Abstract
Despite increasing evidence that high-dose chemotherapy and autologous stem cell transplantation (HDCT/auto-SCT) might improve the survival of patients with high-risk or recurrent solid tumors, therapy effectiveness for bone and soft tissue sarcoma treatment remains unclear. This study retrospectively investigated the feasibility and effectiveness of HDCT/auto-SCT for high-risk or recurrent bone and soft tissue sarcoma. A total of 28 patients (18 high-risk and 10 recurrent) underwent single or tandem HDCT/auto-SCT between October 2004 and September 2014. During follow-up of a median 15.3 months, 18 patients exhibited disease progression and 2 died of treatment-related toxicities (1 veno-occlusive disease and 1 sepsis). Overall, 8 patients remained alive and progression-free. The 3-year overall survival (OS) and event-free survival (EFS) rates for all 28 patients were 28.7% and 26.3%, respectively. In the subgroup analysis, OS and EFS rates were higher in patients with complete or partial remission prior to HDCT/auto-SCT than in those with worse responses (OS, 39.1% vs. 0.0%, P = 0.002; EFS, 36.8% vs. 0.0%, P < 0.001). Therefore, careful selection of patients who can benefit from HDCT/auto-SCT and maximal effort to reduce tumor burden prior to treatment will be important to achieve favorable outcomes in patients with high-risk or recurrent bone and soft tissue sarcomas.
Keywords: Bone and Soft Tissue Sarcoma, Children, High-Dose Chemotherapy, Autologous Stem Cell Transplantation
Graphical Abstract
INTRODUCTION
Pediatric bone and soft tissue sarcomas are heterogeneous tumors of mesenchymal origin that make up approximately 10%–15% of all childhood malignancies (1). The prognosis for children with bone and soft tissue sarcomas has significantly improved with the introduction of effective multi-agent chemotherapy, more accurate radiotherapy delivery, and aggressive surgery for local disease. However, a proportion of children with advanced or recurrent sarcomas still have poor prognoses with conventional therapy (2,3,4). To improve the outcome of these patients, various efforts have been made, such as intensification of chemotherapy by interval compression or adding active agents to the standard therapy, which provided some improvements (5); however, the prognosis of high-risk and recurrent sarcoma remains poor.
High-dose chemotherapy and autologous stem cell transplantation (HDCT/auto-SCT) has been used as consolidation therapy for children with a variety of high-risk or recurrent solid tumors (6,7). The rationale for this treatment is that many of these tumors are sensitive to chemotherapy and radiation, but because of steep dose-response curves to both treatment modalities, relatively small dose reductions can result in sharp decreases in log tumor cell kill (8). Despite increasing evidence that HDCT/auto-SCT might improve the survival of patients with high-risk or recurrent solid tumors, HDCT/auto-SCT efficacy for bone and soft tissue sarcoma treatment is uncertain (9,10,11). Many previous studies investigating HDCT/auto-SCT effectiveness for various advanced bone and soft tissue sarcomas yielded inconclusive results (12,13,14).
With the hypothesis that dose escalation might have benefit in high-risk or relapsed bone and soft tissue sarcomas, HDCT/auto-SCT was performed in a subset of patients. In this study, we retrospectively analyzed the feasibility and effectiveness of HDCT/auto-SCT in children with high-risk or recurrent bone and soft tissue sarcomas.
MATERIALS AND METHODS
Twenty-eight patients with bone and soft tissue sarcomas who received HDCT/auto-SCT between October 2004 and September 2014 at the Pediatric Stem Cell Transplantation Unit of Samsung Medical Center were retrospectively studied.
High-risk sarcomas were defined as those in patients with metastatic disease at diagnosis or for which there was incomplete tumor excision. For Ewing sarcomas, centrally located tumors were considered as high-risk.
Tumor response was categorized according to the Response Evaluation Criteria in Solid Tumors, version 1.1. Toxicities were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4.03.
Overall survival (OS) and event-free survival (EFS) were assessed from the date of first HDCT/auto-SCT. Events were progression of disease, relapse, and death. Survival curves were generated according to the Kaplan-Meier method, and comparisons between survival curves were performed using the log-rank test. P values less than 0.05 were considered statistically significant.
Ethics statement
The study protocol was approved by the institutional review board at Samsung Medical Center, Seoul, Korea (IRB No. 2016-01-053). The need for informed consent was waived by the board.
RESULTS
Patients
Patient characteristics are summarized in Table 1. A total of 28 patients (18 high-risk and 10 recurrent) received single or tandem HDCT/auto-SCT. Eight patients were diagnosed with sarcomas of bone origin, and 20 patients were diagnosed with soft tissue sarcomas. Ewing sarcoma family of tumor was the most common diagnosis followed by osteosarcoma, synovial sarcoma, and desmoplastic small round cell tumor. Of the 18 patients with high-risk sarcomas, 16 had metastases at diagnosis, and the remaining 2 patients had incomplete excision of their primary tumors.
