Abstract
Background
Despite increasing concern regarding health problems as a result of environmental pollutants, no association of toxic heavy metals with sarcopenia has been demonstrated in the general population. We investigated the association of heavy metals, including lead, mercury and cadmium, with sarcopenia in the Korean population.
Methods
Participants included 344 males and 360 females older than 65 years based on data from the fourth and fifth Korea National Health and Nutritional Examination Surveys. Measurements of blood lead, mercury and cadmium levels were performed. To evaluate the cumulative effect of the three heavy metals, subjects were categorized into quartiles. Sarcopenia was defined according to the criteria for the Asia Working Group for Sarcopenia (AWGS) (SMI<5.4 kg/m2 in females and <7.0 kg/m2 in males).
Results
Of 704 elderly persons (344 in males and 360 in females), prevalences of sarcopenia were 26.7% (92/344) in male and 7.5% (27/360) in female. Mean serum levels of lead in sarcopenia group were significantly higher than non-sarcopenia males (P=0.03). After adjustment for confounding factors, odds ratio for sarcopenia were increased with concentration category of lead (P=0.005 and P<0.001), mercury (P=0.001 and P<0.001) and cadmium (P=0.010 and P<0.001) in males and females, respectively.
Conclusions
This study demonstrates that high levels of blood lead, mercury and cadmium increase the prevalence of sarcopenia in both genders of elderly populations.
Keywords: Cadmium, Lead, Mercury, Metals heavy, Sarcopenia
INTRODUCTION
There has been growing concern worldwide regarding health problems resulting from exposure to heavy metals, such as lead, mercury and cadmium. Because toxic heavy metals such as lead, mercury and cadmium are widely dispersed in the environment,[1,2,3] members of the general population, not only those in contaminated areas, are exposed to low doses of heavy metals during their lifetime. These heavy metals are accumulated in the human body due to no mechanism for active excretion of toxic heavy metals.[4]
Long-term exposures of heavy metals are known to associate with development and progression of bone disease.[5,6,7] Although pathogenic mechanisms of lead, mercury and cadmium are still investigating, these heavy metals are toxic to the human body by increasing oxidative stress. This mechanism is possible to explain the relationship between exposure of heavy metals and various metabolic disorders such as hypertension and diabetes.[8] Toxic heavy metals also are known to relate with increasing the risk of osteoporosis.[9,10,11,12,13] Several studies demonstrate that osteoporosis and sarcopenia have similar pathophysiology and risk factors. Therefore, long-term accumulations of these heavy metals may be related with bone and muscles weakness.
Recently, sarcopenia in the elderly is representative disease in muscular changes and an independent risk factor for falls, disability, morbidity, and mortality.[14,15,16,17] As pathophysiologic mechanisms, sarcopenia generally results from a complex bone-muscle interaction in relation to chronic disease and aging. However, there was no report to study relationship of heavy metals and sarcopenia.
Therefore, the purpose of this cross sectional study was to assess the relationship of blood lead, mercury and cadmium levels with sarcopenia and their cumulative effect on skeletal muscles in elderly populations using Korea National Health and Nutritional Examination Surveys.
METHODS
1. Ethics statement
This study's protocol for analysis of the 2008-2011 Korea National Health and Nutritional Examination Surveys (KNHANES) data was reviewed and approved by the Institutional Review Board (Approval No. 2008-04EXP-01-C, 2009-01CON-03-C, 2010-02CON-21-C and 2011-02CON-06-C) of the Korea Centers for Disease Control and Prevention (KCDC). Informed consent was obtained from all of the participants when the 2008, 2009, 2010, and 2011 KNHANES were conducted.
2. Participants
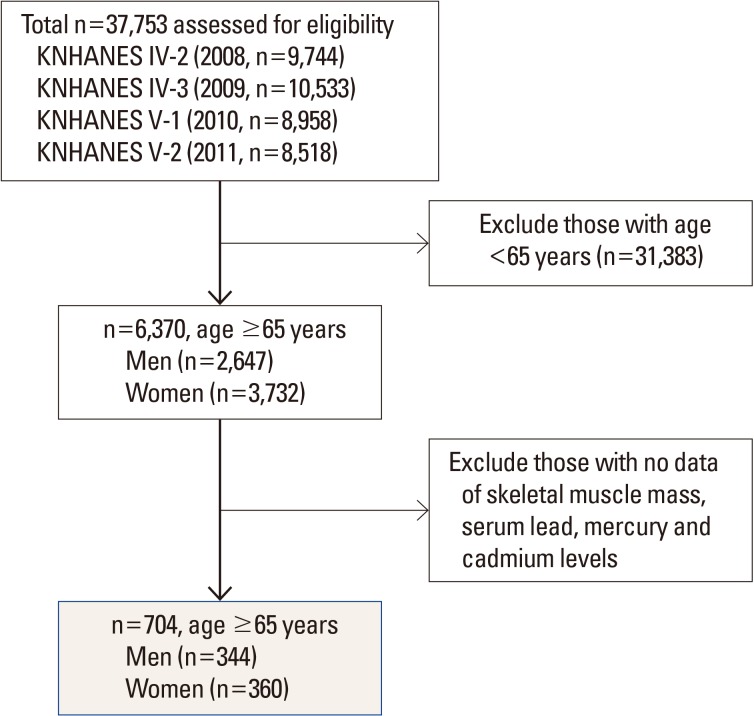
This study was based on data from the KNHANES 2008-2011, which was conducted by the Korea Ministry of Health and Welfare. KNHANES is a nationwide representative cross-sectional survey for the Korean population with a clustered, multistage, stratified, and rolling sampling design. KNHANES comprises three sections: a health interview, a health examination, and a dietary survey. The survey data are collected via household interviews and by direct standardized physical examinations conducted in specially equipped mobile examination centers. We collected data from 37,753 participants from 2008 (n=9,744), 2009 (n=10,533), 2010 (n=8,958), and 2011 (n=8,518). Participants were excluded if they were under the age of 65 years, or if data were not available to evaluate skeletal muscle mass, and serum lead, mercury and cadmium levels. After these exclusions, a total of 704 participants (males 344, females 360) were analyzed in the present study (Fig. 1).
