Abstract
Pesticide formulation includes solvents (methanol and xylene) and antifreeze (ethylene glycol) whose metabolites are anions such as formic acid, hippuric acid, and oxalate. However, the effect of the anion gap on clinical outcome in acute pesticide intoxication requires clarification. In this prospective study, we compared the anion gap and other parameters between surviving versus deceased patients with acute pesticide intoxication. The following parameters were assessed in 1,058 patients with acute pesticide intoxication: blood chemistry (blood urea nitrogen, creatinine, glucose, lactic acid, liver enzymes, albumin, globulin, and urate), urinalysis (ketone bodies), arterial blood gas analysis, electrolytes (Na+, K+, Cl- HCO3-, Ca++), pesticide field of use, class, and ingestion amount, clinical outcome (death rate, length of hospital stay, length of intensive care unit stay, and seriousness of toxic symptoms), and the calculated anion gap. Among the 481 patients with a high anion gap, 52.2% had a blood pH in the physiologic range, 35.8% had metabolic acidosis, and 12.1% had acidemia. Age, anion gap, pesticide field of use, pesticide class, seriousness of symptoms (all P < 0.001), and time lag after ingestion (P = 0.048) were significant risk factors for death in univariate analyses. Among these, age, anion gap, and pesticide class were significant risk factors for death in a multiple logistic regression analysis (P < 0.001). In conclusions, high anion gap is a significant risk factor for death, regardless of the accompanying acid-base balance status in patients with acute pesticide intoxication.
Keywords: Acidosis, Pesticides, Biomarkers, Acid-base Equilibrium
Graphical Abstract
INTRODUCTION
The ingestion of pesticides is a common method of suicide in many Asian countries (1,2,3). Patients who have ingested pesticides are candidates for aggressive therapy because their chance for survival improves when adequate therapy is instituted (4). To provide adequate therapy, it is important to have an in-depth knowledge of clinical markers that can predict the clinical outcome.
Patients with acute pesticide intoxication occasionally suffer acid-base imbalance resulting from various causes (5,6). Unconsciousness, with or without respiratory failure, is a common clinical manifestation that can lead to respiratory acidosis. Metabolic acidosis is also a common clinical feature of acute pesticide intoxication (5,6). Various causes of acute metabolic acidosis are encountered in patients with acute pesticide intoxication. First, acute pesticide intoxication is frequently complicated by circulatory collapse, which causes the accumulation of lactic acid. Second, additives can provide anions when they enter the body, because the metabolites of some additives are anions: for example, ethylene glycol is commonly included as an antifreeze agent, and its metabolites are glycolic acid or oxalate, and the metabolites of the common solvents methanol and xylene are formaldehyde and formic acid (methanol) (7,8) and hippuric acid (xylene) (9).
Individuals with metabolic acidosis usually experience nausea, vomiting, and fatigue. As the acidosis worsens, the patient begins to feel weak and drowsy. Eventually, blood pressure can fall, leading to shock, coma, and death. These symptoms resemble the toxic symptoms of acute pesticide intoxication. This implies that an acid-base imbalance may aggravate the toxic symptoms or exert a synergistic toxicity with the pesticide itself. Therefore, it is important to evaluate the acid-base status in patients with acute pesticide intoxication. Despite its importance, little is known about the incidence and effect of the acid-base imbalance implicated by the anion gap on clinical outcome in acute pesticide intoxication. The current study is designed to observe the effect of the anion gap on clinical outcome and to determine the composition of the unmeasured anions in acute pesticide intoxication.
MATERIALS AND METHODS
A prospective study was performed to identify the potential effects of high anion gap metabolic acidosis on the clinical outcome of acute pesticide intoxication. Between January 2011 and December 2014, 1,331 patients attempted suicide by pesticide ingestion and were treated at the Institute of Pesticide Poisoning at Soonchunhyang University Cheonan Hospital, a tertiary referral center for toxicology patients located in a rural area of Korea. Patients were excluded if they were transferred from another hospital after treatment of the acid-base imbalance with intravenous NaHCO3 administration or after undergoing hemodialysis. Patients who arrived 12 hours or more after pesticide ingestion were also excluded. Using these criteria, 1,058 patients were enrolled in this study. The pesticide type was confirmed by inspecting the pesticide bottle that was presented by the individual accompanying the patient.
Upon admission, patients received standardized medical emergency treatment. Gastric lavage was performed if the patients arrived within 2 hours after ingestion. Hemodialysis or hemoperfusion was initiated early and was based on the chemical characteristics of the pesticide.
Blood samples for arterial blood gas analysis (ABGA) and electrolytes (Na+, K+, Cl- HCO3-, and Ca++) were drawn simultaneously in the emergency room, before the initiation of extracorporeal treatment.
The blood chemistry test included blood urea nitrogen (BUN), creatinine, glucose, lactic acid, liver enzymes, albumin, globulin, and urate. Routine urinalysis included ketone body measurement.
Pesticides were sorted into four groups according to their field of use as herbicides, insecticides, fungicides, and surfactants. Each group was subdivided into various classes of pesticides, based on the classes published by the respective Resistance Action Committees (Specialist Technical Groups of CropLife International: HRAC for herbicides, IRAC for insecticides, FRAC for fungicides, and RRAC for rodenticides). The common name of the pesticide was used, as determined by Technical Committee 81 of the International Organization for Standardization (ISO/TC81). The amount of pesticide ingested was calculated by the number of mouthfuls (1 mouthful = 20 mL), as stated by the patient, or calculated from the remaining amount in the pesticide bottle.
Calculation of the anion gap, corrected for albumin
The serum anion gap was calculated as follows: AG = Na+ – (Cl- + HCO3-) initially. To correct for the albumin effect on the anion gap value, every decrease of 1 g/dL of albumin added 2.5 mmol of anion: (4.5 g/dL – measured serum albumin in g/dL) × 2.5. Therefore, in the results of this manuscript, the term "anion gap" represents the anion gap corrected for albumin. The normal (physiologic) range of the anion gap was defined as 6-14 mEq/L, and an anion gap >14.1 mEq/L was considered high. The physiologic ranges were defined as follows: 7.35-7.45 for pH, 22-28 mmol/L for HCO3-, and 35-45 mmHg for PaCO2. The patients were divided into three groups according to their blood pH value: acidemic (pH < 7.35), physiologic (7.35 ≤ pH ≤ 7.45), and alkalotic (pH > 7.45). Metabolic acidosis was defined as an acidemic pH with low PaCO2 and HCO3-. Metabolic alkalosis was defined as an alkalotic pH with high PaCO2 and HCO3-. Respiratory acidosis was defined as an acidemic pH with high PaCO2 and HCO3-. Respiratory alkalosis was defined as an alkalotic pH with low HCO3- and PaCO2. Finally, acidemia was defined as an acidemic pH with one or both of HCO3- and PaCO2 within the physiologic range.
