Abstract
The Clinical Assessment Interview for Negative Symptoms (CAINS) was developed to overcome the limitations of existing instruments and reflect the current view of negative symptoms. The aim of the present study was to evaluate the reliability and validity of the Korean version of the Clinical Assessment Interview for Negative Symptoms (K-CAINS). Inpatients (n = 49) and outpatients (n = 70) with schizophrenia were recruited from three institutions. The confirmative factor analysis, test-retest reliability, inter-rater reliability, convergent validity, and discriminant validity were assessed. The study group consisted of 71 males (59.7%) and 48 females (40.3%). Their mean age was 42.15 years (SD = 12.2). The K-CAINS was confirmed to be divided into two subscales of 9 items related to "motivation/pleasure" and 4 items related to "expression" in concordance with the original version of the CAINS. The results showed that the K-CAINS had a good inter-rater reliability (ICC = 0.84-0.94), test-retest reliability (r = 0.90, P < 0.001). Convergent validity was proven by demonstrating a significant correlation with the Positive and Negative Syndrome Scale (PANSS) negative subscale, and the Scale for the Assessment of Negative Symptoms (SANS). Discriminant validity was proven by the lack of a significant correlation with the PANSS positive subscale, the Korean version of the Beck depression inventory (BDI), the Korean version of the Calgary depression scale for schizophrenia (K-CDSS), and the Modified Simpson Angus scale (MSAS). The K-CAINS could be a reliable and valid tool to assess the negative symptoms of Korean schizophrenia patients.
Keywords: Schizophrenia, Negative Symptoms, CAINS, Korean
Graphical Abstract
INTRODUCTION
Schizophrenia is a chronic disease that causes dysfunction in various areas and is commonly associated with impairments in social and occupational function. Whereas positive symptoms (hallucination, delusion, disorganized speech, etc.) represent an exaggeration of normal processes, negative symptoms (anhedonia, avolition, and flat affect) are conceptualized as an absence or diminution of normal processes. Negative symptoms are more resistant to treatment (1,2) cause more severe functional impairments (3) and are related to a source of burden for caregivers (4). Despite the clinical importance of negative symptoms, negative symptoms remain inadequately addressed by current clinical reality (5,6). Therefore, the management of negative symptoms is an essential aspect on treatment of schizophrenia. Moreover, negative symptoms are difficult to distinguish from depressive symptoms, drug side effects, and secondary negative symptoms due to positive symptoms (7). Therefore, negative symptoms need to be accurately assessed prior to treatment.
The commonly used negative symptom rating scales include the Scale for the Assessment of Negative Symptoms (SANS) (8), Positive and Negative Syndrome Scale (PANSS) (9), Schedule for Affective Disorders and Schizophrenia (SADS) (10), and the Schedule for the Deficit Syndrome (SDS) (11). However, recent studies have identified significant limitations associated with the current instruments used to assess negative symptoms (12,13). For example, the SANS and the PANSS, which are the most commonly used instruments to assess negative symptoms include items that assess cognitive functioning (12). However, these items do not correlate well with other negative symptoms items and are conceptually distinct from negative symptoms (14,15,16,17). Additionally, existing instruments do not consider the patient's specific circumstances (e.g., a lack of opportunity due to social and economic deprivation, isolation related to geographical location, a lack of social skills, and family conflicts) but instead assess the behavioral results (e.g., observation of behavior during the interview, occupational and interpersonal relationships, and social behavior). However, social and occupational failures that derive from these factors are distinct from those derived from emotional (anhedonia) or motivational (avolition) deficits, which are the core concepts of negative symptoms (18). Therefore, existing instruments that assess only a patient's behavioral results are limited in their ability to assess the core concepts of negative symptoms.
Recent studies suggest that negative symptoms appear to cluster into two components, a diminished expression symptom cluster and an avolition-apathy cluster (19,20). The Clinical Assessment Interview for Negative Symptoms (CAINS) was developed to consider limitations of existing instruments and reflect the current view of negative symptoms (12,21,22,23). The final version of the CAINS consists of nine items related to "motivation/pleasure" factor and four items related to "expression" factor (23). The final version of the CAINS demonstrated strong reliability and validity, and its independence from depression, medication side effects, and cognition which are difficult to distinguish from negative symptoms, especially demonstrated its strong discriminant validity (23). The results of the validation studies of the German (24) and the Chinese version (25) of the CAINS were consistent with those of the original study (23).
