Abstract
It is important to assess psychological distress after a diagnosis for cancer survivors, a population with a high risk for psychological distress. The aim of this study is to assess psychological distress among cancer survivors and to clarify the associated factors. In this cross-sectional analysis, data were obtained from standardized questionnaires administered to 1,163 cancer survivors and 49,243 non-cancer survivors who participated in the Fourth and Fifth Korea National Health and Nutrition Examination Survey (2007-2012). We identified the adjusted rates for psychological distress and assessed factors associated with this kind of distress using multivariate logistic regression. Cancer survivors tended to have a higher adjusted rate of psychological distress than the general population. The current depressive symptom rate for cancer survivors was 16.69%, and the adjusted rate for history of depression in cancer survivors was 15.61%. The adjusted rate for higher level of stress was 25.51% in cancer survivors. Among the cancer survivors, younger subjects, female subjects, and those with limited social support were more prone to psychological distress. In addition, current smokers or risky drinkers, those with chronic diseases, and those with a poor self-perception of their health status were also identified as a high-risk group for psychological distress. As the number of cancer survivors has increased, the importance of assessing psychological distress after a cancer diagnosis should be emphasized among all cancer survivors. Further, psychological supportive care interventions for cancer survivors are needed to improve the survival rate and improve their quality of life.
Keywords: Psychological Status, Cancer Survivors, Distress, Risk Factors, Korean
Graphical Abstract

INTRODUCTION
The number of cancer survivors has progressively increased and is expected to further increase with improved cancer screening, more effective cancer treatment, and steady aging of the population. Currently more than two-thirds of persons diagnosed with cancer are expected to live 5 years or more after diagnosis (1). In Korea, cancer incidence has increased, while overall cancer mortality rates have declined since 2002. The number of cancer survivors in Korea was 1.23 million in 2012 (2). As more adults survive with cancer, it is important to understand how cancer and cancer treatment affects quality of life.
For cancer patients, the diagnosis and treatment of cancer can be considered a multifold stress (3). Many previous reports have given attention to the psychological sequelae of the post cancer experience. Although many long-term cancer survivors can successfully adapt to life after cancer, some cancer survivors experience significant and lasting psychological distress, unfortunately. Fear of recurrence, worries about health, physical change, social isolation, and/or economic problems can develop causing psychological distress in many cancer survivors (1,4). Because psychological distress has been identified as a significant problem for cancer survivors, guidelines provided by the National Comprehensive Cancer Network (NCCN) have emphasized assessment of psychological distress after receiving a cancer diagnosis (5).
The prevalence of psychological distress in cancer survivors ranges from 10% to 40% (6,7). However, cancer survivors' psychological distress is not often estimated by medical staff, and utilization of psychological health services has been low among cancer survivors (8,9). Untreated psychological distress may have long-term harmful consequences on cancer survivors' compliance with treatment, survival rate, and their quality of survivorship (10,11). It is important to evaluate the psychological distress of cancer survivors because such distress is likely to be treatable, and early intervention may improve the overall physical or mental health of cancer survivors. Because of the given challenges related to survivors' psychological distress, many previous studies mainly explored significant variables influencing depression and distress among cancer survivors.
The aim of the present study was to assess psychological distress (current depressive symptoms, history of depression, and the level of stress) among cancer survivors and to clarify the associated factors (sociodemographics, behavioral factors, and clinical variables) in a nationwide sample of cancer survivors. We hypothesized that patient sociodemographic characteristics, such as being a woman, being younger, and having a lower income level, have an influence on psychological distress. These sociodemographic factors have been shown to influence psychological distress in the general population. We also hypothesized that behavioral factors, such as smoking or drinking, would influence this desire as psychological difficulties. Finally, we hypothesized that patient clinical factors, such as comorbidity, cancer type, and time since cancer diagnosis, would influence psychological distress.
MATERIALS AND METHODS
Participants
The Korean National Health and Nutrition Examination Survey (KNHANES) IV (2007–2009) and KNHANES V (2010–2012) were used as data of this study. These represent the general Korean population as nationwide surveys and include comprehensive information on sociodemographics, health status, and health behavior (12). A stratified multistage probability sampling design was used, and face-to-face interviews were conducted at candidates' homes by trained interviewers to gather health information (12). Each participant gave informed consent prior to inclusion in the study.
The initial data for the present study comprised 50,405 candidates who participated in both the health interview and health examination surveys. Of these, 1,163 cancer survivors were selected as the study group, and 49,242 non-cancer survivors were selected as the controls. Cancer survivors in this study were defined as patients from the time of cancer diagnosis through the remaining years of life, as defined by the National Coalition for Cancer Survivorship (NCCS). Participants were reported by self-report on questionnaire. In the questionnaire about their health history, people were asked whether they ever had a diagnosis of cancer such as stomach, liver, colon, breast, cervix, lung, thyroid and others cancer. Participants who answered “yes” were defined as cancer survivors. This study did not require the ethical approval of our Institutional Review Board because the survey data that we analyzed are publicly available.
Associated factors and definition of psychological status
We collected various factors potentially associated with psychological status. These risk factors were divided into three groups: sociodemographic, behavioral, and clinical factors. The sociodemographic factors were age (< 65 years or ≥ 65 years), sex, education level (college education or more, middle/High school education, less than elementary education), household monthly income (< 3,000,000 KRW [approximately 2,557 USD], or ≥ 3,000,000 KRW), area of residence (urban or rural), spouse status (yes or no), and health insurance type (medical assistance or none, government health insurance without private health insurance, government health insurance with private health insurance).
