Abstract
Objective
Examine whether the acute kidney injury (AKI) commonly observed among ultramarathon participants places the individual at risk for subsequent AKI of worse magnitude.
Design
Observational.
Setting
Western States Endurance Run.
Participants
Race finishers with post-race blood studies.
Independent Variable
AKI following one race.
Main Outcome Measures
Extent of AKI in subsequent race.
Results
Among 627 finishes in which serum creatinine values were known, 36.2% met “risk” or “injury” criterion with this group characterized by having faster finish times, greater body weight loss during the race and higher post-race serum creatine kinase and urea nitrogen concentrations when compared with those not meeting the criteria. We identified 38 runners who had undergone post-race blood analyses at multiple races among which 16 (42.1%) met the “risk” or “injury” criterion at the first race. Of those 16 runners, 12 (75%) met the criteria at a subsequent race, an incidence that was higher (p=0.0026) than the overall 36.2% incidence. For most (56.2%) of the 16 runners meeting the criteria at the first race, the subsequent race caused less increase in serum creatinine concentration and decrement in estimated glomerular filtration rate than the first race.
Conclusions
Mild AKI is common in 161-km ultramarathons, but there was no evidence that prior AKI caused greater renal dysfunction from a subsequent exercise stimulus of similar magnitude. This offers some reassurance to runners and their physicians that mild to moderate AKI in the setting of an ultramarathon is not cumulative or without complete recovery of kidney function when stressed.
Keywords: creatine kinase, creatinine, dehydration, endurance exercise, glomerular filtration rate, rhabdomyolysis
Introduction
Some degree of acute kidney injury (AKI) is relatively common in ultramarathon races, with documented incidence being 30–80%.1,2 In the vast majority of cases, such incidents go unnoticed, although hospitalization for AKI after an ultramarathon, albeit rare, does occur.3,4 Due to the nature of these athletic events, the most common etiology of AKI is rhabdomyolysis caused by muscle damage arising from the extraordinary levels of continuous exertion. In fact, in 161-km ultramarathons it is not uncommon to see serum creatine kinase (CK) concentrations well over 20,000 U/L,5–7 which are at the level in which general guidelines not specific to ultramarathon runners would recommend treatment.8,9 The muscle breakdown products from rhabdomyolysis cause renal arteriolar vasoconstriction (20).10 Increased sympathetic tone, intravascular volume depletion and the vasoconstrictive effect of nonsteroidal anti-inflammatory drugs may also contribute to reduced renal perfusion and further increase the risk of AKI during an ultramarathon.
Given the high frequency of mild AKI during an ultramarathon, we asked whether such insults place a runner at risk for a subsequent similar event of the same, or perhaps higher, magnitude. Prior studies have suggested full recovery of resting renal chemistries overnight following a 56-km ultramarathon (Irving et al. MSSE 1990),11 and after 40–80-km stages of a multi-stage ultramarathon with no apparent cumulative effect observed.2 However, other studies have shown abnormalities of resting renal chemistries persisting the day following 100-km12 and 56-km13 ultramarathons, and for several days after a 90-km ultramarathon.14 It is possible that greater kidney insult could result from longer ultramarathons such as the race studied here.
Furthermore, it is possible that resting renal chemistries may not fully reflect renal function under stressed conditions and in the presence of decreased intravascular volume.
In this study, we utilized data from multiple years of post-race blood analyses performed at the 161-km Western States Endurance Run (WSER) in order to track post-race serum creatinine concentrations in runners who met the criteria for AKI at the completion of one race. Our premise was that those with AKI at one race would have greater impairment in renal function after a subsequent race if such injuries are cumulative and without complete recovery of normal renal function during stress. Additionally, we examine the overall incidence of AKI using this large data set to expand upon our prior work.1
Methods
The study utilized data collected at the 2011, 2012 and 2014 WSER. Post-race blood analyses were not performed at the 2013 event resulting in exclusion of this year from the study. The WSER is a 161-km point-to-point foot race which takes place during the last weekend in June. The course is almost entirely on single track mountain trails with 5500 m of cumulative climb and 7000 m of cumulative descent, and a 30 hour time limit for completion. Additional details of the race are provided elsewhere.15–17 Ambient temperature data from weather stations near the course were used to determine the maximum temperature during each race. Institutional review board approval was obtained from the VA Northern California Health Care System with a waiver of consent.