Fig. 1. Selection process of study subjects, KNHANES IV, V (2008-2011). KNHANES, Korea National Health and Nutrition Examination Survey.
3. Health examination survey
A health questionnaire was used to obtain information on age, gender, socioeconomic status, educational status, smoking status (current, former, or never smoker), alcohol intake and moderate physical activity and walking activity (yes or no). Moderate physical activity was 5 or more days of moderate-intensity activity for at least 30 min per day. Walking physical activity was 5 or more days of walking for at least 30 min per day. Body weight and height were measured in light clothing with no shoes, and body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Information on diabetes as a potential confounding factor was examined through the health interview survey.
4. Measurement of lead, mercury and cadmium levels in whole blood
We measured total lead, mercury and cadmium levels in blood. For measurements of blood heavy metal levels, blood samples of individual subjects were collected in standard commercial evacuated tubes containing sodium heparin (Vacutainer; Becton, Dickinson & Co, Franklin Lakes, NJ, USA). Graphite-furnace atomic absorption spectrometry with Zeeman background correction (AAnalyst 600; Perkin Elmer, Wilton, CT, USA) was used for measurement of blood lead and cadmium levels. A gold-amalgam collection method with a DMA-80 (Milestone, Sorisole, Italy) was used for measurement of blood mercury levels. All blood metal analyses were performed at the Neodin Medical Institute, a laboratory certified by the Korean Ministry of Health and Welfare.
5. Measurements of appendicular skeletal muscle mass and definition of sarcopenia
Body composition was measured by whole-body dual energy X-ray absorptiometry (DXA; QDR 4500A, Hologic Inc., Bedford, MA, USA). Bone mineral content, fat mass and lean soft-tissue mass were measured separately for each part of the body, including the arms and legs. The lean soft-tissue masses of the arms and legs were almost equal to the skeletal muscle mass. As absolute muscle mass correlates with height, the skeletal muscle mass index was calculated by the following formula: lean mass (kg)/height2 (m2), which is directly analogous to BMI (weight [kg]/height2 [m2]). Arm skeletal muscle mass index was defined as (arm lean mass [kg]/height2 [m2]). Leg skeletal muscle mass index was defined as (leg lean mass [kg]/height2 [m2]). Appendicular skeletal muscle mass index (SMI) was defined as the sum of the arm SMI and the leg SMI. Sarcopenia was defined according to the criteria for the Asia Working Group for Sarcopenia (AWGS) (SMI <5.4 kg/m2 in females and <7.0 kg/m2 in males).[18]
6. Dietary intake measurement
Dietary intake was assessed by trained staff using a complete 24-hr recall method. Daily intake of energy and protein were calculated by referencing nutrient concentrations in foods according to the Korean Food Composition Table.
7. Biochemical analyses
Blood and urine samples were collected the morning after fasting for at least 8 hr. Collected samples were immediately refrigerated and transported in cold storage (4℃-8℃) to the central laboratory of Neodin Medical Institute (Seoul, Korea) within 24 hr. Transported samples were separated into small aliquots and stored at -70℃.
Serum 25-hydroxy-vitamin D (25-[OH]D), parathyroid hormone, and alkaline phosphatase levels were measured using a gamma counter (1470 Wizard; Perkin Elmer, Turku, Finland), Hitachi Automatic Analyzer 7600 (Hitachi Ltd., Tokyo, Japan) and LIAISON (DiaSorin, Stillwater, MN, USA) with radioimmunoassay (25-hydroxy-vitamin D 125I RIA Kit; DiaSorin), enzymatic (Pureauto S ALP; Sekisui Medical Co., Ltd, Tokyo, Japan) and chemiluminescence immunoassay (N-tact PTH Assay kit; DiaSorin), respectively.
8. Statistical analysis
Complex sample analysis was used in this study to correct the distributions of the cluster sample regarding the primary sampling unit, covariance and significance to correspond with those of the general Korean population. All analyses were carried out using the sample weights of KNHANES.