The parameters for assessing clinical outcome included the death rate, length of hospital stay (days), length of intensive care unit (ICU) stay (days), and the seriousness of the toxic symptoms. The seriousness of the toxic symptoms was evaluated using the Workload Management System for Critical Care Nurses (WMSCN) (10) and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores (11). According to the workload of the ICU nurse, the seriousness of the toxic symptoms was classified using the WMSCN as either Class 1 (patient able to care for themselves, minimal degree of nursing care required), Class 2 (moderate degree of nursing care required), Class 3 (acute degree of nursing required), Class 4 (intensive degree of nursing required), Class 5 (continual nursing required), or Class 6 (intensive nursing from two or more nurses required). The APACHE II score was calculated from the patient’s age and the following 12 routine physiological measurements: PaO2, body temperature, blood pressure, arterial pH, heart rate, respiratory rate, serum Na+ and K+, creatinine, hematocrit, white blood cell (WBC) count, and Glasgow Coma Scale score. These measurements were taken during the first 24 hours after admission.
Comparison of parameters by clinical status (death)
The following parameters were compared between the surviving and non-surviving (deceased) groups: age, volume of pesticides ingested, time lag before arrival at the hospital after ingestion, parameters for clinical outcome, grade of toxic symptoms, and laboratory values, including the complete blood count (CBC), blood chemistry, electrolytes, and ABGA. For the parameters that were significantly different between the surviving and non-surviving groups, an analysis of the risk factors for poor clinical outcome (death) was performed using univariate and multivariate logistic regression analyses.
Statistical analysis
All demographic data are presented as the mean ± SD, unless otherwise noted. Univariate analyses utilized the Student’s t-test for analyzing differences between the two groups and χ2 test for comparing the categorical variables. All variables with a P-value < 0.05 from the univariate analyses were considered statistically significant and were used in the multivariable logistic regression analysis. All data were analyzed using R (v3.1.2) or SPSS version 17.0 for Windows.
Ethics statement
The study was approved by the institutional review board of Soonchunhyang Cheonan Hospital (IRB No. 201502002). Informed consent was waived by the board.
RESULTS
Patient demographics
This study included 668 male and 390 female patients, with a mean age of 56.8 ± 15.8 years (median 56 years, range 13-92 years).
Death rate and acid-base balance status for all patients
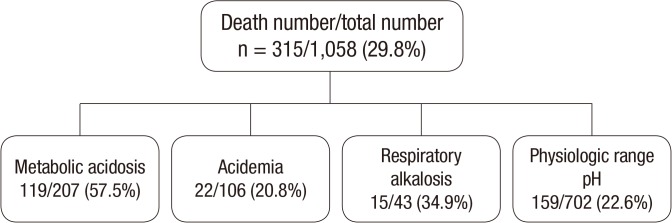
The acid-base balance status was classified as physiologic in 702 of the 1,058 cases (66.4%), metabolic acidosis in 207 cases (19.6%), acidemia in 106 cases (10.0%), and respiratory alkalosis in 43 cases (4.1%). The death rate was significantly higher in the metabolic acidosis group (57.5%) than the acidemia (20.8%) and respiratory alkalosis (34.9%) groups (P < 0.001; Fig. 1).
Fig. 1.
Total number and death rate of the overall patient sample, by acid-base balance status.
Note that the death rate is significantly greater in the metabolic acidosis group compared to the other acid-base balance status groups (P < 0.001).
Association of metabolic acidosis and a high anion gap
Among the 207 patients with metabolic acidosis, 83% (172 patients) had a high anion gap, 12.6% (26 patients) were within the physiologic range, and 4.4% (9 patients) had a low anion gap. Meanwhile, 35.8% (172/481) of the patients with a high anion gap had metabolic acidosis, 12.1% (58/481) had acidemia, and 52.2% (251/481) had a neutral acid-base balance.
Incidence of a high anion gap and death rate
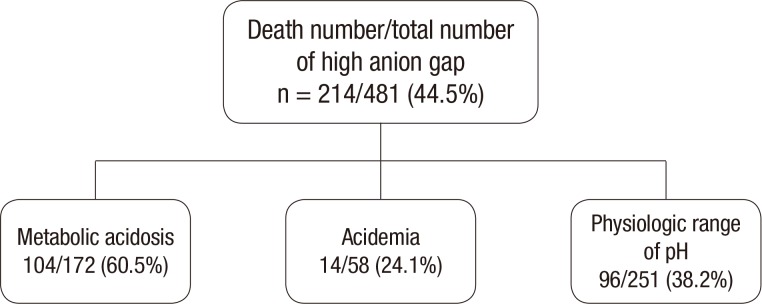
Of the 1,058 total patients, 45.5% (481 patients) had a high anion gap (> 14 mEq/L), 36.6% (387 patients) had an anion gap within the normal range, and 18.0% (190 patients) had a low anion gap. The death rate in the high, normal, and low anion gap group was 44.5%, 19.6%, and 13.2%, respectively (P < 0.001). The death rate in the group of patients with a high anion gap was greater when it was accompanied by metabolic acidosis (60.5%) than acidemia (24.1%) or a pH within the physiologic range (38.2%) (Fig. 2).
Fig. 2.
Total number and death rate of patients with a high anion gap, by acid-base balance status.
Note that the death rate is significantly greater when the high anion gap is accompanied by metabolic acidosis (P < 0.001).
Comparison of parameters between the surviving and deceased groups
Patients in the deceased group were older (P < 0.001), had higher APACHE II and WMSCN scores (P < 0.001), and the time lag after ingestion until arrival at the hospital was longer (P = 0.035) than in the surviving group. Furthermore, the duration of both the ICU (P < 0.005) and total hospital (P < 0.001) stays were shorter in the non-surviving group than the surviving group. Regarding the laboratory parameters, the anion gap, PO2, serum levels of glucose, lactate, BUN, creatinine, and uric acid, and WBC counts were significantly greater in the non-surviving group (all P < 0.001). Meanwhile, the PCO2, HCO3-, BE, serum levels of chloride, calcium, ionized calcium, and K+, and red blood cell (RBC) count (P = 0.011) were greater in the surviving group (all other P values < 0.001; Table 1).