All runners underwent pre-race body weight measurement during registration in the morning on the day before race start. Post-race body weight was measured immediately upon completion of the race. All measurements were made with calibrated battery-operated digital scales (Health o meter, model 349KLX, Boca Raton, FL) placed on solid level surfaces. Each measurement was made with the runner clothed in running wear and shoes, and care was taken to assure that other items, such as jackets, waist packs and hydration vests, were removed and nothing was in the runner’s hands.
Within a few minutes after finishing the race, those runners who were willing to provide a blood sample had blood drawn while seated into heparinized tubes via an antecubital vein. A clinical laboratory performed analyses for serum creatinine, CK and urea nitrogen.
On-line pre-race and post-race questionnaires were used to gather information about running background and recent training of race participants in 2011 and 2014, respectively. With regard to information about years of regular running and years of running ultramarathons, information provided one year was used to generate missing data for other years when possible.
The present study used the RIFLE (Risk, Injury, Failure, Loss and Endstage kidney disease) criteria for AKI.18 Measured post-race serum creatinine at least 1.5 and 2.0 times baseline creatinine defined the “risk” and “injury” criteria, respectively. Since blood was not taken prior to the run, we estimated pre-race serum creatinine concentration. An expected glomerular filtration rate (GFR) of 100 ml/min was used when age was ≤40 years, and 140 minus age was used for those >40 years of age.19 The pre-race expected GFR was then used in the ‘modification of diet in renal disease’ equation20 to back-calculate expected pre-race serum creatinine concentration. Rearrangement of the same equation allowed calculation of post-race estimated GFR. Then, from these calculated GFR values, the pre-race to post-race difference in GFR (ΔGFR) was determined.
Analyses were performed on the full group of runners with post-race blood results as well as the subset with post-race blood results from multiple races. Continuous data underwent normality testing with the D’Agostino-Pearson test. Characteristics of those meeting the criterion for “injury”, those meeting the criterion for “risk” and those not meeting these criteria were compared with the Kruskal-Wallis test followed by Dunn’s Multiple Comparison test when an overall significant effect was identified since all of these variables had skewed data. Comparison of paired data were made with the paired t-test or Wilcoxon matched-pairs signed rank test depending on whether or not the data passed normality testing. Two-way (group x race number) were used to compare the subgroup of runners with data for two races who met the AKI “risk” or “injury” criterion both races with those who only met the criteria the first race. Categorical data were analyzed with the Chi-square or Fisher’s exact test. Correlations were performed with Pearson or Spearman correlation analyses depending on whether or not the data passed normality testing. Statistical significance was set at p<0.05.
Ethical Considerations
This work was based upon data collected during research approved by our institutional review board. No consenting specifically for the present analyses was required.
Results
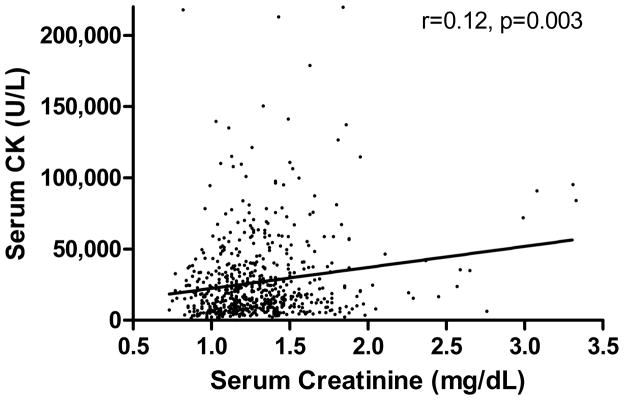
Among all three races, post-race blood was obtained for 627 (accounted for by 585 different runners) of the 922 race finishes. The “risk” criterion was met by 227 (36.2%) among which 31 (4.9%) also met the “injury” criterion. Selected characteristics of those meeting the “injury” criterion, those meeting the “risk” criterion, and those not meeting either criterion are shown in Table 1. Those meeting the “injury” criterion lost more weight during the race and were faster than the others, and those meeting the “risk” criterion lost more weight than those not meeting either criterion. As expected, post-race serum creatinine concentrations varied among groups, as did post-race serum and urea nitrogen. But age, sex, post-race serum CK concentration, running experience and recent training did not vary among groups. Post-race serum CK and creatinine concentrations were significantly correlated (Figure 1).
