 6 years; and a change score B by subtracting the estimated Z scores for perceptual organization at age 11 years from age 6 years. We then subtracted change score B from change score A (see all comparisons in absolute values for changes from 6 to 11 and 11 to 16 in Table 3).
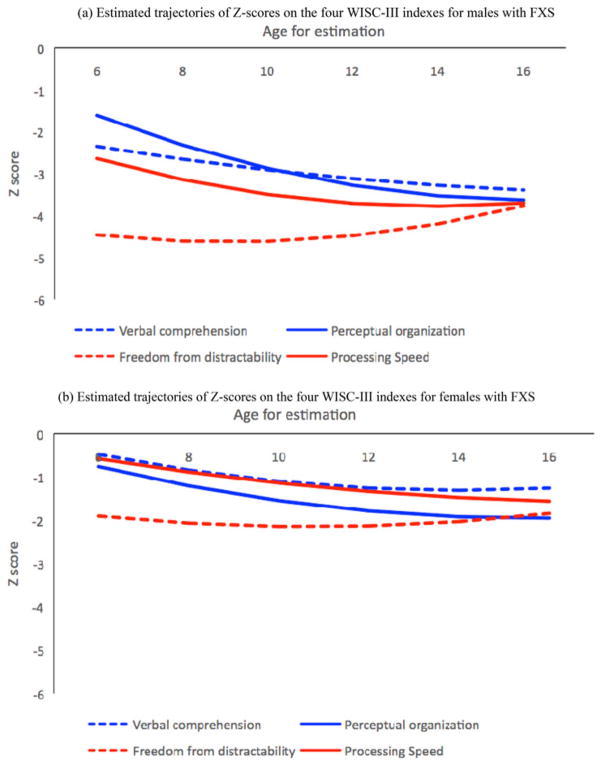
Figure 2.
Estimated trajectories of the four Wechsler Intelligence Scale for Children—Third Edition Z scores according to age (years) based on mixed effects modeling for (a) males and (b) females with fragile X syndrome. The estimated trajectory of quadratic growth for verbal comprehension is compared to that of perceptual organization, and the estimated trajectory of freedom from distractibility is compared to that of processing speed. Key findings include a relative strength for verbal skills in comparison with visuospatial–constructive skills arising in adolescence and a discrepancy between working memory (weakness) and processing speed (strength) in childhood that diminishes in adolescence.
Table 3.
Comparing Z scores between WISC-III indexes among individuals with FXS based on estimated trajectories using mixed effects modeling
| WISC-III Indexes | Males With FXS (N = 114)
|
Females With FXS (N = 70)
|
||
|---|---|---|---|---|
| Estimate | p | Estimate | p | |
| Verbal comprehension vs. perceptual organization | ||||
| Age 6a | −0.398 | .099 | 0.300 | .144 |
| Age 11a | 0.019 | .779 | 0.456 | <.001 |
| Age 16a | 0.351 | .003 | 0.679 | <.001 |
| Change from 6 to 11b | 0.417 | .083 | 0.156 | .516 |
| Change from 11 to 16b | 0.332 | .011 | 0.223 | .117 |
| Processing speed vs. freedom from distractibility | ||||
| Age 6a | 1.378 | <.001 | 1.253 | <.001 |
| Age 11a | 1.042 | <.001 | 0.808 | <.001 |
| Age 16a | −0.027 | .860 | 0.246 | .058 |
| Change from 6 to 11b | 0.337 | .271 | 0.446 | .221 |
| Change from 11 to 16b | 1.069 | <.001 | 0.562 | .009 |
Note: WISC-III, Wechsler Intelligence Scale for Children—Third Edition; FXS, fragile X syndrome.
Difference at age 6, 11, and 16 years: positive scores indicate that verbal comprehension > perceptual organization or processing speed > freedom from distractibility; negative scores indicate the opposite pattern.
Absolute values are reported. For significant changes, refer to Figure 2 (and absolute values of estimates in Table 3) to interpret which index is changing more than the other.
Results
For the first set of analyses, trajectories of Z scores were estimated separately for each WISC-III index for males and for females. Estimated trajectories are overlaid onto observed data in Figure 1. The trajectories for the normative sample would correspond to a horizontal line at Z = 0. In typical development, raw scores increase with age but the standard scaled scores or normative Z scores remain the same.