Table 1. Comparison of parameters between the surviving and deceased groups.
| Parameters | Survivor (n = 743) | Death (n = 315) | P value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | ||
| Age, yr | 55.1 | 15.8 | 54 | 60.4 | 15.1 | 60 | < 0.001 |
| Ingested volume, mL | 120.3 | 116 | 100 | 132.1 | 99.7 | 100 | 0.099 |
| Time lag, hr | 4.9 | 4.3 | 3 | 5.6 | 4.9 | 4 | 0.035 |
| APACH II score | 30.3 | 10 | 29 | 35.2 | 10.2 | 34 | < 0.001 |
| WMSCN score | 3.2 | 0.7 | 3 | 3.4 | 0.7 | 3 | < 0.001 |
| ICU admission, day | 5.9 | 4.6 | 5 | 4.9 | 5.7 | 3 | 0.005 |
| Total admission, day | 9.9 | 7.1 | 7 | 5.1 | 6 | 3 | < 0.001 |
| pH | 7.4 | 0.1 | 7.4 | 7.3 | 0.1 | 7.4 | < 0.001 |
| Anion gap | 11.5 | 7.3 | 11.1 | 18.8 | 9.1 | 18.8 | < 0.001 |
| PCO2, mmHg | 35.5 | 6.1 | 35.5 | 29.4 | 8.6 | 30 | < 0.001 |
| HCO3, mmol/L | 21 | 4.3 | 21.5 | 16.2 | 5.8 | 16.4 | < 0.001 |
| BE, mmol/L | -3.6 | 4.7 | -3 | -8.3 | 6.7 | -7.9 | < 0.001 |
| PO2, mmHg | 86.4 | 22.7 | 85 | 94.9 | 29.5 | 92 | < 0.001 |
| SO2, % | 93.8 | 9.2 | 96 | 93.5 | 10.3 | 97 | 0.636 |
| Na+, mEq/L | 136.2 | 6 | 137 | 136.4 | 6.5 | 137 | 0.639 |
| K+., mEq/L | 4 | 0.6 | 4 | 3.4 | 0.8 | 3.3 | < 0.001 |
| CI-, mEq/L | 103.8 | 5 | 104 | 101.4 | 6.1 | 102 | < 0.001 |
| Ca++, mg/dL | 8.9 | 0.8 | 8.9 | 8.6 | 0.9 | 8.7 | < 0.001 |
| Ionized Ca++, mmol/L | 1 | 0.2 | 1 | 0.9 | 0.2 | 1 | 0.009 |
| Albumin, mg/dL | 4.4 | 0.5 | 4.4 | 4.3 | 0.7 | 4.4 | 0.691 |
| Total protein, g/dL | 7 | 0.7 | 7 | 6.9 | 0.9 | 6.9 | 0.173 |
| A.G Ratio | 1.7 | 0.8 | 1.7 | 1.8 | 0.4 | 1.7 | 0.613 |
| Lactate, mg/dL | 3.1 | 2.5 | 2.3 | 7.3 | 4.4 | 6.6 | < 0.001 |
| Uric acid, mg/dL | 5.3 | 1.9 | 5.2 | 5.9 | 2.3 | 5.4 | < 0.001 |
| BUN, mg/dL | 14.5 | 6.8 | 13.1 | 17.2 | 9.8 | 15 | < 0.001 |
| Creatinine, mg/dL | 0.9 | 0.5 | 0.8 | 1.5 | 1.1 | 1.2 | < 0.001 |
| Glucose, mg/dL | 132.1 | 45.5 | 119 | 174.6 | 60.8 | 165 | < 0.001 |
| Cholesterol, mg/dL | 181.3 | 43.4 | 178 | 173.1 | 54.9 | 165 | 0.034 |
| Triglyceride, mg/dL | 179 | 174.5 | 124 | 180.4 | 203.5 | 115.5 | 0.922 |
| WBC Count, 103/µL | 12.1 | 5.6 | 10.8 | 17.4 | 7.2 | 16.4 | < 0.001 |
| RBC Count, 106/µL | 4.5 | 0.6 | 4.5 | 4.4 | 0.6 | 4.4 | 0.011 |
| Hemoglobin, g/dL | 14.3 | 1.9 | 14.3 | 14 | 2 | 14 | 0.024 |
| Hematocrit, % | 41.5 | 4.9 | 41.6 | 40.7 | 5.5 | 40.6 | 0.03 |
Comparison of categorical variables between the surviving and non-surviving groups
There was no difference in the male/female ratio between the two groups. The pesticide field of use category and the composition of pesticide classes differed between the two groups. The majority of the deceased patients (273/315, 86.5%) ingested bipyridium, while only 25.8% of the surviving patients (192/743) ingested bipyridium (P < 0.001; Table 2).
Table 2. Comparison of variables between the surviving and deceased groups.
| Variables | Survivor (n = 743) | Death group (n = 315) | P value | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Sex | Male | 472 | 63.5 | 196 | 62.2 | 0.807 |
| Female | 271 | 36.5 | 119 | 37.8 | ||
| Field of use | Herbicide | 527 | 70.9 | 292 | 92.7 | < 0.001 |
| Insecticide | 176 | 23.7 | 21 | 6.7 | ||
| Surfactant | 22 | 3 | 1 | 0.3 | ||
| Fungicide | 18 | 2.3 | 1 | 0.3 | ||
| Pesticide class | Bipyridylium | 192 | 25.8 | 273 | 86.7 | < 0.001 |
| Glycine derivative | 180 | 24.2 | 11 | 3.5 | ||
| Organophosphate | 92 | 12.4 | 14 | 4.4 | ||
| Phosphinic acid | 86 | 11.6 | 5 | 1.6 | ||
| Pyrethroid | 53 | 7.1 | 5 | 1.6 | ||
| Surfactant | 21 | 2.8 | 1 | 0.3 | ||
| Chloroacetamide | 18 | 2.4 | 2 | 0.6 | ||
| Others | 101 | 13.6 | 4 | 1.3 | ||
Note that the clinical outcome (death rate) is different according to the pesticide group, categorized by either the "field of use" or "pesticide class". The death rate was incomparably high in the herbicide category, particularly for bipyridylium. The P values are from χ2 tests.