To compare means between the non-sarcopenia and sarcopenia groups, Student's t-test was used and to compare proportions, the χ2 test was used. Multiple logistic regression analysis was conducted to calculate odds ratio and 95% confidence intervals (Cis) for the association between frequency of binge drinking and the presence of sarcopenia after adjustment for demographic variables (age, waist circumference and BMI), which served as covariates. Spearman correlation analysis was performed to investigate the association between heavy metals and variables. The geometric means with standard errors of heavy metals were calculated according to their quartiles. For each heavy metal, subjects were categorized into quartiles. To evaluate the cumulative effect of the three heavy metals, each heavy metal was categorized into 10 groups using the 10th percentiles. The category number of each heavy metal (1-10 assigned to successively increasing categories) was added to make the sum of heavy metal levels, producing a value of 3 to 30, which was itself categorized into quartiles, generating four groups.
Multivariate logistic regression analysis was used to calculate individual heavy metals or sum of heavy metal effects on sarcopenia, adjusting for BMI, waist circumference, and energy intake. All statistical tests were two-tailed, and statistical significance was defined as P<0.05. The statistical analysis was performed using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Baseline characteristics of elderly populations according to the presence of sarcopenia
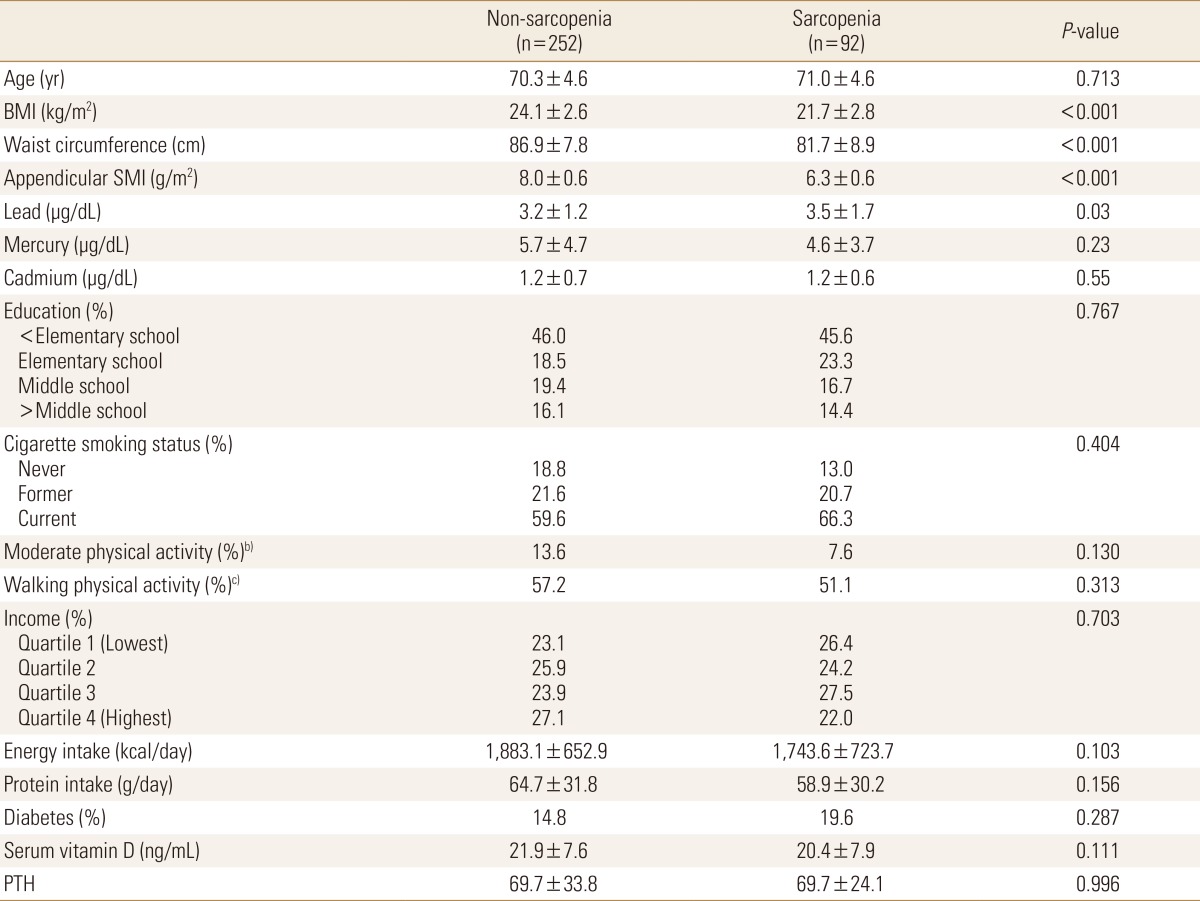
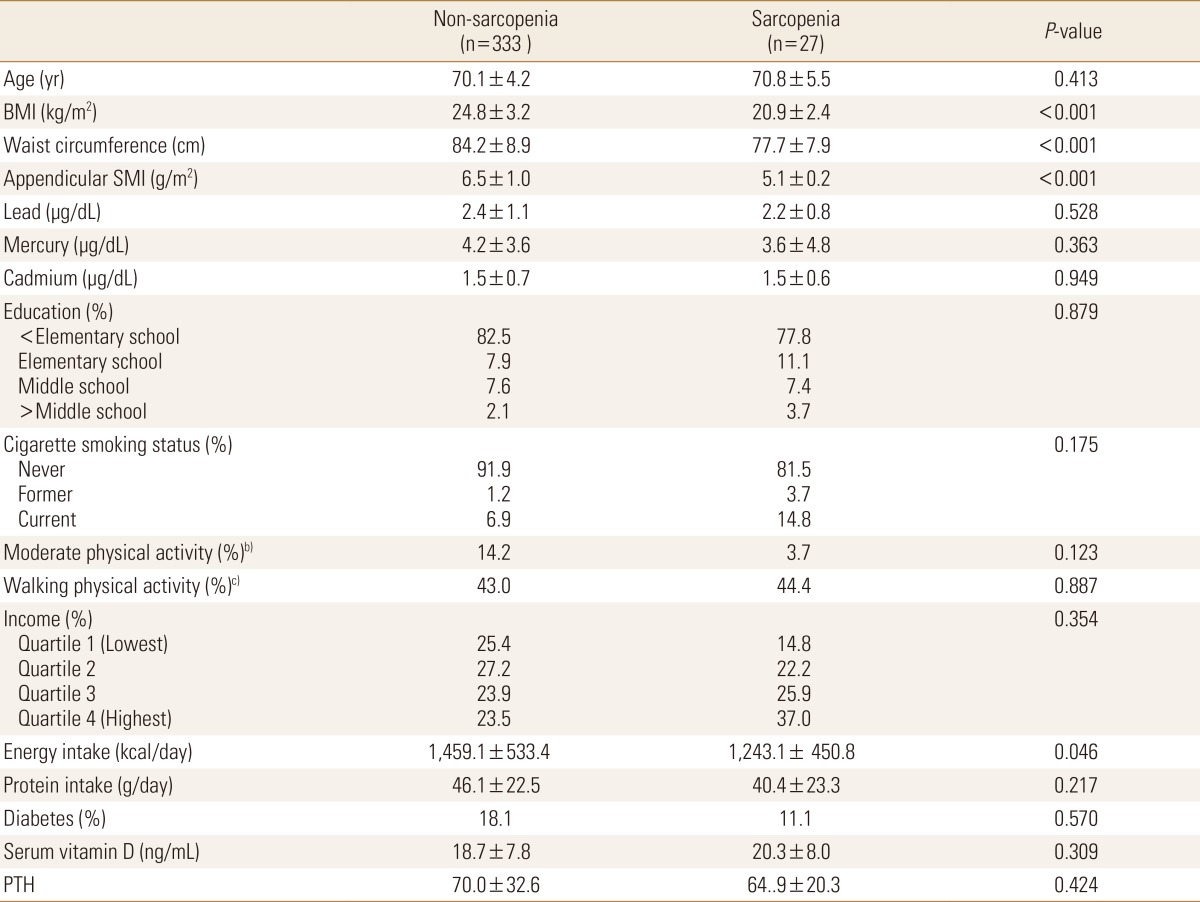
Seven hundreds four persons (344 in male and 360 in female) were enrolled in this study. The mean age were 70.5 years (range, 65-87 years) in male and 72.5 years (range, 65-85 years) in females. Prevalences of sarcopenia were 26.7% (92/344) in male and 7.5% (27/360) in female and baseline values for the elderly populations according to the presence of sarcopenia are shown in Table 1 and 2. BMI (P<0.001), waist circumference (P<0.001), and appendicular SMI (P<0.001) differed significantly between non-sarcopenia and sarcopenia in both genders. Mean serum levels of lead in sarcopenia group were significantly higher than non-sarcopenia males (P=0.03).