The behavioral risk factors included smoking status (nonsmoker or past smoker, current smoker), alcohol consumption (nondrinker or non-risky drinker, risky drinker), physical activity (active, inactive/inadequately active), and self-perceived health status. Risky drinking was defined as alcohol consumption exceeding 3 standard drinks per day (13). Physical activity was classified as follows: active (moderate physical activity for at least 150 minutes per week or vigorous physical activity for at least 75 minutes per week) and inactive/inadequately active (exercised regularly but at levels that were less than sufficient) (14). Self-perceived health status was classified into two levels according to responses to the question “How do you assess your own health status?”: “very good,” “good,” “fair,” and “poor,” “very poor.”
The clinical factors included comorbidities, cancer types (gastric, hepatic, colon cancer, breast, cervical, lung, thyroid, and other cancers), and time since cancer diagnosis (≤ 1 year, 2–4 years, and ≥ 5 years). The comorbidities included hypertension, diabetes, chronic renal disease, coronary artery disease, and lung diseases such as asthma, tuberculosis, and chronic obstructive pulmonary disease (12).
For evaluation of psychological distress, participants were asked to report on current depressive symptoms, history of depression, and the level of stress. Current depressive symptoms were evaluated by one question: “Have you felt constantly sad or hopeless for over 2 weeks in the past one year?” Respondents answered the current depressive symptoms as yes or no. In addition, people were asked whether they ever had a diagnosis of depression. Respondents answered the history of depression as yes or no (15). The level of stress was asked as follows: “How much do you feel stress in your usual life?” The level of stress was reported as none, small, some or extreme. We redichotomized into none/small or some/extreme.
Analysis
We used a weighted population sample to reflect the sampling method and response rate. We calculated the estimated proportions and standard errors for baseline characteristics related to psychological status. The statistical significance was assessed as P value to show differences between groups according to cancer status using logistic regression. We calculated the adjusted rate of current depressive symptom, history of depression, and higher level of stress in non-cancer survivors versus cancer survivors in younger individuals (< 65 years old) and elderly individuals (≥ 65 years old). In addition, we calculated adjusted odds ratio (aOR) using multivariate logistic regression for the risk of all psychological distress. Current model 1 was adjusted for sociodemographic factors. Current model 2 was adjusted for behavioral factors in addition to all variables in current model 1. Further, we performed subgroup analysis among the participants into two categories of younger and elderly cancer survivors to evaluate the effect of cancer type using above multivariate logistic regression. The level of significance was set at P < 0.05. All estimates in the analysis were properly weighted to represent the general Korean population using a complex, multistage, probability sampling design (12). All statistical analyses were performed using STATA 10.0 (StataCorp., College Station, TX, USA).
RESULTS
Participants
Table 1 shows the baseline characteristics of the study population by cancer status. There were significant differences between the non-cancer controls and cancer survivors for all characteristics except health insurance type. In total, 63.33% of the cancer survivors were less than 65 years old and 64.18% were female. The most common cancer type among all patients was gastric cancer (16.95%). The most prevalent cancer types in men were gastric cancer (29.24%), colon cancer (16.58%), and liver cancer (7.99%), whereas for women they were cervical cancer (20.02%), breast cancer (19.57%), and thyroid cancer (13.84%). Among the cancer survivors, 19.51% was diagnosed as having the disease less than 1 year ago, 28.79% were diagnosed between 2 to 4 years ago, and 51.79% were diagnosed more than 5 years ago.
Table 1. Characteristics of non-cancer controls (n = 49,243) versus cancer survivors (n = 1,163).
| Variables | Estimated proportion % (SE) | P value | ||
|---|---|---|---|---|
| Total population | Non-cancer controls | Cancer survivors | ||
| Age, yr | < 0.001 | |||
| < 65 | 88.98 (0.24) | 89.48 (0.23) | 63.33 (1.77) | |