Acid-base balance in the patients with a high anion gap
Among the 481 patients with a high anion gap, 52.2% (251/481) had a pH in the physiologic range, 35.8% (172/481) had metabolic acidosis, i.e., "high anion gap metabolic acidosis", and 12.1% (58/481) had acidemia. The death rate was 60.5% for the high anion gap metabolic acidosis group, 38.2% for the patients with a high anion gap but physiologic pH, and 24.1% for the high anion gap acidemia group (P < 0.001; Table 3).
Table 3. Death rate according to the acid-base balance status and anion gap size.
| Parameters | Death No./total No. | Death rate, % | P value |
|---|---|---|---|
| Acid base balance | < 0.001 | ||
| Metabolic acidosis*,† | 119/207 | 57.5 | |
| Acidemia* | 22/106 | 20.8 | |
| Respiratory alkalosis | 15/43 | 34.9 | |
| Physiologic† | 159/702 | 22.6 | |
| Acid base status with high anion gap | < 0.001 | ||
| Metabolic acidosis*,† | 104/172 | 60.5 | |
| Acidemia* | 14/58 | 24.1 | |
| Neutral† | 96/251 | 38.2 | |
| Anion gap | < 0.001 | ||
| High*,† | 214/481 | 44.5 | |
| Normal* | 76/387 | 19.6 | |
| Low† | 25/190 | 13.2 |
Note that all of the 3 acid-base balance parameters (acid base balance, acid base status with a high anion gap, and the anion gap itself) are significant factors for predicting the death rate in acute pesticide intoxication.
*,†Matched letters indicate significant differences corrected for multiple comparisons, with P values adjusted using the Bonferroni method.
Analysis of risk factors for clinical outcome (death) by univariate and multiple logistic regression analyses
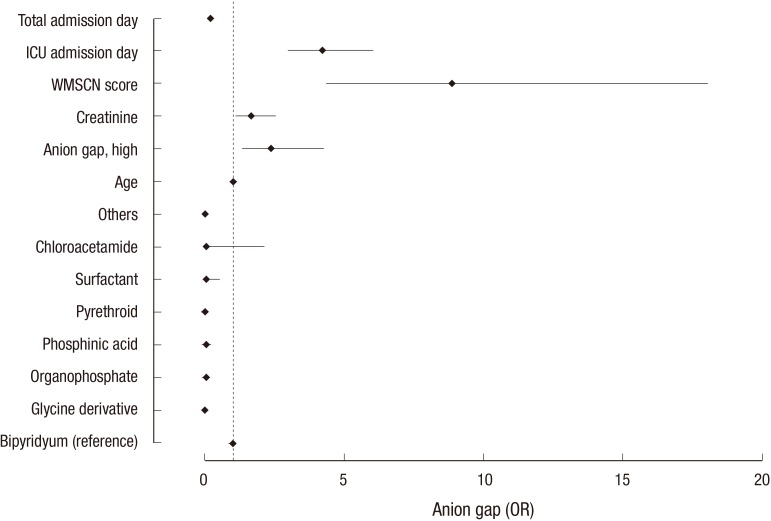
Age, anion gap, serum creatinine, field of use, pesticide class, time lag after ingestion (P = 0.048), APACHE II score, WMSCN score, ICU stay, and overall hospital stay were significant risk factors for death in univariate analyses (all other P values < 0.001; Table 4). Among these risk factors, variables were selected for multivariable logistic regression analyses, and age, anion gap, serum creatinine, pesticide class, WMSCN score, ICU stay, and overall hospital stay were significant risk factors for death in our model (Table 4 and Fig. 3).
Table 4. Analysis of risk factors for clinical outcome (death) using univariate and multivariable logistic regression analyses.
| Variables | Univariate analysis (a) | Multivariable analysis (b) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||||
| Age | 1.02 | 1.01 | 1.03 | < 0.001 | 1.03 | 1.01 | 1.05 | < 0.001 | |
| Sex | 1.05 | 0.79 | 1.38 | 0.747 | |||||
| Anion gap | 3.04 | 2.28 | 4.06 | < 0.001 | 2.38 | 1.34 | 4.25 | < 0.001 | |
| Creatinine | 5.06 | 3.66 | 7.00 | < 0.001 | 1.66 | 1.10 | 2.51 | 0.02 | |
| Field of use class | Herbicide | Reference | |||||||
| Insecticide | 0.2 | 0.12 | 0.32 | < 0.001 | |||||
| Surfactant | 0.08 | 0 | 0.39 | 0.015 | |||||
| Fungicide | 0.1 | 0.02 | 0.5 | 0.026 | |||||
| Pesticide class | Bipyridylium | Reference | Reference | ||||||
| Glycine Derivative | 0.04 | 0.02 | 0.08 | < 0.001 | 0.02 | 0.00 | 0.06 | < 0.001 | |
| Organophosphate | 0.11 | 0.06 | 0.19 | < 0.001 | 0.04 | 0.01 | 0.17 | < 0.001 | |
| Phosphinic acid | 0.04 | 0.02 | 0.09 | < 0.001 | 0.04 | 0.01 | 0.20 | < 0.001 | |
| Pyrethroid | 0.06 | 0.03 | 0.14 | < 0.001 | 0.01 | 0.00 | 0.09 | < 0.001 | |
| Surfactant | 0.03 | 0.01 | 0.17 | 0.001 | 0.06 | 0.01 | 0.53 | 0.01 | |
| Chloroacetamide | 0.09 | 0.01 | 0.33 | 0.002 | 0.05 | 0.00 | 2.13 | 0.11 | |
| Others | 0.03 | 0.02 | 0.07 | < 0.001 | 0.02 | 0.00 | 0.13 | < 0.001 | |
| Ingested volume, mL | 1 | 1 | 1 | 0.1556 | |||||
| Time lag, hr* | 1.03 | 1 | 1.06 | 0.048 | |||||
| APACHE II score | 1.09 | 1.04 | 1.15 | < 0.001 | |||||
| WMSCN score | 1.5 | 1.24 | 1.81 | < 0.001 | 8.87 | 4.37 | 18.03 | < 0.001 | |
| ICU admission day | 0.94 | 0.9 | 0.97 | < 0.001 | 4.23 | 2.98 | 6.02 | 0.001 | |
| Total admission day | 0.79 | 0.75 | 0.82 | < 0.001 | 0.22 | 0.16 | 0.31 | 0.001 | |
Note that age, anion gap, serum creatinine, pesticide class, Workload Management System for Critical Care Nurses (WMSCN) score, and admission day are significant risk factors for death (P < 0.001). Both the number of patients and the death rate were incomparably higher in the herbicide (bipyridylium) category than in the other pesticide categories. Therefore, the death rates in the other pesticide groups are presented in reference to the herbicide (bipyridylium) category.
APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, intensive care unit.
*time lag: arrival to the hospital after ingestion of pesticide.
Fig. 3.
Odds ratios (ORs) of the significant clinical variables from the multivariable logistic regression analysis.
The clinical outcome refers to the death rate. Note that the anion gap is an independent risk factor that predicts death. The ORs, 95% confidence intervals (CIs), and P values for each variable are presented in Table 4.
ICU, intensive care unit; WMSCN, Workload Management System for Critical Care Nurses.
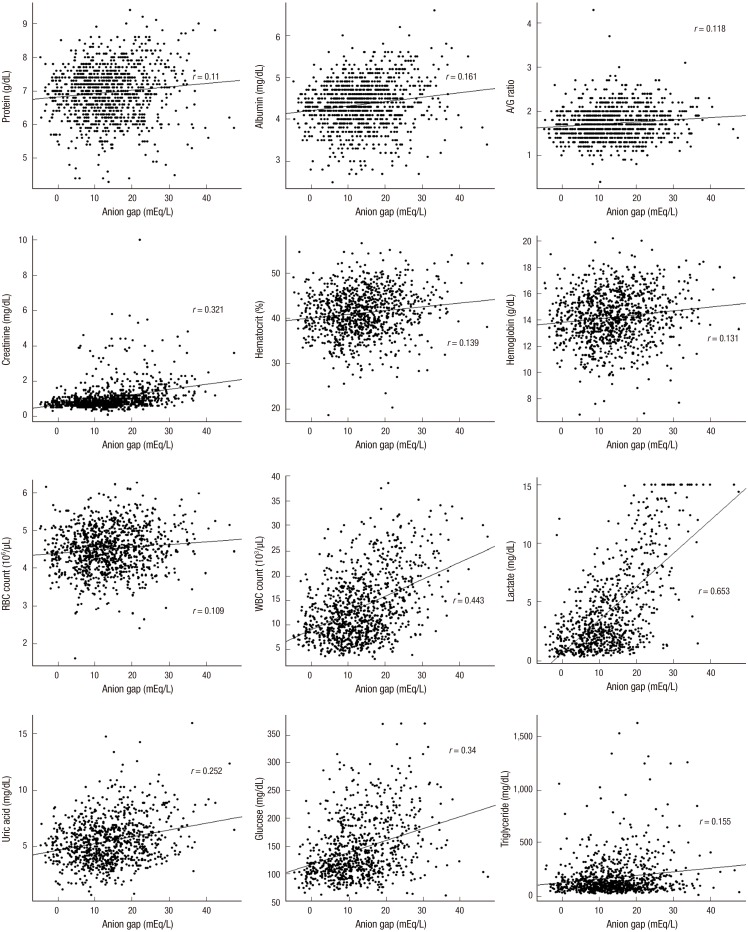
Correlation between the anion gap and other parameters (Fig. 4)
Fig. 4.
Relationship of the anion gap with parameters, including blood chemistry and complete blood cell count.
Note that there was a significant relationship (P < 0.001) with all of the parameters: plasma levels of protein and albumin, albumin/globulin ratio, serum creatinine levels, hematocrit, hemoglobin, RBC and WBC counts, lactate, uric acid, glucose, and triglycerides.
The anion gap values were positively correlated with Na+, ionized Ca++, albumin, total protein, A/G ratio, lactate, uric acid, BUN, creatinine, glucose, cholesterol, triglyceride, WBC count, RBC count, hemoglobin, and hematocrit. Meanwhile, there was a negative correlation between the anion gap and CI-, K+, and Ca++.
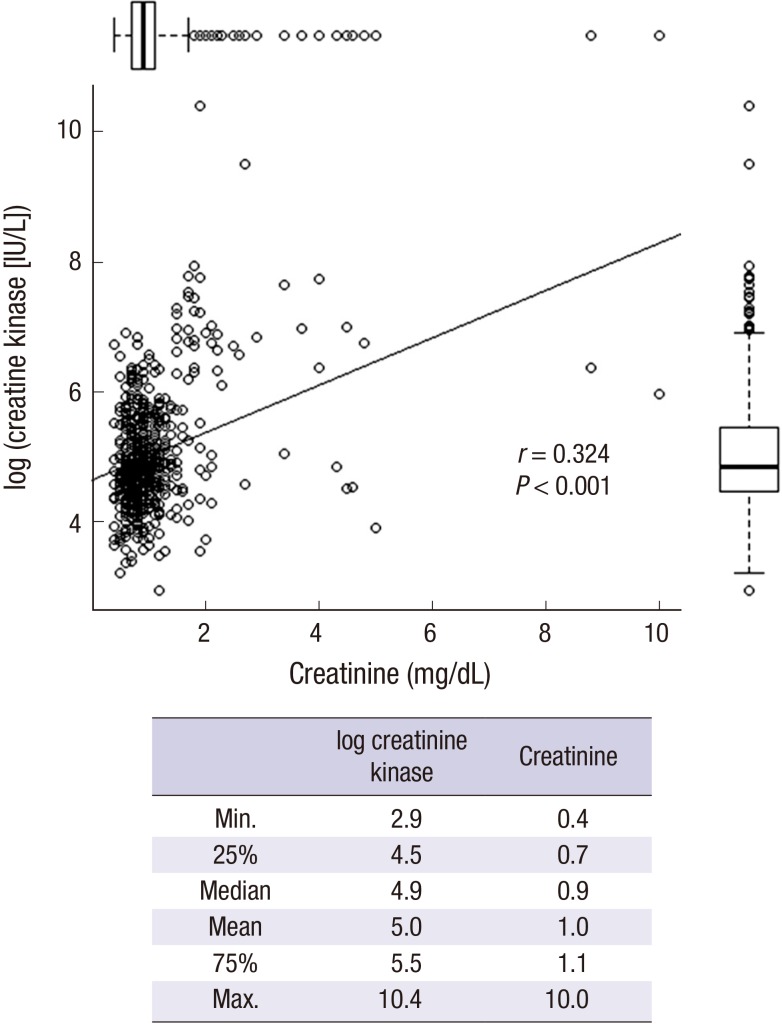
In addition, there was a significant correlation between the serum creatinine and creatine kinase levels (Fig. 5).