Table 1. Baseline characteristics and heavy metal levels of elderly males according to the presence of sarcopeniaa).
a)All values are presented as means±standard deviation and percentage distribution of participant, as appropriate. Significance was compared between non-sarcopenia and sarcopenia using Student's t-test or Chi-square test. The unweighted sample size was presented in the table, but the results presented reflect the weighted sample. b)Moderate physical activity was 5 or more days of moderate-intensity activity of at least 30 min per day. c)Walking physical activity was 5 or more days of walking of at least 30 min per day.
BMI, body mass index; SMI, skeletal muscle index; BMD, bone mass density; PTH, parathyroid hormone.
Table 2. Baseline characteristics and heavy metal levels of elderly females according to the presence of sarcopeniaa).
a)All values are presented as means±SD and percentage distribution of participant, as appropriate. Significance was compared between non-sarcopenia and sarcopenia using Student's t-test or Chi-square test. The unweighted sample size was presented in the table, but the results presented reflect the weighted sample. b)Moderate physical activity was 5 or more days of moderate-intensity activity of at least 30 min per day. c)Walking physical activity was 5 or more days of walking of at least 30 min per day.
BMI, body mass index; SMI, skeletal muscle index; BMD, bone mass density; PTH, parathyroid hormone.
2. Correlations of lead, mercury and cadmium levels with clinical variables
Most variables showed correlations with lead, mercury and cadmium levels. Males showed higher concentrations of blood lead, mercury and cadmium than females. Smoking was closely correlated with blood lead (r=0.355) and mercury (r=0.196) levels. Correlations were observed among the three heavy metals: r=0.160 and r=0.092 for lead and mercury, lead and cadmium, respectively (Table 3).
Table 3. Correlation coefficients between heavy metals and clinical variables.