For participants with FXS, all trajectories were associated with negative Z score values, which indicate that our group of participants with FXS received lower raw scores relative to the WISC-III normative sample across the developmental period. Three scenarios were observed: Z score trajectories decreased, increased, or stabilized, corresponding to a widening, narrowing, or steady gap between participants with FXS in comparison with the WISC-III normative sample, respectively. A widening gap between participants with FXS and the normative sample (i.e., a decreasing trajectory of Z scores for participants with FXS) reveals an increasing disparity from the normative sample, indicating that participants with FXS did not keep pace with the typical rate of development. A narrowing gap between participants with FXS and the normative sample (i.e., an increasing Z score trajectory for participants with FXS) reveals that skills of participants with FXS were improving at a rate above that of the normative sample. A narrowing gap could be attributed to delayed onset or delayed mastery of a skill for participants with FXS. A stable gap between the two groups (i.e., a stable trajectory of Z scores for participants with FXS) indicates that the groups are developing at the same rate over a period of time.
We followed the developmental trajectory of our group of participants with FXS relative to the WISC-III normative sample from age 6 to 11 years and age 11 to 16 years based on estimated Z scorestrajectories (see change scores from 6 to 11 and 11 to 16 in Table 2). Overall, verbal comprehension, perceptual organization, and processing speed Z scores were marked by a widening gap from the normative sample, while the freedom from distractibility Z scores showed a narrowing gap. Specifically significant p values in Table 2 indicate that, for males, verbal comprehension and perceptual organization Z scores revealed a widening gap from the normative sample from 6 to 16 years. For females, these two indexes showed an increasing gap from the normative sample from 6 to 11 years that remained wide but stable from 11 to 16 years. The processing speed Z scores also showed an increasing gap from the normative sample from 6 to 11 years for males that remained wide but stable from 11 to 16 years. For females, this index showed a widening gap from the normative sample from 6 to 16 years. The freedom from distractibility Z scores showed a markedly different trajectory compared with the other three indexes. For both males and females, the freedom from distractibility Z scores showed a wide but steady gap from the normative sample that narrowed from 11 to 16 years. This was the only index showing a narrowing gap from the normative sample in adolescence.
For the second set of analyses, trajectories of two WISC-III indexes were modeled simultaneously for direct comparisons across domains (Figure 2). We compared Z scores at ages 6, 11, and 16 years (see comparisons at ages 6, 11, and 16 in Table 3) and change in Z score from ages 6 to 11 years and from 11 to 16 years for verbal comprehension Z scores versus perceptual organization Z scores, and processing speed Z scores versus freedom from distractibility Z scores (see comparisons in absolute values for changes from 6 to 11 and 11 to 16 in Table 3). Overall, the perceptual organization Z scores were lower than the verbal comprehension scores in adolescence; and freedom from distractibility scores were lower than processing speed scores in childhood, but no difference was observed in adolescence. Specifically, significant p values in Table 3 indicate that, for males, perceptual organization Z scores and verbal comprehension Z scores were not significantly different at ages 6 and 11 years; perceptual organization Z scores decreased more than verbal comprehension Z scores from 11 to 16 years, and by age 16 years, perceptual organization Z scores were significantly lower than verbal comprehension Z scores. For females, perceptual organization Z scores were significantly lower than verbal comprehension Z scores at ages 11 and 16 years, but the two indexes did not differ significantly in terms of change over time. For both males and females, freedom from distractibility Z scores were significantly lower than processing speed Z scores at ages 6 and 11 years; from 11 to 16 years, freedom from distractibility Z scores showed a narrowing gap relative to the normative sample, while processing speed Z scores showed a wide but stable (males) or widening gap (females) relative to the normative sample, and by age 16 years, there was no significant difference between the two indexes.
Discussion
The aims of this study were to (a) assess the development of specific cognitive domains during childhood and adolescence and (b) investigate potential strengths and weaknesses in cognitive development of individuals with FXS. Specifically, we sought to compare verbal versus visuospatial–constructive processing and mental arithmetic/working memory with processing speed. With a longitudinal design, we employed the indexes of the WISC-III to measure these four cognitive domains and used Z scores to overcome floor effects associated with standard scores. Z scores also allow for direct comparison of cognitive domains to one another.
With respect to our first aim, overall, verbal comprehension, perceptual organization (visuospatial–constructive skills), and processing speed indexes were marked by a widening gap from the normative sample, while freedom from distractibility (working memory) showed a narrowing gap. Specifically, trajectories of Z scores indicated a widening gap between individuals with FXS relative to the WISC-III normative sample in childhood (from age 6 to 11) for verbal comprehension, perceptual organization, and processing speed indexes. In adolescence (from ages 11 to 16), a widening gap from the normative sample was observed for verbal comprehension and perceptual organization (males) and processing speed (females), while a wide but stable gap was observed for verbal comprehension and perceptual organization (females) and processing speed (males). We observed a different trajectory for freedom from distractibility, which showed a wide but stable gap from the normative sample in childhood and a narrowing gap in adolescence. Key findings related to our second aim include a relative strength for verbal skills in comparison with visuospatial–constructive skills arising in adolescence and a discrepancy between working memory and processing speed in childhood that fades in adolescence.