Table 1. Patient characteristics.
| Characteristics | n = 28 |
|---|---|
| Sex | |
| Male/female | 18/10 |
| Median age at transplant, yr (range) | 16.3 (1.3–21.4) |
| Diagnoses, No. (%) | |
| Ewing sarcoma family | 11 (39.3) |
| Osteosarcoma | 6 (21.4) |
| Synovial sarcoma | 3 (10.7) |
| Desmoplastic small round cell tumor | 3 (10.7) |
| Rhabdomyosarcoma | 2 (7.1) |
| Others* | 3 (10.7) |
| Treatment prior to transplant, No. (%) | |
| Chemotherapy alone | 8 (28.6) |
| Chemotherapy + Surgery | 10 (35.7) |
| Chemotherapy + Radiotherapy | 6 (21.4) |
| Chemotherapy + Surgery + Radiotherapy | 4 (14.3) |
| Primary site of tumor, No. (%) | |
| Soft tissue origin | 20 (71.4) |
| Bone origin | 8 (28.6) |
| Cause of HDCT/auto-SCT, No. (%) | |
| High-risk tumor | 18 (64.3) |
| Recurrent tumor | 10 (35.7) |
| Tumor status prior to HDCT/auto-SCT, No. (%) | |
| Complete response | 15 (53.6) |
| Partial response | 5 (17.9) |
| Stable disease | 2 (7.1) |
| Progressive disease | 6 (21.4) |
HDCT/auto-SCT, high-dose chemotherapy and autologous stem cell transplantation.
*Others included malignant peripheral nerve sheath tumor (n = 1), epithelioid inflammatory myofibroblastic sarcoma (n = 1), and epithelioid sarcoma (n = 1).
HDCT/auto-SCT
Patients received multimodal treatment, including chemotherapy, radiotherapy, and surgical resection, before receiving HDCT/auto-SCT as a consolidation treatment (Table 1). Single and tandem HDCT/auto-SCT were initially planned for 5 and 23 patients, respectively. Among the 23 patients intended to have tandem HDCT/auto-SCT, only 14 patients underwent the second HDCT/auto-SCT, and the remaining 9 did not have the second HDCT/auto-SCT due to tumor progression after the first HDCT/auto-SCT (n = 3), parent refusal (n = 3), poor general condition (n = 2), and toxic death during the first HDCT/auto-SCT (n = 1).
A variety of HDCT regimens were employed (Table 2). We allowed a 12-week interval between the first and second HDCT/auto-SCT to prevent toxic deaths in the second HDCT/auto-SCT.
Table 2. High-dose chemotherapy regimens.
| Regimens | Drugs | Dose | Schedule | Total dose |
|---|---|---|---|---|
| First HDCT regimens | ||||
| CTE | Carboplatin | 500 mg/m2/day | Days -8, -7, and -6 | 1,500 mg/m2 |
| Thiotepa | 300 mg/m2/day | Days -5, -4, and -3 | 900 mg/m2 | |
| Etoposide | 250 mg/m2/day | Days -5, -4, and -3 | 750 mg/m2 | |
| MEC | Melphalan | 35 mg/m2/day | Days -7, -6, -5, and -4 | 140 mg/m2 |
| Etoposide | 60 mg/kg/day | Day -3 | 60 mg/kg | |
| Carboplatin | 500 mg/m2/day | Days -4, -3, and -2 | 1,500 mg/m2 | |
| CEC | Carboplatin | 650 mg/m2/day | Days -7, -6, and -5 | 1,950 mg/m2 |
| Etoposide | 650 mg/m2/day | Days -7, -6, and -5 | 1,950 mg/m2 | |
| Cyclophosphamide | 1,800 mg/m2/day | Days -4, -3, and -2 | 5,400 mg/m2 | |
| Second HDCT regimens | ||||
| CM | Cyclophosphamide | 1,500 mg/m2/day | Days -8, -7, -6, and -5 | 6,000 mg/m2 |
| Melphalan | 60 mg/m2/day | Days -4, -3, and -2 | 180 mg/m2 | |
| TBI-CM | Total body irradiation | 3.33 Gy/day | Days -9, -8, and -7 | 9.99 Gy |
| Cyclophosphamide | 1,000 mg/m2/day | Days -6, -5, and -4 | 3,000 mg/m2 | |
| Melphalan | 60 mg/m2/day | Days -3, and -2 | 120 mg/m2 |
Hematologic recovery
For tandem HDCT/auto-SCT, roughly half of the collected stem cells were infused for marrow rescue during each HDCT/auto-SCT. A median of 2.4 × 106 CD34+ cells/kg (range, 1.1–17.0) were infused for the first HDCT/auto-SCT, and the median times required to reach an absolute neutrophil count (ANC) of 0.5 × 109/L and a platelet count of 20 × 109/L without transfusion over the previous 7 days were 10 days (range, 8–13) and 23 days (range, 14–48), respectively. For the second HDCT/auto-SCT, a median of 3.2 × 106 CD34+ cells/kg (range, 1.1–17.0) were infused, and the median times required to reach an absolute neutrophil count (ANC) of 0.5 × 109/L and a platelet count of 20 × 109/L was 10 days (range, 9–13) and 23 days (range, 16–251), respectively. For the 14 patients who received tandem HDCT/auto-SCT, there was no statistical difference of time to engraftment of either neutrophils or platelets between the first and second HDCT/auto-SCT (ANC: 10.0 vs. 10.5 days, P = 0.541; platelet: 21.0 vs. 26.0 days, P = 0.438, respectively).