| ≥ 65 | 11.02 (0.24) | 10.52 (0.23) | 36.67 (1.77) | |
| Sex | < 0.001 | |||
| Male | 50.16 (0.23) | 50.44 (0.24) | 35.82 (1.77) | |
| Female | 48.84 (0.23) | 49.56 (0.24) | 64.18 (1.77) | |
| Education | < 0.001 | |||
| ≥ College | 24.29 (0.39) | 24.41 (0.39) | 18.45 (1.57) | |
| Middle/High school | 43.91 (0.38) | 43.91 (0.38) | 43.95 (1.82) | |
| ≤ Elementary school | 31.80 (0.33) | 31.68 (0.33) | 37.60 (1.67) | |
| Monthly income, thousand KRW | < 0.001 | |||
| ≥ 3,000 | 42.86 (0.67) | 43.03 (0.67) | 34.18 (1.90) | |
| < 3,000 | 57.14 (0.67) | 56.97 (0.67) | 65.82 (1.90) | |
| Area of residence | < 0.001 | |||
| Urban | 81.28 (1.16) | 81.37 (1.16) | 76.94 (1.82) | |
| Rural | 18.72 (1.16) | 18.63 (1.16) | 23.06 (1.82) | |
| Spouse status | < 0.001 | |||
| Yes | 90.10 (0.22) | 90.30 (0.21) | 81.07 (1.42) | |
| No | 9.90 (0.22) | 9.70 (0.21) | 18.93 (1.42) | |
| Health insurance(HI) type | < 0.001 | |||
| Medical assistance or none | 3.47 (0.19) | 3.43 (0.19) | 5.82 (0.93) | |
| Government HI without private HI | 23.81 (0.38) | 23.45 (0.37) | 41.95 (1.91) | |
| Government HI with private HI | 72.72 (0.41) | 73.12 (0.41) | 52.22 (1.95) | |
| Smoking | < 0.001 | |||
| non or past smoker | 70.19 (0.32) | 66.98 (0.33) | 79.95 (1.42) | |
| current smoker | 29.81 (0.32) | 30.02 (0.33) | 20.05 (1.42) | |
| Alcohol | < 0.001 | |||
| Non risky | 55.03 (0.35) | 54.56 (0.36) | 77.49 (1.64) | |
| Risky drinking* | 44.97 (0.35) | 45.44 (0.36) | 22.51 (1.64) | |
| Physical activity† | - | |||
| Active | 14.30 (0.27) | 14.44 (0.27) | 8.01 (1.03) | |
| Inactive/inadequately active | 85.70 (0.27) | 85.56 (0.27) | 91.99 (1.03) | |
| Self-perceived health status | < 0.001 | |||
| Very good/good/fair | 84.03 (0.26) | 84.52 (0.26) | 59.29 (1.74) | |
| Poor/very poor | 15.97 (0.26) | 15.48 (0.26) | 40.71 (1.74) | |
| Chronic disease‡ | < 0.001 | |||
| No | 82.82 (0.27) | 83.06 (0.27) | 70.42 (1.55) | |
| Yes | 17.18 (0.27) | 16.94 (0.27) | 29.58 (1.55) | |
| Cancer type | - | |||
| Others | 31.72 (1.70) | |||
| Gastric | 16.95 (1.34) | |||
| Hepatic | 3.57 (0.72) | |||
| Colon | 9.43 (1.05) | |||
| Breast | 12.56 (1.19) | |||
| Cervical | 12.85 (1.21) | |||
| Lung | 2.19 (0.42) | |||
| Thyroid | 10.73 (1.24) | |||
| Time since cancer diagnosis | - | |||
| ≤ 1 years | 19.51 (1.61) | |||
| 2-4 years | 28.79 (1.74) | |||
| ≥ 5 years | 51.70 (1.88) | |||
| Current depressive symptoms | < 0.001 | |||
| No | 87.78 (0.21) | 87.94 (0.21) | 79.93 (1.45) | |
| Yes | 12.22 (0.21) | 12.06 (0.21) | 20.07 (1.45) | |
| History of depression | < 0.001 | |||
| No | 89.41 (0.20) | 89.63 (0.20) | 79.15 (1.47) | |
| Yes | 10.59 (0.20) | 10.37 (0.20) | 20.85 (1.47) | |
| Level of stress | < 0.001 | |||
| None/Small | 74.06 (0.27) | 74.08 (0.27) | 72.89 (1.63) | |
| Some/Extreme | 25.93 (0.27) | 25.92 (0.27) | 27.11 (1.63) | |
*Risk drinking is defined as consuming more than 3 standard drinks per day on occasion; †Physical activity was classified as no physical activity/inadequately active group, active group (moderate physical activity for at least 150 minutes per week or vigorous physical activity for at least 75 minutes per week); ‡Chronic diseases were hypertension, diabetes, chronic renal disease, coronary artery disease, and lung disease such as asthma, tuberculosis, chronic obstructive pulmonary disease.
Adjusted rates for psychological distress in non-cancer survivors versus cancer survivors, in younger individuals, and in elderly individuals.
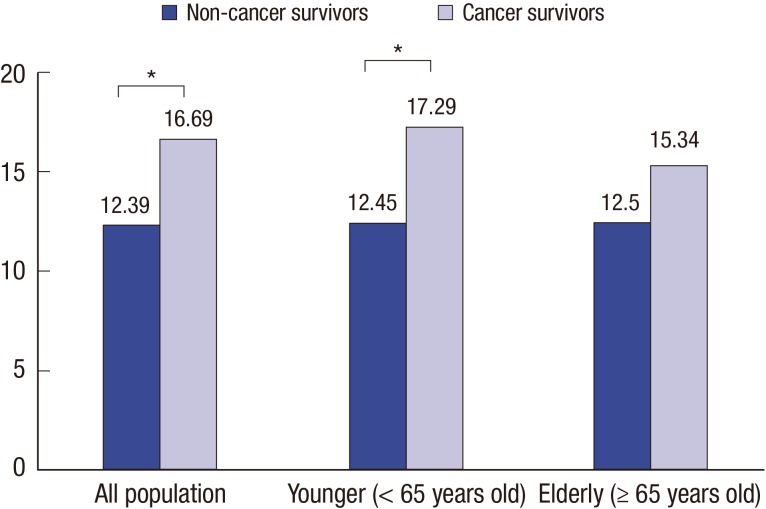
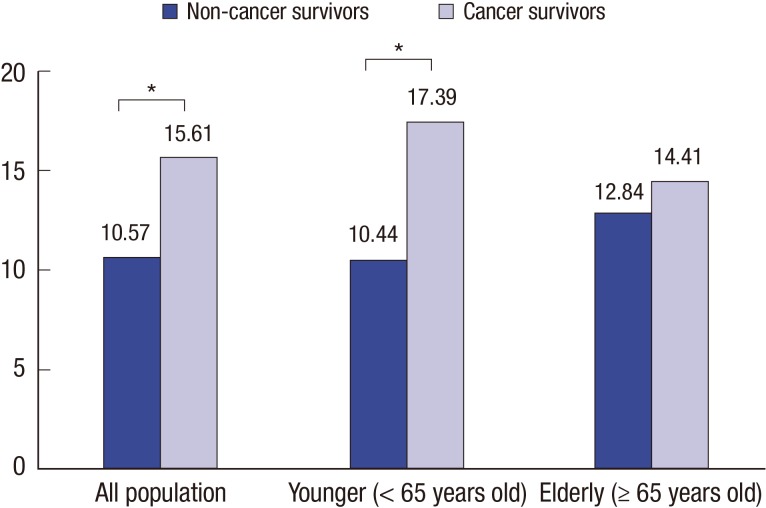
After adjusting for sociodemographic factors (age, sex, educational level, monthly income, spouse status, residential area, and health insurance types), current depressive symptoms rates for non-cancer survivors and cancer survivors were 12.39% and 16.69%, respectively (P < 0.05); and those for younger non-cancer survivors and cancer survivors were 12.45% and 17.29%, respectively, (P < 0.05) (Fig. 1). The adjusted rate for history of depression showed cancer survivors' history was significantly higher than non-cancer survivors (10.57% in non-cancer survivors, 15.61% in cancer survivors). It was consistent in younger population, not older population (Fig. 2). There was not significant differences of the adjusted rate for higher level of stress between non-cancer survivors and cancer survivors (P = 0.95). However, there was significant difference in elderly population. The rates were 16.25% in non cancer survivors and 21.35% in cancer survivors, respectively (P < 0.05) (Fig. 3).