Development within cognitive domains
Our findings that Z scores for verbal comprehension, perceptual organization, and processing speed showed a widening gap from the normative sample in childhood, specifically from ages 6 to 11, support results from previous studies (Dykens et al., 1989; Fisch et al., 1996, 1999, 2002, 2010; Kover et al., 2013; Skinner et al., 2005). Our results concerning adolescence differ from previous studies that observed declines in standardized IQ scores in adolescence (Fisch et al., 1996, 1999, 2002, 2010) that should be analogous to a widening gap in Z scores between FXS relative to the normative sample. However, we found that the disparity from the normative sample stabilized or faded in adolescence. Specifically, we found a steady (males: processing speed, females: verbal comprehension and perceptual organization) or narrowing gap (males and females: freedom from distractibility) between our group of participants with FXS and the WISC-III normative sample. Fitting our data to nonlinear trends instead of linear trends made it possible to detect changes in Z score trajectories from a widening gap in childhood to a narrowing or stable gap in adolescence. Studies to date have predominantly used test–retests or cross-sectional designs that could only be examined with discrepancy scores assuming linear trends. In addition, many studies have lacked data covering the entire childhood and adolescence age range, which can impede detection of nonlinear trends. Thus, methodological issues preventing the modeling of nonlinear trends in cognitive development may have artificially inflated declines that were stabilization of scores or plateauing.
Finding a widening gap between our group of participants with FXS relative to the WISC-III normative sample in childhood followed by stabilization or narrowing of that gap in adolescence supports the notion that cognitive development of individuals with FXS is dynamic and may not follow a predictable course from childhood to adolescence in many with this condition (Cornish et al., 2013). Underconnectivity of large-scale brain networks, including decreased functional connectivity in the salience network, left executive control network, language network, and visuospatial network, may underlie the cognitive deficits observed throughout childhood and adolescence in FXS (Hall et al., 2013).
Developmental profiles: Comparison between cognitive domains
Our second aim was to assess the presence of strengths and weaknesses within the cognitive profile of individuals with FXS within the context of a longitudinal study, a comparison that has yet to be established. Overall, our results suggest that the pattern of strengths and weaknesses is dynamic throughout childhood and adolescence. Specifically, we found a relative strength for verbal skills in comparison with visuoconstructive skills and improvements in working memory compared with processing speed, all arising in adolescence.
Our results shed light on the mixed findings present in the literature regarding relative strengths for verbal skills versus a weakness for visuospatial–constructive skills (Cornish et al., 1998; Curfs et al., 1989; Fisch, 2006; Lachiewicz et al., 2006; Simon et al., 1995). For males, these two cognitive domains were similar in childhood, followed by the emergence of a relative strength for verbal skills in comparison with visuospatial–constructive skills in adolescence. This discrepancy appeared in adolescence because perceptual organization Z scores decreased more than verbal comprehension Z scores. For females, verbal skills were also a relative strength. The trajectory for verbal comprehension was above that of perceptual organization throughout development, although the discrepancy was only statistically significant in adolescence. Impaired visuospatial–constructive processing may stem from functional abnormalities in the magnocellular/dorsal pathway (the “where” stream of visual processing) of individuals with FXS (Kogan, Bertone, et al., 2004; Kogan, Boutet, et al., 2004). Structural and functional abnormalities of the inferior parietal lobe (Gothelf et al., 2008; Hallahan et al., 2011; Kwon et al., 2001) and superior parietal lobe (Kwon et al., 2001) and reduced white matter connectivity within the post-central gyrus (Barnea-Goraly et al., 2003) may also contribute to visuospatial impairments associated with FXS.