Fig. 5.
Correlation between serum creatinine and creatine kinase levels.
The boxes near the X and Y axes represent the interquartile range (25%–75%). The bar in the boxplot represents the median value. The mean, median, 25%, 75%, minimum, and maximum values are presented in the right table. There was a significant positive correlation (r = 0.324, P < 0.001), between serum creatinine and creatine kinase levels.
DISCUSSION
Disease severity may be expressed using quantitative measurement parameters, such as the APACHE and WMSCN scores, or the duration of the hospital stay, particularly the ICU stay. However, these parameters may have limitations to their use in patients with acute pesticide intoxication, for the following reasons: first, during the first several days, many patients, particularly those with acute paraquat intoxication, show no specific symptoms, leading to a low APACHE or WMSCN score even though the final mortality is higher than that with other pesticides (12). Second, the hospital stay is short when the patient dies early. For these reasons, we focused on the death rate as a conclusive clinical parameter in the current study.
Drug-induced acid-base disorder can be classified into five different categories based on the pathophysiology: 1) metabolic acidosis caused by acid overload, which may occur through the accumulation of acids by endogenous mechanisms, 2) base loss: proximal renal tubular acidosis caused by drugs, 3) alkalosis resulting from acid and/or chloride loss via renal or extrarenal (e.g., laxative drugs) mechanisms; 4) exogenous bicarbonate loads: milk-alkali syndrome, overshoot alkalosis after bicarbonate therapy or citrate administration; and 5) respiratory acidosis or alkalosis resulting from drug-induced depression of the respiratory center or neuromuscular impairment (13).
Of the 1,058 patients, 702 had an acid-base imbalance (33.6%); 172 of the 1,058 cases had metabolic acidosis (16.3%), followed by acidemia (5.5%), and respiratory alkalosis (4.1%). There were no respiratory acidosis cases in this study, perhaps because the blood samples for ABGA were drawn after respiratory resuscitation was performed as needed. The death rate was significantly higher in the group of patients with metabolic acidosis (57.5%) than other groups of patients with an acid-base imbalance. In particular, the death rate was greater when metabolic acidosis was associated with a high anion gap (60.5%) (Table 3).
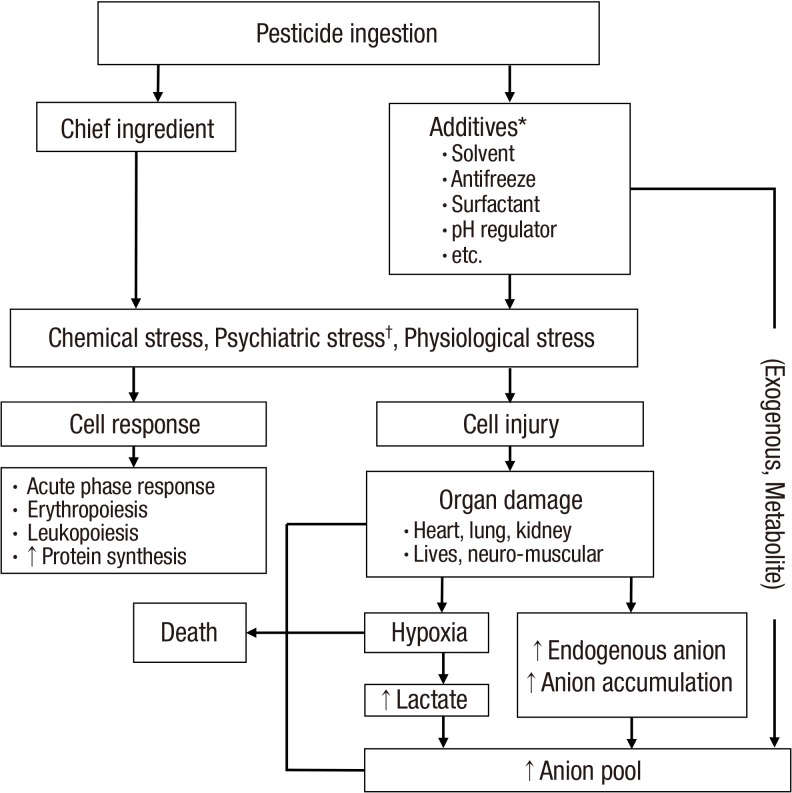
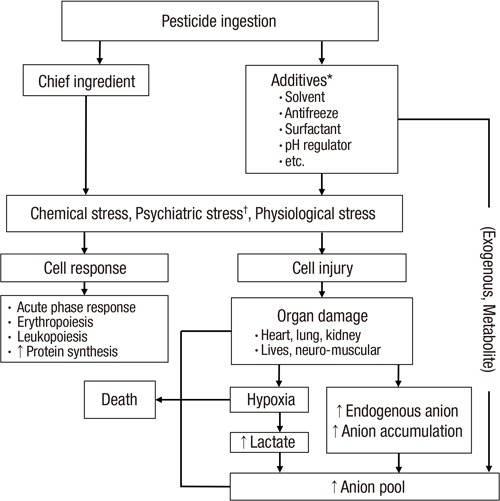
The mechanism behind the high death rate in the metabolic acidosis group likely has a complicated background. It is unclear which factors play a dominant role, such as the acidosis itself and the underlying pathophysiology of the metabolic acidosis, during the process of a grave clinical outcome. It is widely accepted that acidosis itself is critical for maintaining a normal physiology in the human body. In same way, the underlying pathophysiology of metabolic acidosis is also a critical factor in the human body. Taken together, we present the proposed mechanisms for both anion production and the high death rate in acute pesticide intoxication in Fig. 6.
Fig. 6.
Simplified overview of anion production and proposed mechanism of death in acute pesticide intoxication.
Arrows indicate the proposed sequence of events.
*Not all of the pesticide formulations include all of the additives presented in this figure; †Psychiatric stress is included because all of the subjects committed suicide in a state of agitation.
The majority (83%) of the metabolic acidosis patients, though not all, had a high anion gap. The anion gap levels varied: 45.5% (481/1,058 cases) were in the high anion gap group, 36.6% (387/1,058) were in the group with a normal anion gap range, and 18.0% (190/1,058) were in the low anion gap group. Meanwhile, among the 481 patients in the high anion gap group, 35.8% had metabolic acidosis, 12.1% acidemia, and 52.2% a neutral acid-base balance. These results suggest that the anion gap may be a more comprehensive biological marker than other parameters of the acid-base imbalance.