Table 1.
Comparison of selected characteristics of race finishers meeting the AKI “injury” criterion (n=31), those meeting the “risk” but not the “injury” criterion (n=196), and those not meeting either criteria (n=400). Sample sizes for the data obtained by questionnaire were 21–24, 94–117 and 200–247 and for the three groups.
| Characteristic | Meeting “Injury” Criterion | Meeting “Risk” Criterion | Not Meeting Either Criteria |
|---|---|---|---|
| Age (years) | 42 (38 – 49) | 42 (36 – 48) | 41 (35 – 49) |
| Sex (% men) | 83.9 | 78.1 | 83.5 |
| Duration of regular running (years) | 10 (6 – 22) | 12 (7 – 22) | 13 (7 – 23) |
| Duration of running ultramarathons (years) | 6 (4 – 9) | 6 (4 – 10) | 5 (3 – 9) |
| 161-km ultramarathons completed (number) | 3 (1 – 4) | 3 (2 – 6) | 2 (1 – 6) |
| 161-km ultramarathons not finished (number) | 1 (0 – 1) | 0 (0 – 1) | 0 (0 – 1) |
| Average training week running distance (km) | 97 (72 – 129) | 97 (72 – 113) | 97 (80 – 113) |
| Highest training week running distance (km) | 129 (80 – 172) | 100 (80 – 139) | 100 (80 – 141) |
| Longest single run distance (km) | 100 (84 – 150) | 121 (86 – 159) | 106 (80 – 145) |
| Finish placing (number) | 107 (38 – 227)* | 158 (68 – 244) | 183 (101 – 244) |
| Finish time (hours) | 23.53 (19.80 – 27.96)* | 25.64 (21.86 – 28.64) | 26.84 (23.13 – 28.60) |
| Body weight change (%) | −2.8 (−3.8 – −0.3)*** †† | −0.9 (−2.6 – 0.6)** | −0.4 (−1.6 – 1.0) |
| Serum creatinine (mg/dL) | 1.98 (1.85 – 2.57)*** ††† | 1.46 (1.39 – 1.61)*** | 1.15 (1.03 – 1.26) |
| Serum urea nitrogen (mg/dL) | 42 (34 – 53)*** ††† | 28 (23 – 36)*** | 22 (18 – 26) |
| Serum creatinine kinase (U/L) | 23,800 (10,163 – 56,700) | 15,850 (8,300 – 35,500) | 18,100 (9,100 – 32,443) |
Data are reported as median and interquartile range or percentage. The average and highest training week distances and longest single run distance were for the 3 months prior to the race.
p<0.05,
p<0.01,
p<0.001 compared with the group not meeting “risk” or “injury” criterion
p<0.01,
p<0.001 compared with group meeting “risk” criterion
Figure 1.
Relationship between serum CK and creatinine concentrations for 667 161-km ultramarathon finishes.
There were 38 finishers who had undergone post-race blood analyses after at least two races. For four runners having three races with post-race blood studies, the first race and the other race with the most comparable finish time were selected for comparison. Among these 38 runners, 16 (42.1%) met the “risk” criterion of which 2 (5.3%) also met the “injury” criterion in the first race, and 18 (47.4%) met the “risk” criterion of which 3 (7.9%) also met the “injury” criterion in the second race. These incidences for this subset of 38 runners meeting the “risk” or “injury” criteria were similar (p=0.49 and 0.17) to the 36.2% meeting the criteria when considering the full group of 627 finishes.
Considering the 16 runners meeting the “risk” or “injury” criterion in the first race, mean (±SD) age at the time of the first race was 41 ± 10 years, 75% were men, and the mean interval between the two races was 1.5 years. Of these 16 runners who met the “risk” or “injury” criterion in the first race, 12 (75%) met the “risk” criterion (including 3 who met the “injury” criterion) in the second race. This 75% incidence of meeting the “risk” or “injury” criterion was higher than the 36.2% meeting the criteria when considering the full group of 627 finishes (p=0.0026) and the 42.1% meeting the criteria in the first race among the subset of 38 runners with post-race blood analyses after at least two races (p=0.038).