We also found improvements in working memory during adolescence, as measured by the Freedom From Distractibility Index, which was the only index to show a narrowing gap between our group of participants with FXS and the WISC-III normative sample for males and females. Like previous studies (Hessl et al., 2009; Mazzocco, 2001; Munir et al., 2000; Ornstein et al., 2008; Rivera et al., 2002; van der Molen et al., 2010), we found overall negative Z scores on the Freedom From Distractibility Index, which measures mental arithmetic and working memory skills. In contrast with previous cross-sectional studies, our longitudinal design revealed a wide but stable gap between our group of participants with FXS relative to the normative sample in childhood followed by a narrowing of that gap in adolescence for both males and females with FXS. Freedom from distractibility was a relative weakness in comparison with processing speed in childhood, but these domains were comparable by age 16 years. The initial discrepancy faded by age 16 because, during adolescence, freedom from distractibility Z scores increased for males and females while processing speed Z scores stabilized for males and decreased for females. Distinct trajectories for freedom from distractibility compared with processing speed suggest that working memory/mental manipulation of auditory information and attention/processing speed skills seem more independent for children with FXS than in the typical population. In the WISC-III normative sample, Z scores for these two cognitive domains are equal to 0 at all ages by definition, which presumably translates to Z scores trajectories of overlapping flat lines. In our sample, the Z scores trajectories of these two cognitive domains are in opposite directions and almost cross over in adolescence. In a sample of children with FXS who were younger than our participants, Cornish et al. (2013) also found greater improvements in working memory compared with attention skills in a longitudinal study. Thus, the discrepant developmental trajectories of working memory and attention may be a hallmark of the cognitive developmental profile of children and adolescent with FXS.
The unique trajectory of the Freedom From Distractibility Index could be related to atypical brain development associated with FXS. In childhood, freedom from distractibility was the index with the lowest Z scores, possibly because solving the tasks included in this index (arithmetic and digit span) requires involvement of frontostriatal circuits known to be atypical in FXS (Haas et al., 2009; Hoeft et al., 2010). Specifically, atypical activation of middle frontal gyri (Kwon et al., 2001) of individuals with FXS has been reported during spatial working-memory tasks and greater enlargement of this area during early childhood (Hoeft et al., 2010). However, in adolescence, freedom from distractibility Z scores increased (narrowing gap compared with the normative sample), which is surprising given that FXS is associated with aberrant maturation of the middle frontal gyri during adolescence (Bray et al., 2011). Alternatively, a narrowing gap from the normative sample in adolescence for this index could be related to the development of the caudate nucleus, a structure also involved in spatial working memory. Although the caudate nucleus is enlarged in individuals with FXS, its rate of growth during adolescence is similar for individuals with FXS and typical development (Bray et al., 2011).
Moving toward disorder-specific cognitive developmental profiles
Previous research has shown that the strengths and weaknesses within the cognitive profile of individuals with FXS are different than individuals with other neurodevelopmental disorders such as Down syndrome (Kogan et al., 2009) or Williams syndrome (Fisch et al., 2010). Recently, Sansone et al. (2014) described strengths and weaknesses within the cognitive profile of individuals with FXS and individuals with autism spectrum disorders (ASD) using the Z score method. They found that individuals in both groups (FXS or ASD) showed a relative weakness in verbal working memory. However, individuals with FXS showed a relative strength in perceptual knowledge, whereas individuals with ASD showed a relative strength in spatial reasoning and verbal quantitative reasoning. Comparison of individuals with FXS and individuals with idiopathic intellectual disabilities and developmental delays has shown neural correlates of FXS that are independent of level of intellectual functioning, including aberrant morphology of the caudate nucleus (Peng et al., 2013) and white matter microstructure (Green et al., 2015), atypical resting state functional connectivity (Hall, Jiang, Reiss, & Greicius, 2013), and metabolite differences in the caudate nucleus (Bruno et al., 2013). These results taken together with our findings support further investigation of a potential disorder-specific effect on cognition associated with FXS. Future research should include appropriate comparison groups of individuals with intellectual and developmental disabilities in order to determine whether the cognitive strengths and weaknesses identified with Z scores are specific to FXS, or are also present in those with intellectual disabilities in general.
Further, we argue that it is important to consider disorder-specific cognitive development in addition to disorder-specific cognitive profile. Future research should aim to compare disorder-specific cognitive developmental profiles across different disorders with longitudinal designs and metrics allowing direct comparison of cognitive domains. For example, Cornish et al. (2007) found that for children with FXS, selective and sustained attention increased more with age compared to inhibition skills, while inhibition skills increased more than selective and sustained attention for children with Down syndrome, which indicates potential disorder-specific developmental profiles (Cornish et al., 2007).
Cognitive developmental profiles could also be explored within the population of FXS, that is, subgroup-specific cognitive developmental profile. Romano et al. (2014) identified subgroups within a sample of children with FXS that differ on neuroanatomy, IQ, and autism symptomatology. These subgroups may also differ in terms of cognitive developmental profile. The impact of FMRP methylation on cognitive developmental profiles also deserves further attention because the effects of methylation change throughout development, specifically until puberty (Golder et al., 2013), and may have an age-specific impact on cognitive developmental profiles.
