Abstract
Kidney length is the most useful parameter for clinical measurement of kidney size, and is useful to distinguish acute kidney injury from chronic kidney disease. In this prospective observational study of 437 normal children aged between 0 and < 13 years, kidney length was measured using sonography. There were good correlations between kidney length and somatic values, including age, weight, height, and body surface area. The rapid growth of height during the first 2 years of life was intimately associated with a similar increase in kidney length, suggesting that height should be considered an important factor correlating with kidney length. Based on our findings, the following regression equation for the reference values of bilateral kidney length for Korean children was obtained: kidney length of the right kidney (cm) = 0.051 × height (cm) + 2.102; kidney length of the left kidney (cm) = 0.051 × height (cm) + 2.280. This equation may aid in the diagnosis of various kidney disorders.
Keywords: Kidney, Length, Height, Child, Ultrasonography
Graphical Abstract
INTRODUCTION
Congenital anomalies of the kidney and urinary tract are relatively common, accounting for approximately 20%-30% of all anomalies identified in the prenatal period (1). Among the congenital anomalies of the kidney and urinary tract, reflux nephropathy, obstructive uropathy, and renal agenesis/hypoplasia/dysplasia are responsible for 30%-50% of end-stage renal disease in children (2). Renal hypoplasia is defined as congenitally small kidneys with a reduced number of nephrons but normal architecture, and can be clinically diagnosed based on renal ultrasonography findings as a small-sized kidney of 2 standard deviations below the expected mean of an age-matched normal individual (3). In addition, mild renal hypoplasia at the lower expected normal range is associated with hypertension and chronic kidney disease (CKD) in adults (3). Therefore, it is important to clinically diagnose renal hypoplasia in children.
To diagnose renal hypoplasia in children, the size of the kidneys should be measured. While renal volume is the most accurate parameter of kidney size, kidney length is the most useful parameter for clinical measurement of kidney size because it is simple to obtain and is minimally affected by interobserver variability (4,5). Kidney length is also useful to distinguish patients with acute kidney injury from those with CKD (6). It is known that the renal length and calculated volume are influenced by the overall body size of the individual, including the age, height, weight, and body surface area (BSA) (7,8,9). Since 1980, several researchers have reported the renal length in normal children; however, Korean reference data from national studies are lacking. Therefore, we conducted the present study to determine the renal size in children by ultrasound. Here, we present our results along with a review of the available literature on kidney size measurements.
MATERIALS AND METHODS
This prospective observational study involved 437 healthy children aged less than 13 years who were born at full-term and were examined in the National Health Screening Program for infants and children in Korea, who visited for vaccination or were discharged after admission for unrelated health problems between July 2012 and May 2014. Subjects whose weight-for-age and height-for-age were in the 5th to 95th percentiles based on the 2007 Korean National Growth Charts (10), without any clinical history of precocious puberty; urinary tract infection; chronic illness, including neurologic, oncologic, immunologic, gastroenterologic, or nephrologic conditions; and abnormal kidney ultrasonography findings (e.g., single kidney, dysplastic kidney, renal cyst, hydronephrosis, ureter duplication) were included.
The examinations were prospectively carried out using kidney ultrasonography by two pediatric radiologists. All 437 patients were scanned using an iU22 ultrasound machine (Philips, Bothell, WA, USA) with 4-9 MHz convex transducers. The largest longitudinal dimension of both kidneys was measured in the longitudinal view, passing through the renal hilum, with the subjects in the prone or supine oblique position (Fig. 1).
Fig. 1.
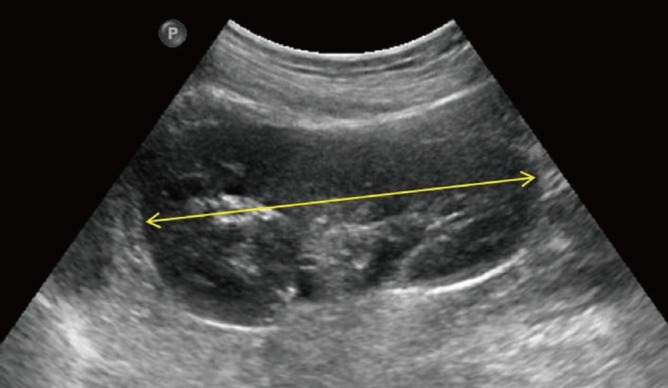
Maximum longitudinal length of the left kidney passing through the renal hilum (yellow arrow).
The relationships of renal length with age, sex, body weight, height, BSA, and body mass index (BMI) were analyzed, and renal growth curves according to age, height, weight and BSA were obtained.
Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was considered at P < 0.05. Linear regression analysis was used to assess the correlations of renal length with age, sex, body weight, height, BSA, and BMI. Right-left asymmetry in renal length was assessed using the paired t-test.
Ethics statement
The study protocol was approved by the institutional review board in Jeju National University Hospital (IRB file no. 2012-05-007-004). Written informed consent was obtained from all subjects.
RESULTS
There were 247 (56.5%) and 190 (43.5%) males and females, respectively. The age distribution ranged from 3 days to 12.7 years. There were 228 (52.2%) and 209 (47.8%) subjects aged less and more than 2 years, respectively. The left kidney was significantly longer than the right (P < 0.001); the mean values of the right and left kidneys were 6.8 ± 1.5 cm and 7.0 ± 1.5 cm, respectively.
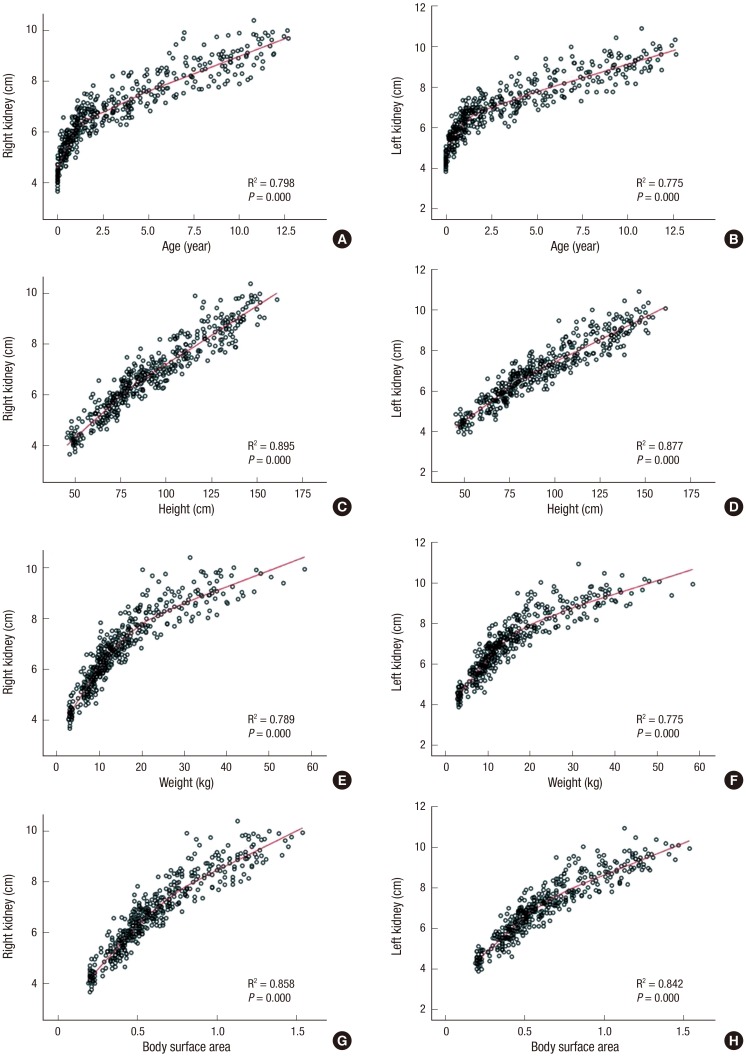
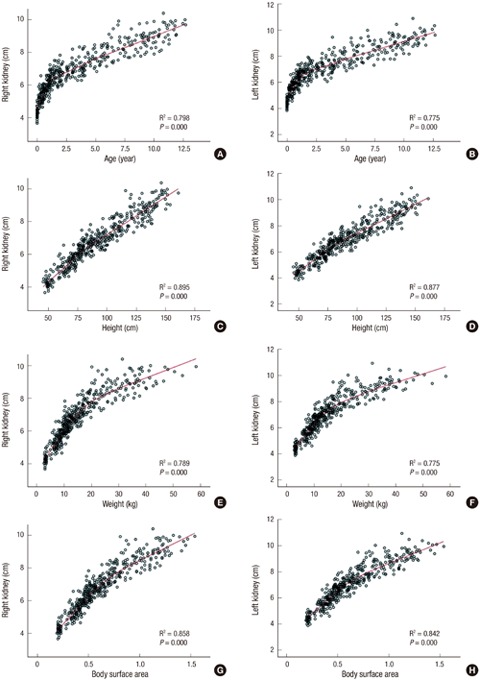
There was no consistent difference in kidney length according to sex. However, there were good correlations between kidney length and other somatic values, including age, weight, height, and BSA (Fig. 2; linear regression analysis, P < 0.01 for all). On the other hand, the correlation between BMI and kidney length was not significant, with low R2 correlation coefficients of 0.093 and 0.097 for the right and left kidney, respectively.
