Abstract
Objective
HIV infection leads to age-related conditions in relatively young persons. HIV-associated neurocognitive disorders (HAND) are considered among the most prevalent of these conditions. To study the mechanisms underlying this disorder, researchers need an accurate method for measuring biological aging. Here we apply a recently developed measure of biological aging, based on DNA methylation, to the study of biological aging in HIV+ brains.
Design
Retrospective analysis of tissue bank specimens and pre-mortem data.
Methods
58 HIV+ adults underwent medical and neurocognitive evaluation within one year of death. DNA was obtained from occipital cortex and analyzed with the Illumina Infinium Human Methylation 450K platform. Biological age determined via the Epigenetic Clock was contrasted with chronological age to obtain a measure of age acceleration, which was then compared between those with HAND and neurocognitively normal individuals.
Results
The HAND and neurocognitively normal groups did not differ with regard to demographic, histologic, neuropathologic, or virologic variables. HAND was associated with accelerated aging relative to neurocognitively normal individuals, with average relative acceleration of 3.5 years. Age acceleration did not correlate with pre-mortem neurocognitive functioning or HAND severity.
Conclusions
This is the first study to demonstrate that the epigenetic age of occipital cortex samples is associated with HAND status in HIV+ individuals pre-mortem. While these results suggest that the increased risk of a neurocognitive disorder due to HIV might be mediated by an epigenetic aging mechanism, future studies will be needed to validate the findings and dissect causal relationships and downstream effects.
Keywords: HIV-associated neurocognitive disorder, HANA, HAND, epigenetic, HIV, epigenetic clock
INTRODUCTION
Although antiretroviral therapy for HIV infection is highly effective at preventing AIDS-related complications, treated patients are at a significant risk for a number of diseases typically associated with aging, including cardiovascular disease, osteoporosis, cancer, cognitive impairment, and frailty (Deeks, 2011; Desquilbet et al., 2011; Fausto et al., 2006; Grinspoon & Carr, 2005; Kirk et al., 2013; Lucas et al., 2007; Martin & Volberding, 2010; M. Silverberg et al., 2009; M. J. Silverberg et al., 2011; Thomas & Doherty, 2003; Triant, Lee, Hadigan, & Grinspoon, 2007; Wendelken & Valcour, 2012; Womack et al., 2011). Among the aging HIV+ population, it has become evident that the incidence of HIV-Associated Non-AIDS (HANA) conditions is increasing (Greene M, 2013). HANA conditions affect virtually every organ system and have as a common theme an association with advancing age and a pathogenesis likely based on chronic inflammation. Arguably, the most prevalent HANA condition is HIV-associated neurocognitive disorder (HAND), a spectrum syndrome characterized clinically by neurocognitive impairment and biologically by chronic immune activation, oxidative stress, and excitotoxicity (Gannon, Khan, & Kolson, 2011; Kraft-Terry, Buch, Fox, & Gendelman, 2009). Early studies indicated that older individuals were at increased risk for HAND (Cherner et al., 2004). Additional evidence that HAND is due at least partially to accelerated aging includes studies of CSF metabolomics (Cassol, Misra, Dutta, Morgello, & Gabuzda, 2014), in vitro analysis in astrocytes(Ojeda et al., 2014), and neurophysiological studies (Chang, Holt, Yakupov, Jiang, & Ernst, 2013; Holt, Kraft-Terry, & Chang, 2012). Still, in clinical samples telomere length has not been consistently associated with neurocognitive impairment or other indicators of neuroHIV (Giesbrecht et al., 2014; Malan-Muller et al., 2013). Clinico-pathological studies have found age-related pathology in relatively young HIV+ cases, including reduced β-amyloid (Aβ)-42 levels in cerebral spinal fluid (Brew, Pemberton, Blennow, Wallin, & Hagberg, 2005) and increased Aβ deposits in brain tissue sections (Soontornniyomkij, Moore, et al., 2012b).
In order to effectively investigate accelerated aging in HIV, an accurate method for quantifying biological age is needed. Telomere length, which relates to cellular senescence, has been the most popular method for studying biological aging. An alternative and remarkably more accurate method is enabled by the epigenetic process of DNA methylation (DNAm). DNAm is probably the most widely studied epigenetic mechanism, and is a chemical process by which methyl groups are added to the DNA molecule. Methylation sometimes modifies the function of the DNA, e.g. by suppressing gene transcription. While it is well known that DNAm is essential for normal development, a large body of literature demonstrates that it also plays an important role in aging (Jung & Pfeifer, 2015; Yuan et al., 2015). DNAm levels are particularly promising biomarkers of aging since chronological age (i.e., the calendar years that have passed since birth) has a profound effect on DNAm levels in most human tissues and cell types (Alisch et al., 2012; Bell et al., 2012; Bollati et al., 2009; Christensen et al., 2009; Day et al., 2013; Hernandez et al., 2011; S Horvath et al., 2012; Johansson, Enroth, & Gyllensten, 2013; Numata et al., 2012; Rakyan et al., 2010; A. E. Teschendorff et al., 2010; Vivithanaporn et al.). Several recent studies support measuring accelerated aging effects using DNAm levels (Bocklandt et al., 2011; Hannum et al., 2013; S Horvath, 2013a). The recently developed biomarker of aging (referred to as Epigenetic Clock (S. Horvath, 2013)) is an attractive biomarker of aging for the following reasons: a) it is more highly correlated with chronological age than previous biomarkers (Boks et al., 2009; S. Horvath et al., 2014), b) it applies to most tissues, cell types, and fluids that contain human DNA (with the exception of sperm), c) it relates, to some extent, to biological age since DNAm age of blood is predictive of all-cause mortality even after adjusting for chronological age and a variety of known risk factors (Marioni, Shah, McRae, Chen, et al., 2015). Similarly, markers of physical and mental fitness are also found to be associated with the epigenetic age of blood (lower abilities associated with age acceleration) (Marioni, Shah, McRae, Ritchie, et al., 2015).