Toxicities
Acute grade 3–4 toxicities during HDCT/auto-SCT are summarized in Table 3. Frequent toxicities were fever, stomatitis, hypokalemia, elevated liver enzymes, and diarrhea. Two treatment-related mortalities (7.1%) were noted during the first HDCT/auto-SCT, and those deaths were attributed to hepatic veno-occlusive disease (n = 1) and sepsis (n = 1). There were no toxic deaths during the second HDCT/auto-SCT.
Table 3. Acute grade 3–4 toxicities during HDCT/auto-SCT.
| Parameters | First HDCT/auto-SCT (n = 28) | Second HDCT/auto-SCT (n = 14) |
|---|---|---|
| ≥ 1 episode of fever (≥ 38.0℃) | 27 (96.4%) | 10 (71.4%) |
| Days of fever (≥ 38.0℃), median (range) | 5.5 (1-18) | 2 (0-8) |
| Positive blood culture | 3 (10.7%) | 1 (7.1%) |
| Stomatitis | 18 (64.3%) | 3 (21.4%) |
| Vomiting | 6 (21.4%) | 2 (14.3%) |
| Diarrhea | 9 (32.1%) | 3 (21.4%) |
| Liver enzyme elevation | 12 (42.9%) | 1 (7.1%) |
| Hyperbilirubinemia | 5 (17.9%) | 1 (7.1%) |
| Renal insufficiency | 0 (0%) | 0 (0%) |
| Hypokalemia | 13 (46.4%) | 5 (35.7%) |
| Hyperkalemia | 0 (0%) | 0 (0%) |
| Hepatic veno-occlusive disease | 1 (3.6%) | 0 (0%) |
| Treatment-related mortality | 2 (7.1%) | 0 (0%) |
HDCT/auto-SCT, high-dose chemotherapy and autologous stem cell transplantation.
Tumor response
Tumor responses before and after HDCT/auto-SCT were summarized in Table 4. Among the 15 patients who achieved complete remission (CR) to prior therapy, all evaluable patients except 1 (patient #17) who showed progressive disease (PD) after first HDCT/auto-SCT remained CR shortly after the HDCT/auto-SCT, and 5 of them still alive without disease.
Table 4. Detailed treatment flow for individual patients.
| Patient # | Diagnosis | Sex | Age, yr | Stage | Pre-HDCT treatment | Tumor status | 1st HDCT regimen | 2nd HDCT regimen | Event, mon* | Outcome, mon* | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prior to 1st HDCT | After 1st HDCT | 3 months after 2nd HDCT | |||||||||||
| HR | 1 | Epithelioid sarcoma | F | 13.6 | 4 | CTx + Surgery + RTx | SD | PD | — | MEC | — | Progression (2) | DOD (25) |
| 2 | Osteosarcoma | F | 14.7 | 4B | CTx + RTx | PD | PD | — | MEC | — | Progression (3) | DOD (9) | |
| 3 | Ewing | M | 0.6 | 1 | CTx | CR | CR | CR | CTE | CM | — | NED (66) | |
| 4 | Osteosarcoma | M | 14.3 | 4 | CTx + Surgery + RTx | CR | — | — | MEC | — | TRM (2) | DOC (2) | |
| 5 | Ewing | M | 17.1 | 4 | CTx + Surgery | CR | CR | — | MEC | — | Relapse (5) | DOD (13) | |
| 6 | Ewing | M | 14.6 | 4 | CTx + Surgery | PR | SD | — | MEC | — | — | NED (57) | |
| 7 | DSRCT | M | 17.0 | 4 | CTx | PR | PD | SD | CTE | CM | Progression (5) | DOD (9) | |
| 8 | Ewing | M | 16.0 | 4 | CTx + Surgery | CR | CR | CR | CTE | CM | Relapse (17) | DOD (47) | |
| 9 | Ewing | M | 16.2 | 4 | CTx + Surgery | CR | CR | CR | CTE | CM | Relapse (17) | DOD (24) | |
| 10 | Ewing | F | 8.3 | 4 | CTx + Surgery | PD | — | — | CTE | — | TRM (0) | DOC (0) | |
| 11 | DSRCT | M | 14.4 | 4 | CTx + Surgery | CR | CR | — | CTE | — | Relapse (7) | DOD (16) | |
| 12 | Synovial sarcoma | F | 16.9 | 4 | CTx + Surgery + RTx | PD | PD | PD | CTE | CM | Progression (2) | Follow up loss (18) | |
| 13 | RMS | M | 16.8 | 4 | CTx + RTx | CR | CR | CR | CTE | CM | Relapse (8) | Follow up loss (25) | |