Fig. 2.
Renal growth curve according to the child’s age, height, weight, and body surface area.
The renal length curve for age showed different patterns before and after 20 months of age (Fig. 2). In other words, the kidney was growing rapidly during the first 24 months, while the growth rate slowed down thereafter. The strongest influencing factor among these somatic values was height. Using this parameter, the following linear regression equation was obtained: right kidney length (cm) = 0.051 × height (cm) + 2.102; left kidney length (cm) = 0.051 × height (cm) + 2.280.
DISCUSSION
Sonography can be easily and safely performed, without radiation exposure in children. Renal sonography can provide important information regarding for example renal anomalies and indirect renal function. For this reason, accurate reference values of renal length measured by sonography are important in children. A major strength of the present study is the analysis of a pediatric healthy cohort; therefore, the measurement values can be considered representative. Moreover, the most valuable point of the present study is that almost 50% of the enrolled children were aged less than 24 months; hence, more accurate reference values could be obtained for these subjects.
Many studies have shown that height is the strongest factor related with kidney length (7,11,12,13). Furthermore, according to the current study, the renal growth curve might be biphasic, with 20 months as its starting point, indicating that height should be considered an important factor correlating with kidney length (Fig. 2). In fact, a previous study concluded that the reason for this important relationship is that the rapid growth in height during the first 2 years of life is intimately associated with a similar increase in kidney length (7). Our renal growth curve showed a biphasic pattern for the relationship with age, and a linear pattern for the relationship with height. Therefore, our linear regression equation for bilateral renal length according to height is simpler than similar equations from prior studies (11,13,14).
Of note, our study was a descriptive study. Therefore, there was no null hypothesis about pre-specified estimated 95% confidence intervals and total width (precision) for determining the appropriate sample size. The tolerance probability is 0.900 that a sample size of 437 from approximately 85,000 children aged less than 13 years on Jeju Island, according to the 2010 Census, will produce a two-sided 95% confidence interval with a distance from the mean to the limits that is ≤ 0.060 if the population standard deviation is 0.600.
In terms of renal growth according to age, it is known that the renal volume changes around 1 year of age (9), with a rapid but gradually decreasing growth rate observed in the initial 7 months after birth, followed by a constant and slower rate (15). In infants under 12 months, kidney length has been reported to be poorly estimated with an age-based simple formula (16). In other studies, the renal length has been shown to increase rapidly until 24 months, while the growth rate is reduced thereafter (13,17), whereas our data showed a statistically significant difference before and after 20 months of age.
The reference values for the renal lengths according to age are presented in Tables 1 and 2. Compared with in a previous study (13), our data provide more accurate values in subjects aged less than 24 months, owing to the fact that these subjects were subdivided according to the age distribution. According to our study, the average renal lengths tended to be slightly larger than the previously reported values (13), by as much as 1-2 mm in subjects aged less than 24 months. Especially, these differences tended to be greater in subjects aged more than 4-5 years. It can be speculated that renal growth is affected by the proportion of their current height, which was taller in the present study (18). Of note, although our findings differed from those obtained in an Indian study in 2012, they showed no differences compared to the American reference values in 2011 (16,19). We believe that this lack of difference is due to the fact that the height growth of Korean children has recently been reported to be similar to that of western children (16,18).
Table 1. Longitudinal lengths of both kidneys in individuals aged less than 24 months.
| Age, mon | No. | Site | Minimum, cm | Maximum, cm | Mean, cm | SD |
|---|---|---|---|---|---|---|
| < 1 | 36 | Rt | 3.66 | 5.07 | 4.31 | 0.31 |
| Lt | 3.85 | 5.08 | 4.47 | 0.30 | ||
| 1-3 | 22 | Rt | 4.29 | 6.04 | 5.13 | 0.42 |
| Lt | 4.35 | 5.81 | 5.25 | 0.39 | ||
| 3-5 | 14 | Rt | 4.89 | 6.57 | 5.43 | 0.43 |
| Lt | 4.89 | 6.59 | 5.56 | 0.47 | ||
| 5-7 | 29 | Rt | 4.65 | 6.09 | 5.43 | 0.43 |
| Lt | 4.57 | 6.68 | 5.63 | 0.49 | ||
| 7-9 | 15 | Rt | 5.15 | 6.05 | 5.64 | 0.27 |
| Lt | 5.14 | 6.40 | 5.81 | 0.39 | ||
| 9-11 | 16 | Rt | 5.32 | 6.60 | 5.89 | 0.39 |
| Lt | 5.50 | 6.96 | 6.13 | 0.47 | ||
| 11-15 | 42 | Rt | 4.99 | 7.24 | 6.23 | 0.46 |
| Lt | 5.24 | 7.36 | 6.40 | 0.52 | ||
| 15-18 | 21 | Rt | 5.70 | 7.51 | 6.54 | 0.44 |
| Lt | 5.76 | 7.47 | 6.67 | 0.40 | ||
| 18-24 | 33 | Rt | 5.86 | 7.83 | 6.69 | 0.48 |
| Lt | 5.77 | 7.93 | 6.84 | 0.53 |
SD, standard deviation; Rt, right; Lt, left.