We recently found evidence that HIV infection is associated with accelerated epigenetic aging in both brain and peripheral blood mononuclear cells (S. Horvath & Levine, 2015). Specifically, brains of those infected with HIV demonstrated age acceleration of 7.4 years compared to uninfected controls, and 5.2 years in peripheral blood mononuclear cells. While our previous article shows that HIV infected (HIV+) individuals have accelerated aging in brain tissue, it remains to be seen if this is clinically relevant. In the current article, we test the hypothesis that individuals with HAND exhibit accelerated epigenetic aging. Toward this end, we measured the DNAm age acceleration using the Epigenetic Clock in post-mortem brain tissue of HIV+ individuals who were characterized pre-mortem with regards to neurocognitive, medical, and virologic variables.
METHODS
Participants
This study was conducted in accordance with the University of California, Los Angeles Medical Institutional Review Board. Data and biological samples from 58 individuals enrolled in either the National Neurological AIDS Bank (NNAB) or California NeuroAIDS Tissue Network (CNTN) were used. The NNAB and CNTN are member sites of the National NeuroAIDS Tissue Consortium(Morgello et al., 2001) (NNTC.org). Post-mortem findings of non-HIV related neurological disease was exclusionary, as was diagnosis substance dependence within one year of death. Participant characteristics for this cohort are shown in Table 1. All clinical lab work was performed by CLIA certified clinical labs.
Table 1.
Group comparisons (neurologically normal vs. HAND)
| Variable | Normal Mean (SD) |
HAND Mean (SD) |
Significance Testing (ANOVA) |
|---|---|---|---|
| Education | 13.2 (1.9) | 12.6 (3) | P = .463 |
| Age at Death | 46.5 (10.4) | 45.7 (9) | P = .798 |
| Duration of Infection | 18.5 (9.3) | 22.8 (6.1) | P = .056 |
| CPE | 8.4 (2.8) | 9.2 (5.9) | P = .631 |
| Global Clinical Rating* | 3.5 (1.2) | 6.1 (1.6) | P < .001 |
| Nadir CD4** | N=10 25 (34) | N=18 33 (55) | P = .694 |
| Plasma Viral Load at Diagnosis |
109281 (226683) | 162469 (252499) | P = .474 |
| Log Plasma VL at Diagnosis | 3.5 (1.6) | 4.2 (1.3) | P = .104 |
| CD4 at Diagnosis | 128 (150) | 109 (141) | P = .663 |
| CSF Viral Load at Death** | N=4, 21345 (39189) | N=14, 70650 (228220) | P = .679 |
| Log RNA VL Frontal Cortex** | N=8, 3.2 (1) | N=18, 4.3 (1.5) | P = .065 |
| Log DNA VL Frontal Cortex** | N=8, 3.1 (.4) | N=18, 4 (1.4) | P = .122 |
| Variable |
Normal Number (Percentage) |
HAND Number (Percentage) |
Significance Testing (chi-square) |
| Male Gender | 11 (73%) | 35 (81%) | p = .487 |
| European American Ethnicity | 10 (67%) | 29 (67%) | p = .931 |
| African American Ethnicity | 4 (27%) | 11 (26%) | -- |
| Percent on HAART | 13 (87%) | 33 (77%) | p = .712 |
Variables
HAND Diagnosis
All cases were, within one year of death, diagnosed as either neurocognitively normal or with HAND per established research criteria (Antinori et al., 2007; "Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder. The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders," 1996). This was based on either the previous 1996 American Academy of Neurology criteria if their last pre-mortem evaluation occurred prior to the publication of the newer HAND research criteria (“Frascati)” in 2007, or according to the Frascati criteria if after 2007. The NNAB and CNTN diagnostic criteria prior to 2007 included a “subsyndromic” impairment classification that is essentially identical to Asymptomatic Neurocognitive Impairment (ANI) according to the Frascati criteria. Because the criteria for MCMD and HAD (AAN) remained essentially identical to MND and HAD (Antinori et al 2007), we were able to group participants from both diagnostic eras. The diagnosis of HAND depends largely on neurocognitive functioning, which was determined with a comprehensive battery of validated tests described previously. Tests are grouped by neurocognitive domain (e.g., learning, memory, attention, motor), and a measure of global neurocognitive functioning is also determined based on all test scores. The influence of age acceleration on domain and global neurocognitive functioning scores were examined in exploratory analyses.
CNS Penetration Effectiveness (CPE)
We calculated the CPE, a score that is based on the pharmacokinetic and pharmacodynamic characteristics of antiretroviral medications (Letendre, 2011). CPE scores for the regimen reported at the time of neurocognitive testing were calculated. Higher scores indicate a regimen with increased penetration of the blood brain barrier.
HIV Disease Measures
Peripheral blood was drawn from living participants via venipuncture and stored in EDTA and heparinized tubes and then assayed for viral load using the Roche Amplicor Assay and by flow cytometry for CD4+ subsets. Reliable assays cannot be conducted on post mortem subjects because venipuncture cannot be collected after the heart has ceased beating and the peripheral blood has coagulated. Several measures of virologic status were evaluated. This included CD4+ cell count and HIV plasma viral load measured at the last pre-mortem visit within one year of death, as well as duration of HIV infection. CSF viral load at time of death was also available for a subset of individuals. In addition, HIV DNA and RNA levels as measured in medial frontal cortex were available for a subset of participants, described next.
Brain HIV Viral Load (DNA & RNA)
For a subset of individuals (8 neurocognitively normal and 18 HAND), HIV DNA and RNA was quantified from the dorsolateral frontal cortex, as described previously (Gelman et al., 2013). Briefly, HIV RNA and DNA were quantified using cDNA and total genomic DNA using HIV gag/pol primer and probe sequences by PCR(Palmer et al., 2003). The reaction contained 4 µl of cDNA or 1 µg of total DNA, 12.5 µl of Sigma JumpStart Taq ReadyMix (Cat. No. D7440, Sigma, St. Louis, MO, USA), 3.5 µl of 25 mmol/L MgCl2, 0.8 µl of 10 µmol/L HIV primer mix and 0.5 µl of 10 µmol/L HIV probe adjusted to 25 µl. Conditions were 2 min. at 95°C, 40 cycles of 15 sec. at 95°C and 60 sec. at 60°C. Real time PCR was run using an Eppendorf RealPlex (Hamburg, Germany). Copies per µg of total RNA were calculated with a standard curve using a previously quantified HIV-positive RNA primary standard(Gelman et al., 2012).