Understanding the strengths and weaknesses within the cognitive developmental profile of individuals with intellectual disabilities has important clinical implications for academic and vocational planning (American Psychiatric Association, 2013). It is often challenging for clinicians and caregivers to identify strengths and weaknesses within the cognitive profile of individuals with intellectual impairments or disabilities. Our findings support the need for additional research on cognitive developmental profiles of individuals with intellectual disabilities, which could translate to disorder- and age-specific interventions based on developmental variation in the pattern of these cognitive strengths and weaknesses. It is possible that cognitive domains identified here as strengths could be more amenable to improvements with interventions. Alternatively, early interventions focused on weaknesses could alter developmental trajectories and change the profile of cognitive strengths and weaknesses throughout childhood and adolescence.
Methodological challenges for comparison of cognitive domains
The pattern of strengths and weaknesses that we identified within the cognitive profile of individuals with FXS in the present study would have been missed with standardized IQ scores due to floor effects. We do not wish to imply that the WISC-III and similar tests should not be used as diagnostic tools to assess intellectual disability. However, we argue, like Hessl et al. (2009), that standard scores may obscure potential strengths and weaknesses within the cognitive profile of individuals with FXS. Our findings underscore the importance of measuring two processes simultaneously and contrasting them on a comparable metric, such as Z scores, to obtain an accurate description of the cognitive developmental profile of individuals with FXS. This practice is common within the field of clinical neuropsychology (Strauss, Spreen, & Spreen, 2006).
We note that the interpretation of our Z-score data is limited by the use of the WISC-III. Given that data collection started in 1997 before publication of the WISC-IV in 2003 (Wechsler, 2003), we were constrained to the use of the WISC-III throughout the duration of the study. We recognize that the normative data published in the WISC-III is somewhat outdated, given that our study continued until 2011. Interpretation of our findings is also limited by the inclusion of several raw scores of 0 to calculate Z scores. As explained in the methods, raw scores of 0 were included as per WISC-III standard administration procedure for subtests that were attempted but could not be completed due to behaviors preventing test administration (e.g., minimal expressive language or limited attention span) or the participant’s inability to provide an accurate response or to understand the task. Alternatively, the scores could have been treated as missing or the participants could have been removed from the sample, both of which would have resulted in higher mean Z scores (Z scores closer to 0). Finally, interpretation of our findings is also limited by a participation bias in favor of families with median and mean household income above the nationwide average.
Conclusion
In conclusion, our findings reveal a dynamic cognitive developmental profile associated with FXS. The use of Z scores and a longitudinal design revealed nonlinear trajectories for verbal comprehension, perceptual organization, processing speed/attention, and working memory relative to a normative sample. Comparing these trajectories showed that strengths and weaknesses within the cognitive profile of individuals with FXS change during childhood and adolescence.
Acknowledgments
We thank the participants and their families. This research was made possible thanks to a collaboration with NCS Pearson Inc. Support for this research was provided by National Institute of Mental Health Grants MH064708 and MH50047, the Canel Family Fund (Principal Investigator grants to A.L.R.), and the Fonds Québécois pour la Recherche sur la Société et la Culture (postdoctoral fellowship to E.-M.Q).
Appendix A
Parameter estimates of quadratic growth trajectories
| WISC-III Index and Parameter Estimate | Males With FXS
|
Females With FXS
|
||||
|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | |
| Verbal comprehension | ||||||
| Intercept | −2.349 | 0.149 | <.001 | −0.478 | 0.259 | .065 |
| Age | −0.164 | 0.059 | .006 | −0.209 | 0.099 | .036 |
| Age2 | 0.006 | 0.005 | .280 | 0.013 | 0.008 | .127 |
| Perceptual organization | ||||||
| Intercept | −1.608 | 0.104 | <.001 | −1.608 | 0.104 | <.001 |
| Age | −0.392 | 0.053 | <.001 | −0.392 | 0.053 | <.001 |
| Age2 | 0.019 | 0.005 | <.001 | 0.019 | 0.005 | <.001 |
| Freedom from distractibility | ||||||
| Intercept | −4.459 | 0.212 | <.001 | −1.902 | 0.317 | .000 |
| Age | −0.111 | 0.076 | .143 | −0.104 | 0.097 | .283 |
| Age2 | 0.018 | 0.006 | .005 | 0.011 | 0.008 | .143 |
| Processing speed | ||||||
| Intercept | −2.639 | 0.249 | <.001 | −0.579 | 0.250 | .020 |
| Age | −0.286 | 0.092 | .002 | −0.168 | 0.079 | .033 |
| Age2 | 0.018 | 0.007 | .013 | 0.007 | 0.006 | .264 |
Note: WISC-III, Wechsler Intelligence Scale for Children—Third Edition; FXS, fragile X syndrome.
