Abstract
Background
The best options of several bisphosphonates for prevention of osteoporotic fractures in postmenopausal women remain controversial. We determined which bisphosphonate provides better efficacy in prevention of osteoporotic fractures using a decision analysis tool, in terms of quality of life.
Methods
A decision analysis model was constructed containing final outcome score and the probability of vertebral and hip fracture within 1 year. Final outcome was defined as health-related quality of life, and was used as an utility in the decision tree. Probabilities were obtained by literature review, and health-related quality of life was evaluated by consensus committee. A roll back tool was used to determine the best bisphosphonate, and sensitivity analysis was performed to compensate for decision model uncertainty.
Results
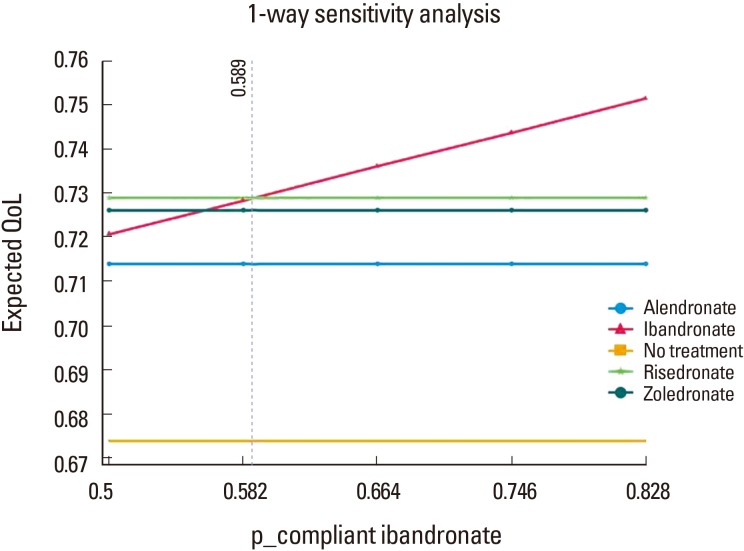
The decision model favored bisphosphonate with higher compliance in terms of quality of life. In one-way sensitivity analysis, ibandronate was more beneficial than the others, when probability of compliance on ibandronate was above 0.589.
Conclusions
In terms of quality of life, the decision analysis model showed that compliance was most important for patients in real world, regardless of type of bisphosphonate.
Keywords: Diphosphonates, Fractures bone, Osteoporosis postmenopausal, Patient compliance
INTRODUCTION
Bisphosphonate is effective for the treatment of osteoporosis, and prevention of osteoporotic fractures. Practically, several types of bisphosphonates have been available in prevention of osteoporotic fractures.
Although the efficacy of several bisphosphonates in elderly patients have been previously presented in prospective randomized clinical trials (RCTs),[1,2,3,4] the optimal choice of bisphosphonate for osteoporotic patients remains controversial. Although RCT are believed to provide the highest level of evidence regarding the merits of procedures, RCT does not provide a decision guide which bisphosphonate should be choose. Moreover, there was no RCT that with head-to-head comparison of bisphosphonate.
On the other hands, decision analysis is a quantitative method to determine which option provides the better outcomes based on current evidence.[5,6]
Therefore, in this study, we determined, using decision analysis, which bisphosphonate provides the better outcome for prevention of osteoporotic fractures, in terms of quality of life.
METHODS
1. Model design
A decision tree, depicting the probabilities and outcomes (utilities) of each bisphosphonate for the prevention of osteoporotic fracture in postmenopausal women, was developed using decision analysis software (TreeAge Pro 2011; TreeAge Software Inc., Williamstown, MA, USA).