DNA Processing for Methylation Analysis
Tissue Processing, DNA extraction, and Genotyping
Frozen occipital cortex samples were shipped to the University of California Los Angeles - Biological Samples Processing Core from the NNAB and CNTN for DNA extraction. The Autopure LS nucleic acid purification instrument was used for extracting DNA. Samples were quantified using OD 260/280. Extracted DNA was then genotyped. Prior to methylation assaying, the samples were checked for concentration by Quant-iT ds DNA Assay kit (Invitrogen) and for quality by agarose gel. Medial frontal cortex was also processed for a subset of cases (2 neurocognitively normal and 5 HAND).
DNA Methylation
Methylation analysis was performed with the Illumina Infinium HumanMethylation450 BeadChip, which measures bisulfite-conversion-based, single-CpG resolution DNAm levels at 485577 CpG sites in the human genome. The standard protocol of Illumina methylation assays quantifies methylation levels by the β value using the ratio of intensities between methylated (signal A) and un-methylated (signal B) alleles. Specifically, the β value is calculated from the intensity of the methylated (M corresponding to signal A) and un-methylated (U corresponding to signal B) alleles, as the ratio of fluorescent signals β = Max(M,0)/[Max(M,0) + Max(U,0) + 100]. Thus, β values range from 0 (completely un-methylated) to 1 (completely methylated) (Dunning, Barbosa-Morais, Lynch, Tavare, & Ritchie, 2008).
The Epigenetic Clock
The epigenetic clock is based on the DNAm levels of 353 CpGs (S Horvath, 2013a). Predicted age, referred to as DNAm age, correlates with chronological age in sorted cell types (CD4+ cells, monocytes, B cells, glial cells, neurons) and tissues and organs including whole blood, brain, breast, kidney, liver, lung, and saliva (S Horvath, 2013a). By construction, the epigenetic clock (and software) applies to data generated using the Illumina 450K (S Horvath, 2013a). Mathematical details and software tutorials for the epigenetic clock can be found in the Additional files of (S Horvath, 2013a). An online age calculator can be found at our webpage (http://labs.genetics.ucla.edu/horvath/htdocs/dnamage/) (S Horvath, 2013b). The epigenetic clock software implements a data normalization step that repurposes the Beta Mixture Quantile dilation normalization method from Teschendorff (Andrew E. Teschendorff et al., 2013) so that it automatically references each sample to a gold standard (details can be found in Additional file 1 from (S Horvath, 2013a)). We defined an epigenetic measure of age acceleration by regressing DNAm age on chronological age and forming residuals. By definition, this measure of age acceleration is not correlated with chronological age. Separate measures of epigenetic age acceleration were calculated for occipital cortex and the subset of medial frontal cortex samples.
Statistical methods
Student T-tests were employed to compare the two groups (HAND vs. non-HAND) on variables that exhibited a normal distribution (e.g. various immunologic and virologic variables). In order to compare age acceleration between the two groups (e.g. HAND vs controls), we employed the Kruskal Wallis test, which is a non-parametric group comparison test. Robust measures of correlation (e.g. biweight mid-correlations or Spearman’s rho) were used to examine the relationship between age acceleration and ordinal variables of interest, including HCV infection, age at death, CPE score, neuropathological findings, and HIV-related measures.
DNAm age acceleration residuals were calculated for each case. Separate residuals were calculated for occipital cortex and the subset of medial frontal cortex samples. This residual value was then used in statistical tests. Student T-tests were employed to compare the two groups (HAND vs. non-HAND) on demographic, immunologic, and virologic variables. In order to compare age acceleration between the two groups, we employed the Kruskal Wallis test to compare individuals diagnosed with HAND within one year of death to those diagnosed as neurocognitively normal. The Kruskal Wallis test was also used to assess group differences in those cases where both variables showed non-normal distribution. Biweight mid-correlation or Pearson correlation were used to examine the relationship between age acceleration and other variables of interest, including HCV infection, age at death, CPE score, neurocognitive functioning, HAND severity, neuropathological findings, and HIV-related measures.
RESULTS
Group differences are summarized in Table 1. The two groups did not differ with regards to virologic or demographic variables, although there was a notable trend for greater duration of infection among the HAND group (p = 0.056). As expected, the HAND group demonstrated greater neurocognitive impairment (p < .001).
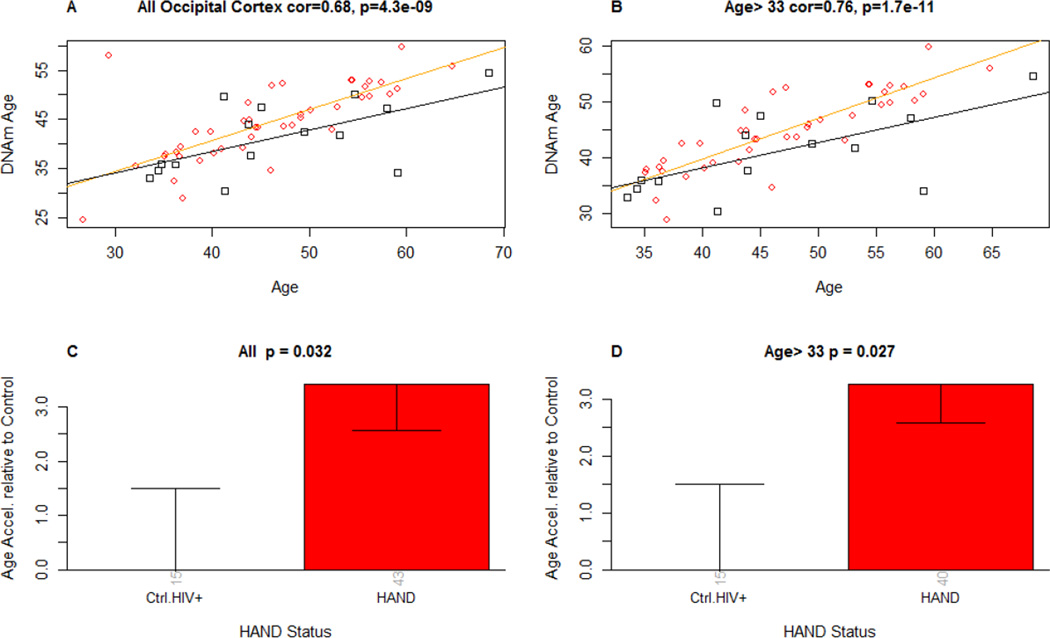
Epigenetic age showed greater acceleration in the HAND group, as shown by the regression lines in Figure 1 (panel A). The overall correlation between DNAm age and chronological age was highly significant (r = 0.68, p < .0001). Kruskal Wallis testing confirmed that individuals diagnosed with HAND within one year of death had greater accelerated epigenetic aging as compared to those who were diagnosed as neurocognitively normal (p = 0.032) (Figure 1, panel C). As can be seen in panel A, there was an extreme outlier in the HAND group. When analyses were therefore restricted to individuals over 33 (thereby excluding this outlier and 2 other HAND cases), the correlation between DNAm age and chronological age were even stronger (r = 0.76), and the group differences more robust (p = 0.027).