Moreover, effective translation and verification of the reliability and validity of measurements must take precedence when seeking to apply a measurement developed for one area to another having a different culture and language.
Thus, we have translated the final version of the CAINS (23) and initiated standardization research in this study to verify and take complementary measures of its reliability and validity. The present study aims to make K-CAINS available for the study, diagnosis and treatment of schizophrenia patients in Korea.
MATERIALS AND METHODS
Participants
The participants consisted of 119 patients with schizophrenia, recruited in inpatient (n = 49, 41.2%) and outpatient (n = 70, 58.8%) at 3 medical centers (Department of Psychiatry, Catholic University of Daegu School of Medicine; Department of Psychiatry, Keimyung University Dongsan Medical Center; Department of Psychiatry, Bugok National Hospital). The patients were diagnosed with schizophrenia using the Korean version of Mini-International Neuropsychiatric Interview (K-MINI) (26). The study group consisted of 71 males (59.7%) and 48 females (40.3%). Their mean age was 42.15 years (SD = 12.2), their mean duration of illness was 13.4 years (SD = 0.7), and their mean number of episode was 4.02 (SD = 4.28). The exclusion criteria were a mood episode within the past month, substance dependence in the past 6 months, substance abuse in the past month, a history of head injury or neurological disorder, or a clinically severe medical condition. All participants provided written informed consent.
Procedures
Prior to the translation, we obtained permission from the author of the final version CAINS (23) for the translation and standardized study in Korea. The Korean version of the Clinical Assessment Interview for Negative Symptoms (K-CAINS) was developed via a forward-backward translation procedure (27). Two authors of the Korean version who are native speakers of Korean with a high level of fluency in English, independently translated 13 items of the original (English) version of the CAINS. Both translated versions were compared and after discussing, the first Korean version of the CAINS was obtained. The preliminary Korean version was back-translated to English by native English speakers without reference to the CAINS. Finally, the translators compared the back-translated version and original version of the CAINS and discussed the translation difference. The Korean version of the CAINS was administered to a pilot group of 10 schizophrenia patients to identify any latent problems in translation. After the interview, the patients were asked about each item. The patients demonstrated a good understanding of each item. The final Korean version of the Clinical Assessment Interview for Negative Symptoms (K-CAINS) was then completed.
Three clinical raters with medical doctorate degrees and highly clinical experienced psychiatrists were trained in the administration of the CAINS interview. Training included an overview of the CAINS, the interview procedure, each item's anchor point, education regarding the assessment guide and ratings of 5 patient's videotaped interviews.
The interviews were conducted in two sessions approximately 4 weeks apart (mean = 27.4 days, SD = 3.7). In the first session, the K-CAINS, Positive and Negative Syndrome Scale (PANSS) (9), Scale for the Assessment of Negative Symptoms (SANS) (8), Korean version of Brief Psychiatric Rating Scale (K-BPRS) (28), Korean version of the Calgary Depression Scale for Schizophrenia (K-CDSS) (29), the Korean version of the Beck Depression inventory (K-BDI) (30), and Modified Simpson Angus Scale (MSAS) (31) were administered to all participants. The CAINS interviews were videotaped, and 51 videos were rated independently by 2 other raters to evaluate inter-rater reliability. Videotapes were only used to evaluate inter-rater reliability and were then discarded. In the second session, the K-CAINS and K-BPRS (28) were re-administered to evaluate the test-retest reliability.