| 14 | Osteosarcoma | F | 10.9 | 1A | CTx + Surgery | CR | CR | CR | CTE | CM | — | NED (18) | |
| 15 | Ewing | M | 18.7 | 4 | CTx + RTx | SD | SD | PD | CTE | CM | Progression (2) | DOD (9) | |
| 16 | DSRCT | M | 9.7 | 3 | CTx + RTx | CR | CR | — | CTE | — | — | NED (14) | |
| 17 | Inflammatory myofibroblastic tumor | M | 16.3 | 4 | CTx + Surgery | CR | PD | — | CTE | — | Relapse (3) | DOD (8) | |
| 18 | Synovial sarcoma | F | 11.8 | 4 | CTx + RTx | PR | SD | SD | CTE | TBI-CM | Progression (6) | AWD (6) | |
| Relapse | 19 | Ewing | F | 5.7 | 4 | CTx + RTx | PD | PD | — | CEC | — | Progression (1) | DOD (3) |
| 20 | Ewing | M | 13.5 | 4 | CTx | PD | SD | — | CTE | — | Progression (5) | DOD (5) | |
| 21 | MPNST | M | 13.7 | 1 | CTx + Surgery + RTx | PR | PD† | — | CTE | — | — | NED (37) | |
| 22 | Osteosarcoma | M | 14.5 | 4B | CTx | CR | CR | CR | CTE | CM | — | NED (29) | |
| 23 | Ewing | M | 11.6 | 4B | CTx | PR | SD | SD | CTE | CM | — | NED (24) | |
| 24 | Ewing | M | 7.7 | 4 | CTx + Surgery | CR | CR | — | CTE | — | Relapse (7) | DOD (11) | |
| 25 | RMS | M | 0.3 | 4 | CTx | CR | CR | CR | CTE | CM | Relapse (12) | AWD (24) | |
| 26 | Osteosarcoma | F | 16.0 | 4B | CTx + Surgery | CR | CR | CR | CTE | CM | Relapse (12) | Follow up loss (17) | |
| 27 | Osteosarcoma | F | 14.5 | 1B | CTx | SD | SD | — | CTE | — | Progression (5) | DOD (11) | |
| 28 | Synovial sarcoma | F | 15.4 | 4 | CTx + Surgery | CR | CR | CR | CTE | CM | — | NED (14) | |
HDCT, high-dose chemotherapy; HR, high-risk; CTx, chemotherapy; RTx, radiotherapy; SD, stable disease; PD, progressive disease; MEC, melphalan + etoposide + carboplatin; DOD, died of disease; Ewing, Ewing sarcoma family; CR, complete remission; CTE, carboplatin + thiotepa + etoposide; CM, cyclophosphamide + melphalan; NED, no evidence of disease; TRM, treatment-related mortality; DOC, died from other cause; PR, partial remission; RMS, rhabdomyosarcoma; DSRCT, desmoplastic small round-cell tumor; TBI, total body irradiation; AWD, alive with disease; CEC, carboplatin + etoposide + cyclophosphamide; MPNST, malignant peripheral nerve sheath tumor.
*Months from date of last high-dose chemotherapy and autologous stem cell transplantation. †This patient showed increased enhancing lesion after the HDCT/auto-SCT and seemed to have PD, but the lesion decreased without any treatment. The increased enhancing lesion was retrospectively suspected as radiotherapy related change.
Five patients achieved partial remission (PR) before HSCT/auto-SCT, and 2 of them (patient #6 and #23) showed stable disease (SD) after the HDCT and both of them are alive without tumor progression with 57 and 24 months of follow up duration, respectively. One patient (patient #21) with malignant peripheral nerve sheath tumor (MPNST) in the brain showed increased enhancing lesion after the HDCT/auto-SCT and seemed to have PD, but the lesion decreased without any treatment showing long-term survival of the patient. The increased enhancing lesion in the brain was retrospectively suspected as radiotherapy related change. Overall, 3 of 5 who showed PR to prior therapy remained alive without progression after HDCT/auto-SCT. Among the 8 patients showed SD or PD after prior therapy, 3 patients showed SD during HDCT but finally all of them showed disease progression.