FIGURE 1.
Panel A and B: DNA methylation age (y-axis) versus chronological age in A) all brain samples and B) those brains from individuals who died past age 33 (this analysis was undertaken due to the outlier shown in panel A). Red circles and black squares in the scatter plot denote samples from HAND and neurocognitively normal HIV+ cases, respectively. The orange line depicts a linear regression line through HAND samples. Similarly, the black solid line corresponds to a regression line of DNA methylation age on chronological age in neurocognitively normal samples. For each sample, age acceleration is defined as the vertical distance to the relevant regression line (i.e. DNA methylation age minus the expected value based on HIV-samples).
Panels C: Mean age acceleration (y-axis) versus HAND status in all brain samples. Each bar plot depicts one standard error around the mean value and reports the Kruskal Wallis (non-parametric) group comparison test p-value. Panel D: Analogous results can be obtained when restricting the analysis to those over age 33 (due to outlier values in younger cases). The number of samples in each group is reported by the rotated number under each bar.
Correlation analysis did not reveal any significant associations between age acceleration and HCV infection (r = 0.0076, p = 0.95), age at the time of death (r = −0.008, p = 0.94), CPE score (r = 0.06, p = 0.57), nadir CD4+ cell count (r = 0.1, p = 0.42), CD4+ cell count as measured at death (r = 0.049, p = 0.63), or plasma viral load at the time of death (r = −0.14, p = 0.16). No correlation between global neurocognitive functioning (r = 0.22, p = 0.10) or HAND severity (r = 0.21, p = 0.11) was observed. Further, no correlations between individual neurocognitive domains and age acceleration were observed (data not shown). Conversely, duration of HIV infection was correlated with age acceleration (r = 0.28, p = 0.038).
We also examined the potential confounder of comorbid neuropathological conditions. As shown in Table 2, the HAND group had a disproportionate number of additional neuropathological findings (e.g., HIV encephalitis). However, we found no significant correlations between age acceleration and presence or severity of these neuropathological findings.
Table 2.
Neurocognitive diagnosis and Neuropathological findings
| Variable | Normal N |
HAND N |
Significance Testing |
|---|---|---|---|
| Asymptomatic Neurocognitive Impairment | n/a | 12 | -- |
| Minor Cognitive/Motor Disorder | n/a | 20 | -- |
| HIV-Associated Dementia | n/a | 11 | -- |
| Neuropathological Findings | Correlation with Age Acceleration* | ||
| Cryptococcus | 0 | 2 | R = .203, p = .126 |
| CMV Infection | 0 | 2 | R = −.102, p = .448 |
| HIV Encephalitis | 0 | 3 | R = −.137, p = .304 |
| White Matter Forebrain Lesion | 1 | 6 | R = −.058, p = .663 |
| Periventricular Siderophages | 12 | 25 | R = −.173, p = .195 |
| Microglial nodule encephalitis | 0 | 2 | R = −.192, p = .149 |
| Perivascular Mononuclear Cells | 7 | 9 | R = −.111, p = .408 |
The rate of comorbid medical conditions did not differ between those with and without HAND. Specifically, for those cases for which these data were available, we did not observe differences in incidence rates of hypertension (Χ2 = 2.6, p = 0.11), hyperlipidemia (Χ2 = 2.9, p = 0.09), chronic renal disease (Χ2 = 0.026, p = 0.87), diabetes, (Χ2 = 0.363, p = 0.55), viral hepatitis (Χ2 = .077, p = 0.78), end stage liver disease (Χ2 = 0.84, p = 0.36), chronic obstructive pulmonary disease (Χ2 = 0.535, p = 0.47), non-AIDS defining cancer and (Χ2 = 1.13, p = 0.29), and tobacco smoking (Χ2 = 3.71, p = 0.054). Because of the trends seen for tobacco smoking and hyperlipidemia, we also compared age acceleration between those with and without these two comorbidities using the Kruskal Wallis test. We found no significant difference tobacco users and non-users (p = 0.18) or individuals with or without hyperlipidemia (p = 0.81).
A small subset of cases (N = 7) had DNAm age acceleration and HIV RNA and DNA derived from medial frontal cortex. Based on this small sample, we did not observe a correlation between age acceleration and either RNA (r = −0.14, p = 0.79) or DNA (r = 0.14, p = 0.79). There was also no association between age acceleration in frontal cortex and global neurocognitive functioning (r = 0.22, p = 0.53) or HAND severity (r = 0.11, p = 0.77).
Because duration of infection was significantly correlated with age acceleration (r = 0.28, p = 0.038), we carried out a logistic regression analysis to determine the contribution of chronological age, duration of infection, and age acceleration to HAND status. Neither chronological age (p = 0.85) nor duration of infection (p = 0.08) predicted HAND status. However, age acceleration did (p = 0.04).
Finally, due to the possibility that difference in methylation were driven by difference in cellular composition(Jaffe & Irizarry, 2014), we examined the association between accelerated aging and various histopathology markers. These included Human leukocyte antigen (HLA-DR – marker of macrophages), Allograft inflammatory factor 1 (AIF-1, a marker of microglia), and Glial fibrillary acidic protein (GFAP – a marker of astrocytes). We also examine amyloid-β deposition, as it is associated with both HIV and aging (Achim, Adame, Dumaop, Everall, & Masliah, 2009; Green et al., 2005; Soontornniyomkij, Moore, et al., 2012a). These markers were quantified in frontal cortex, frontal white matter, hippocampus, and putamen. Histopathology and quantification procedures for these were described previously(Soontornniyomkij et al., 2010; Soontornniyomkij, Soontornniyomkij, et al., 2012; Toggas & Mucke, 1996). No correlations between age acceleration and any marker in any region were observed (data not shown).