Data analysis
Summary for sociodemographic characteristics variables were performed using descriptive analysis, the value of mean ± SD (standard deviation) presented for quantitative variables and the value of frequency (percent) for qualitative variables (Table 1). To assess the internal consistency of the K-CAINS, Cronbach's α (32) was calculated for both the subscale and total scale. We conducted a confirmatory factor analysis (33) of the CAINS items to confirm that the structure of the K-CAINS was the same as that of the original version of the CAINS using structural equation. To check consistency between measured variables in structural equation, construct reliability was obtained and the value of > 0.7 indicated good construct reliability. To check accuracy of measured concept in structural equation, construct validity was obtained and the value of the average variance extracted (AVE) > 0.5 indicated good construct validity. To check validity of item discrimination in structural equation, discriminant validity was obtained and the value of the average variance extracted (AVE) > the squared multiple correlation (SMC) indicated good discriminant validity (Table 2). Additionally, we calculated goodness of fit for model such as χ2, df, NFI, IFI, TLI, CFI, RMSEA, AIC. To assess the inter-rater reliability of 3 raters, the intraclass correlation coefficient (ICC) (34) was calculated. Convergent validity was assessed by examining the correlation between the K-CAINS and negative symptom scales (SANS and PANSS negative subscale). Discriminant validity was assessed by examining the correlation between the CAINS and the PANSS positive subscale, PANSS general psychopathology subscale (9), Korean version of the Beck Depression inventory (K-BDI) (30), Korean version of the Calgary Depression Scale for Schizophrenia (K-CDSS) (29), and Modified Simpson Angus Scale (MSAS) (31). All test 2-sided and P value of < 0.05 indicated statistical significance. All statistical analyses were performed using IBM SPSS version 19.0 and IBM SPSS AMOS 19.0.
Table 1. Sociodemographic characteristics of participants with schizophrenia (n = 119).
| Parameters | No. | % |
|---|---|---|
| Male | 71 | 59.7 |
| Has a job | 19 | 16 |
| Inpatients | 49 | 41.2 |
| Marital status | ||
| Married | 23 | 19.3 |
| Never married | 81 | 68.1 |
| Widowed | 1 | 0.8 |
| Separated | 2 | 1.7 |
| Divorced | 9 | 7.6 |
| Remarriage | 3 | 2.5 |
| SES | ||
| High | 1 | 0.8 |
| Middle | 61 | 51.3 |
| Low | 57 | 47.9 |
| Mean | SD | |
| Age, yr | 42.3 | 12.2 |
| Education | 11.6 | 2.9 |
| Duration of illness, yr | 13.5 | 9.7 |
| Number of hospitalizations | 4 | 4.2 |
Table 2. Confirmatory factor analysis for the K-CAINS Items and goodness of fit for model.
| Measured variables | Construct reliability | AVE | Correlation coefficient | SMC | |
|---|---|---|---|---|---|
| 1. Social: Family Relationships | 0.883 | 0.802 | 0.704 | 0.496 | |
| 2. Social: Friendships | |||||
| 3. Social: Past Week Pleasure | |||||
| 4. Social: Expected Pleasure | |||||
| 5. Work & School: Motivation | |||||
| 6. Work & School: Expected Pleasure | |||||
| 7. Recreation: Motivation | |||||
| 8. Recreation: Past Week Pleasure | |||||
| 9. Recreation: Expected Pleasure | |||||
| 10. Expression: Facial | 0.891 | 0.858 | |||
| 11. Expression: Vocal Prosody | |||||
| 12. Expression: Gestures | |||||
| 13. Expression: Speech | |||||
| Statistic | χ2(P) | df | NFI | IFI | |
| Value | 212.776 (0.000) | 64 | 0.820 | 0.867 | |
| Statistic | TLI | CFI | RMSEA | AIC | |
| Value | 0.806 | 0.863 | 0.140 | 292.776 | |
CAINS, Clinical Assessment Interview for Negative Symptoms; AVE, Average Variance Extracted; SMC, squared multiple correlation.
Ethics statement
This study was approved by the Daegu Catholic University Medical Center institutional review board (DCUMC IRB approval No. CR-14-130). Written informed consent was given from all the individual participants.