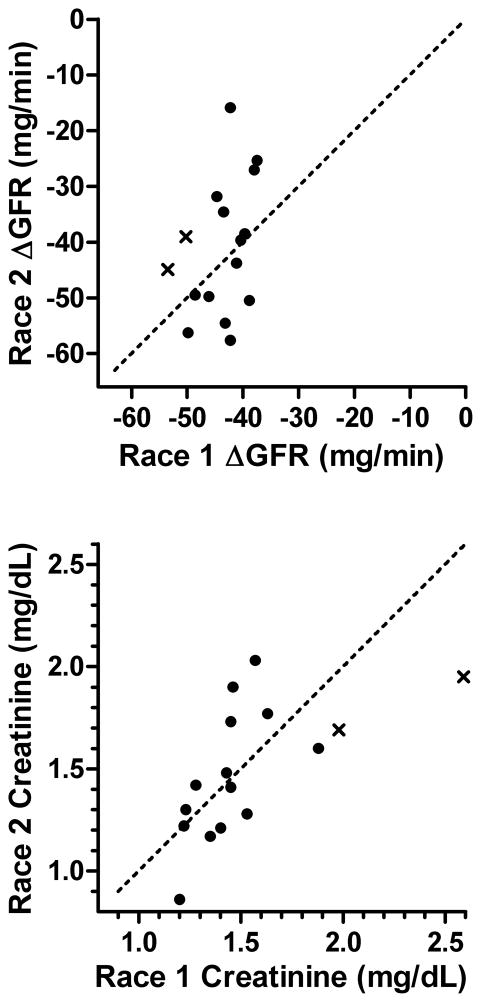
Individual data from the two races for ΔGFR and post-race serum creatinine for the 16 runners meeting the “risk” or “injury” criterion in the first race are compared in Figure 2. There were no statistical differences between races for either variable (p>0.3). Furthermore, it is evident from these plots that most (56.2%) of the individuals showed a lower ΔGFR at the second race compared with the first, and that both runners meeting the “injury” criterion in the first race showed a lower ΔGFR and serum creatinine concentration at the second race. Correlation analyses revealed no association between races for ΔGFR (p=0.16) but a strong direct association for post-race serum creatinine concentration (r=0.72, p=0.003).
Figure 2.
Relationship between the change in GFR (ΔGFR = post-race estimated GFR - pre-race expected GFR) and post-race serum creatinine concentration between two races for 16 runners meeting the “risk” (●) and “injury” (x) criteria in the first race. The line of identity is indicated by the dashed line. Note that a less adverse effect on renal function for race 2 compared with race 1 is denoted by individual data points above the line of identity for ΔGFR and below the line of identity for post-race creatinine.
Among the 16 race finishers meeting the AKI “risk” or “injury” criterion in the first race, the group who met either criterion in the second race were compared with those not meeting either criterion in the second race (Table 2). There were no statistical group, race number or interaction effects for maximum race temperature, finish placing or time, percentage body weight change, and post-race serum CK concentration. As expected, there were significant race number effects for age and years of running experience, but no group or interaction effects. For serum urea nitrogen, the interaction effect was nearly significant (p=0.06) but group and race number effects were not evident.
Table 2.
Selected characteristics of race finishers meeting the AKI “risk” or “injury” criterion in the first race who met either criterion in the second race (n=12) compared with those not meeting either criterion in the second race (n=4). Sample sizes for the data obtained by questionnaire were 11 and 4 for the two groups. There were no statistical group, race number or interaction effects, except the expected race number effects for age and years of running experience.
| Characteristic | Meeting AKI Criteria in Second Race | Not Meeting AKI Criteria in Second Race | ||
|---|---|---|---|---|
| Race 1 | Race 2 | Race 1 | Race 2 | |
| Age (years) | 42 (36 – 50) | 36 (28 – 41) | ||
| Sex (% men) | 75 | 75 | ||
| Duration of regular running (years) | 11 (8 – 26) | 13 (9 – 15) | ||
| Duration of running ultramarathons (years) | 7 (4 – 9) | 6 (4 – 11) | ||
| Maximum race temperature (°C) | 28.0 (28.0 – 28.0) | 22.0 (22.0 – 31.7) | 28.0 (28.0 – 28.0) | 22.0 (22.0 – 29.3) |
| Finish placing (number) | 44 (17 – 200) | 41 (18 – 236) | 36 (8 – 122) | 45 (20 – 196) |
| Finish time (hours) | 20.36 (17.56 – 27.25) | 19.81 (17.74 – 27.73) | 19.62 (16.38 – 24.49) | 19.84 (17.91 – 26.85) |
| Body weight change (%) | −1.4 (−2.8 – −0.4) | −2.0 (−3.5 – −0.2) | 0.1 (−1.4 – 2.1) | −0.4 (−2.7 – 2.2) |
| Serum urea nitrogen (mg/dL) | 27 (22 – 41) | 36 (28 – 44) | 29 (22 – 36) | 24 (20 – 28) |
| Serum creatinine kinase (U/L) | 8,550 (5,575 – 24,850) | 15,000 (5,425 – 23,905) | 14,350 (7,925 – 29,175) | 19,124 (11,087 – 35,300) |
Data are reported as percentage or as median and interquartile range to allow comparison with data for the larger sample even though many data sets were normally distributed.
