Abstract
Multiple endocrine neoplasia 1 (MEN 1) is a rare genetic disorder classically characterized by a predisposition to tumors of the parathyroid glands, anterior pituitary gland, and pancreatic islet cells (1). In addition to exhibiting these characteristic tumors, MEN 1 patients also have an increased propensity for other tumors such as carcinoids, adrenal adenomas, angiofibromas, and lipomas (1, 2, 3). Although MEN 1 is rare, with a prevalence of approximately 2 per 100,000 people, recognition of this syndrome is extremely important for both patient treatment and evaluation of family members (1, 4). The tumors of MEN 1 are usually benign; however, malignancy of some carcinoid, islet cell, and gastrointestinal tract tumors can cause mortality (5, 6, 7, 8). Diagnosis of MEN 1 is usually made by a combination of history and physical examination, biochemical serum testing, and various imaging modalities (9, 10). We present a classic case of MEN 1 with unique presentation and diagnosis using predominantly nuclear imaging in order to emphasize the role of nuclear imaging in diagnosing and treating MEN 1.
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; MEN 1, multiple endocrine neoplasia 1; EGD, esophagogastroduodenoscopy
Case report
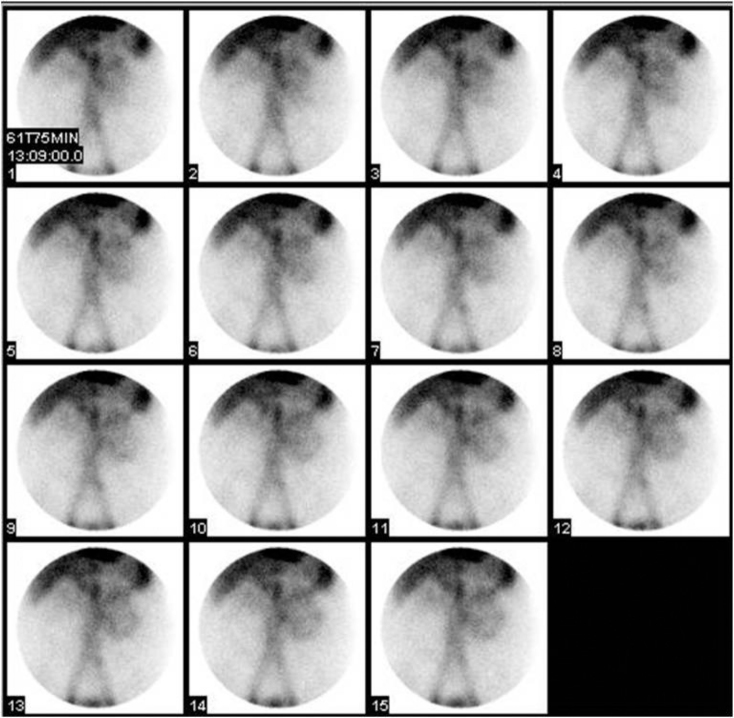
The patient, a 37-year-old male with a history of peptic ulcer disease, presented to the emergency department after syncope. At the time of presentation, the patient’s hemoglobin was 6.8 g/dl, and stool guaiac was positive. Although the patient was not grossly bleeding per rectum, given the patient’s history of peptic ulcer disease, the patient underwent an esophagogastroduodenoscopy (EGD) in the emergency department to evaluate for upper gastrointestinal bleeding. EGD revealed no gross lesions in the esophagus; however, enlarged gastric folds, duodenal erosion, and two umbilicated nodules were found in the duodenum with no evidence of active bleeding. After receiving four units of packed red blood cells and not responding appropriately, the patient underwent a nuclear medicine gastrointestinal bleeding scan for evaluation of a bleeding source. Images show bleeding in the distribution of the small bowel (Fig. 1).
Fig. 1.
37-year-old male with MEN 1. The patient was injected with 24.8 mCi Tc-99m-labeled red blood cells, and images of the abdomen were acquired in the anterior projection for 90 minutes. Images show bleeding in the distribution of the small bowel during 61 to 75 minutes of imaging.
Given the results of the bleeding scan, the patient underwent a repeat EGD that showed umbilicated nodules in the duodenum with blood at the second, third, and fourth portions of the duodenum. The patient was then taken for laparotomy for bleeding duodenal ulcers and underwent duodenectomy of the first and second portions of the duodenum. The pathology report from the duodenal nodules revealed small-bowel mucosa showing focal gastric foveolar metaplasia, marked Brunner gland hyperplasia, and focal small nests of neuroendocrine cells staining positive for chromogranin, synaptophysin, and gastrin. These findings were suspicious for an underlying carcinoid or gastrinoma. CT scan of the abdomen and pelvis on the same day as the laparotomy revealed small-to-borderline enlarged mesenteric lymph nodes measuring up to 1.3 × 1.0 cm. Biopsy of a peripancreatic lymph node revealed metastatic, well-differentiated gastrinoma.