RESULTS
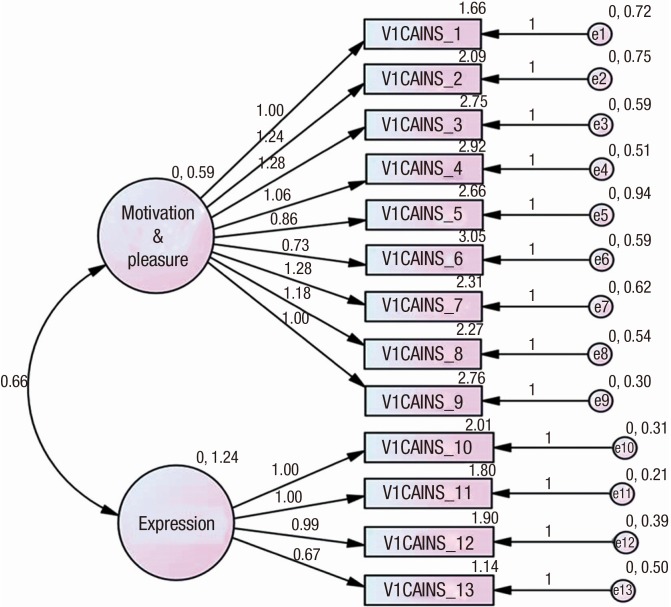
The confirmative factor analysis was conducted to confirm that the K-CAINS was divided into two subscales, like the original (English) version of the CAINS (Fig. 1). The construct reliability of the motivation/pleasure subscale and the expression subscale was 0.883 and 0.891, respectively. Both of these values exceeded 0.7, indicating good construct reliability. The average variance extracted (AVE) of both subscales were 0.802 and 0.858, i.e., more than 0.5, indicating construct validity. Additionally, the AVEs of both subscales were exceeded the SMC value of 0.496, indicating discriminant validity. The values of goodness of fit for model were as follow (χ2 [P] = 212.776[0.000], df = 64, NFI = 0.820, IFI = 0.867, TLI = 0.806, CFI = 0.863, RMSEA = 0.140, AIC = 292.776) (Table 2). The results of the factor analysis of the K-CAINS were consistent with those of previous studies (23,24,25) in which two-dimensional structures were extracted.
Fig. 1.
Figure of structural equation model of K-CAINS.
Cronbach's α value revealed that the K-CAINS had a good internal consistency in the subscales of ‘motivation/pleasure' (Cronbach's α = 0.90) and ‘expression' (Cronbach's α = 0.91) as well as in the whole scale (Cronbach's α = 0.93) (Table 3).
Table 3. Test-retest (K-CAINS).
| Coefficients | Test | Retest |
|---|---|---|
| Mean (SD) | 28.2 (10.7) | 27.6 (10.4) |
| Cronbach's α | 0.933 | 0.926 |
| Pearson correlation (P value) | 0.907 (< 0.001) | |
| Intraclass correlation coefficient (P value) | 0.951 (< 0.001) |
CAINS, Clinical Assessment Interview for Negative Symptoms.
For the 98 participants who completed the K-CAINS in two sessions conducted one month apart, the test-retest reliability for the motivation/pleasure subscale, expression subscales and total scale were 0.89 (P < 0.001), 0.87 (P < 0.001) and 0.90 (P < 0.001) (Table 3), respectively, as measured by Pearson's correlation r (35). The test-retest reliability of the K-BPRS was 0.92 (P < 0.001), which was similar to the result for the K-CAINS.
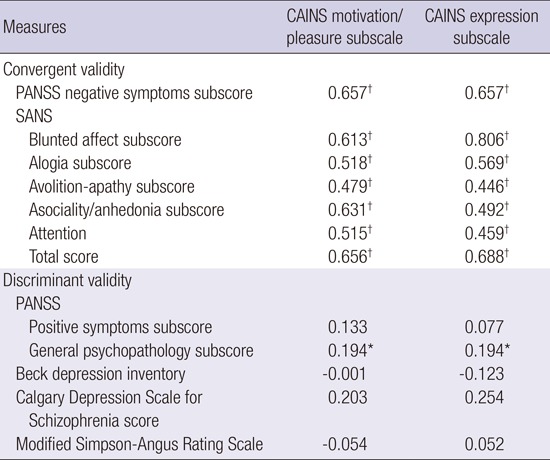
Convergent validity was assessed by examining the correlations between the K-CAINS scales and the negative symptom subscales of the PANSS, and SANS. Both the motivation/pleasure subscale and the expression subscale significantly correlated with the negative symptom subscale of the PANSS (motivation/pleasure: r = 0.657, P < 0.001; expression: r = 0.657, P < 0.001), the SANS total score (motivation/pleasure: r = 0.65, P < 0.001; expression: r = 0.68, P < 0.001) and all subscales, indicating the good convergent validity of the K-CAINS (Table 4).