Fig. 1.
Adjusted rate for current depressive symptoms in non-cancer survivors vs. cancer survivors.
Adjusted for patient characteristics (age, sex, educational level, monthly income, spouse status, residential area, and health insurance types).
* P value < 0.05.
Fig. 2.
Adjusted rate for history of depression in non-cancer survivors vs. cancer survivors.
Adjusted for patient characteristics (age, sex, educational level, monthly income, spouse status, residential area, and health insurance types).
* P value < 0.05.
Fig. 3.
Adjusted rate higher level of stress in non-cancer survivors vs. cancer survivors.
Adjusted for patient characteristics (age, sex, educational level, monthly income, spouse status, residential area, and health insurance types).
* P value < 0.05.
Factors associated with current depressive symptoms, history of depression, and of higher level of stress in cancer survivors
Table 2 shows the multivariate logistic regression analysis, adjusted for only sociodemographic variables (Current model 1), and including behavioral factors (Current model 2). In current model 1, current depressive symptoms among all cancer survivors was significantly higher for the following characteristics: female gender (adjusted odds ratio [aOR], 2.10; 95% confidence interval [CI], 1.40–3.15), low monthly income (aOR, 1.77; 95% CI, 1.12–2.79), current smoker (aOR, 1.66; 95% CI, 1.01–2.72), poor self-perceived health status (aOR, 3.59; 95% CI, 2.39–5.38), and the presence of a chronic disease (aOR, 1.66; 95% CI, 1.66–2.38). History of depression was lower in cancer survivors aged 65 or older (aOR, 0.47; 95% CI, 0.30–0.73). However, history of depression was found to be higher for the following characteristics: female gender (aOR, 2.48; 95% CI, 1.59–3.86), unmarried (aOR, 1.82; 95% CI, 1.15–2.91), risky drinker (aOR, 1.61; 95% CI, 1.03–2.50), poor self-perceived health status (aOR, 2.43; 95% CI, 1.68–3.53), and the presence of a chronic disease (aOR, 1.88; 95% CI, 1.30–2.72). Stress was higher in current smoker (aOR, 1.69; 95% CI, 1.05–2.75), and was also higher for group with reported poor self-perceived health status (aOR, 2.36; 95% CI, 1.16–3.36). In subgroup analysis of elderly cancer survivors, current depressive symptoms and history of depression were lower in thyroid cancer survivors (aOR, 0.054; 95% CI 0.01–0.49, aOR, 0.17; 95% CI, 0.03–0.94). The analysis of current model 2 showed the similarity of results compared with current model 1.
Table 2. Factors associated with current depressive symptoms, history of depression and level of stress among cancer survivors (n = 1,163) using multivariate logistic regression analysis.
| Variables | Model | aOR (95% CI) | ||
|---|---|---|---|---|
| Current depressive symptoms | History of depression | Higher level of Stress | ||
| Age, yr | ||||
| < 65 | 1 | 1 | 1 | |
| ≥ 65 | Model 1* | 0.88 (0.56–1.37) | 0.47 (0.30–0.73) | 1.00 (0.69–1.46) |
| Model 2† | 0.96 (0.01–1.59) | 0.51 (0.32–0.81) | 1.09 (0.72–1.63) | |
| Sex | ||||
| Male | 1 | 1 | 1 | |
| Female | Model 1 | 2.10 (1.40–3.15) | 2.48 (1.59–3.86) | 1.43 (0.97–2.12) |
| Model 2 | 2.72 (1.66–4.47) | 2.88 (1.68–4.93) | 1.78 (1.10–2.87) | |
| Education | ||||
| ≥ College | 1 | 1 | 1 | |
| Middle/High school | Model 1 | 0.83 (0.46–1.48) | 1.01 (0.57–1.81) | 0.58 (0.35–0.95) |
| Model 2 | 0.72 (0.38–1.34) | 0.91 (0.50–1.64) | 0.58 (0.34–0.98) | |
| ≤ Elementary school | Model 1 | 1.46 (0.78–2.73) | 1.67 (0.88–3.54) | 0.98 (0.56–1.71) |
| Model 2 | 1.30 (0.68–2.51) | 1.63 (0.86–3.07) | 0.93 (0.52–1.67) | |
| Monthly income, thousand KRW | ||||
| ≥ 3,000 | 1 | 1 | 1 | |
| < 3,000 | Model 1 | 1.77 (1.12–2.79) | 1.44 (0.92–2.26) | 1.06 (0.71–1.59) |
| Model 2 | 1.68 (1.03–2.73) | 1.46 (0.90–2.38) | 0.92 (0.60–1.41) | |
| Area of residence | ||||
| Urban | 1 | 1 | 1 | |
| Rural | Model 1 | 1.08 (0.70–1.66) | 1.13 (0.74–1.74) | 1.64 (1.11–2.44) |
| Model 2 | 1.04 (0.66–1.64) | 1.13 (0.73–1.73) | 1.71 (1.15–2.56) | |
| Spouse status | ||||
| Yes | 1 | 1 | 1 | |
| No | Model 1 | 0.99 (0.61–1.62) | 1.82 (1.15–2.91) | 0.94 (0.61–1.46) |
| Model 2 | 0.85 (0.52–1.42) | 1.58 (1.00–2.50) | 0.65 (0.41–1.04) | |
| Health insurance(HI) type | ||||