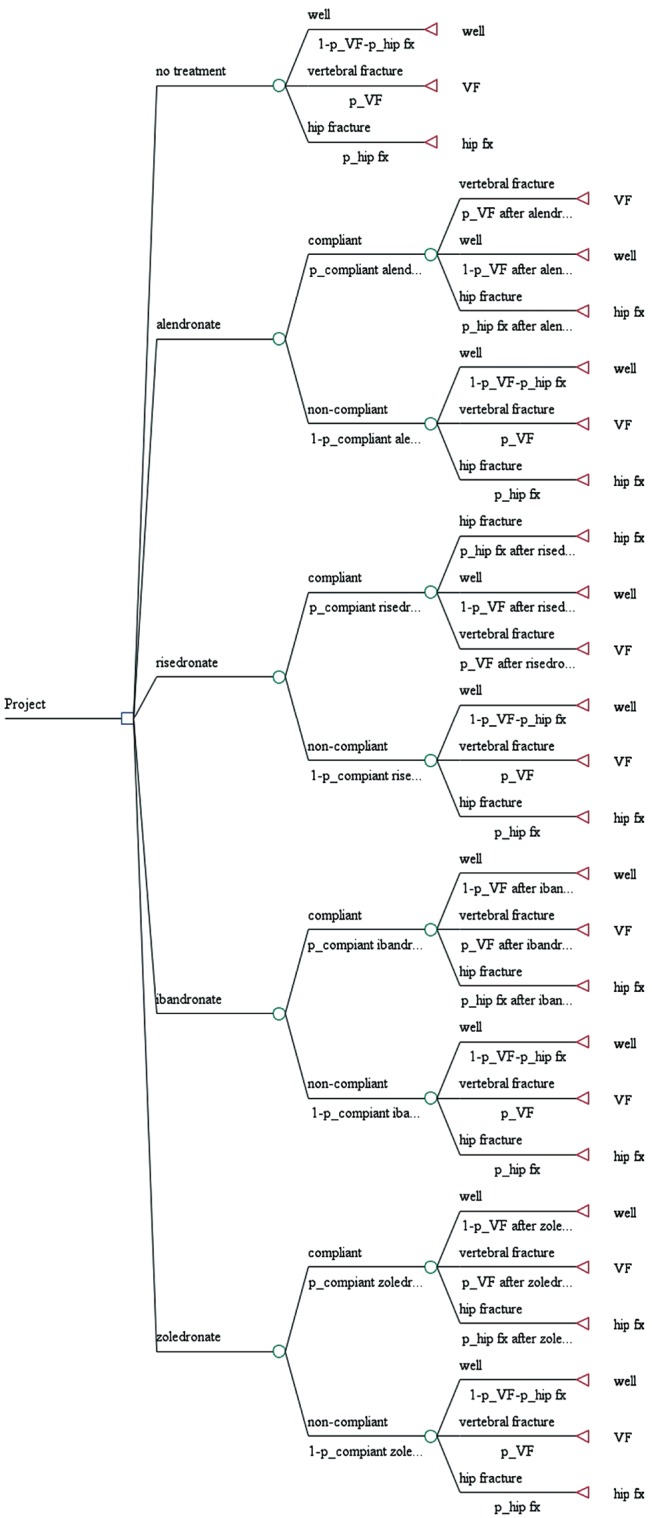
In root node, that is a small square on the left side and represents the first set of decision alternatives, postmenopausal women face a choice; which bisphosphonate is administrated or not to them. In the model, moving from left to right, the tree divides to branch at chance nodes, each of which represents an opportunity for a patient to enter into one or more health states (well, vertebral fracture, or hip fracture) after an administration. Each terminal node of the tree has a corresponding health state and has an associated utility value (Fig. 1).
Fig. 1. Decision tree with probability and utility variables. VF, vertebral fracture; fx, fracture.
2. Event probabilities
We conducted a literature review to identify baseline probability estimates for each chance node. Among original studies which included outcomes to prevent vertebral and hip fracture in postmenopausal women during at least 1-year follow-up, early RCTs with placebo were selected, because there was no study with head-to-head comparison. Finally, 4 original articles were selected for each bisphosphonate.[1,2,3,4] The percentage of reduced risk of vertebral fracture and hip fracture were reviewed (Table 1).
Table 1. Probabilities of vertebral fracture and hip fracture in each bisphosphonate.