Discussion
The unusual degree of stress of an ultramarathon, even on the body of a healthy individual, puts the participant at risk for harm to several organ systems. The renal system seems particularly vulnerable for injury in these events given the extent of muscle damage,1,3,21 the prevalent use of nonsteroidal anti-inflammatory drugs,5,22,23 and the ravages of intravascular volume shifts resulting from sweating and frequent occurrences of gastrointestinal fluid losses and inadequate fluid replacement. Since elevated serum creatinine, and hence attenuated GFR, occurs at least transiently in a relatively high percentage of ultramarathon runners, it is valuable to know whether such an event puts these individuals at risk for a similar or worse event during subsequent exercise. This has practical importance in that a runner may wish to limit his or her ultra-endurance sports participation if it is known that each insult causes successive and cumulative renal damage.
The primary purpose of the current study was to determine whether runners who showed some renal dysfunction at the conclusion of an ultramarathon were more likely to have similar or worse dysfunction in a subsequent ultramarathon of similar stress. While several studies have shown resting renal chemistries normalize after an ultramarathon,2,11,14 it is possible that resting renal chemistries may not fully reflect kidney function under stress. We are unaware of any prior work that has examined renal function after an ultramarathon in athletes who had AKI after a prior ultramarathon of similar stress. We now show, in a relatively small group of runners taking part in a 161-km ultramarathon, evidence that those with renal dysfunction (as assessed by serum creatinine) after one ultramarathon are more likely than other runners to have dysfunction of a similar magnitude after a subsequent ultramarathon of comparable stress, but that there is no evidence of dysfunction of higher magnitude occurring in the subsequent race. This finding should be reassuring to athletes and clinicians caring for endurance athletes. The findings also provides further rationale for avoiding overzealous rehydration due to concern for the renal system, which can place athletes with mild hyponatremia at risk for becoming acutely symptomatic.24–26
There are several likely reasons for our findings, the most obvious being that the post-race elevation in serum creatinine in the runners meeting the “risk” or “injury” criterion was due to transient changes in renal perfusion from intravascular volume depletion, and vasoconstrictive effects of increased sympathetic tone and nonsteroidal anti-inflammatory drugs (if used). It is also possible that some of the runners can be undergoing acute renal arteriolar vasoconstriction as a result of muscle breakdown products from rhabdomyolysis.10 This seems to be supported by a direct relationship between post-race serum CK and creatinine concentrations as identified in our present study and past work.1 Since there is no intrinsic renal damage in this scenario, the creatinine change is reversible and would not be expected to reflect a permanent change in kidney function.
The present work also reports on a large data set (n=667) of 161-km ultramarathon finishes showing the AKI “risk” criterion was met in 36.2% of the finishes, among which 4.9% also met the “injury” criterion. This was comparable to our prior analysis reporting that 34% met either AKI criterion among a subset of 207 of these finishes.1 A comparison of selected characteristics of those meeting the AKI criteria showed that these individuals finished faster, had greater body weight loss during the race and had higher post-race serum CK and urea nitrogen concentrations when compared with those not meeting the AKI criteria. However, among the subset of runners with data for multiple races, there were no statistical differences in finish time or place, weight change and post-race serum CK and urea nitrogen concentrations between those meeting the AKI criteria both races and those only meeting the criteria the first race, probably owing to the small sample size.