Events and survival
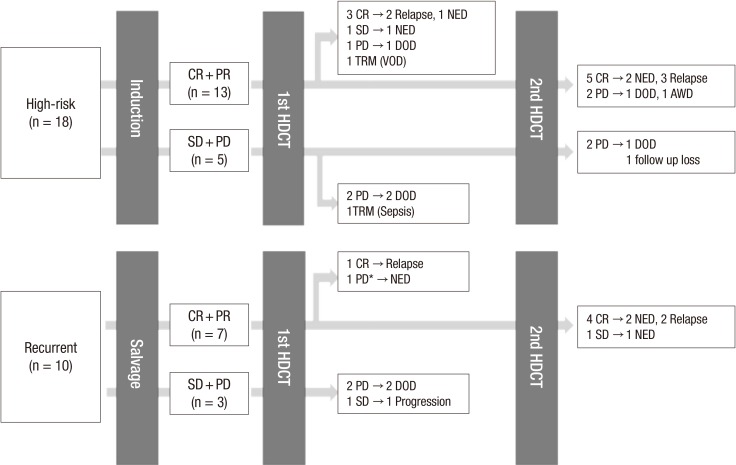
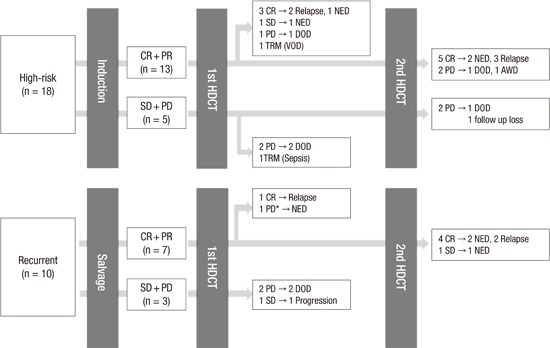
Summary of treatment flow and outcome was illustrated in Fig. 1. The median follow-up duration from the first HDCT/auto-SCT for all patients was 15.3 months (range, 0.0–66.3 months). Eighteen patients exhibited disease progression and 2 patients died of treatment-related toxicities. Overall, 8 patients remained alive without disease. By disease category, 3 of 11 patients with Ewing sarcoma family of tumor, 2 of 6 with osteosarcoma, 1 of 3 with synovial sarcoma, 1 of 3 with desmoplastic small round cell tumor, and 1 of 3 with other sarcomas were alive without disease. Of them, 1 patient (patient #16), who was diagnosed with desmoplastic small round cell tumor, had a huge pelvic mass with multiple peritoneal seedings and received chemotherapy, surgery, and whole abdominal radiotherapy followed by single HDCT/auto-SCT. One patient (patient #28) with synovial sarcoma relapsed with lung metastases 18 months after previous treatment and received salvage chemotherapy and surgery followed by tandem HDCT/auto-SCT. Detailed patient information is shown in Table 4.
Fig. 1.
Overview of treatment flow and outcome. Treatment flow and outcome of all patients were illustrated. Overall, 8 patients (4 high-risk and 4 recurrent tumor) who achieved complete remission to prior therapy remained progression-free after high dose chemotherapy.
CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; HDCT, high-dose chemotherapy; NED, no evidence of disease; DOD, died of disease; TRM, treatment-related mortality; AWD, alive with disease.
*This patient showed increased enhancing lesion after the HDCT/auto-SCT and seemed to have PD, but the lesion decreased without any treatment. The increased enhancing lesion was retrospectively suspected as radiotherapy related change.
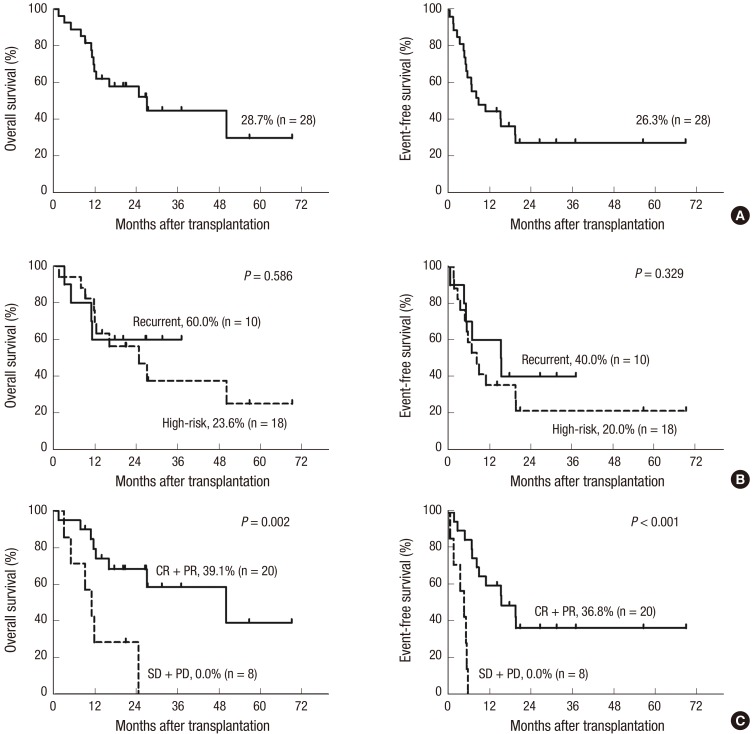
The 3-year OS and EFS rates for all patients were 28.7% (95% confidence interval [CI], 23.1%–45.7%) and 26.3% (95% CI, 13.4%–34.5%), respectively (Fig. 2A). There were no differences in OS and EFS between patients with tumors of bone origin or those with soft tissue sarcomas (OS: 50.0% vs. 28.3%, P = 0.776; EFS: 37.5% vs. 20.6%, P = 0.578). There were also no differences in OS and EFS between high-risk and recurrent tumors (OS: 23.6% vs. 60.0%, P = 0.586; EFS: 20.0% vs. 40.0%, P = 0.329) (Fig. 2B). However, tumor status prior to HDCT/auto-SCT was a significant predictor of outcomes after HDCT/auto-SCT. The OS and EFS rates were significantly higher for patients who achieved CR or PR to prior therapy compared to those for patients with SD or PD after previous treatment (OS: 39.1% vs. 0.0%, P = 0.002; EFS: 36.8% vs. 0.0%, P < 0.001) (Fig. 2C).