DISCUSSION
HAND may be the most prevalent manifestations of accelerated aging due to HIV, and is among those conditions collectively referred to as HANA conditions. The biological mechanisms underlying the clinical manifestation of accelerated aging in HIV+ subjects remain elusive. We recently demonstrated accelerated aging in the brains and blood cells of HIV+ individuals. In this study, we have shown that this accelerated epigenetic aging in post-mortem brain tissue has clinical relevance, as it is strongly associated with HAND diagnosed within one year of death. Based on the available data, this was not due to peripheral virologic measures (CD4+ cell count and viral load), the activity of antiretroviral medications in the CNS, or neuropathological or medical comorbidities. It is also interesting that while the HAND group as a whole exhibited accelerated aging in brain tissue, this measure was not correlated with HAND severity or neurocognitive functioning. Instead, the results implicate duration of infection as a possible determinant of accelerated aging. These findings suggest to us that chronic, low level HIV replication in brain reservoirs perpetuates pathological processes resulting in accelerated age as measured epigenetically; however, such changes are not reliably captured by neuropsychological instruments. As suggested in the past (e.g., (Levine, Panos, & Horvath, 2014), this supports the need to implement intermediate level phenotypes (e.g., neurophysiological and histopathological) in studies of HAND pathogenesis, rather than relying solely on the neurocognitive manifestations.
Studies of dynamic cellular processes including transcription and translation have revealed potential targets for use as biomarkers or drug development in neuroHIV (Levine, Horvath, et al., 2013; Levine, Miller, et al., 2013; Pendyala & Fox; Pulliam et al., 2011; Winkler, Chaudhuri, & Fox, 2012). The quantification of epigenetic processes on a whole-genome scope and the delineation of their role in disease have become increasingly feasible in recent years. In particular, DNAm has become recognized as a significant determinant in cellular processes, and one that is highly variable as a function of disease status. Despite its potential role in HAND, there are no published studies of DNAm in this context. Indeed, there are very few studies overall on epigenetics and HAND(Desplats et al., 2013; Kazantsev & Thompson, 2008; Mukerjee et al., 2011; Narasipura, Kim, & Al-Harthi, 2014; Saiyed et al., 2011; Sun, Spencer, Chen, Li, & Davie, 2003; Tatro et al., 2010), as recently reviewed (Kallianpur & Levine, 2014; Levine et al., 2014). To our knowledge there have been no peer-reviewed studies examining global (i.e., genome-wide) DNAm in the context of HAND (Perez-Santiago J, 2012). As such, this nascent area of investigation may indeed yield important information about the neuropathogenesis of HAND.
The results of this study should be interpreted with the following caveats. It may be that the cause of death influenced brain methylation. Unfortunately, cause of death was not collected in a consistent evaluable way in this multi-site study. Regardless, cause of death is often times unreliable due to the multiple comorbidities of the participants and the fact that the condition that ultimately led to death (e.g., pneumonia) may not be related to the condition that precipitated it (e.g., hepatitis). More relevant, considering that we have examined brain DNA, are the neuropathological findings that are consistently gathered according to a standard protocol. We did not find any of the brain pathologies to be the cause of the age acceleration differences between the HAND and neurocognitively normal groups. In addition, the relationship between viral load and age acceleration was not fully addressed, as plasma virologic measures at the time of death are generally not available for NNTC cases. The results of the brain and CSF VL analyses on subsets of the current sample suggest that accelerated aging may be associated with greater viral replication. Larger samples will be needed to address this. Furthermore, noted by Jaffe and Irizarry (Jaffe & Irizarry, 2014), cellular composition may affect DNAm profiles within tissue. While we did not observe an association between age acceleration and various histological markers, the markers were quantified in several brain regions outside of the region used to derive the epigenomic data (occipital lobe), and only a subset of cell types were measured indirectly through these methods. Finally, we have used the term "accelerated epigenetic aging" for the sake of consistency with previous articles (S. Horvath, Garagnani, et al., 2015; S. Horvath & Levine, 2015; S. Horvath, Mah, et al., 2015); however, the term "premature aging" may be more appropriate since our limited sample size did not allow us to assess whether there is an actual increase in the rate of change
With an accurate method for determining biological age and accelerated aging in brain tissue, it is now possible to examine biological correlates and behavioral consequences of accelerated aging in the context of HIV. We previously found that HIV infection is associated with accelerated biological aging in the brains of infected individuals (S. Horvath & Levine, 2015), and here we show that accelerated aging is also associated with HAND. Additional studies will aid in elucidating the direction of this association since our data do not lend themselves for addressing whether accelerated epigenetic aging causes HAND, or vice versa. We believe that applying this methodology to histopathological studies, where DNA methylation is assayed in adjacent regions to tissue undergoing histopathological characterization and/or HIV viral load assay, will further aid in the explication age-related neuropathogenesis due to HIV. Further, applying the Epigenetic Clock to the study of HANA pathogenesis in general may enable an accurate measure of age acceleration that can then be examined in association with relevant biological and clinical factors. One exciting possibility is that changes in DNAm may play a role in the onset and progression of neurodegenerative diseases (Migliore & Coppede, 2009). As such, targeting this epigenetic process with effective compounds opens a new area of preventative and therapeutic approaches for neuroHIV.
ACKNOWLEDGEMENTS
Research results presented in this review were made possible by the following NIH grants: R01MH096648; National Neurological AIDS Bank (U24MH100929, 1U01MH083500, and R24-NS38841); California NeuroAIDS Tissue Network (U24-MH100928 and R24-MH59745); and the University of California - Los Angeles Center for AIDS Research/Clinical and Translational Science Institute grant (UL1TR000124).Our deepest gratitude to the volunteers enrolled in the National Neurological AIDS Bank and California NeuroAIDS Tissue Network. Our thanks to Ben Gouaux, Seth Sherman, and Lucas Barwick for their assistance in compiling the data.
Footnotes
CONFLICT OF INTEREST STATEMENT
No conflicts of interest to report
Contributor Information
Andrew J. Levine, Department of Neurology, David Geffen School of Medicine at the University of California, Los Angeles, ajlevine@mednet.ucla.edu.
Austin Quach, Department of Human Genetics, David Geffen School of Medicine at the University of California, Los Angeles.