Table 4. Convergent validity and Discriminant validity of the K-CAINS scale.
| Measures | CAINS motivation/pleasure subscale | CAINS expression subscale |
|---|---|---|
| Convergent validity | ||
| PANSS negative symptoms subscore | 0.657† | 0.657† |
| SANS | ||
| Blunted affect subscore | 0.613† | 0.806† |
| Alogia subscore | 0.518† | 0.569† |
| Avolition-apathy subscore | 0.479† | 0.446† |
| Asociality/anhedonia subscore | 0.631† | 0.492† |
| Attention | 0.515† | 0.459† |
| Total score | 0.656† | 0.688† |
| Discriminant validity | ||
| PANSS | ||
| Positive symptoms subscore | 0.133 | 0.077 |
| General psychopathology subscore | 0.194* | 0.194* |
| Beck depression inventory | -0.001 | -0.123 |
| Calgary Depression Scale for | 0.203 | 0.254 |
| Schizophrenia score | ||
| Modified Simpson-Angus Rating Scale | -0.054 | 0.052 |
CAINS, Clinical Assessment Interview for Negative Symptoms; PANSS, Positive and Negative Syndrome Scale; SANS, Scale for the Assessment of Negative Symptoms.
* P < 0.05; † P < 0.001.
Discriminant validity was assessed by examining the correlation between the K-CAINS and positive subscale of the PANSS, general psychopathology of the PANSS, K-BDI, K-CDSS, and MSAS. Neither the motivation/pleasure subscale nor the expression subscale significantly correlated with the positive symptoms subscale of the PANSS (motivation/pleasure: r = 0.13, P = 0.15; expression: r = 0.12, P = 0.19), depression symptoms scale (K-BDI: motivation/pleasure: r = -0.001, P = 0.99; expression: r = -0.12, P = 0.18), (K-CDSS) (motivation/pleasure: r = 0.20, P = 0.21; expression: r = 0.25, P = 0.11) or medication side effect scale (MSAS) (motivation/pleasure: r = -0.05, P = 0.63; expression: r = 0.52, P = 0.64), indicating good discriminant validity. Although both subscales of the K-CAINS correlated with the general psychopathology of the PANSS (motivation/pleasure: r = 0.19, P = 0.03; expression: r = 0.52, P = 0.03), the SANS also significantly correlates with the general psychopathology subscale of the PANSS (r = 0.42, P < 0.001), and this latter correlation is stronger than that of the K-CAINS (Table 4).
The item-total score correlation ranged from 0.60 to 0.70 in the subscale of motivation/pleasure and from 0.72 to 0.82 in the subscale of expression (Table 5).
Table 5. Descriptive statistics and inter-rater agreement for K- CAINS items.
| Items | Mean | SD | Skewness | Item-total correlation | ICC |
|---|---|---|---|---|---|
| 1. Social: Family Relationships | 1.66 | 1.15 | 0.28 | 0.72 | 0.90 |
| 2. Social: Friendships | 2.09 | 1.29 | -0.07 | 0.76 | 0.93 |
| 3. Social: Past Week Pleasure | 2.75 | 1.25 | -0.86 | 0.77 | 0.90 |
| 4. Social: Expected Pleasure | 2.92 | 1.09 | -1.12 | 0.72 | 0.90 |
| 5. Work & School: Motivation | 2.66 | 1.17 | -0.63 | 0.60 | 0.90 |
| 6. Work & School: Expected Pleasure | 3.05 | 0.95 | -0.69 | 0.61 | 0.84 |
| 7. Recreation: Motivation | 2.31 | 1.26 | -0.37 | 0.79 | 0.92 |
| 8. Recreation: Past Week Pleasure | 2.27 | 1.17 | -0.21 | 0.78 | 0.91 |
| 9. Recreation: Expected Pleasure | 2.76 | 0.94 | -1.07 | 0.78 | 0.90 |
| 10. Expression: Facial | 2.01 | 1.25 | 0.01 | 0.78 | 0.93 |
| 11. Expression: Vocal Prosody | 1.80 | 1.21 | 0.19 | 0.82 | 0.92 |
| 12. Expression: Gestures | 1.90 | 1.27 | 0.19 | 0.78 | 0.92 |
| 13. Expression: Speech | 1.14 | 1.03 | 0.68 | 0.72 | 0.94 |
CAINS, Clinical Assessment Interview for Negative Symptoms.