Fig. 2.
Survival graph of all patients. The overall survival (OS) and event-free survival (EFS) rates, which were calculated from the date of transplantation, for all 28 patients are 28.7% (95% confidence interval [CI], 23.1%–45.7%) and 26.3% (95% CI, 13.4%–34.5%), respectively (A). There are no differences in OS and EFS between high-risk and recurrent tumors (B). The OS and EFS rates are significantly higher in patients who achieved complete remission (CR) or partial remission (PR) to prior therapy compared with those in patients who had stable disease (SD) or progressive disease (PD) after prior therapy (C).
DISCUSSION
The HDCT/auto-SCT treatment strategy is based on the hypothesis that dose escalation might improve survival of children with high-risk or recurrent/refractory solid tumors. This strategy has shown clinical benefit in some children with high-risk or recurrent solid tumors. Neuroblastoma is the model disease for which patients benefit from HDCT/auto-SCT. With the introduction of HDCT/auto-SCT, neuroblastoma patient outcomes significantly improved in many studies (15,16,17,18). Our institute also has reported several studies using HDCT/auto-SCT in patients with neuroblastoma and brain tumors, and these studies showed feasible outcomes and tolerable treatment-related toxicities (19,20,21).
The clinical and biologic characteristics of bone and soft tissue sarcoma are different from those of neuroblastoma or pediatric brain tumor, and therefore, the success of HDCT/autoSCT in these tumors would not guarantee the usefulness of HDCT/autoSCT in bone and soft tissue sarcoma. However, the options available in the setting of high-risk or recurrent sarcoma are quite limited. For this reason, HDCT/auto-SCT was used to treat patients with high-risk or recurrent bone and soft tissue sarcoma, expecting improved survival, and the clinical outcomes from this treatment were reviewed in this study. In our study, the toxicities of single or tandem HDCT/auto-SCT were tolerable showing 7.1% of treatment-related mortality. The 3-year OS and EFS rates for all patients were 28.7% and 26.3%, respectively, and patients who achieved CR or PR prior to HDCT/auto-SCT had better outcomes.
Many previous studies investigated HDCT/auto-SCT effectiveness for various advanced bone and soft tissue sarcomas but yielded inconclusive results (12,13,14), and there were studies that showed promising results for HDCT/auto-SCT. Matsubara et al. (22) reported the results of HDCT/auto-SCT in 22 patients with advanced rhabdomyosarcoma, and 8 (36.4%) were alive without evidence of disease. The 5-year OS rate was 70% for the 14 patients who were in CR at the time of HDCT/auto-SCT. In another study of patients with Ewing tumors (23), tandem (n = 13) or single (n = 7) HDCT/auto-SCT was performed in 20 patients, yielding OS and EFS rates of 45% and 47%, respectively. For osteosarcoma, Hong et al. (24) reported the result of HDCT/auto-SCT in 19 patients with high-risk osteosarcoma, and the OS and EFS were 78.3% and 67.4%, at a median follow-up of 31 months from HDCT/auto-SCT. In a study of 36 patients with desmoplastic small round cell tumors treated with HDCT/auto-SCT (25), the 3-year OS was 57% in patients who achieved CR before HDCT/auto-SCT.
In our cohort, patients with CR or PR prior to transplant had better outcomes from HDCT/auto-SCT. Similarly, the data from the French group showed significantly better outcomes for adult patients with advanced soft tissue sarcomas who achieved CR before HDCT/auto-SCT (26). In addition, Kasper et al. (27) reported that patients with advanced sarcoma who had no evidence of disease before HDCT/auto-SCT gained survival benefits. Therefore, pre-HDCT/auto-SCT disease status may be one of the most important factors in predicting outcomes for patients with advanced stage or recurrent sarcomas. In our study, some patients proceeded to HDCT/auto-SCT despite progressive disease after prior therapy because there was no further option for the patients. However, since patients achieving a CR or PR prior to transplant had better outcomes after HDCT/autoSCT in our study, it would be better to attempt to decrease tumor burden before HDCT/auto-SCT. HDCT/auto-SCT can be carefully employed as consolidation therapy for patients who achieved CR or PR with prior therapy. A new therapeutic approach to decrease tumor burden, such as targeted therapy, could be considered in patients who cannot achieve a CR or PR with conventional treatment.
The frequent toxicities during the HDCT/auto-SCT in this study were mucositis-related toxicity, isolated elevated liver enzymes and fever, which were manageable without complications. Toxic death occurred in 2 patients during the first HDCT/auto-SCT showing 7.1% of toxic death rate. This data suggests the feasibility of HDCT/auto-SCT with tolerable toxicities. Half of our patients underwent tandem HDCT/auto-SCT. Definite conclusions regarding tandem HDCT could not be made because of the small number of patients, but toxicities were tolerable during the second HDCT/auto-SCT, yielding no treatment-related mortality.