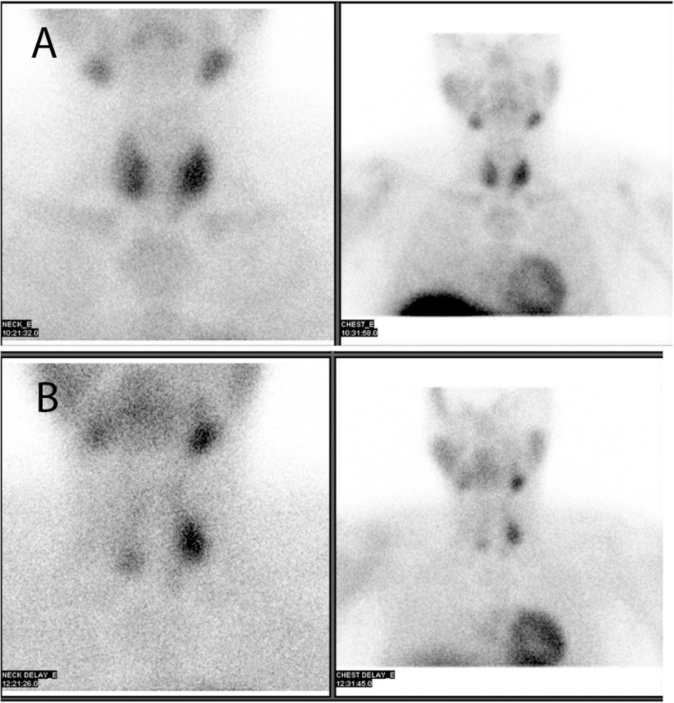
In addition to the gastrointestinal bleeding, which was thought to be due to a gastrin-secreting tumor, the patient had an elevated calcium level (11.5 mg/dl) on routine lab tests. Parathyroid hormone level was investigated at this point and found to be markedly elevated, at 470 pg/ml. The patient subsequently underwent ultrasound of the thyroid that showed two discrete nodules posterior to the left thyroid lobe. One nodule was posterior to the upper pole of the left lobe and measured approximately 1.4 × 1.9 × 1.7 cm. The second, well-defined hypoechoic nodule was located posterior to the mid and lower pole of the left lobe and measured 2.0 × 1.5 × 1.9 cm. These findings were suggestive of multiple parathyroid adenomas. The patient next underwent a nuclear medicine parathyroid scan. Delayed images showed a large persistent focus of increased radiotracer uptake in the mid-left thyroid lobe, and a smaller persistent focus of increased radiotracer uptake in the lower pole of the right thyroid lobe. Two questionable foci of less intense persistent tracer uptake were also noted in the upper pole of the right thyroid lobe and the lower pole of the left thyroid lobe. Findings were consistent with multiple parathyroid adenomas or parathyroid hyperplasia (Fig. 2).
Fig. 2A & B.
37-year-old male with MEN 1. The patient was injected with 25.8 mCi Tc-99m sestamib, i and images of the neck and chest in the anterior view were acquired at 10 minutes and at 2 hours. Early images (2A) show tracer uptake in both lobes of the thyroid gland, more prominent in the left mid thyroid and lower pole of right thyroid. Delayed images (2B) demonstrate a large persistent focus of increased radiotracer uptake in the mid left thyroid lobe and a smaller persistent focus of increased radiotracer uptake in the lower pole of the right thyroid lobe. Two questionable foci of less intense persistent tracer uptake also appear in the upper pole of the right thyroid lobe and the lower pole of the left thyroid lobe. Findings are consistent with multiple parathyroid adenomas or parathyroid hyperplasia.