We acknowledge some limitations to this study, the most significant being that pre-race serum creatinine concentration was not measured, thus we were required to impute the values expected of normal individuals from the GFR formulae.20 Given the healthy state required of these runners, it is highly likely that the vast majority had a normal serum creatinine at the start of each race as supported by prior evidence for normal resting renal chemistries in ultramarathon runners.2,11–14 We recognize that since creatinine (but not GFR) is roughly proportional to muscle mass,27 highly muscular individuals could have confounded the data in that an underestimation of pre-race serum creatinine concentration could have resulted in an incorrect determination that a runner met the “risk” or “injury” criterion for AKI. In other words, if pre-race creatinine was actually higher than estimated at the first race, then some runners might have been incorrectly included in the group meeting the “risk” or “injury” criterion after that race.
This is not likely to have been a common issue since the typical ultramarathon runner in this event is not overly muscular.28,29 Furthermore, we interpret the lack of any apparent change in post-race serum creatinine concentrations between the first and second races, which appeared to be of similar stress given statistically similar maximum ambient temperatures and race finish times, as important support for our conclusion that runners had full recovery from AKI of the first race. Of course, another limitation is the small sample size for the multiple year comparison component of this study and our lack of knowledge about the use of nonsteroidal anti-inflammatory drugs by each specific runner. We also have no knowledge of the extent of prior AKI or intervening episodes of AKI that might have occurred between the first and second races examined in this study.
Conclusions
From this work, we conclude that it is common for 161-km ultramarathon runners to meet AKI criteria after a race. Those most likely to meet the criteria finish faster, have greater body weight loss during the race and have higher post-race serum CK and urea nitrogen concentrations when compared with those not meeting the criteria. Most importantly though, there was no evidence that those with prior AKI had greater renal dysfunction with a subsequent exercise stimulus of similar magnitude. This should offer some reassurance to runners and their physicians that mild to moderate AKI in the setting of an ultramarathon is not cumulative and is likely associated in the majority of cases with complete recovery not necessitating aggressive intervention. A prospective study with baseline pre-race parameters would be the optimal approach for confirmation of our findings.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Northern California Health Care System. The work was also supported by the Western States Endurance Run Foundation. We thank Sutter Auburn Faith Hospital and Sierra Nevada Memorial Hospital for laboratory services, and Lodi Health for phlebotomy services. The contents reported here do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Hoffman MD, Stuempfle KJ, Fogard K, et al. Urine dipstick analysis for identification of runners susceptible to acute kidney injury following an ultramarathon. J Sports Sci. 2013;31:20–31. doi: 10.1080/02640414.2012.720705. [DOI] [PubMed] [Google Scholar]
- 2.Lipman GS, Krabak BJ, Waite BL, et al. A prospective cohort study of acute kidney injury in multi-stage ultramarathon runners: the Biochemistry in Endurance Runner Study (BIERS) Res Sports Med. 2014;22:185–192. doi: 10.1080/15438627.2014.881824. [DOI] [PubMed] [Google Scholar]
- 3.Bruso JR, Hoffman MD, Rogers IR, et al. Rhabdomyolysis and hyponatremia: a cluster of five cases at the 161-km 2009 Western States Endurance Run. Wilderness Environ Med. 2010;21:303–308. doi: 10.1016/j.wem.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Seedat YK, Aboo N, Naicker S, et al. Acute renal failure in the “Comrades Marathon” runners. Ren Fail. 1989–1990;11(4):209–212. doi: 10.3109/08860228909054933. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman MD, Fogard K. Factors related to successful completion of a 161-km ultramarathon. Int J Sports Physiol Perform. 2011;6:25–37. doi: 10.1123/ijspp.6.1.25. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman MD, Ingwerson JL, Rogers IR, et al. Increasing creatine phosphokinase concentration at the 161-km Western States Endurance Run. Wilderness Environ Med. 2012;23:56–60. doi: 10.1016/j.wem.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Nieman DC, Henson DA, Davis JM, et al. Quercetin ingestion does not alter cytokine changes in athletes competing in the Western States Endurance Run. J Interferon Cytokine Res. 2007;27:1003–1011. doi: 10.1089/jir.2007.0050. [DOI] [PubMed] [Google Scholar]