The average intraclass correlation coefficient (ICC) between the three raters was 0.96 (P < 0.001) for the CAINS motivation/pleasure subscale, 0.95 (P < 0.001) for the CAINS expression subscale, and 0.96 (P < 0.001) for the total scale. The ICC for all CAINS items exceeded 0.90 (P < 0.001) (Table 5).
DISCUSSION
The results of the present study demonstrated that the Korean version of the CAINS is valid and reliable. The Korean version of the CAINS (K-CAINS) was also confirmed to be divided into two subscales (motivation/pleasure subscale and expression subscale), in concordance with previous studies (23,24,25). It showed a high internal consistency in both the motivation/pleasure subscale and expression subscale (Cronbach's α = 0.90 and 0.91). This finding was similar to that of the original study (23) (Cronbach's α = 0.76 and 0.88), and the validation study (Cronbach's α = 0.85 and 0.90) of the CAINS performed in China (25). In addition, the K-CAINS showed a high internal consistency in item-total correlation (0.60-0.82), which was similar to the results of the validation study of the CAINS performed in China (0.50-0.79).
The scores were similar between the three tested institutions and between the test and retest, which were conducted one month apart. This similarity was more pronounced than that reported in previous studies. We attribute this similarity to our familiarity with the existing study, which sufficiently discussed before the research was carried out.
The Korean Version of the CAINS (K-CAINS) showed high convergent validity with scales evaluating the negative symptoms (each subscale of the SANS and the total and negative symptoms subscale of the PANSS). In addition, K-CAINS showed good discriminant validity, as indicated by a no significant correlation with scales that evaluate positive symptoms, depressive symptoms, and side effects of medication. Additionally, the Korean version of the CAINS (K-CAINS) was also confirmed to be effectively divided into two subscales like the final version of the CAINS (23), as indicated by a confirmatory factor analysis that utilized the structural equation.
The original study only assessed outpatients (23). In the present study, inpatients were included to demonstrate the availability of K-CAINS in different circumstances and in patients who have relatively severe psychotic symptoms. In fact, we found that the inpatient groups had more severe psychotic symptoms, as indicated by significantly higher scores than the PANSS total score (82.78 vs. 57.84, P < 0.05), positive symptoms subscale (20.22 vs. 11.97, P < 0.001), and general psychopathology (42.41 vs. 27.53, P < 0.05) of outpatients. However, the PANSS negative symptoms subscale did not significantly differ between these groups. In other words, the present study demonstrated the validity and reliability of the CAINS, even in a population (inpatient group) that is different from those examined in the original study.
In the correlation analysis to evaluate the discriminant validity of the CAINS, only the CAINS and the general psychopathology subscale of the PANSS showed significant correlation. However, the SANS also significantly correlates with the general psychopathology subscale of the PANSS, and this correlation is stronger than that of the CAINS. A previous validation study of the Chinese version of the CAINS showed similar results to those of the present study but demonstrated a stronger correlation than that observed in the present study (motivation/pleasure r = 0.466, P < 0.01; expression r = 0.527, P < 0.01). We postulate that this result is due to the wide range of conditions that are included in the PANSS general psychopathology subscale. Moreover, some items of general psychopathology subscale of the PANSS (‘motor retardation', ‘disturbance of volition', ‘active social avoidance') were conceptually closer to negative symptoms.
In the CAINS evaluation, evaluating the expression subscale can be considered more subjective than evaluating the motivation/pleasure subscale because the expression subscale is only assessed by the rater's observation during interview, whereas the motivation/pleasure subscale consists of specific questions to the patients. However, the current study showed a high degree of coincidence between raters in both the expression subscale and motivation/pleasure subscale, which suggests that the CAINS is not subject to this potential limitation.
In this study, the original study (23), studies performed in Germany (24) and studies performed in China (25), drug history, such as the type of drug or drug response were not investigated. In future studies, the validity and reliability of the K-CAINS needs to be proven in patient groups divided according to drug history (taking or not taking drugs and the response to treatment).