Table 2. Longitudinal lengths of both kidneys in individuals aged between 2 and 13 years.
| Age, yr | No. | Site | Minimum, cm | Maximum, cm | Mean, cm | SD |
|---|---|---|---|---|---|---|
| 2-3 | 31 | Rt | 5.96 | 7.66 | 6.79 | 0.43 |
| Lt | 6.07 | 7.84 | 7.07 | 0.43 | ||
| 3-4 | 35 | Rt | 6.29 | 8.33 | 7.06 | 0.54 |
| Lt | 6.07 | 9.46 | 7.27 | 0.71 | ||
| 4-5 | 23 | Rt | 6.58 | 8.46 | 7.53 | 0.5 |
| Lt | 7.06 | 8.67 | 7.77 | 0.5 | ||
| 5-6 | 22 | Rt | 6.83 | 8.53 | 7.78 | 0.49 |
| Lt | 6.89 | 9.4 | 7.93 | 0.68 | ||
| 6-7 | 15 | Rt | 7.36 | 9.92 | 8.39 | 0.85 |
| Lt | 7.49 | 9.99 | 8.38 | 0.8 | ||
| 7-8 | 17 | Rt | 7.69 | 8.94 | 8.36 | 0.44 |
| Lt | 7.32 | 9.21 | 8.51 | 0.54 | ||
| 8-9 | 13 | Rt | 7.77 | 9.75 | 8.63 | 0.61 |
| Lt | 7.76 | 9.79 | 8.68 | 0.61 | ||
| 9-10 | 22 | Rt | 7.89 | 9.9 | 8.83 | 0.49 |
| Lt | 7.77 | 10.44 | 9.09 | 0.61 | ||
| 10-11 | 15 | Rt | 7.9 | 10.4 | 8.96 | 0.71 |
| Lt | 8.17 | 10.9 | 9.19 | 0.65 | ||
| 11-13 | 16 | Rt | 8.51 | 10 | 9.47 | 0.44 |
| Lt | 8.82 | 10.34 | 9.55 | 0.48 |
SD, standard deviation; Rt, right; Lt, left.
The other values of age and weight also showed good correlations with kidney length in children from 2 to 12 years of age in the present study. The left kidney was significantly longer than the right, as has been previously reported (11,13,14). On the other hand, there were no significant sex-specific differences.
In conclusion, we here reported the reference values of kidney length for Korean children. We believe that these data may help facilitate the diagnosis of renal hypoplasia or pediatric CKD and help estimate the appropriate donor kidney length in children by our simple linear regression equation in which only the height of the donor needs to be applied.
Footnotes
Funding: This study was supported by a grant from the Jeju National University Hospital Research Fund (2012).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Literature review: Han S, Lee MS, Choi GM, Han KH. Study concept and design: Choi GM, Han KH. Data collection and/or processing: Oh MS, Hwang G, Han S, Kang HS, Kim SH, Kim YD, Kang KS, Shin KS, Han KH. Statistical analysis and/or interpretation: Oh MS, Hwang G, Han KH. Writing: Han S, Han KH. Critical revision and final approval: all authors.