The traditional risk factors for death are the toxicity of the chief ingredients, ingestion amount, time lag to hospital arrival, and comorbidities of the patients. In addition to these factors, our results showed that the death rate was significantly higher in the high anion gap group than the normal or low anion gap groups, regardless of the accompanying acid-base balance status. This finding suggests that the anion gap is a surrogate marker of the grave pathophysiologic process following acute pesticide intoxication.
An increase in the anion gap is most often due to an increase in unmeasured anions, and less commonly to a decrease in cations such as Ca++, Mg++, and K+. The term "anion gap" implies unmeasured anions. However, some measured anions, such as lactate and uric acid, are included in the anion gap. Therefore, the definition of the anion gap as the unmeasured anions is a misnomer in the current study. The anion gap value is negatively correlated with Ca++ and K+ (unfortunately, Mg++ was not included in the current study), suggesting that a decrease in cations is one factor increasing the anion gap in our patients. The anion gap may decrease with a decrease in anionic albumin (14); the albumin levels were fairly low in some cases (data not shown). We believe that as a negative acute phase reactant, albumin levels decrease in some patients. However, we corrected the anion levels for low albumin to minimize its effect on the anion gap. With normal serum albumin, a high anion gap is usually due to non-chloride containing acids that contain inorganic (phosphate, sulfate), organic (ketoacids, lactate, uremic organic anions), exogenous (ingested toxins with organic acid production), or unidentified anions. In our study, there was a significant positive correlation between the anion gap values and the serum levels of both lactate and uric acid, suggesting that these two anions are significant components of the "anion pool". However, the correlation coefficient was 0.32 (P < 0.001) for lactic acid and 0.01 (P < 0.001) for uric acid, suggesting that these two anions do not account for everything in the anion pool in our patients.
Serum creatinine levels were positively correlated with creatine kinase levels (Fig. 6). Collectively, with our previous observation of early kidney injury markers in pesticide intoxication (15,16) and the relatively short time lag between pesticide ingestion and arrival to the emergency room, we believe that serum creatinine levels do not always represent the degree of renal function deterioration in our patients. There could be two possible causes of high serum creatinine levels; one possible cause is pre-existing renal failure and the other is rhabdomyolysis or hyper-creatine kinase-emia (17,18). However, pre-existing renal failure was excluded in the current study based on the patient’s history.
Our previously published article (19) showed that pesticide intoxication is a frequent cause of rhabdomyolysis and is more common among men than women. The volume of pesticide ingested, and not the degree of human toxicity, is the main factor influencing the incidence of rhabdomyolysis. The most reliable test for the diagnosis of rhabdomyolysis is the blood creatine kinase level. The concentrations increase steadily for 12 hours after the original muscle injury, remain elevated for 1–3 days, and then gradually decrease. Levels 5 times above the upper limit of normal indicate rhabdomyolysis. Mild increases without kidney impairment are referred to as hyper-creatine kinase-emia, in which high serum creatinine and creatine kinase levels can be associated regardless of renal function (17,18). Based on this, we believe that rhabdomyolysis or hyper-creatine kinase-emia (17,18) was the cause of the high serum creatinine levels in the present study.
Severe triglyceridemia can lead to an underestimation of the Na+ and Cl- concentrations, lowering the anion gap. However, in the current study, triglyceridemia levels had a positive relationship with the anion gap value. This suggests that the anions directly or indirectly inhibit lipoprotein lipase or alter hepatic lipoprotein synthesis.
The glucose level, WBC count, RBC count, and total protein were also positively correlated with the anion gap value. We hypothesize that psychiatric or chemical stress stimulates not only the hematopoietic pathway (20,21) but also the production of acute phase reactant proteins or immunoglobulin (22,23). The relationship between the glucose level and the anion gap may be related to ketoacid accumulation. However, results of the urinalysis dip stick test for ketone bodies were negative for all patients, and only a few patients were diabetic (retrospectively analyzed). Therefore, we believe that the glucose level increased via the stimulation of sympathetic activity or an altered balance of glucose regulating hormones, which influenced the anion levels as well.
This study has some limitations. First, pesticides include various additives that may have anion metabolites. Unfortunately, the precise formulation of each pesticide is not available, as manufacturers do not reveal the formulation to the general population, and the details of the additives were not obtained in each patient. Second, the acid-base balance is more informative when it is measured sequentially (24). However, we measured the ABGA only once, because the majority of patients were treated with extracorporeal elimination early, to correct both the electrolyte and acid-base imbalance. Further research is necessary focusing on the changes in the acid-base balance. Third, the time lag to arrive at the hospital after ingestion was heterogeneous. Therefore, the degree of correction and the compensation process of the acid-base imbalance may differ between patients. Fourth, underlying diseases of the lungs and kidneys were not encountered. Finally, paraquat poisoning accounts for > 85% of the deaths in our cohort and this would have masked any possible effects seen for other compounds (25).
Even with these limitations, our study shows that a high anion gap is commonly observed in acute pesticide intoxication. It is a significant risk factor for death, regardless of the accompanying acid-base balance status, in patients with acute pesticide intoxication. Our results suggest that treatment modalities that decrease the anion pool, such as hemodialysis, must be applied early in acute pesticide intoxication.
Footnotes
Funding: This work was conducted with the support of the "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01083201)" Rural Development Administration, Republic of Korea.
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study concept & design: Gil HW, Hong SY. Acquisition of data: Lee JW, Hwang IW, Moon HJ, Kim KH. Data analysis and interpretation: Park SY. Drafting and revision: Hong SY. Final approval: all authors.