David J. Moore, Department of Psychiatry, University of California, San Diego School of Medicine
Cristian L. Achim, Department of Psychiatry, University of California, San Diego School of Medicine
Virawudh Soontornniyomkij, Department of Psychiatry, University of California, San Diego School of Medicine
Eliezer Masliah, Departments of Neuroscience and Pathology, University of California, San Diego School of Medicine
Elyse J. Singer, Department of Neurology, David Geffen School of Medicine at the University of California, Los Angeles
Benjamin Gelman, Department of Pathology, University of Texas Medical Branch
Natasha Nemanim, Department of Neurology, David Geffen School of Medicine at the University of California, Los Angeles.
Steve Horvath, Departments of Human Genetics and Biostatistics, David Geffen School of Medicine at the University of California, Los Angeles
REFERENCES
- Achim CL, Adame A, Dumaop W, Everall IP, Masliah E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4(2):190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alisch RS, Barwick BG, Chopra P, Myrick LK, Satten GA, Conneely KN, Warren ST. Age-associated DNA methylation in pediatric populations. Genome Res. 2012;22(4):623–632. doi: 10.1101/gr.125187.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JT, Tsai P-C, Yang T-P, Pidsley R, Nisbet J, Glass D The Mu, T.C. Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population. PLoS Genet. 2012;8(4):e1002629. doi: 10.1371/journal.pgen.1002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocklandt S, Lin W, Sehl ME, Sanchez FJ, Sinsheimer JS, Horvath S, Vilain E. Epigenetic predictor of age. PLoS ONE. 2011;6(6):e14821. doi: 10.1371/journal.pone.0014821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boks MP, Derks EM, Weisenberger DJ, Strengman E, Janson E, Sommer IE, Ophoff RA. The Relationship of DNA Methylation with Age, Gender and Genotype in Twins and Healthy Controls. PLoS One. 2009;4(8):e6767. doi: 10.1371/journal.pone.0006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollati V, Schwartz J, Wright R, Litonjua A, Tarantini L, Suh H, Baccarelli A. Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mechanisms of Ageing and Development. 2009;130(4):234–239. doi: 10.1016/j.mad.2008.12.003. doi: http://dx.doi.org/10.1016/j.mad.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65(9):1490–1492. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- Cassol E, Misra V, Dutta A, Morgello S, Gabuzda D. Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS. 2014 doi: 10.1097/QAD.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Holt JL, Yakupov R, Jiang CS, Ernst T. Lower cognitive reserve in the aging human immunodeficiency virus-infected brain. Neurobiol Aging. 2013;34(4):1240–1253. doi: 10.1016/j.neurobiolaging.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Ellis RJ, Lazzaretto D, Young C, Mindt MR, Atkinson JH, Heaton RK. Effects of HIV-1 infection and aging on neurobehavioral functioning: preliminary findings. AIDS. 2004;18(Suppl 1):S27–S34. [PubMed] [Google Scholar]
- Christensen B, Houseman E, Marsit C, Zheng S, Wrensch M, Wiemels J, Kelsey K. Aging and Environmental Exposures Alter Tissue-Specific DNA Methylation Dependent upon CpG Island Context. PLoS Genet. 2009;5(8):e1000602. doi: 10.1371/journal.pgen.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder. The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders. Neurology. 1996;47(5):1247–1253. doi: 10.1212/wnl.47.5.1247. [DOI] [PubMed] [Google Scholar]
- Day K, Waite L, Thalacker-Mercer A, West A, Bamman M, Brooks J, Absher D. Differential DNA methylation with age displays both common and dynamic features across human tissues that are influenced by CpG landscape. Genome Biology. 2013;14(9):R102. doi: 10.1186/gb-2013-14-9-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG. HIV Infection, Inflammation, Immunosenescence, and Aging. Annual Review of Medicine. 2011;62(1):141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Dumaop W, Smith D, Adame A, Everall I, Letendre S, Masliah E. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology. 2013;80(15):1415–1423. doi: 10.1212/WNL.0b013e31828c2e9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, Margolick JB. A Frailty-Related Phenotype Before HAART Initiation as an Independent Risk Factor for AIDS or Death After HAART Among HIV-Infected Men. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66A(9):1030–1038. doi: 10.1093/gerona/glr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning M, Barbosa-Morais N, Lynch A, Tavare S, Ritchie M. Statistical issues in the analysis of Illumina data. BMC Bioinformatics. 2008;9(1):85. doi: 10.1186/1471-2105-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto A, Bongiovanni M, Cicconi P, Menicagli L, Ligabò EV, Melzi S, Monforte AdA. Potential predictive factors of osteoporosis in HIV-positive subjects. Bone. 2006;38(6):893–897. doi: 10.1016/j.bone.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol. 2011;24(3):275–283. doi: 10.1097/WCO.0b013e32834695fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Chen T, Johnson KM, Jennings K, Freeman DH, Jr, Soukup VM. Prefrontal dopaminergic and enkephalinergic synaptic accommodation in HIV-associated neurocognitive disorders and encephalitis. J Neuroimmune Pharmacol. 2012;7(3):686–700. doi: 10.1007/s11481-012-9345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Morgello S, Masliah E, Commins D, Achim CL, Soukup VM. Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J Acquir Immune Defic Syndr. 2013;62(5):487–495. doi: 10.1097/QAI.0b013e31827f1bdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht CJ, Thornton AE, Hall-Patch C, Maan EJ, Cote HC, Money DM, Pick N. Select neurocognitive impairment in HIV-infected women: associations with HIV viral load, hepatitis C virus, and depression, but not leukocyte telomere length. PLoS One. 2014;9(3):e89556. doi: 10.1371/journal.pone.0089556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19(4):407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- Greene M J. A. C. L. H. W. V. V. MAnagement of human immunodeficiency virus infection in advanced age. Jama. 2013;309(13):1397–1405. doi: 10.1001/jama.2013.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon S, Carr A. Cardiovascular Risk and Body-Fat Abnormalities in HIV-Infected Adults. New England Journal of Medicine. 2005;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Zhang K. Genome-wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Molecular Cell. 2013;49(2):359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez DG, Nalls MA, Gibbs JR, Arepalli S, van der Brug M, Chong S, Singleton AB. Distinct DNA methylation changes highly correlated with chronological age in the human brain. Human Molecular Genetics. 2011;20(6):1164–1172. doi: 10.1093/hmg/ddq561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JL, Kraft-Terry SD, Chang L. Neuroimaging studies of the aging HIV-1-infected brain. J Neurovirol. 2012;18(4):291–302. doi: 10.1007/s13365-012-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013a;14(R115) doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. Webpage: http://labs.genetics.ucla.edu/horvath/dnamage. 2013b