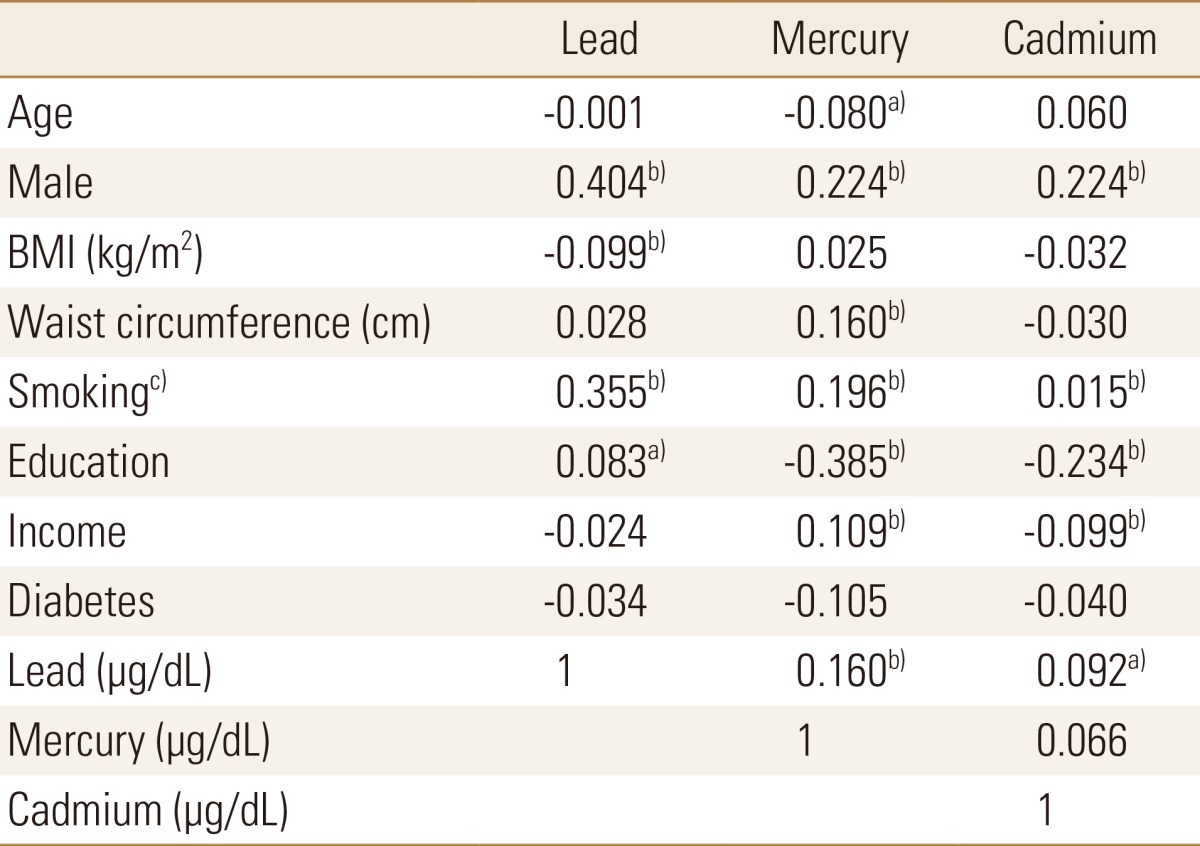
Age, BMI, waist circumference, blood lead, mercury and cadmium level are continuous variables.
a)P<0.05. b)P<0.01. c)Smoking status was divided into three categories: never, former and current smokers.
BMI, body mass index.
3. Prevalence of sarcopenia according to heavy metal category
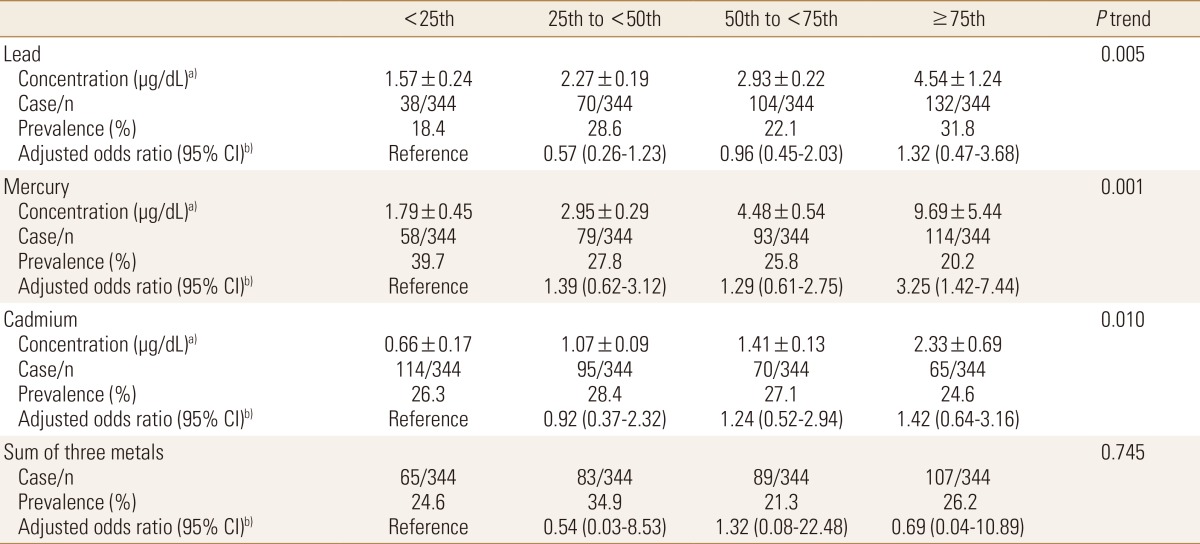
When no variable was adjusted, the prevalence of sarcopenia increased with increasing lead concentration category. However, the prevalence of sarcopenia decreased with increasing mercury and cadmium concentration category.