In this study, the condition of subjects may have changed in the time interval between the examination and re-examination, which was one month and longer than the two-week intervals reported in a previous study (23). However, the BPRS and CAINS motivation/pleasure subscale and expression subscale did not significantly differ upon test-retesting, as examined by a paired T-test; in other words, symptoms remained relatively stable over a period of one month. Composition of the subjects might explain this finding as over half of the subjects were chronic outpatients. Furthermore, in the case of hospitalized patients (n = 49), most subjects (n = 40) were chronic patients hospitalized for long periods at the national hospital. Therefore, the patients likely remained relatively stable with no change in their condition.
The CAINS provides a clear and comprehensive measurement and has the advantages that the distinction of anchor points and evaluation of each item are easy. In addition, negative symptoms can be distinguished from depressive symptoms and drug side effects. This multi-center study demonstrates that the Korean version of the Clinical Assessment Interview for Negative Symptoms (K-CAINS) is reliable and valid. Thus, K-CAINS could be a useful tool for the diagnosis, treatment, and clinical study of schizophrenia in Korea.
ACKNOWLEDGMENT
The authors thank Dae-Hee Lee for data collection and management.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study concept and coordination: Woo JM. Study design: Woo JM, Jung SI. Data collection: Woo JM, Kim YT, Jung SI. Analysis and interpretation of data: Kwak SG, Jung SI, Woo JM. Writing manuscript: Jung SI, Woo JM. Discussion and manuscript revision: Woo JM, Kim YT, Jung SI.
References
- 1.Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, Himelhoch S, Fang B, Peterson E, Aquino PR, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36:71–93. doi: 10.1093/schbul/sbp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkpatrick B, Kopelowicz A, Buchanan RW, Carpenter WT., Jr Assessing the efficacy of treatments for the deficit syndrome of schizophrenia. Neuropsychopharmacology. 2000;22:303–310. doi: 10.1016/S0893-133X(99)00122-0. [DOI] [PubMed] [Google Scholar]
- 3.Strauss GP, Harrow M, Grossman LS, Rosen C. Periods of recovery in deficit syndrome schizophrenia: a 20-year multi-follow-up longitudinal study. Schizophr Bull. 2010;36:788–799. doi: 10.1093/schbul/sbn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Provencher HL, Mueser KT. Positive and negative symptom behaviors and caregiver burden in the relatives of persons with schizophrenia. Schizophr Res. 1997;26:71–80. doi: 10.1016/S0920-9964(97)00043-1. [DOI] [PubMed] [Google Scholar]
- 5.Murphy BP, Chung YC, Park TW, McGorry PD. Pharmacological treatment of primary negative symptoms in schizophrenia: a systematic review. Schizophr Res. 2006;88:5–25. doi: 10.1016/j.schres.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery SA, van Zwieten-Boot B. ECNP consensus meeting. Negative, depressive and cognitive symptoms of schizophrenia. Nice, March 2004. Eur Neuropsychopharmacol. 2007;17:70–77. doi: 10.1016/j.euroneuro.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Newcomer JW, Faustman WO, Yeh W, Csernansky JG. Distinguishing depression and negative symptoms in unmedicated patients with schizophrenia. Psychiatry Res. 1990;31:243–250. doi: 10.1016/0165-1781(90)90093-k. [DOI] [PubMed] [Google Scholar]
- 8.Andreasen NC. The scale for the assessment of negative symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989:49–58. [PubMed] [Google Scholar]
- 9.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 10.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 11.Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT., Jr The schedule for the deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30:119–123. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard JJ, Kring AM, Horan WP, Gur R. Toward the next generation of negative symptom assessments: the collaboration to advance negative symptom assessment in schizophrenia. Schizophr Bull. 2011;37:291–299. doi: 10.1093/schbul/sbq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull. 2006;32:259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayers SL, Curran PJ, Mueser KT. Factor structure and construct validity of the scale for the assessment of negative symptoms. Psychol Assess. 1996;8:269–280. [Google Scholar]