References
- 1.Weerasinghe M, Konradsen F, Eddleston M, Pearson M, Gunnell D, Hawton K, Jayamanne S, Pabasara C, Jayathilaka T, Dissanayaka K, et al. Risk factors associated with purchasing pesticide from shops for self-poisoning: a protocol for a population-based case-control study. BMJ Open. 2015;5:e007822. doi: 10.1136/bmjopen-2015-007822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal S. Suicide in India. Br Med Bull. 2015;114:127–134. doi: 10.1093/bmb/ldv018. [DOI] [PubMed] [Google Scholar]
- 3.Gao X, Duan L, Yang C, Ye P, Ji C, Wang Y, Deng X, Jin Y, Er Y, Wang L. Analysis on the characteristics of self-inflicted injury/suicide based on the Chinese National Injury Surveillance System from 2006 to 2013. Zhonghua Liu Xing Bing Xue Za Zhi. 2015;36:17–19. [PubMed] [Google Scholar]
- 4.Cho HD, Kim NY, Gil HW, Jeong DS, Hong SY. Comparison of families with and without a suicide prevention plan following a suicidal attempt by a family member. J Korean Med Sci. 2015;30:974–978. doi: 10.3346/jkms.2015.30.7.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon JM, Chun BJ. Predicting acute complicated glyphosate intoxication in the emergency department. Clin Toxicol (Phila) 2010;48:718–724. doi: 10.3109/15563650.2010.488640. [DOI] [PubMed] [Google Scholar]
- 6.Lee HL, Chen KW, Chi CH, Huang JJ, Tsai LM. Clinical presentations and prognostic factors of a glyphosate-surfactant herbicide intoxication: a review of 131 cases. Acad Emerg Med. 2000;7:906–910. doi: 10.1111/j.1553-2712.2000.tb02069.x. [DOI] [PubMed] [Google Scholar]
- 7.Gil HW, Jeong MH, Park JS, Choi HW, Kim SY, Hong SY. An outbreak of food borne illness due to methomyl pesticide intoxication in Korea. J Korean Med Sci. 2013;28:1677–1681. doi: 10.3346/jkms.2013.28.11.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gil HW, Hong JR, Song HY, Hong SY. A case of methanol intoxication caused by methomyl pesticide ingestion. Hum Exp Toxicol. 2012;31:1299–1302. doi: 10.1177/0960327112459532. [DOI] [PubMed] [Google Scholar]
- 9.Bahrami A, Jonidi-Jafari A, Mahjub H. Environmental exposure to xylenes in drivers and petrol station workers by urinary methylhippuric acid. J Res Health Sci. 2008;8:61–68. [PubMed] [Google Scholar]
- 10.Time oriented score system (TOSS): a method for direct and quantitative assessment of nursing workload for ICU patients. Italian Multicenter Group of ICU research (GIRTI) Intensive Care Med. 1991;17:340–345. doi: 10.1007/BF01716193. [DOI] [PubMed] [Google Scholar]
- 11.Del Bufalo C, Morelli A, Bassein L, Fasano L, Quarta CC, Pacilli AM, Gunella G. Severity scores in respiratory intensive care: APACHE II predicted mortality better than SAPS II. Respir Care. 1995;40:1042–1047. [PubMed] [Google Scholar]
- 12.Gil HW, Hong JR, Jang SH, Hong SY. Diagnostic and therapeutic approach for acute paraquat intoxication. J Korean Med Sci. 2014;29:1441–1449. doi: 10.3346/jkms.2014.29.11.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitterer D, Schwab M, Alscher MD, Braun N, Latus J. Drug-induced acid-base disorders. Pediatr Nephrol. 2015;30:1407–1423. doi: 10.1007/s00467-014-2958-5. [DOI] [PubMed] [Google Scholar]
- 14.Emmett M. Approach to the patient with a negative anion gap. Am J Kidney Dis. 2016;67:143–150. doi: 10.1053/j.ajkd.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Gil HW, Yang JO, Lee EY, Hong SY. Clinical implication of urinary neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in patients with acute paraquat intoxication. Clin Toxicol (Phila) 2009;47:870–875. doi: 10.3109/15563650903306651. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Gil HW, Yang JO, Lee EY, Hong SY. The clinical features of acute kidney injury in patients with acute paraquat intoxication. Nephrol Dial Transplant. 2009;24:1226–1232. doi: 10.1093/ndt/gfn615. [DOI] [PubMed] [Google Scholar]
- 17.Nozuma S, Okamoto Y, Higuchi I, Yuan J, Hashiguchi A, Sakiyama Y, Yoshimura A, Higuchi Y, Takashima H. Clinical and electron microscopic findings in two patients with mitochondrial myopathy associated with rpisodic hyper-creatine kinase-emia. Intern Med. 2015;54:3209–3214. doi: 10.2169/internalmedicine.54.5444. [DOI] [PubMed] [Google Scholar]
- 18.Finsterer J, Stöllberger C, Kovacs GG. Asymptomatic hyper-creatine-kinase-emia as sole manifestation of inclusion body myositis. Neurol Int. 2013;5:34–36. doi: 10.4081/ni.2013.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JS, Seo MS, Gil HW, Yang JO, Lee EY, Hong SY. Incidence, etiology, and outcomes of rhabdomyolysis in a single tertiary referral center. J Korean Med Sci. 2013;28:1194–1199. doi: 10.3346/jkms.2013.28.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao JL, Baltimore D. Regulation of stress-induced hematopoiesis. Curr Opin Hematol. 2015;22:286–292. doi: 10.1097/MOH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González-Murillo Á, Fernández L, Baena S, Melen GJ, Sánchez R, Sánchez-Valdepeñas C, Segovia JC, Liou HC, Schmid R, Madero L, et al. The NFKB inducing kinase modulates hematopoiesis during stress. Stem Cells. 2015;33:2825–2837. doi: 10.1002/stem.2066. [DOI] [PubMed] [Google Scholar]
- 22.Zulkifli I, Najafi P, Nurfarahin AJ, Soleimani AF, Kumari S, Aryani AA, O’Reilly EL, Eckersall PD. Acute phase proteins, interleukin 6, and heat shock protein 70 in broiler chickens administered with corticosterone. Poult Sci. 2014;93:3112–3118. doi: 10.3382/ps.2014-04099. [DOI] [PubMed] [Google Scholar]
- 23.Argente MJ, García ML, Birlanga V, Muelas R. Relationship between cortisol and acute phase protein concentrations in female rabbits. Vet J. 2014;202:172–175. doi: 10.1016/j.tvjl.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Kraut JA, Nagami GT. The serum anion gap in the evaluation of acid-base disorders: what are its limitations and can its effectiveness be improved? Clin J Am Soc Nephrol. 2013;8:2018–2024. doi: 10.2215/CJN.04040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JW, Hwang IW, Kim JW, Moon HJ, Kim KH, Park S, Gil HW, Hong SY. Common pesticides used in suicide attempts following the 2012 paraquat ban in Korea. J Korean Med Sci. 2015;30:1517–1521. doi: 10.3346/jkms.2015.30.10.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]