In male, after adjustment for BMI and waist circumference, odds ratios were increased with increasing lead (P=0.005), mercury (P=0.001) and cadmium (P=0.010) concentration. However, the sum of heavy metals showed no association with the prevalence of sarcopenia (Table 4).
Table 4. Adjusted odds ratios and 95% confidence intervals (CI) of prevalent sarcopenia in males according to heavy metal category.
a)Concentration, geometric mean±standard error. b)Adjusted for body mass index and waist circumference.
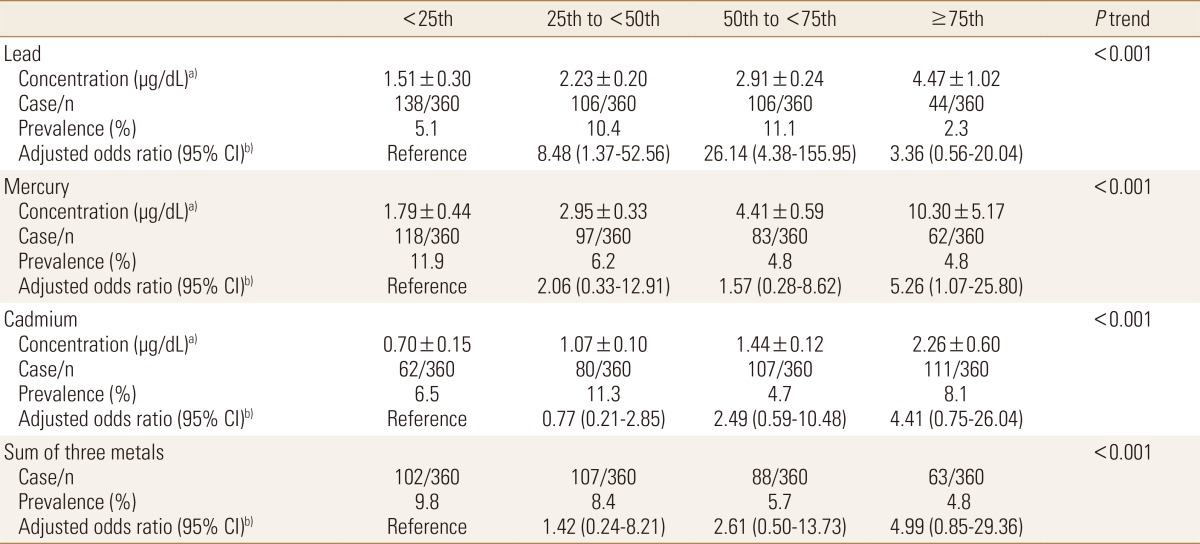
In female, after adjustment for BMI, waist circumference and energy intake, the odds ratios were increased with increasing concentration category of lead (P<0.001), mercury (P<0.001) and cadmium (P<0.001), and the sum of the three heavy metals (P<0.001) (Table 5).
Table 5. Adjusted odds ratios and 95% confidence interval (CI) of prevalent sarcopenia in females according to heavy metal category.
a)Concentration, geometric mean±standard error. b)Adjusted for body mass index, waist circumference and energy intake.
DISCUSSION
Accumulations of heavy metals in human body are known to cause of public health problems. Of heavy metals, lead, mercury and cadmium are representative toxic metals and related researches have been reported. This study shows that odds ratio for sarcopenia, after adjustment, were increased with concentration category of lead (P=0.005 and P<0.001), mercury (P=0.001 and P<0.001) and cadmium (P=0.001 and P<0.001) in males and females, respectively. Therefore, we found that elderly populations with sarcopenia had high serum levels of heavy metals in both genders.
Recently, sarcopenia, which is defined as the age-related decrease in muscle mass, have been widely investigated in a variety of fields because sarcopenia is known to a major cause of impairment of physical function, limitation of mobility, falls, osteoporosis, and hospitalization.[14,15,16,17,19,20] Although its cause is not completely understood, sarcopenia generally results from a complex bone-muscle interaction in relation to chronic disease and aging.[21,22,23] Therefore, effects of heavy metals in bone metabolism are also important to muscular metabolism in elderly populations.