- 15.White L, Harvey PD, Opler L, Lindenmayer JP, The PANSS Study Group Empirical assessment of the factorial structure of clinical symptoms in schizophrenia. A multisite, multimodel evaluation of the factorial structure of the positive and negative syndrome scale. Psychopathology. 1997;30:263–274. doi: 10.1159/000285058. [DOI] [PubMed] [Google Scholar]
- 16.van der Gaag M, Cuijpers A, Hoffman T, Remijsen M, Hijman R, de Haan L, van Meijel B, van Harten PN, Valmaggia L, de Hert M. The five-factor model of the positive and negative syndrome scale I: confirmatory factor analysis fails to confirm 25 published five-factor solutions. Schizophr Res. 2006;85:273–279. doi: 10.1016/j.schres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Harvey PD, Koren D, Reichenberg A, Bowie CR. Negative symptoms and cognitive deficits: what is the nature of their relationship? Schizophr Bull. 2006;32:250–258. doi: 10.1093/schbul/sbj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellack AS, Sayers M, Mueser KT, Bennett M. Evaluation of social problem solving in schizophrenia. J Abnorm Psychol. 1994;103:371–378. doi: 10.1037//0021-843x.103.2.371. [DOI] [PubMed] [Google Scholar]
- 19.Strauss GP, Horan WP, Kirkpatrick B, Fischer BA, Keller WR, Miski P, Buchanan RW, Green MF, Carpenter WT., Jr Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res. 2013;47:783–790. doi: 10.1016/j.jpsychires.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32:238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ. Development and psychometric validation of the clinical assessment interview for negative symptoms (CAINS) Schizophr Res. 2011;132:140–145. doi: 10.1016/j.schres.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The clinical assessment interview for negative symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170:165–172. doi: 10.1176/appi.ajp.2012.12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engel M, Fritzsche A, Lincoln TM. Validation of the German version of the clinical assessment interview for negative symptoms (CAINS) Psychiatry Res. 2014;220:659–663. doi: 10.1016/j.psychres.2014.07.070. [DOI] [PubMed] [Google Scholar]
- 25.Chan RC, Shi C, Lui SS, Ho KK, Hung KS, Lam JW, Wang Y, Cheung EF, Yu X. Validation of the Chinese version of the clinical assessment interview for negative symptoms (CAINS): a preliminary report. Front Psychol. 2015;6:7. doi: 10.3389/fpsyg.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu SW, Kim YS, Noh JS, Oh KS, Kim CH, Namkoong K, Chae JH, Lee GC, Jeon SI, Min KJ, et al. Validity of Korean version of the mini-international neuropsychiatric interview. Anxiety Mood. 2006;2:50–55. [Google Scholar]
- 27.Koller M, Aaronson NK, Blazeby J, Bottomley A, Dewolf L, Fayers P, Johnson C, Ramage J, Scott N, West K, EORTC Quality of Life Group Translation procedures for standardised quality of life questionnaires: the European organisation for research and treatment of cancer (EORTC) approach. Eur J Cancer. 2007;43:1810–1820. doi: 10.1016/j.ejca.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 28.Kim MK, Lee BK, Jeon YW. Reliability of Korean brief psychiatric rating scale(BPRS): comparison of interrater reliability between the two rating methods and correlation of BPRS and SCL-90 self-report test. Korean J Clin Psychol. 2003;22:685–698. [Google Scholar]
- 29.Kim YK, Won SD, Lee KM, Choi HS, Jang HS, Lee BH, Han CS. A study on the reliability and validity of the Korean version of the Calgary depression scale for schizophrenia(K-CDSS) J Korean Neuropsychiatr Assoc. 2005;44:446–455. [Google Scholar]
- 30.Han HM, Yeom TH, Shin YW, Kim KH, Yoon DJ, Chung KJ. A standardization study of Beck depression inventory in Korea. J Korean Neuropsychiatr Assoc. 1986;25:487–500. [Google Scholar]
- 31.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 32.Zinbarg RE, Revelle W, Yovel I, Li W. Cronbach's α, Revelle's β, and McDonald's ω H: their relations with each other and two alternative conceptualizations of reliability. Psychometrika. 2005;70:123–133. [Google Scholar]
- 33.Thompson B. Exploratory and Confirmatory Factor Analysis: Understanding Concepts and Applications. Washington, D.C.: American Psychological Association; 2004. [Google Scholar]
- 34.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 35.Ahlgren P, Jarneving B, Rousseau R. Requirements for a cocitation similarity measure, with special reference to Pearson's correlation coefficient. J Am Soc Inf Sci Technol. 2003;54:550–560. [Google Scholar]