- Horvath S, Erhart W, Brosch M, Ammerpohl O, von Schonfels W, Ahrens M, Hampe J. Obesity accelerates epigenetic aging of human liver. Proc Natl Acad Sci U S A. 2014;111(43):15538–15543. doi: 10.1073/pnas.1412759111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Garagnani P, Bacalini MG, Pirazzini C, Salvioli S, Gentilini D, Franceschi C. Accelerated epigenetic aging in Down syndrome. Aging Cell. 2015;14(3):491–495. doi: 10.1111/acel.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Levine AJ. HIV-1 Infection Accelerates Age According to the Epigenetic Clock. J Infect Dis. 2015 doi: 10.1093/infdis/jiv277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Mah V, Lu AT, Woo JS, Choi OW, Jasinska AJ, Coles LS. The cerebellum ages slowly according to the epigenetic clock. Aging (Albany NY) 2015;7(5):294–306. doi: 10.18632/aging.100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Zhang Y, Langfelder P, Kahn R, Boks M, van Eijk K, Ophoff RA. Aging effects on DNA methylation modules in human brain and blood tissue. Genome Biology. 2012;13:R97. doi: 10.1186/gb-2012-13-10-r97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe A, Irizarry R. Accounting for cellular heterogeneity is critical in epigenome-wide association studies. Genome Biology. 2014;15(2):R31. doi: 10.1186/gb-2014-15-2-r31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson A, Enroth S, Gyllensten U. Continuous Aging of the Human DNA Methylome Throughout the Human Lifespan. PLoS One. 2013;8(6):e67378. doi: 10.1371/journal.pone.0067378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Pfeifer GP. Aging and DNA methylation. BMC Biol. 2015;13:7. doi: 10.1186/s12915-015-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallianpur AR, Levine AJ. Host Genetic Factors Predisposing to HIV-Associated Neurocognitive Disorder. Curr HIV/AIDS Rep. 2014 doi: 10.1007/s11904-014-0222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nature Reviews Drug Discovery. 2008;7(10):854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- Kirk GD, Mehta SH, Astemborski J, Galai N, Washington J, Higgins Y, Thomas DL. HIV, Age, and the Severity of Hepatitis C Virus–Related Liver DiseaseA Cohort Study. Annals of Internal Medicine, N/A. 2013 doi: 10.7326/0003-4819-158-9-201305070-00604. (N/A), N/A-N/A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft-Terry SD, Buch SJ, Fox HS, Gendelman HE. A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron. 2009;64(1):133–145. doi: 10.1016/j.neuron.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Top Antivir Med. 2011;19(4):137–142. [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Horvath S, Miller EN, Singer EJ, Shapshak P, Baldwin GC, Langfelder P. Transcriptome analysis of HIV-infected peripheral blood monocytes: gene transcripts and networks associated with neurocognitive functioning. J Neuroimmunol. 2013;265(1–2):96–105. doi: 10.1016/j.jneuroim.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Miller JA, Shapshak P, Gelman B, Singer EJ, Hinkin CH, Horvath S. Systems analysis of human brain gene expression: mechanisms for HIV-associated neurocognitive impairment and common pathways with Alzheimer's disease. BMC Med Genomics. 2013;6(1):4. doi: 10.1186/1755-8794-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Panos SE, Horvath S. Genetic, transcriptomic, and epigenetic studies of HIV-associated neurocognitive disorder. J Acquir Immune Defic Syndr. 2014;65(4):481–503. doi: 10.1097/QAI.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, Mehta SH, Atta MG, Kirk GD, Galai N, Vlahov D, Moore RD. End-stage renal disease and chronic kidney disease in a cohort of African-American HIV-infected and at-risk HIV-seronegative participants followed between 1988 and 2004. AIDS. 2007;21(18):2435–2443. doi: 10.1097/QAD.0b013e32827038ad. [DOI] [PubMed] [Google Scholar]
- Malan-Muller S, Hemmings SM, Spies G, Kidd M, Fennema-Notestine C, Seedat S. Shorter telomere length - A potential susceptibility factor for HIV-associated neurocognitive impairments in South African women [corrected] PLoS One. 2013;8(3):e58351. doi: 10.1371/journal.pone.0058351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, Deary IJ. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25. doi: 10.1186/s13059-015-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Shah S, McRae AF, Ritchie SJ, Muniz-Terrera G, Harris SE, Deary IJ. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int J Epidemiol. 2015 doi: 10.1093/ije/dyu277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Volberding P. HIV and Premature Aging: A Field Still in Its Infancy. Annals of Internal Medicine. 2010;153(7):477–479. doi: 10.7326/0003-4819-153-7-201010050-00013. [DOI] [PubMed] [Google Scholar]
- Migliore L, Coppede F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat Res. 2009;667(1–2):82–97. doi: 10.1016/j.mrfmmm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Morgello S, Gelman B, Kozlowski P, Vinters H, Masliah E, Cornford M, Singer E. The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol. 2001;27(4):326–335. doi: 10.1046/j.0305-1846.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Mukerjee R, Chang JR, Del Valle L, Bagashev A, Gayed MM, Lyde RB, Sawaya BE. Deregulation of microRNAs by HIV-1 Vpr protein leads to the development of neurocognitive disorders. J Biol Chem. 2011;286(40):34976–34985. doi: 10.1074/jbc.M111.241547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasipura SD, Kim S, Al-Harthi L. Epigenetic regulation of HIV-1 latency in astrocytes. J Virol. 2014;88(5):3031–3038. doi: 10.1128/JVI.03333-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata S, Ye T, Hyde, Thomas M, Guitart-Navarro X, Tao R, Wininger M, Lipska, Barbara K. DNA Methylation Signatures in Development and Aging of the Human Prefrontal Cortex. The American Journal of Human Genetics. 2012;90(2):260–272. doi: 10.1016/j.ajhg.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda D, Lopez-Costa JJ, Sede M, Lopez EM, Berria MI, Quarleri J. Increased in vitro glial fibrillary acidic protein expression, telomerase activity, and telomere length after productive human immunodeficiency virus-1 infection in murine astrocytes. J Neurosci Res. 2014;92(2):267–274. doi: 10.1002/jnr.23294. [DOI] [PubMed] [Google Scholar]
- Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, Coffin JM. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41(10):4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala G, Fox HS. Proteomic and metabolomic strategies to investigate HIV-associated neurocognitive disorders. Genome Med. 2(3):22. doi: 10.1186/gm143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Santiago J PN, Letendre S, Ellis R, Gouaux B, Moore D, LeBlanc S, Rajagopal N, Zhang K, Woelk C. DNA methylation correlates with neurological decline in HIV-infected individuals; Paper presented at the 19th Conference on Retroviruses and Opportunistic Infections; Seattle, Washington. 2012. [Google Scholar]
- Pulliam L, Rempel H, Sun B, Abadjian L, Calosing C, Meyerhoff DJ. A peripheral monocyte interferon phenotype in HIV infection correlates with a decrease in magnetic resonance spectroscopy metabolite concentrations. AIDS. 2011;25(14):1721–1726. doi: 10.1097/QAD.0b013e328349f022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakyan VK, Down TA, Maslau S, Andrew T, Yang TP, Beyan H, Spector TD. Human aging-associated DNA hypermethylation occurs preferentially at bivalent chromatin domains. Genome Res. 2010;20(4):434–439. doi: 10.1101/gr.103101.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiyed ZM, Gandhi N, Agudelo M, Napuri J, Samikkannu T, Reddy PV, Nair MP. HIV-1 Tat upregulates expression of histone deacetylase-2 (HDAC2) in human neurons: implication for HIV-associated neurocognitive disorder (HAND) Neurochemistry International. 2011;58(6):656–664. doi: 10.1016/j.neuint.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg M, Chao C, Leyden W, Xu L, Tang B, Horberg M, Abrams D. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23(17):2337–2345. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg MJ, Chao C, Leyden WA, Xu L, Horberg MA, Klein D, Abrams DI. HIV Infection, Immunodeficiency, Viral Replication, and the Risk of Cancer. Cancer Epidemiology Biomarkers & Prevention. 2011;20(12):2551–2559. doi: 10.1158/1055-9965.EPI-11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B, Tatro ET, Umlauf A, Achim CL. Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS. 2012a;26(18):2327–2335. doi: 10.1097/QAD.0b013e32835a117c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B, Tatro ET, Umlauf A, Achim CL. Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS. 2012b doi: 10.1097/QAD.0b013e32835a117c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Risbrough VB, Young JW, Wallace CK, Soontornniyomkij B, Jeste DV, Achim CL. Short-term recognition memory impairment is associated with decreased expression of FK506 binding protein 51 in the aged mouse brain. Age (Dordr) 2010;32(3):309–322. doi: 10.1007/s11357-010-9145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Soontornniyomkij B, Moore DJ, Gouaux B, Masliah E, Tung S, Achim CL. Antioxidant sestrin-2 redistribution to neuronal soma in human immunodeficiency virus-associated neurocognitive disorders. J Neuroimmune Pharmacol. 2012;7(3):579–590. doi: 10.1007/s11481-012-9357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JM, Spencer VA, Chen HY, Li L, Davie JR. Measurement of histone acetyltransferase and histone deacetylase activities and kinetics of histone acetylation. Methods. 2003;31(1):12–23. doi: 10.1016/s1046-2023(03)00083-5. [DOI] [PubMed] [Google Scholar]
- Tatro ET, Scott ER, Nguyen TB, Salaria S, Banerjee S, Moore DJ, Everall IP. Evidence for Alteration of Gene Regulatory Networks through MicroRNAs of the HIV-infected brain: novel analysis of retrospective cases. PLoS One. 2010;5(4):e10337. doi: 10.1371/journal.pone.0010337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D, Beck S. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics. 2013;29(2):189–196. doi: 10.1093/bioinformatics/bts680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschendorff AE, Menon U, Gentry-Maharaj A, Ramus SJ, Weisenberger DJ, Shen H, Widschwendter M. Age-dependent DNA methylation of genes that are suppressed in stem cells is a hallmark of cancer. Genome Res. 2010;20(4):440–446. doi: 10.1101/gr.103606.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Doherty SM. HIV infection--a risk factor for osteoporosis. Journal of acquired immune deficiency syndromes (1999) 2003;33(3):281–291. doi: 10.1097/00126334-200307010-00001. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Mucke L. Transgenic models in the study of AIDS dementia complex. Curr Top Microbiol Immunol. 1996;206:223–241. doi: 10.1007/978-3-642-85208-4_12. [DOI] [PubMed] [Google Scholar]
- Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased Acute Myocardial Infarction Rates and Cardiovascular Risk Factors among Patients with Human Immunodeficiency Virus Disease. The Journal of Clinical Endocrinology & Metabolism. 2007;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivithanaporn P, Heo G, Gamble J, Krentz H, Hoke A, Gill M, Leistung C. Neurologic disease burden in treated HIV/AIDS predicts survival. Neurology. 2010;75(13):1150–1158. doi: 10.1212/WNL.0b013e3181f4d5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken L, Valcour V. Impact of HIV and aging on neuropsychological function. Journal of neurovirology. 2012;18(4):256–263. doi: 10.1007/s13365-012-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler JM, Chaudhuri AD, Fox HS. Translating the brain transcriptome in neuroAIDS: from non-human primates to humans. J Neuroimmune Pharmacol. 2012;7(2):372–379. doi: 10.1007/s11481-012-9344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack JA, Goulet JL, Gibert C, Brandt C, Chang CC, Gulanski B for the Veterans Aging Cohort Study Project, T. Increased Risk of Fragility Fractures among HIV Infected Compared to Uninfected Male Veterans. PLoS One. 2011;6(2):e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T, Jiao Y, de Jong S, Ophoff RA, Beck S, Teschendorff AE. An integrative multi-scale analysis of the dynamic DNA methylation landscape in aging. PLoS Genet. 2015;11(2):e1004996. doi: 10.1371/journal.pgen.1004996. [DOI] [PMC free article] [PubMed] [Google Scholar]