| Medical assistance or none | 1 | 1 | 1 | |
| Government HI without private HI | Model 1 | 0.61 (0.29–1.24) | 1.16 (0.51–2.64) | 0.42 (0.21–0.83) |
| Model 2 | 1.22 (0.52–3.51) | 1.50 (0.62–3.64) | 0.58 (0.26–1.28) | |
| Government HI with private HI | Model 1 | 0.65 (0.31–1.38) | 0.89 (0.39–2.04) | 0.52 (0.25–1.08) |
| Model 2 | 1.41 (0.57–3.51) | 1.32 (0.55–3.16) | 0.76 (0.33–1.76) | |
| Smoking | ||||
| Non or past smoker | 1 | 1 | 1 | |
| Current smoker | Model 1 | 1.66 (1.01–2.72) | 1.22 (0.69–2.13) | 1.69 (1.05–2.75) |
| Model 2 | 1.57 (0.91–2.69) | 1.19 (0.65–2.16) | 1.73 (1.04–2.87) | |
| Alcohol | ||||
| Non risky | 1 | 1 | 1 | |
| Risky drinking | Model 1 | 1.47 (0.91–2.36) | 1.61 (1.03–2.50) | 1.30 (0.85–1.98) |
| Model 2 | 1.39 (0.82–2.35) | 1.68 (1.03–2.73) | 1.26 (0.80–2.00) | |
| Physical activity | ||||
| Active | 1 | 1 | 1 | |
| Inactive/inadequately active | Model 1 | 0.97 (0.43–2.18) | 0.67 (0.34–1.34) | 0.64 (0.34–1.18) |
| Model 2 | 0.98 (0.46–2.10) | 0.64 (0.32–1.29) | 0.62 (0.33–1.17) | |
| Self-perceived health status | ||||
| Very good/good/fair | 1 | 1 | 1 | |
| Poor/very poor | Model 1 | 3.59 (2.39–5.38) | 2.43 (1.68–3.53) | 2.36 (1.66–3.36) |
| Model 2 | 3.70 (2.41–5.69) | 2.49 (1.72–3.60) | 2.38 (1.66–3.42) | |
| Chronic disease | ||||
| No | 1 | 1 | 1 | |
| Yes | Model 1 | 1.66 (1.16–2.38) | 1.88 (1.30–2.72) | 1.10 (0.79–1.54) |
| Model 2 | 1.39 (0.94–2.06) | 1.54 (1.03–2.30) | 0.88 (0.62–1.25) | |
| Cancer type | ||||
| Others | 1 | 1 | 1 | |
| Gastric | Model 1 | 1.14 (0.65–1.98) | 1.13 (0.61–2.10) | 1.20 (0.71–2.05) |
| Model 2 | 1.31 (0.74–2.31) | 1.08 (0.55–2.11) | 1.33 (0.78–2.27) | |
| Hepatic | Model 1 | 0.71 (0.25–2.01) | 0.63 (0.15–2.63) | 0.99 (0.40–2.42) |
| Model 2 | 0.83 (0.26–2.72) | 0.61 (0.12–3.00) | 1.10 (0.40–2.99) | |
| Colon | Model 1 | 0.82 (0.40–1.67) | 1.43 (0.73–2.81) | 0.72 (0.36–1.45) |
| Model 2 | 0.80 (0.37–1.74) | 1.48 (0.76–2.90) | 0.71 (0.32–1.59) | |
| Breast | Model 1 | 0.98 (0.55–1.76) | 1.37 (0.75–2.50) | 1.42 (0.81–2.47) |
| Model 2 | 1.16 (0.62–2.17) | 1.38 (0.75–2.54) | 1.34 (0.75–2.40) | |
| Cervical | Model 1 | 1.20 (0.64–2.27) | 0.99 (0.54–1.85) | 1.18 (0.65–2.13) |
| Model 2 | 1.48 (0.73–2.97) | 1.09 (0.58–2.06) | 1.26 (0.65–2.44) | |
| Lung | Model 1 | 0.87 (0.35–2.16) | 1.90 (0.68–5.29) | 1.54 (0.57–4.13) |
| Model 2 | 0.80 (0.29–2.19) | 1.77 (0.54–5.75) | 1.71 (0.57–5.10) | |
| Thyroid | Model 1 | 0.58 (0.27–1.14) | 0.80 (0.40–1.61) | 1.36 (0.68–2.72) |
| Model 2 | 0.64 (0.29–1.40) | 0.83 (0.41–1.69) | 1.51 (0.72–3.20) | |
| Time since cancer diagnosis | ||||
| ≤ 1 years | 1 | 1 | 1 | |
| 2-4 years | Model 1 | 0.69 (0.40–1.20) | 0.69 (0.40–1.20) | 0.61 (0.36–1.04) |
| Model 2 | 0.72 (0.39–1.31) | 0.76 (0.43–1.35) | 0.64 (0.35–1.14) | |
| ≥ 5 years | Model 1 | 1.02 (0.62–1.66) | 0.77 (0.47–1.25) | 0.92 (0.12–0.82) |
| Model 2 | 1.14 (0.67–1.93) | 0.84 (0.50–1.41) | 1.07 (0.62–1.86) | |
Statistically significant results were written in bold.
aOR, adjusted odds ratio; CI, confidence interval.
*Current model 1 was adjusted for sociodemographic factors; †Current model 2 was adjusted for behavioral factors in addition to all variables in current model 1.
DISCUSSION
The aim of this study was to investigate cancer patients' psychological distress and to identify sociodemographic, behavioral, clinical, and psychological correlates. Our results from a large, population-based sample of Korean adults showed that cancer survivors were more vulnerable to psychological illness in comparison with adults with no cancer history. Previous studies have also suggested that more than 10% of cancer patients have experienced depression (16), and a larger proportion of cancer survivors have experienced depressive symptoms (17) and distress. In general, mental distress can disturb quality of life, and incur economic costs related to an increased medical burden (1). The negative effects of persistent psychological distress can result in cancer patients' lower compliance to treatment, screening programs, and recommendations for lifestyle modification (18). Therefore, it is important to preferentially assess and manage this high-risk group vulnerable to psychological distress.