Footnotes
Standardization data from the Wechsler Intelligence Scale, Third Edition (WISC-III). Copyright 1990 NCS Pearson, Inc. Used with permission. All rights reserved.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: Author; 2013. [Google Scholar]
- Bailey DB, Jr, Hatton DD, Skinner M. Early developmental trajectories of males with fragile X syndrome. American Journal on Mental Retardation. 1998;103:29–39. doi: 10.1352/0895-8017(1998)103<0029:EDTOMW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Hatton DD, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of Autism and Developmental Disorders. 2001;31:165–174. doi: 10.1023/A:1010747131386. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Eliez S, Hedeus M, Menon V, White CD, Moseley M, et al. White matter tract alterations in fragile X syndrome: Preliminary evidence from diffusion tensor imaging. American Journal of Medical Genetics. 2003;118:81–88. doi: 10.1002/ajmg.b.10035. [DOI] [PubMed] [Google Scholar]
- Bray S, Hirt M, Jo B, Hall SS, Lightbody AA, Walter E, et al. Aberrant frontal lobe maturation in adolescents with fragile X syndrome is related to delayed cognitive maturation. Biological Psychiatry. 2011;70:852–858. doi: 10.1016/j.biopsych.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno J, Walter Shelly E, Quintin EM, Rostami M, Patnaik S, Spielman D, et al. Aberrant basal ganglia metabolism in fragile X syndrome a magnetic resonance spectroscopy study. Journal of Neurodevelopmental Disorders. 2013;5:1–9. doi: 10.1186/1866-1955-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Cole V, Longhi E, Karmiloff-Smith A, Scerif G. Mapping developmental trajectories of attention and working memory in fragile X syndrome: Developmental freeze or developmental change? Development and Psychopathology. 2013;25:365–376. doi: 10.1017/S0954579412001113. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. The nature of the spatial deficit in young females with fragile-X syndrome: A neuropsychological and molecular perspective. Neuropsychologia. 1998;36:1239–1246. doi: 10.1016/S0028-3932(97)00162-0. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. The spatial cognition in males with fragile X syndrome: Evidence for a neuropsychological phenotype. Cortex. 1999;35:263–271. doi: 10.1016/s0010-9452(08)70799-8. [DOI] [PubMed] [Google Scholar]
- Cornish K, Scerif G, Karmiloff-Smith A. Tracing syndrome-specific trajectories of attention across the lifespan. Cortex. 2007;43:672–685. doi: 10.1016/s0010-9452(08)70497-0. [DOI] [PubMed] [Google Scholar]
- Curfs LM, Schreppers-Tijdin G, Wiegers A, Borghgraef M, Fryns JP. Intelligence and cognitive profile in the fra(X) syndrome: A longitudinal study in 18 fra(X) boys. Journal of Medical Genetics. 1989;26:443–446. doi: 10.1136/jmg.26.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens EM, Hodapp RM, Ort S, Finucane B, Shapiro LR, Leckman JF. The trajectory of cognitive development in males with fragile X syndrome. Journal of the American Academy of Child & Adolescent Psychiatry. 1989;28:422–426. doi: 10.1097/00004583-198905000-00020. [DOI] [PubMed] [Google Scholar]
- Fisch GS. Cognitive–behavioral profiles of females with the fragile X mutation. American Journal of Medical Genetics. 2006;140A:673–677. doi: 10.1002/ajmg.a.31113. [DOI] [PubMed] [Google Scholar]
- Fisch GS, Carpenter N, Holden JJ, Howard-Peebles PN, Maddalena A, Borghgraef M, et al. Longitudinal changes in cognitive and adaptive behavior in fragile X females: A prospective multicenter analysis. American Journal of Medical Genetics. 1999;83:308–312. [PubMed] [Google Scholar]
- Fisch GS, Carpenter N, Howard-Peebles PN, Holden JJ, Tarleton J, Simensen R. The course of cognitive-behavioral development in children with the FMR1 mutation, Williams-Beuren syndrome, and neurofibromatosis type 1: The effect of gender. American Journal of Medical Genetics. 2010;152A:1498–1509. doi: 10.1002/ajmg.a.33412. [DOI] [PubMed] [Google Scholar]
- Fisch GS, Simensen RJ, Schroer RJ. Longitudinal changes in cognitive and adaptive behavior scores in children and adolescents with the fragile X mutation or autism. Journal of Autism and Developmental Disorders. 2002;32:107–114. doi: 10.1023/a:1014888505185. [DOI] [PubMed] [Google Scholar]