References
- 1.Queisser-Luft A, Stolz G, Wiesel A, Schlaefer K, Spranger J. Malformations in newborn: results based on 30,940 infants and fetuses from the Mainz congenital birth defect monitoring system (1990-1998) Arch Gynecol Obstet. 2002;266:163–167. doi: 10.1007/s00404-001-0265-4. [DOI] [PubMed] [Google Scholar]
- 2.Seikaly MG, Ho PL, Emmett L, Fine RN, Tejani A. Chronic renal insufficiency in children: the 2001 Annual Report of the NAPRTCS. Pediatr Nephrol. 2003;18:796–804. doi: 10.1007/s00467-003-1158-5. [DOI] [PubMed] [Google Scholar]
- 3.Cain JE, Di Giovanni V, Smeeton J, Rosenblum ND. Genetics of renal hypoplasia: insights into the mechanisms controlling nephron endowment. Pediatr Res. 2010;68:91–98. doi: 10.1203/PDR.0b013e3181e35a88. [DOI] [PubMed] [Google Scholar]
- 4.Emamian SA, Nielsen MB, Pedersen JF. Intraobserver and interobserver variations in sonographic measurements of kidney size in adult volunteers. A comparison of linear measurements and volumetric estimates. Acta Radiol. 1995;36:399–401. [PubMed] [Google Scholar]
- 5.Schlesinger AE, Hernandez RJ, Zerin JM, Marks TI, Kelsch RC. Interobserver and intraobserver variations in sonographic renal length measurements in children. AJR Am J Roentgenol. 1991;156:1029–1032. doi: 10.2214/ajr.156.5.2017927. [DOI] [PubMed] [Google Scholar]
- 6.Faubel S, Patel NU, Lockhart ME, Cadnapaphornchai MA. Renal relevant radiology: use of ultrasonography in patients with AKI. Clin J Am Soc Nephrol. 2014;9:382–394. doi: 10.2215/CJN.04840513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinkel E, Ertel M, Dittrich M, Peters H, Berres M, Schulte-Wissermann H. Kidney size in childhood. Sonographical growth charts for kidney length and volume. Pediatr Radiol. 1985;15:38–43. doi: 10.1007/BF02387851. [DOI] [PubMed] [Google Scholar]
- 8.Konuş OL, Ozdemir A, Akkaya A, Erbaş G, Celik H, Işik S. Normal liver, spleen, and kidney dimensions in neonates, infants, and children: evaluation with sonography. AJR Am J Roentgenol. 1998;171:1693–1698. doi: 10.2214/ajr.171.6.9843315. [DOI] [PubMed] [Google Scholar]
- 9.Han BK, Babcock DS. Sonographic measurements and appearance of normal kidneys in children. AJR Am J Roentgenol. 1985;145:611–616. doi: 10.2214/ajr.145.3.611. [DOI] [PubMed] [Google Scholar]
- 10.Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, Oh K, Jang MJ, Hwang SS, Yoo MH, et al. 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr. 2008;51:1–25. [Google Scholar]
- 11.Kim JH, Kim MJ, Lim SH, Kim J, Lee MJ. Length and volume of morphologically normal kidneys in Korean children: ultrasound measurement and estimation using body size. Korean J Radiol. 2013;14:677–682. doi: 10.3348/kjr.2013.14.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zerin JM, Blane CE. Sonographic assessment of renal length in children: a reappraisal. Pediatr Radiol. 1994;24:101–106. doi: 10.1007/BF02020164. [DOI] [PubMed] [Google Scholar]
- 13.Kim IO, Cheon JE, Lee YS, Lee SW, Kim OH, Kim JH, Kim HD, Sim JS. Kidney length in normal Korean children. J Korean Soc Ultrasound Med. 2010;29:181–188. [Google Scholar]
- 14.Lee MJ, Son MK, Kwak BO, Park HW, Chung S, Kim KS. Kidney size estimation in Korean children with Technesium-99m dimercaptosuccinic acid scintigraphy. Korean J Pediatr. 2014;57:41–45. doi: 10.3345/kjp.2014.57.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesrobian HG, Laud PW, Todd E, Gregg DC. The normal kidney growth rate during year 1 of life is variable and age dependent. J Urol. 1998;160:989–993. doi: 10.1097/00005392-199809020-00005. [DOI] [PubMed] [Google Scholar]
- 16.Akhavan A, Brajtbord JS, McLeod DJ, Kabarriti AE, Rosenberg HK, Stock JA. Simple, age-based formula for predicting renal length in children. Urology. 2011;78:405–410. doi: 10.1016/j.urology.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Haugstvedt S, Lundberg J. Kidney size in normal children measured by sonography. Scand J Urol Nephrol. 1980;14:251–255. doi: 10.3109/00365598009179571. [DOI] [PubMed] [Google Scholar]
- 18.Ryoo NY, Shin HY, Kim JH, Moon JS, Lee CG. Change in the height of Korean children and adolescents: analysis from the Korea National Health and Nutrition Survey II and V. Korean J Pediatr. 2015;58:336–340. doi: 10.3345/kjp.2015.58.9.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otiv A, Mehta K, Ali U, Nadkarni M. Sonographic measurement of renal size in normal Indian children. Indian Pediatr. 2012;49:533–536. doi: 10.1007/s13312-012-0120-7. [DOI] [PubMed] [Google Scholar]