First, in this study the influence of various sociodemographic factors on psychological distress were explored in specific cancer survivors, as has been done in research on the general population. It is important to note age and sex differences regarding psychological distress. Persons aged 65 years and older were less likely to have a history of depression. The various results of relationship between age and depression were shown in previous studies. Fear of recurrences loss of interpersonal relationships, or disconnection of social career may be more likely to affect the younger population than the elderly population (20). However, it is also less likely to have opportunities to be diagnosed as depression in elderly group compared with younger group due to lower health service accessibility. So, the need for effective screening for depression among elderly cancer survivors should be considered. On the one hand, women were more likely than men to experience depression. Regarding gender differences, most epidemiological studies of depression have demonstrated higher incidence rates among females. A possible explanation for this finding could be in female subjects in paid employment, on the other hand, men were more easily in paid employment than female subjects, relatively (21). However, it should be more comprehensively understood in terms of social role or circumstance. Lower social support such as lower income level or not having a spouse resulted in high depressive symptoms and/or history of depression. Additionally, our findings provide support to the contention that socio-ecological functions, including economic and family problems, impact survivors' psychological distress. It is important to develop accessible and reasonable support systems for cancer survivors and provide proper psychological care. In previous studies, lower social support predicted larger increases in depressive symptoms (22,23,24). These findings emphasized evidence supporting the routine screening of depression, especially for low-income cancer survivors. Further, active screening for depression in high-risk groups, such as low-income and medically underserved populations, should be conducted as an appropriate healthcare service (19,25,26).
Among the behavioral factors, current smoker, risky drinker, and self-perceived poor health status were significantly associated with poor psychological status. Comorbidity was also associated with poor psychological status. Previous studies suggest that modifiable health behaviors, such as smoking and drinking, are strong indicators of psychological distress within cancer survivors (27,28,29). These findings cannot be notable causality from this cross-sectional data, while life behaviors intervention efforts have considerable. For example, interventions targeting smoking cessation have been shown to decrease psychological distress, as well as risk of cancer related outcomes, such as recurrence and treatment complications (30,31). Further, although there are no current guidelines to classify cancer survivors' drinking levels, the results of the current study suggest avoiding excessive alcohol consumption. We recommend that further studies explore the association between health behaviors and psychological distress among cancer survivors. There is scarce data on psychological distress and the self-perceived health status of cancer survivors. However, self-perceived health status has been considered an independent factor related to life style modification (32) or management of one's chronic disease (33). Perceiving one's health status as poor can potentially result in psychological distress. Psychological distress would inversely influence poor self-perceived health status of cancer survivors, because the subjective health status could be affected by psychological factors as well as physical factors. Previous studies have documented the relationship between comorbidities and quality of life or depression (1,34,35). They found that cancer survivors with more comorbid conditions had difficulty with psychological adjustment. The results of the present study are consistent with these previous studies.
Thyroid cancer patients in the subgroup analysis were less likely to have current depressive symptoms and a history of depression than other cancer type patients. Few previous studies have examined depression in thyroid cancer patients comparing other cancer types. Poor prognosis was identified as a predictor of depression (36), and thyroid cancer is considered as having a relatively good prognosis with a high 5-year survival rate. Some data reported depression was not much higher in thyroid cancer patients than in the general population (37). However, these results cannot explain all the causal relationship between thyroid cancer and depression, there is a need for more analysis including a large number of cancer survivors with various cancer types.
This study has several limitations that should be considered. First, given the cross-sectional nature of the surveys, causal relationships between psychological distress and other independent variables cannot be determined. Future studies should expand a prospective design to evaluate the risks of psychological distress among cancer survivors. Second, because much information was collected from self-reported questionnaires, reporting bias cannot be excluded. There was the possibility of recall bias in the question about the history depression. The hard experience such cancer diagnosis and treatment could affect to the answer. So, the group without diagnosis of depression could be included to depression group among cancer survivors. Third, we could not collect the information on more specific cancer types and the type of cancer treatment. We could not assess the types of other cancer due to limitation of data. So, there might be the inappropriateness as reference of other cancers because other cancers showed various prognosis with poor to good level. And, some patients may not receive surgery or chemotherapy depending on cancer type or clinical situation. Future research will be required to more specifically investigate the mechanism that creates psychological distress in cancer survivors according to cancer treatments. Additionally, research at the individual level is needed to clarify the clinical etiologies or outcomes of this increased distress. Fourth, distribution of cancer type in this study is slightly different from the data of the Korea Central Cancer Registry. According to national statistics, the most prevalent cancer among all individuals in Korea is thyroid cancer. The most common cancers among male patients are gastric and colon cancers, whereas the most prevalent cancers among female patients are thyroid and breast cancers (2).
Despite these potential limitations, the present study is one of the few to evaluate in detail psychological distress among Korean cancer survivors using a nationally representative data set. This study showed that psychological distress is more common in cancer survivors than non-cancer survivors after adjusting for certain sociodemographic factors. This study identified that cancer survivors, like non-cancer survivors, shared psychological distress risk factors, such as younger age, female gender, lower socioeconomic status, and certain behavioral factors and comorbidities. The American Society of Clinical Oncology (ASCO) has emphasized screening, assessment, and care of psychosocial distress in all cancer survivors. Psychosocial supportive care interventions have been defined for patients experiencing symptoms of depression and/or anxiety, based on optimal screening and assessment (38). Prompt diagnosis and early, efficacious management of those manifesting significant psychological symptoms have proven useful to help cancer survivors and their families, overcoming psychological distress, and improve mood or quality of life. A strong rapport between patient and physician will also help to assess psychological distress and determine the appropriate treatment strategy (16). In addition, it is important for physicians to regularly reassess the patient's psychological status to determine whether a treatment modality is effective or not. Due to increased survivorship, understanding cancer survivors' psychological distress and problems with mental health is important to advance the quality of survivorship. Our results provide timely appropriate information about psychological status among cancer survivors with various characteristics. Health care providers need to pay more attention to psychological distress during treatment and after treatment. Targeting cancer survivors who have elevated risk factors for psychological distress as identified in this study is essential for providing clinical care and efficient interventions to improve survivors' psychological wellbeing.