- Fisch GS, Simensen R, Tarleton J, Chalifoux M, Holden JJ, Carpenter N, et al. Longitudinal study of cognitive abilities and adaptive behavior levels in fragile X males: A prospective multicenter analysis. American Journal of Medical Genetics. 1996;64A:356–361. doi: 10.1002/(SICI)1096-8628(19960809)64:2<356::AID-AJMG24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Golder DE, Inaba Y, Shi EZ, Skinner C, Bui QM, Francis D, et al. Relationships between age and epi-genotype of the FMR1 exon 1/intron 1 boundary are consistent with non-random X-chromosome inactivation in FM individuals, with the selection for the unmethylated state being most significant between birth and puberty. Human Molecular Genetics. 2013;22:1516–1524. doi: 10.1093/hmg/ddt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Furfaro JA, Hoeft F, Eckert MA, Hall SS, O’Hara R, et al. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP) Annals of Neurology. 2008;63:40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T, Barnea-Goraly N, Raman M, Hall SS, Lightbody AA, Bruno JL, et al. Specific effect of the fragile-X mental retardation-1 gene (FMR1) on white matter microstructure. British Journal of Psychiatry. 2015 doi: 10.1192/bjp.bp.114.151654. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Barnea-Goraly N, Lightbody AA, Patnaik SS, Hoeft F, Hazlett H, et al. Early white-matter abnormalities of the ventral frontostriatal pathway in fragile X syndrome. Developmental Medicine & Child Neurology. 2009;51:593–599. doi: 10.1111/j.1469-8749.2009.03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Burns DD, Lightbody AA, Reiss AL. Longitudinal changes in intellectual development in children with fragile X syndrome. Journal of Abnormal Child Psychology. 2008;36:927–939. doi: 10.1007/s10802-008-9223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Jiang H, Reiss AL, Greicius MD. Identifying large-scale brain networks in fragile X syndrome. JAMA Psychiatry. 2013;70:1215–1223. doi: 10.1001/jamapsychiatry.2013.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan BP, Craig MC, Toal F, Daly EM, Moore CJ, Ambikapathy A, et al. In vivo brain anatomy of adult and males with fragile X syndrome: An MRI study. NeuroImage. 2011;54:16–24. doi: 10.1016/j.neuroimage.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Hessl D, Nguyen DV, Green C, Chavez A, Tassone F, Hagerman RJ, et al. A solution to limitations of cognitive testing in children with intellectual disabilities: The case of fragile X syndrome. Journal of Neurodevelopmental Disorders. 2009;1:33–45. doi: 10.1007/s11689-008-9001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Carter JC, Lightbody AA, Cody Hazlett H, Piven J, Reiss AL. Region-specific alterations in brain development in one- to three-year- old boys with fragile X syndrome. Proceedings of the National Academy of Science. 2010;107:9335–9339. doi: 10.1073/pnas1002762107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan CS, Bertone A, Cornish K, Boutet I, Der Kaloustian VM, Andermann E, et al. Integrative cortical dysfunction and pervasive motion perception deficit in fragile X syndrome. Neurology. 2004;63:1634–1639. doi: 10.1212/01.WNL.0000142987.44035.3B. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Boutet I, Cornish K, Graham GE, Berry-Kravis E, Drouin A, et al. A comparative neuropsychological test battery differentiates cognitive signatures of fragile X and Down syndrome. Journal of Intellectual Disability Research. 2009;53:125–142. doi: 10.1111/j.1365-2788.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Boutet I, Cornish K, Zangenehpour S, Mullen KT, Holden JJ, et al. Differential impact of the FMR1 gene on visual processing in fragile X syndrome. Brain. 2004;127:591–601. doi: 10.1093/brain/awh069. [DOI] [PubMed] [Google Scholar]
- Kover ST, Pierpont EI, Kim JS, Brown WT, Abbeduto L. A neurodevelopmental perspective on the acquisition of nonverbal cognitive skills in adolescents with fragile X syndrome. Developmental Neuropsychology. 2013;38:445–460. doi: 10.1080/87565641.2013.820305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H, Menon V, Eliez S, Warsofsky IS, White CD, Dyer-Friedman J, et al. Functional neuroanatomy of visuospatial working memory in fragile X syndrome: Relation to behavioral and molecular measures. American Journal of Psychiatry. 2001;158:1040–1051. doi: 10.1176/appi.ajp.158.7.1040. [DOI] [PubMed] [Google Scholar]