Our study has the inherent limitations of being a retrospective study with small patient number. High-risk patients showed similar outcome with recurrent patients, but it can be partly because of the limitation of retrospective study in that high-risk patients who showed poor response to prior therapy could be selectively preceded to HDCT/auto-SCT in practical clinical setting. A prospective study with specific criteria for enrollment and larger patient number is needed to confirm the effectiveness of HDCT/auto-SCT in patients with bone and soft tissue sarcomas.
In conclusion, HDCT/auto-SCT in patients with high-risk or recurrent bone and soft tissue sarcomas was feasible, and patients might benefit from tandem HDCT/auto-SCT if they can achieve CR or PR prior to HDCT/auto-SCT. Therefore, careful selection of patients who can benefit from HDCT/auto-SCT with maximal effort to reduce tumor burden prior to HDCT/auto-SCT will be required to improve outcomes for patients with high-risk or recurrent bone and soft tissue sarcomas.
Footnotes
DISCLOSURE: There are no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study design: Lee JW. Data acquisition: Choi YB, Yi ES, Yoo KH. Data analysis: Choi YB, Sung KW, Koo HH. Writing: Choi YB. Review and revision: Lee JW, Sung KW, Koo HH. Approval of final manuscript: all authors.
References
- 1.HaDuong JH, Martin AA, Skapek SX, Mascarenhas L. Sarcomas. Pediatr Clin North Am. 2015;62:179–200. doi: 10.1016/j.pcl.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 3.Meyers PA. High-dose therapy with autologous stem cell rescue for pediatric sarcomas. Curr Opin Oncol. 2004;16:120–125. doi: 10.1097/00001622-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Miser JS, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA, et al. Treatment of metastatic Ewing's sarcoma or primitive neuroectodermal tumor of bone: evaluation of combination ifosfamide and etoposide--a Children's Cancer Group and Pediatric Oncology Group study. J Clin Oncol. 2004;22:2873–2876. doi: 10.1200/JCO.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 5.Weigel BJ, Lyden E, Anderson JR, Meyer WH, Parham DM, Rodeberg DA, Michalski JM, Hawkins DS, Arndt CA. Intensive multiagent therapy, including dose-compressed cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, irinotecan, and radiation, in patients with high-risk rhabdomyosarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2016;34:117–122. doi: 10.1200/JCO.2015.63.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim NK, Kim HS, Suh CO, Kim HO, Lyu CJ. Clinical results of high-dose chemotherapy followed by autologous peripheral blood stem cell transplantation in children with advanced stage rhabdomyosarcoma. J Korean Med Sci. 2012;27:1066–1072. doi: 10.3346/jkms.2012.27.9.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JE, Kang J, Yoo KH, Sung KW, Koo HH, Lim DH, Shin HJ, Kang HJ, Park KD, Shin HY, et al. Efficacy of high-dose chemotherapy and autologous stem cell transplantation in patients with relapsed medulloblastoma: a report on the Korean Society for Pediatric Neuro-Oncology (KSPNO)-S-053 study. J Korean Med Sci. 2010;25:1160–1166. doi: 10.3346/jkms.2010.25.8.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frei E, 3rd, Canellos GP. Dose: a critical factor in cancer chemotherapy. Am J Med. 1980;69:585–594. doi: 10.1016/0002-9343(80)90472-6. [DOI] [PubMed] [Google Scholar]
- 9.Burke MJ, Walterhouse DO, Jacobsohn DA, Duerst RE, Kletzel M. Tandem high-dose chemotherapy with autologous peripheral hematopoietic progenitor cell rescue as consolidation therapy for patients with high-risk Ewing family tumors. Pediatr Blood Cancer. 2007;49:196–198. doi: 10.1002/pbc.21182. [DOI] [PubMed] [Google Scholar]
- 10.Loschi S, Dufour C, Oberlin O, Goma G, Valteau-Couanet D, Gaspar N. Tandem high-dose chemotherapy strategy as first-line treatment of primary disseminated multifocal Ewing sarcomas in children, adolescents and young adults. Bone Marrow Transplant. 2015;50:1083–1088. doi: 10.1038/bmt.2015.118. [DOI] [PubMed] [Google Scholar]
- 11.Nath SV, Prince HM, Choong PF, Toner GC. Durable remissions are rare following high dose therapy with autologous stem cell transplantation for adults with “paediatric” bone and soft tissue sarcomas. Int Semin Surg Oncol. 2005;2:12. doi: 10.1186/1477-7800-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasper B, Lehnert T, Bernd L, Mechtersheimer G, Goldschmidt H, Ho AD, Egerer G. High-dose chemotherapy with autologous peripheral blood stem cell transplantation for bone and soft-tissue sarcomas. Bone Marrow Transplant. 2004;34:37–41. doi: 10.1038/sj.bmt.1704520. [DOI] [PubMed] [Google Scholar]