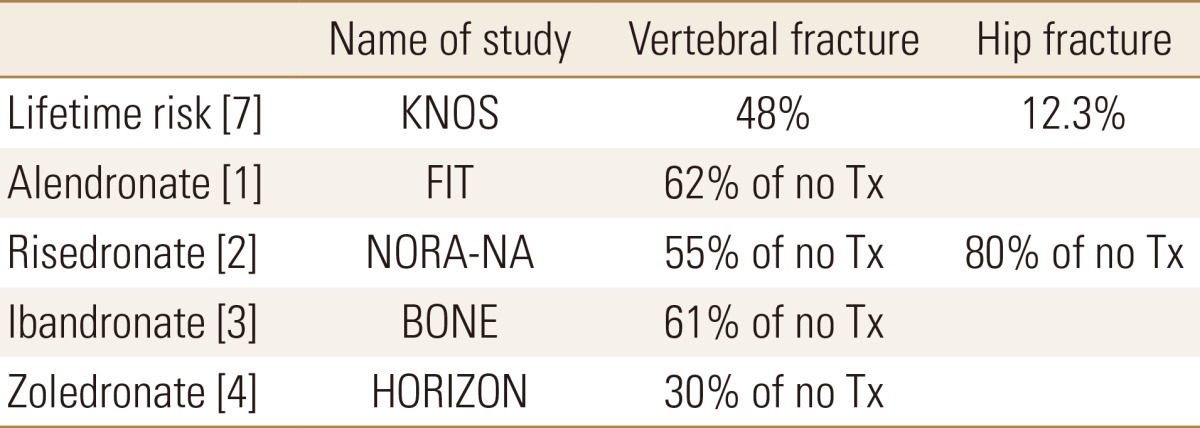
Tx, treatment; KNOS, Korean Nationwide-databased Osteoporosis Study; FIT, fracture intervention trial; NORA, National Osteoporosis Risk Assessment.
For probability of vertebral fracture and hip fracture in decision tree, the lifetime risks at 50 years old in Korea were used.[7]
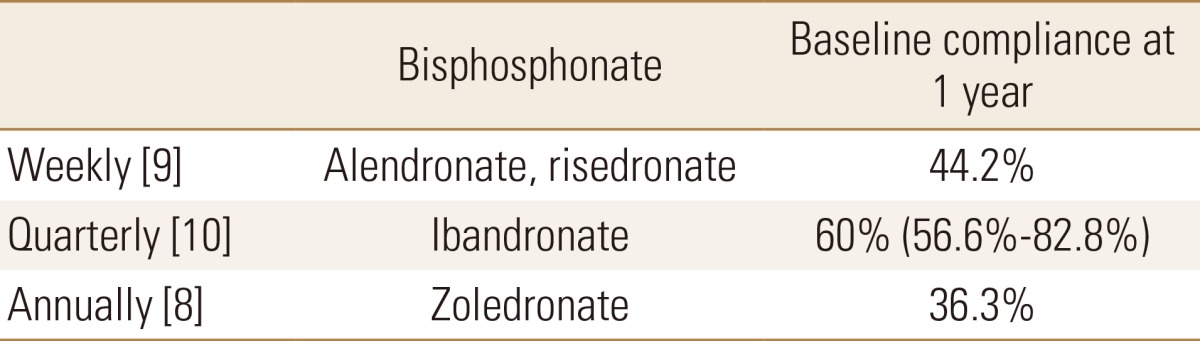
To obtain the whole efficacy of reducing the risk of osteoporotic fracture, the patients should take the medication for a whole year. However, unlike RCTs, the compliance on bisphosphonate was not 100% in real world. The compliance on bisphosphonate has been well known to depend on interval of medication.[8,9] After the interval of medication was categorized with weekly, quarterly, and annually administration, additional literature review was performed to obtain probability of compliant use for each bisphosphonate in real world (Table 2).[8,9,10]
Table 2. Probabilities of compliant use for each bisphosphonate.
3. Health utilities
Quality of life after each event was used as utilities in decision tree. Quality of life were determined in consensus committee, which included 3 orthopedic surgeons with 24 (KHK), 16 (YCH), and 8 (YKL), years of experience. Quality of life on a scale ranged from 0 (death) to 1.0 (perfect health). Hip fracture was defined as a score of 0.3, and vertebral fracture for 0.5 in consensus committee.