- Lachewicz AM, Dawson DV, Spiridigliozzi GA, McConkie-Rosell A. Arithmetic difficulties in females with the fragile X premutation. Journal of Medical Genetics. 2006;140A:665–672. doi: 10.1002/ajmg.a.31082. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. 2. New York: Wiley; 2002. [Google Scholar]
- Loesch DZ, Huggins RM, Bui QM, Epstein JL, Taylor AK, Hagerman RJ. Effect of the deficits of fragile X mental retardation protein on cognitive status of fragile X males and females assessed by robust pedigree analysis. Journal of Developmental and Behavioral Pediatrics. 2002;23:416–423. doi: 10.1097/00004703-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM. Math learning disability and math LD subtypes: Evidence from studies of Turner syndrome, fragile X syndrome, and neurofibromatosis type 1. Journal of Learning Disabilities. 2001;34:520–533. doi: 10.1177/002221940103400605. [DOI] [PubMed] [Google Scholar]
- Munir F, Cornish KM, Wilding J. Nature of the working memory deficit in fragile X syndrome. Brain and Cognition. 2000;44:387–401. doi: 10.1006/brcg.1999.1200. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 7. Los Angeles: Author; 1998–2014. [Google Scholar]
- Ornstein PA, Schaaf JM, Hooper SR, Hatton DD, Mirrett P, Bailey DB. Memory skills of boys with fragile X syndrome. American Journal of Mental Retardation. 2008;113:453–465. doi: 10.1352/2008.113:453-465. [DOI] [PubMed] [Google Scholar]
- Peng D, Kelley R, Quintin EM, Raman M, Thompson P, Reiss AL. Cognitive and behavioral correlates of caudate subregion shape variation in fragile X syndrome. Human Brain Mapping. 2013;35:2861–2868. doi: 10.1002/hbm.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Population Studies Center. Zip code characteristics: Mean and median household income (2006–2010 US Census data) Ann Arbor, MI: University of Michigan, Institute for Social Research, Population Studies Center; 2015. Retrieved on June 1, 2015, from http://www.psc.isr.umich.edu/dis/census/Features/tract2zip/index.html. [Google Scholar]
- Rivera SM, Menon V, White CD, Glasser B, Reiss AL. Functional brain activation during arithmetic processing in females with fragile X syndrome is related to FMR1 protein expression. Human Brain Mapping. 2002;16:206–218. doi: 10.1002/hbm.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano D, Nicolau M, Quintin EM, Mazaika P, Lightbody AA, Hazlett H, et al. Topological methods reveal high and low functioning neuro-phenotypes within fragile X syndrome. Human Brain Mapping. 2014;35:4904–4915. doi: 10.1002/hbm.22521. doi:0.1002/hbm.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F, Heitz D, Tarleton J, MacPherson J, Malmgren H, Dahl N, et al. A multicenter study on genotype-phenotype correlations in the fragile X syndrome, using direct diagnosis with probe StB12.3: The first 2,253 cases. American Journal of Human Genetics. 1994;55:225–237. [PMC free article] [PubMed] [Google Scholar]
- Sansone SM, Schnieder A, Bickel E, Berry-Kravis E, Prescott C, Hessl D. Improving IQ measurement in intellectual disabilities using true deviation from population norms. Journal of Neurodevelopmental Disorders. 2014 doi: 10.1186/1866-1955-6-16. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon EW, Rappaport DA, Papka M, Wodruff-Pak DS. Fragile-X and Down’s syndrome: Are there syndrome-specific cognitive profiles at low IQ levels? Journal of Intellectual Disability Research. 1995;30:326–330. doi: 10.1111/j.1365-2788.1995.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Skinner M, Hooper S, Hatton DD, Roberts J, Mirrett P, Schaaf J, et al. Mapping nonverbal IQ in young boys with fragile X syndrome. American Journal of Medical Genetics. 2005;132A:25–32. doi: 10.1002/ajmg.a.30353. [DOI] [PubMed] [Google Scholar]
- Strauss E, Spreen EMS, Spreen O. A compendium of neuro-psychological tests. New York: Oxford University Press; 2006. [Google Scholar]
- van der Molen MJW, Huizinga M, Huizenga HM, Ridderinkhof KR, Van der Molen MW, Hamel BJC, et al. Profiling fragile X syndrome in males: Strengths and weaknesses in cognitive abilities. Research in Developmental Disabilities. 2010;31:426–439. doi: 10.1016/j.ridd.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-H. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]