Of three representative heavy metals, relationship between bone metabolism and lead are well established, which is accumulated more than 90% of lead in bone.[24,25] Several human studies have demonstrated that lead interfered with bone formation and bone strength and increase the risk of osteoporosis and bone fracture.[26,27,28,29] Campbell and Auinger [30] reported negative association between lead exposure and BMD and resulted in osteoporosis in adult humans using data from the Third National Health and Nutrition Examination Survey (NHANES III) in the USA. Several animal studies confirmed that lead affects osteoblast and osteoclast function.[11,31,32,33] In addition, mercury influences calcium metabolism and affects bone.[10] Suzuki et al. [34] conducted an animal study to examine the effects of heavy metals such as cadmium and mercury on calcium homeostasis, plasma calcium and calcitonin in goldfish. They found that mercury influenced calcium metabolism and affected bone. However, effect of Mercury in bone metabolism are still controversial.[35]
Recently, exposure to cadmium has attracted increasing attention as a risk factor for osteoporosis. Wallin et al.[13] examined the associations between low-level cadmium exposure, from diet and smoking, and BMD and incident fractures in 936 elderly males from the Swedish cohort of the Osteoporotic Fractures in Men (MrOS) study. They found that even a relatively low cadmium level increases the risk of low BMD and osteoporosis-related fractures in elderly males. Engström et al.[9] investigated the association between low environmental cadmium exposure and BMD and fracture risk in 2,688 elderly females using a Swedish Mammography cohort. They found that long-term, low-level exposure to Cd from food (mainly cereals, vegetables and potatoes), is associated with a higher risk of osteoporosis and fractures. Chen et al.[36] found a strong relationship between blood Cd level and decreased BMD, especially in females. In addition, they determined that blood Pb level might be related to low BMD in males. They suggested that Cd and Pb might have an interactive effect on bone.
These effects of bone metabolism by accumulations of three heavy metals in human body are also affect in muscular mechanism and resulted in sarcopenia. Although no direct study between toxic heavy metals and sarcopenia has been conducted, the findings of this cross sectional study might be one of explanations of relationship between heavy metals and sarcopenia.
This study had several limitations. First, it did not evaluate the causality between heavy metal exposure and sarcopenia. Prospectively designed studies are mandatory to clarify this relationship. Second, because blood levels of lead, mercury and cadmium differ among ethnic groups, this result might not be able to be extrapolated to other ethnic groups. Third, although lead, mercury and cadmium levels in blood are widely used and well-established biomarkers of exposure, and those blood concentrations may be correlated with chronic accumulated exposure in the general population with stable environmental exposure to heavy metals, they reflect mainly recent exposure. Therefore, they could underestimate cumulative exposure. In particular, we measured total mercury only in whole blood, including both the inorganic and organic forms, without differentiating other forms of mercury. Although total mercury in blood may be a reliable biomarker of mercury exposure, we could not infer which form is the major contributor to blood mercury and by which route exposure occurs. Fourth, we did not measure all environmental exposure to heavy metals; thus, the possibility of a positive relationship between other heavy metals with sarcopenia remains. Therefore, the relationship between heavy metals and sarcopenia should be verified in further longitudinal studies. Finally, although a definition of sarcopenia has been established, debate is ongoing. We used the definition of the AWGS group.[18]
In conclusion, this study demonstrates that high levels of blood lead, mercury and cadmium increase the prevalence of sarcopenia in both genders of elderly populations.
Footnotes
No potential conflict of interest relevant to this article was reported.
The authors thank the Korea Centers for Disease Control and Prevention, who performed the KNHANES.
References
- 1.Henson MC, Chedrese PJ. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp Biol Med (Maywood) 2004;229:383–392. doi: 10.1177/153537020422900506. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Scott Clark C. Lead loadings in household dust in Delhi, India. Indoor Air. 2009;19:414–420. doi: 10.1111/j.1600-0668.2009.00605.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosin A. The long-term consequences of exposure to lead. Isr Med Assoc J. 2009;11:689–694. [PubMed] [Google Scholar]
- 4.Mercier M. International approach of the assessment of chemical risks. J Hyg Epidemiol Microbiol Immunol. 1990;34:1–7. [PubMed] [Google Scholar]
- 5.Nogawa K, Kido T. Biological monitoring of cadmium exposure in itai-itai disease epidemiology. Int Arch Occup Environ Health. 1993;65:S43–S46. doi: 10.1007/BF00381306. [DOI] [PubMed] [Google Scholar]
- 6.Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- 7.Vig EK, Hu H. Lead toxicity in older adults. J Am Geriatr Soc. 2000;48:1501–1506. [PubMed] [Google Scholar]
- 8.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Engström A, Michaëlsson K, Vahter M, et al. Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women. Bone. 2012;50:1372–1378. doi: 10.1016/j.bone.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Jin GB, Inoue S, Urano T, et al. Induction of anti-metallothionein antibody and mercury treatment decreases bone mineral density in mice. Toxicol Appl Pharmacol. 2002;185:98–110. doi: 10.1006/taap.2002.9531. [DOI] [PubMed] [Google Scholar]