- 8.Clarkson PM, Eichner ER. Exertional rhabdomyolysis: Does elevated blood creatine kinase foretell renal failure? Curr Sports Med Rep. 2006;5:57–60. doi: 10.1007/s11932-006-0030-3. [DOI] [PubMed] [Google Scholar]
- 9.Terpilowski J, Criddle L. Rhabdomyolysis following a gunshot wound and one trauma center’s protocol and guidelines. J Emerg Nurs. 2004;30:36–41. doi: 10.1016/j.jen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Holt SG, Moore KP. Pathogenesis and treatment of renal dysfunction in rhabdomyolysis. Intensive Care Med. 2001;27:803–811. doi: 10.1007/s001340100878. [DOI] [PubMed] [Google Scholar]
- 11.Irving RA, Noakes TD, Burger SC, et al. Plasma volume and renal function during and after ultramarathon running. Med Sci Sports Exerc. 1990;22:581–587. doi: 10.1249/00005768-199010000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Kao WF, Hou SK, Chiu YH, et al. Effects of 100-km ultramarathon on acute kidney injury. Clin J Sport Med. 2015;25:49–54. doi: 10.1097/JSM.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 13.Holtzhausen LM, Noakes TD, Kroning B, et al. Clinical and biochemical characteristics of collapsed ultra-marathon runners. Med Sci Sports Exerc. 1994;26:1095–1101. [PubMed] [Google Scholar]
- 14.Irving RA, Noakes TD, Raine RI, et al. Transient oliguria with renal tubular dysfunction after a 90 km running race. Med Sci Sports Exerc. 1990;22:756–761. doi: 10.1249/00005768-199012000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman MD, Stuempfle KJ, Rogers IR, et al. Hyponatremia in the 2009 161-km Western States Endurance Run. Int J Sports Physiol Perform. 2011;7:6–10. doi: 10.1123/ijspp.7.1.6. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman MD, Wegelin JA. The Western States 100-Mile Endurance Run: participation and performance trends. Med Sci Sports Exerc. 2009;41:2191–2198. doi: 10.1249/MSS.0b013e3181a8d553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parise C, Hoffman MD. Influence of temperature and performance level on pacing a 161-km trail ultramarathon. Int J Sports Physiol Perform. 2011;6:243–251. doi: 10.1123/ijspp.6.2.243. [DOI] [PubMed] [Google Scholar]
- 18.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granerus G, Aurell M. Reference values for 51Cr-EDTA clearance as a measure of glomerular filtration rate. Scand J Clin Lab Invest. 1981;41:611–616. doi: 10.3109/00365518109090505. [DOI] [PubMed] [Google Scholar]
- 20.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 21.Hoffman MD, Stuempfle KJ, Sullivan K, et al. Exercise-associated hyponatremia with exertional rhabdomyolysis: importance of proper treatment. Clin Nephrol. 2014 Jun 16; doi: 10.5414/CN108233. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.McAnulty SR, Owens JT, McAnulty LS, et al. Ibuprofen use during extreme exercise: effects on oxidative stress and PGE2. Med Sci Sports Exerc. 2007;3:1075–1079. doi: 10.1249/mss.0b13e31804a8611. [DOI] [PubMed] [Google Scholar]
- 23.Nieman DC, Dumke CL, Henson DA, et al. Muscle damage is linked to cytokine changes following a 160-km race. Brain Behav Immun. 2005;19:398–403. doi: 10.1016/j.bbi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Bennett BL, Hew-Butler T, Hoffman MD, et al. Wilderness Medical Society practice guidelines for treatment of exercise-associated hyponatremia: 2014 update. Wilderness Environ Med. 2014;25(4 Suppl):S30–42. doi: 10.1016/j.wem.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Bennett BL, Hew-Butler T, Hoffman MD, et al. In reply to Clinical practice guidelines for treatment of exercise-associated hyponatremia. Wilderness Environ Med. 2013;24:468–471. doi: 10.1016/j.wem.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman MD, Rogers IR, Joslin J, et al. Managing collapsed or seriously ill participants of ultra-endurance events in remote environments. Sports Med. 2014 Oct 19; doi: 10.1007/s40279-014-0270-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Wasung ME, Chawla LS, Madero M. Biomarkers of renal function, which and when? Clinica Chimica Acta. 2015;438:350–357. doi: 10.1016/j.cca.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman MD. Anthropometric characteristics of ultramarathoners. Int J Sports Med. 2008;29:808–811. doi: 10.1055/s-2008-1038434. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman MD, Lebus DK, Ganong AC, et al. Body composition of 161-km ultramarathoners. Int J Sports Med. 2010;31:106–109. doi: 10.1055/s-0029-1241863. [DOI] [PubMed] [Google Scholar]