- 13.Meyers PA, Krailo MD, Ladanyi M, Chan KW, Sailer SL, Dickman PS, Baker DL, Davis JH, Gerbing RB, Grovas A, et al. High-dose melphalan, etoposide, total-body irradiation, and autologous stem-cell reconstitution as consolidation therapy for high-risk Ewing's sarcoma does not improve prognosis. J Clin Oncol. 2001;19:2812–2820. doi: 10.1200/JCO.2001.19.11.2812. [DOI] [PubMed] [Google Scholar]
- 14.Sauerbrey A, Bielack S, Kempf-Bielack B, Zoubek A, Paulussen M, Zintl F. High-dose chemotherapy (HDC) and autologous hematopoietic stem cell transplantation (ASCT) as salvage therapy for relapsed osteosarcoma. Bone Marrow Transplant. 2001;27:933–937. doi: 10.1038/sj.bmt.1703023. [DOI] [PubMed] [Google Scholar]
- 15.Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, Klingebiel T, Kremens B, Schilling FH, Schrappe M, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 16.George RE, Li S, Medeiros-Nancarrow C, Neuberg D, Marcus K, Shamberger RC, Pulsipher M, Grupp SA, Diller L. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006;24:2891–2896. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- 17.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 18.Pritchard J, Cotterill SJ, Germond SM, Imeson J, de Kraker J, Jones DR. High dose melphalan in the treatment of advanced neuroblastoma: results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Pediatr Blood Cancer. 2005;44:348–357. doi: 10.1002/pbc.20219. [DOI] [PubMed] [Google Scholar]
- 19.Sung KW, Lee SH, Yoo KH, Jung HL, Cho EJ, Koo HH, Lee SK, Kim J, Lim DH, Suh YL, et al. Tandem high-dose chemotherapy and autologous stem cell rescue in patients over 1 year of age with stage 4 neuroblastoma. Bone Marrow Transplant. 2007;40:37–45. doi: 10.1038/sj.bmt.1705691. [DOI] [PubMed] [Google Scholar]
- 20.Sung KW, Lim H, Lee SH, Yoo KH, Koo HH, Kim JH, Suh YL, Joung YS, Shin HJ. Tandem high-dose chemotherapy and autologous stem cell transplantation for anaplastic ependymoma in children younger than 3 years of age. J Neurooncol. 2012;107:335–342. doi: 10.1007/s11060-011-0745-8. [DOI] [PubMed] [Google Scholar]
- 21.Sung KW, Son MH, Lee SH, Yoo KH, Koo HH, Kim JY, Cho EJ, Lee SK, Choi YS, Lim DH, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk neuroblastoma: results of SMC NB-2004 study. Bone Marrow Transplant. 2013;48:68–73. doi: 10.1038/bmt.2012.86. [DOI] [PubMed] [Google Scholar]
- 22.Matsubara H, Makimoto A, Higa T, Kawamoto H, Takayama J, Ohira M, Yokoyama R, Beppu Y, Takaue Y. Possible benefits of high-dose chemotherapy as intensive consolidation in patients with high-risk rhabdomyosarcoma who achieve complete remission with conventional chemotherapy. Pediatr Hematol Oncol. 2003;20:201–210. [PubMed] [Google Scholar]
- 23.Rosenthal J, Bolotin E, Shakhnovits M, Pawlowska A, Falk P, Qian D, Oliver C, Sato J, Miser J, Forman S. High-dose therapy with hematopoietic stem cell rescue in patients with poor prognosis Ewing family tumors. Bone Marrow Transplant. 2008;42:311–318. doi: 10.1038/bmt.2008.169. [DOI] [PubMed] [Google Scholar]
- 24.Hong CR, Kang HJ, Kim MS, Ju HY, Lee JW, Kim H, Kim HS, Park SH, Park KD, Park JD, et al. High-dose chemotherapy and autologous stem cell transplantation with melphalan, etoposide and carboplatin for high-risk osteosarcoma. Bone Marrow Transplant. 2015;50:1375–1378. doi: 10.1038/bmt.2015.145. [DOI] [PubMed] [Google Scholar]
- 25.Cook RJ, Wang Z, Arora M, Lazarus HM, Kasow KA, Champagne MA, Saber W, van Besien KM, Hale GA, Copelan EA, et al. Clinical outcomes of patients with desmoplastic small round cell tumor of the peritoneum undergoing autologous HCT: a CIBMTR retrospective analysis. Bone Marrow Transplant. 2012;47:1455–1458. doi: 10.1038/bmt.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blay JY, Bouhour D, Ray-Coquard I, Dumontet C, Philip T, Biron P. High-dose chemotherapy with autologous hematopoietic stem-cell transplantation for advanced soft tissue sarcoma in adults. J Clin Oncol. 2000;18:3643–3650. doi: 10.1200/JCO.2000.18.21.3643. [DOI] [PubMed] [Google Scholar]
- 27.Kasper B, Dietrich S, Mechtersheimer G, Ho AD, Egerer G. Large institutional experience with dose-intensive chemotherapy and stem cell support in the management of sarcoma patients. Oncology. 2007;73:58–64. doi: 10.1159/000120629. [DOI] [PubMed] [Google Scholar]