- 11.Klein RF, Wiren KM. Regulation of osteoblastic gene expression by lead. Endocrinology. 1993;132:2531–2537. doi: 10.1210/endo.132.6.8504755. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H, Asakawa A, Li JB, et al. Zinc as an appetite stimulator - the possible role of zinc in the progression of diseases such as cachexia and sarcopenia. Recent Pat Food Nutr Agric. 2011;3:226–231. doi: 10.2174/2212798411103030226. [DOI] [PubMed] [Google Scholar]
- 13.Wallin M, Barregard L, Sallsten G, et al. Low-level cadmium exposure is associated with decreased bone mineral density and increased risk of incident fractures in elderly men: the MrOS Sweden study. J Bone Miner Res. 2016;31:732–741. doi: 10.1002/jbmr.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanitallie TB. Frailty in the elderly: contributions of sarcopenia and visceral protein depletion. Metabolism. 2003;52:22–26. doi: 10.1016/s0026-0495(03)00297-x. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd BD, Williamson DA, Singh NA, et al. Recurrent and injurious falls in the year following hip fracture: a prospective study of incidence and risk factors from the Sarcopenia and Hip Fracture study. J Gerontol A Biol Sci Med Sci. 2009;64:599–609. doi: 10.1093/gerona/glp003. [DOI] [PubMed] [Google Scholar]
- 16.Yoon HK, Park C, Jang S, et al. Incidence and mortality following hip fracture in Korea. J Korean Med Sci. 2011;26:1087–1092. doi: 10.3346/jkms.2011.26.8.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frisoli A, Jr, Chaves PH, Ingham SJ, et al. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: results from the Womens Health and Aging Study (WHAS) II. Bone. 2011;48:952–957. doi: 10.1016/j.bone.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Kwon HJ, Ha YC, Park HM. Prevalence of sarcopenia in the Korean woman based on the Korean national health and nutritional examination surveys. J Bone Metab. 2016;23:23–26. doi: 10.11005/jbm.2016.23.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990s–991s. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 21.Kaji H. Interaction between muscle and bone. J Bone Metab. 2014;21:29–40. doi: 10.11005/jbm.2014.21.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patil R, Uusi-Rasi K, Pasanen M, et al. Sarcopenia and osteopenia among 70-80-year-old home-dwelling Finnish women: prevalence and association with functional performance. Osteoporos Int. 2013;24:787–796. doi: 10.1007/s00198-012-2046-2. [DOI] [PubMed] [Google Scholar]
- 23.Verschueren S, Gielen E, O'Neill TW, et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos Int. 2013;24:87–98. doi: 10.1007/s00198-012-2057-z. [DOI] [PubMed] [Google Scholar]
- 24.Alfvén T, Järup L, Elinder CG. Cadmium and lead in blood in relation to low bone mineral density and tubular proteinuria. Environ Health Perspect. 2002;110:699–702. doi: 10.1289/ehp.110-1240916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barry PS. A comparison of concentrations of lead in human tissues. Br J Ind Med. 1975;32:119–139. doi: 10.1136/oem.32.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton JD, O'Flaherty EJ. Effects of lead exposure on skeletal development in rats. Fundam Appl Toxicol. 1994;22:594–604. doi: 10.1006/faat.1994.1066. [DOI] [PubMed] [Google Scholar]
- 27.Goyer RA, Epstein S, Bhattacharyya M, et al. Environmental risk factors for osteoporosis. Environ Health Perspect. 1994;102:390–394. doi: 10.1289/ehp.94102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Escribano A, Revilla M, Hernández ER, et al. Effect of lead on bone development and bone mass: a morphometric, densitometric, and histomorphometric study in growing rats. Calcif Tissue Int. 1997;60:200–203. doi: 10.1007/s002239900214. [DOI] [PubMed] [Google Scholar]
- 29.Potula V, Kleinbaum D, Kaye W. Lead exposure and spine bone mineral density. J Occup Environ Med. 2006;48:556–564. doi: 10.1097/01.jom.0000222556.89044.90. [DOI] [PubMed] [Google Scholar]
- 30.Campbell JR, Auinger P. The association between blood lead levels and osteoporosis among adults--results from the third national health and nutrition examination survey (NHANES III) Environ Health Perspect. 2007;115:1018–1022. doi: 10.1289/ehp.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Y, Fu D, Liu Z. Effect of lead on apoptosis in cultured rat primary osteoblasts. Toxicol Ind Health. 2012;28:136–146. doi: 10.1177/0748233711407956. [DOI] [PubMed] [Google Scholar]
- 32.Pounds JG, Long GJ, Rosen JF. Cellular and molecular toxicity of lead in bone. Environ Health Perspect. 1991;91:17–32. doi: 10.1289/ehp.919117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puzas JE, Sickel MJ, Felter ME. Osteoblasts and chondrocytes are important target cells for the toxic effects of lead. Neurotoxicology. 1992;13:783–788. [PubMed] [Google Scholar]
- 34.Suzuki N, Yamamoto M, Watanabe K, et al. Both mercury and cadmium directly influence calcium homeostasis resulting from the suppression of scale bone cells: the scale is a good model for the evaluation of heavy metals in bone metabolism. J Bone Miner Metab. 2004;22:439–446. doi: 10.1007/s00774-004-0505-3. [DOI] [PubMed] [Google Scholar]
- 35.Cho GJ, Park HT, Shin JH, et al. The relationship between blood mercury level and osteoporosis in postmenopausal women. Menopause. 2012;19:576–581. doi: 10.1097/gme.0b013e3182377294. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Wang K, Wang Z, et al. Effects of lead and cadmium co-exposure on bone mineral density in a Chinese population. Bone. 2014;63:76–80. doi: 10.1016/j.bone.2014.02.017. [DOI] [PubMed] [Google Scholar]