4. Statistical analysis
TreeAge Pro 2011 (TreeAge Software Inc.) was used to construct the decision analysis. Final quality of life was calculated using a "roll back" technique, and sensitivity analysis was conducted to assess the uncertainty of the decision tree model. Threshold values were defined as point of intersection at which preferred bisphosphonate changed. The variables with greatest uncertainty were the probabilities of compliance of bisphosphonate. The probabilities of compliance of ibandronate ranged from 56.6% to 82.8% (Table 2).[10]
This study was exempted from institutional review board (IRB) review because it did not involve human subjects.
RESULTS
When performing the roll back, the expected values for risedronate, Ibandronate, and zoledronate were 0.75, whereas the expected values for alendronate were 0.71. Meanwhile the expected values of no treatment were 0.67. Accordingly, the decision model showed that risedronate, ibandronate, and zoledronate was the better than alendronate in terms of quality of life.
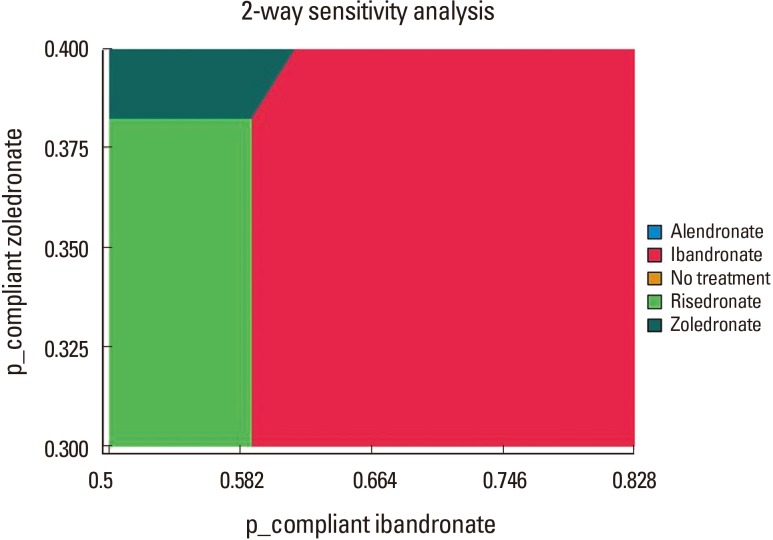
In addition, one-way sensitivity analysis on the probabilities showed that ibandronate was superior to another bisphosphonates, when the probability of compliance on ibandronate was more than 0.589 (Fig. 2). Two-way sensitivity analysis on compliance on ibandronate and zoledronate showed that bisphosphonate with higher compliance was superior to another bisphosphonate, when the probability of compliance was more than 0.589 (Fig. 3).
Fig. 2. One-way sensitivity analysis on the compliance of ibandronate.
Fig. 3. Two-way sensitivity analysis on the compliance of ibandronate and zoledronate.
DISCUSSION
On the decision analysis model presented that bisphosphonate with higher compliance is a better choice than another bisphosphonate in terms of quality of life. Key factor is compliance of each bisphosphonate in this study. Furthermore, the sensitivity analyses showed that our decision model was relatively stable.
Although the efficacy of each bisphosphonate in elderly patients have been previously reported,[1,2,3,4] the study does not provide a decision guide due to a lack of uniform clinical outcome measures. In the present study, the quality of life was used to unify the clinical outcomes of each scenario.
Poor compliance is a key-limiting factor in treatment of osteoporosis, which is asymptomatic silent chronic disease until osteoporotic fractures occur.[11,12,13,14,15] Higher age, low socioeconomic status, poor awareness on osteoporosis, complex method of ingestion and adverse effect such as gastroesophageal irritation have been well-known risk factors for low compliance with bisphosphonate.[16,17,18]
New type of bisphosphonate with various dose intervals have introduced to overcome poor compliance, and bisphosphonates with longer interval showed higher compliance.[8,9,19]
Although several types of bisphosphonate have been presented to have higher efficacy to prevent osteoporotic fracture, compliance on it was revealed to be key factor in this decision analysis study.