ACKNOWLEDGMENT
This study was based on Korea National Health and Nutritional Examination Survey, Ministry of Health and Welfare, Republic of Korea. We gratefully acknowledge the numerous investigators involved in the collection and management of data.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Research conception & design: Choi KH, Park SM. Data analysis and interpretation: Choi KH. Statistical analysis: Choi KH, Park SM. Critical revision of the manuscript: Choi KH, Park SM. Approval of final manuscript: all authors.
References
- 1.Hoffman KE, McCarthy EP, Recklitis CJ, Ng AK. Psychological distress in long-term survivors of adult-onset cancer: results from a national survey. Arch Intern Med. 2009;169:1274–1281. doi: 10.1001/archinternmed.2009.179. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, Lee KH. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47:127–141. doi: 10.4143/crt.2015.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, Meader N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12:160–174. doi: 10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 4.Harrison SE, Watson EK, Ward AM, Khan NF, Turner D, Adams E, Forman D, Roche MF, Rose PW. Primary health and supportive care needs of long-term cancer survivors: a questionnaire survey. J Clin Oncol. 2011;29:2091–2098. doi: 10.1200/JCO.2010.32.5167. [DOI] [PubMed] [Google Scholar]
- 5.Donovan KA, Jacobsen PB. Progress in the implementation of NCCN guidelines for distress management by member institutions. J Natl Compr Canc Netw. 2013;11:223–226. doi: 10.6004/jnccn.2013.0029. [DOI] [PubMed] [Google Scholar]
- 6.Zabora J. BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Chochinov HM, Wilson KG, Enns M, Lander S. Prevalence of depression in the terminally ill: effects of diagnostic criteria and symptom threshold judgments. Am J Psychiatry. 1994;151:537–540. doi: 10.1176/ajp.151.4.537. [DOI] [PubMed] [Google Scholar]
- 8.Fallowfield L, Ratcliffe D, Jenkins V, Saul J. Psychiatric morbidity and its recognition by doctors in patients with cancer. Br J Cancer. 2001;84:1011–1015. doi: 10.1054/bjoc.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Passik SD, Dugan W, McDonald MV, Rosenfeld B, Theobald DE, Edgerton S. Oncologists' recognition of depression in their patients with cancer. J Clin Oncol. 1998;16:1594–1600. doi: 10.1200/JCO.1998.16.4.1594. [DOI] [PubMed] [Google Scholar]
- 10.Valdimarsdóttir U, Helgason AR, Fürst CJ, Adolfsson J, Steineck G. The unrecognised cost of cancer patients' unrelieved symptoms:a nationwide follow-up of their surviving partners. Br J Cancer. 2002;86:1540–1545. doi: 10.1038/sj.bjc.6600271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtz ME, Kurtz JC, Given CW, Given B. Relationship of caregiver reactions and depression to cancer patients' symptoms, functional states and depression--a longitudinal view. Soc Sci Med. 1995;40:837–846. doi: 10.1016/0277-9536(94)00249-s. [DOI] [PubMed] [Google Scholar]
- 12.Choi KH, Park SM, Lee K, Lee JH, Park JS. Influenza vaccination and associated factors among Korean cancer survivors : a cross-sectional analysis of the Fourth & Fifth Korea National Health and Nutrition Examination Surveys. J Korean Med Sci. 2014;29:1061–1068. doi: 10.3346/jkms.2014.29.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganry O, Baudoin C, Fardellone P. Effect of alcohol intake on bone mineral density in elderly women: The EPIDOS Study. Epidémiologie de l'Ostéoporose. Am J Epidemiol. 2000;151:773–780. doi: 10.1093/oxfordjournals.aje.a010277. [DOI] [PubMed] [Google Scholar]
- 14.Denlinger CS, Ligibel JA, Are M, Baker KS, Demark-Wahnefried W, Dizon D, Friedman DL, Goldman M, Jones L, King A, et al. Survivorship: healthy lifestyles, version 2.2014. J Natl Compr Canc Netw. 2014;12:1222–1237. doi: 10.6004/jnccn.2014.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim SM, Kim HC, Lee S. Psychosocial impact of cancer patients on their family members. Cancer Res Treat. 2013;45:226–233. doi: 10.4143/crt.2013.45.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao G, Okoro CA, Li J, White A, Dhingra S, Li C. Current depression among adult cancer survivors: findings from the 2010 Behavioral Risk Factor Surveillance System. Cancer Epidemiol. 2014;38:757–764. doi: 10.1016/j.canep.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho J, Choi EK, Kim SY, Shin DW, Cho BL, Kim CH, Koh DH, Guallar E, Bardwell WA, Park JH. Association between cancer stigma and depression among cancer survivors: a nationwide survey in Korea. Psychooncology. 2013;22:2372–2378. doi: 10.1002/pon.3302. [DOI] [PubMed] [Google Scholar]
- 18.Arrieta O, Angulo LP, Núñez-Valencia C, Dorantes-Gallareta Y, Macedo EO, Martínez-López D, Alvarado S, Corona-Cruz JF, Oñate-Ocaña LF. Association of depression and anxiety on quality of life, treatment adherence, and prognosis in patients with advanced non-small cell lung cancer. Ann Surg Oncol. 2013;20:1941–1948. doi: 10.1245/s10434-012-2793-5. [DOI] [PubMed] [Google Scholar]