Although decision analysis is a useful tool to apply evidence-based medicine to make informed clinical decisions, the obtained results depend on the level of the selected studies and the validity of the used utilities in the model.
Several limitations of the present study should be considered. First, the utilities used in this study, which were physicians-derived, need to be validated with respect to their values to patients and the clinical meaning of utility differences. Second, for reason of simplicity, our model included only vertebral and hip fracture, despite the fact that other osteoporotic fractures could also affect quality of life. Third, compliance might be more diverse than those included in this study, and such considerations could alter expected clinical outcomes. To overcome these limitations, we conducted sensitivity analyses with a broad range of clinically pertinent values, which presented the relatively stability of our decision model. Fourth, we did not include another type of bisphosphonate such as combination with vitamin D.
Despite these limitations, the present study presented that compliance play a key role for better quality of life in treatment of osteoporotic patients, regardless type of bisphosphonate.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Black DM, Cummings SR, Karpf DB, et al. Fracture Intervention Trial Research Group. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 2.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 3.Chesnut CH, 3rd, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 4.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 5.Brauer CA, Bozic KJ. Using observational data for decision analysis and economic analysis. J Bone Joint Surg Am. 2009;91(Suppl 3):73–79. doi: 10.2106/JBJS.H.01537. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein J. Decision analysis. J Bone Joint Surg Am. 1997;79:1404–1414. doi: 10.2106/00004623-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Park C, Ha YC, Jang S, et al. The incidence and residual lifetime risk of osteoporosis-related fractures in Korea. J Bone Miner Metab. 2011;29:744–751. doi: 10.1007/s00774-011-0279-3. [DOI] [PubMed] [Google Scholar]
- 8.Lee YK, Nho JH, Ha YC, et al. Persistence with intravenous zoledronate in elderly patients with osteoporosis. Osteoporos Int. 2012;23:2329–2333. doi: 10.1007/s00198-011-1881-x. [DOI] [PubMed] [Google Scholar]
- 9.Cramer JA, Amonkar MM, Hebborn A, et al. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005;21:1453–1460. doi: 10.1185/030079905X61875. [DOI] [PubMed] [Google Scholar]
- 10.Cooper A, Drake J, Brankin E. Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006;60:896–905. doi: 10.1111/j.1742-1241.2006.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell RG, Watts NB, Ebetino FH, et al. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 12.Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122:S14–S21. doi: 10.1016/j.amjmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Siris ES, Selby PL, Saag KG, et al. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009;122:S3–S13. doi: 10.1016/j.amjmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Hadji P, Claus V, Ziller V, et al. GRAND: the German retrospective cohort analysis on compliance and persistence and the associated risk of fractures in osteoporotic women treated with oral bisphosphonates. Osteoporos Int. 2012;23:223–231. doi: 10.1007/s00198-011-1535-z. [DOI] [PubMed] [Google Scholar]
- 15.Landfeldt E, Ström O, Robbins S, et al. Adherence to treatment of primary osteoporosis and its association to fractures--the Swedish Adherence Register Analysis (SARA) Osteoporos Int. 2012;23:433–443. doi: 10.1007/s00198-011-1549-6. [DOI] [PubMed] [Google Scholar]
- 16.Kamatari M, Koto S, Ozawa N, et al. Factors affecting long-term compliance of osteoporotic patients with bisphosphonate treatment and QOL assessment in actual practice: alendronate and risedronate. J Bone Miner Metab. 2007;25:302–309. doi: 10.1007/s00774-007-0768-6. [DOI] [PubMed] [Google Scholar]
- 17.Kertes J, Dushenat M, Vesterman JL, et al. Factors contributing to compliance with osteoporosis medication. Isr Med Assoc J. 2008;10:207–213. [PubMed] [Google Scholar]
- 18.Weycker D, Macarios D, Edelsberg J, et al. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007;18:271–277. doi: 10.1007/s00198-006-0230-y. [DOI] [PubMed] [Google Scholar]
- 19.Cramer JA, Gold DT, Silverman SL, et al. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023–1031. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]