- 19.Ell K, Sanchez K, Vourlekis B, Lee PJ, Dwight-Johnson M, Lagomasino I, Muderspach L, Russell C. Depression, correlates of depression, and receipt of depression care among low-income women with breast or gynecologic cancer. J Clin Oncol. 2005;23:3052–3060. doi: 10.1200/JCO.2005.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Momino K, Akechi T, Yamashita T, Fujita T, Hayahi H, Tsunoda N, Miyashita M, Iwata H. Psychometric properties of the Japanese version of the concerns about recurrence scale (CARS-J) Jpn J Clin Oncol. 2014;44:456–462. doi: 10.1093/jjco/hyu032. [DOI] [PubMed] [Google Scholar]
- 21.Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- 22.Hughes S, Jaremka LM, Alfano CM, Glaser R, Povoski SP, Lipari AM, Agnese DM, Farrar WB, Yee LD, Carson WE, 3rd, et al. Social support predicts inflammation, pain, and depressive symptoms: longitudinal relationships among breast cancer survivors. Psychoneuroendocrinology. 2014;42:38–44. doi: 10.1016/j.psyneuen.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang CY, Hsu MC. Social support as a moderator between depressive symptoms and quality of life outcomes of breast cancer survivors. Eur J Oncol Nurs. 2013;17:767–774. doi: 10.1016/j.ejon.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Han KH, Hwang IC, Kim S, Bae JM, Kim YW, Ryu KW, Lee JH, Noh JH, Sohn TS, Shin DW, et al. Factors associated with depression in disease-free stomach cancer survivors. J Pain Symptom Manage. 2013;46:511–522. doi: 10.1016/j.jpainsymman.2012.10.234. [DOI] [PubMed] [Google Scholar]
- 25.Carlson LE, Bultz BD. Cancer distress screening. Needs, models, and methods. J Psychosom Res. 2003;55:403–409. doi: 10.1016/s0022-3999(03)00514-2. [DOI] [PubMed] [Google Scholar]
- 26.Trask PC. Assessment of depression in cancer patients. J Natl Cancer Inst Monogr. 2004;2004:80–92. doi: 10.1093/jncimonographs/lgh013. [DOI] [PubMed] [Google Scholar]
- 27.Boyes AW, Girgis A, D'Este C, Zucca AC. Flourishing or floundering? Prevalence and correlates of anxiety and depression among a population-based sample of adult cancer survivors 6 months after diagnosis. J Affect Disord. 2011;135:184–192. doi: 10.1016/j.jad.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Shinn EH, Basen-Engquist K, Thornton B, Spiess PE, Pisters L. Health behaviors and depressive symptoms in testicular cancer survivors. Urology. 2007;69:748–753. doi: 10.1016/j.urology.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duffy SA, Ronis DL, Valenstein M, Fowler KE, Lambert MT, Bishop C, Terrell JE. Depressive symptoms, smoking, drinking, and quality of life among head and neck cancer patients. Psychosomatics. 2007;48:142–148. doi: 10.1176/appi.psy.48.2.142. [DOI] [PubMed] [Google Scholar]
- 30.Browman GP, Wong G, Hodson I, Sathya J, Russell R, McAlpine L, Skingley P, Levine MN. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328:159–163. doi: 10.1056/NEJM199301213280302. [DOI] [PubMed] [Google Scholar]
- 31.Chelghoum Y, Danaïla C, Belhabri A, Charrin C, Le QH, Michallet M, Fiere D, Thomas X. Influence of cigarette smoking on the presentation and course of acute myeloid leukemia. Ann Oncol. 2002;13:1621–1627. doi: 10.1093/annonc/mdf269. [DOI] [PubMed] [Google Scholar]
- 32.Park JJ, Park HA. Prevalence of cigarette smoking among adult cancer survivors in Korea. Yonsei Med J. 2015;56:556–562. doi: 10.3349/ymj.2015.56.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi KH, Park SM, Lee K, Kim KH, Park JS, Han SH. Prevalence, awareness, control, and treatment of hypertension and diabetes in Korean cancer survivors: a cross-sectional analysis of the Fourth and Fifth Korea National Health and Nutrition Examination Surveys. Asian Pac J Cancer Prev. 2013;14:7685–7692. doi: 10.7314/apjcp.2013.14.12.7685. [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Son BH, Hwang SY, Han W, Yang JH, Lee S, Yun YH. Fatigue and depression in disease-free breast cancer survivors: prevalence, correlates, and association with quality of life. J Pain Symptom Manage. 2008;35:644–655. doi: 10.1016/j.jpainsymman.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Vissers PA, Thong MS, Pouwer F, Zanders MM, Coebergh JW, van de Poll-Franse LV. The impact of comorbidity on health-related quality of life among cancer survivors: analyses of data from the PROFILES registry. J Cancer Surviv. 2013;7:602–613. doi: 10.1007/s11764-013-0299-1. [DOI] [PubMed] [Google Scholar]
- 36.Moubayed SP, Sampalis JS, Ayad T, Guertin L, Bissada E, Gologan OE, Soulières D, Lambert L, Filion E, Nguyen-Tan PF, et al. Predicting depression and quality of life among long-term head and neck cancer survivors. Otolaryngol Head Neck Surg. 2015;152:91–97. doi: 10.1177/0194599814557772. [DOI] [PubMed] [Google Scholar]
- 37.Tagay S, Herpertz S, Langkafel M, Erim Y, Bockisch A, Senf W, Görges R. Health-related quality of life, depression and anxiety in thyroid cancer patients. Qual Life Res. 2006;15:695–703. doi: 10.1007/s11136-005-3689-7. [DOI] [PubMed] [Google Scholar]
- 38.Andersen BL, DeRubeis RJ, Berman BS, Gruman J, Champion VL, Massie MJ, Holland JC, Partridge AH, Bak K, Somerfield MR, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32:1605–1619. doi: 10.1200/JCO.2013.52.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]