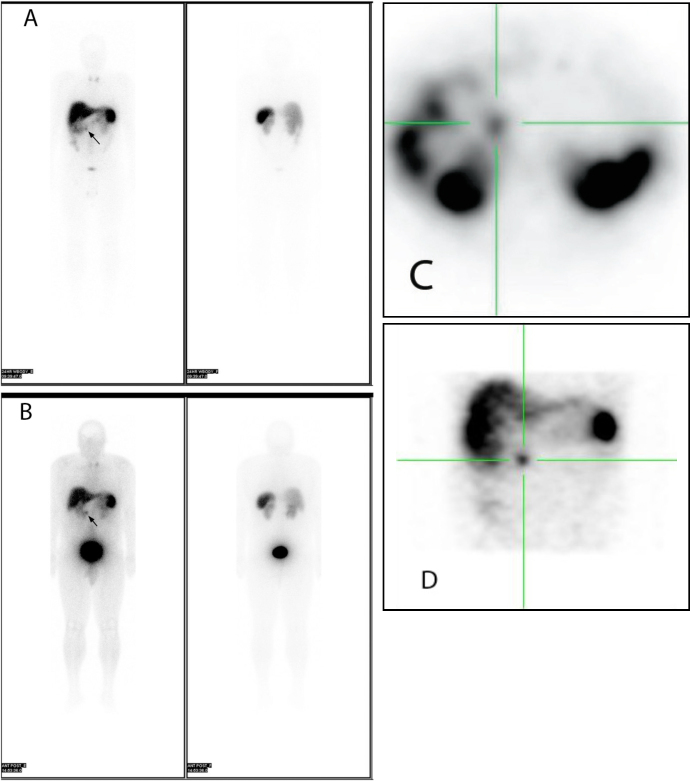
Given the findings of gastrinoma and parathyroid pathology, a diagnosis of MEN 1 was suspected. A prolactin level was drawn and returned elevated, at 46.4 ng/ml. Subsequently, MRI of the head with contrast revealed an area of diminished enhancement in the pituitary gland to the right of midline, measuring 4 mm, with no mass effect on adjacent structures, suggestive of a pituitary microadenoma. The patient also underwent an octreotide scan; results were consistent with metastatic somatostatin-receptor-positive malignancy involving the lymph nodes in the porta hepatis region (Fig. 3).
Fig. 3A-D.
37-year-old male with MEN 1. The patient was injected with 6.4 mCi In-111 octreotide, and images were acquired in the anterior and posterior projections at 4 hours (3A) and 24 hours (3B). SPECT images were also obtained at 24 hours (3C, axial, and 3D, coronal). Findings are consistent with metastatic somatostatin-receptor-positive malignancy involving the lymph nodes either in the peripancreatic or porta hepatis region. In addition, diffusely increased radiotracer uptake in the neck likely corresponds to the parathyroid pathology seen on the prior parathyroid scan.
Discussion
The clinical presentation of MEN 1 depends on the endocrine organs involved, as well as the level of the hormones secreted (1). Therefore, the presentation varies between patients and may reflect elevated levels of one or multiple hormones. Although the initial clinical manifestation of MEN 1 is usually hyperparathyroidism, some patients may present with symptoms of Zollinger-Ellison syndrome prior to symptoms of primary hyperparathyroidism (8, 9, 11, 12). The symptoms of hyperparathyroidism include musculoskeletal complaints, generalized weakness, altered mental status, and in rare cases nephrolithiasis, whereas the symptoms from a gastrin-secreting tumor consist of abdominal pain, diarrhea, or complications of peptic ulcer disease, such as ulcer perforation or bleeding (1). In this case, the patient with a history of peptic ulcer disease presented with syncope, anemia, and gastrointestinal bleeding, which are consistent with Zollinger-Ellison syndrome.
Although this patient exhibited symptoms only from Zollinger-Ellison syndrome, patients may also present with symptoms of pituitary tumors, including headache and visual-field defects. Prolactinomas, which are the most common pituitary tumor in MEN 1, can present differently in male and female patients. Male patients may exhibit erectile dysfunction or decreased libido, while female patients may develop amenorrhea and galactorrhea. Other pituitary tumors such as growth-hormone-secreting tumors may result in acromegaly, while cortisol-secreting tumors may present as Cushing syndrome. In addition, nonsecreting pituitary tumors may also occur (1, 10, 13, 14).
Due to the many possible combinations of tumors and hormone levels in MEN 1, patients may present with a myriad of symptoms as described above, most of which are nonspecific. Therefore, diagnosis of MEN 1 is difficult and should include extensive history, physical examination, biochemical serum testing, and (depending on these results) focused imaging studies. Because the workup of MEN 1 is specific to each individual case, there is no standard protocol or algorithm for diagnosing MEN 1, and therefore physicians do not always take full advantage of the benefits of nuclear imaging. Although MRI, CT, and ultrasound may provide sufficient anatomical information, nuclear imaging can provide additional functional detail (10). Nuclear imaging is frequently used for the diagnosis of sporadic parathyroid adenomas, parathyroid hyperplasia, and neuroendocrine tumors; however, nuclear imaging is not always used in the diagnosis of MEN 1 syndrome.
Numerous radiotracers are available for endocrine tumor imaging, including Tc-99m sestamibi, In-111 pentetreotide, flurodeoxyglucose (F-18 FDG), I-131 meta-iodobenzylguanidine (I-131 MIBG), and Ga-67 (15). Each of these tracers has advantages and disadvantages. Tc-99m sestamibi, for example, concentrates in cells with high mitochondrial content and is very useful in detecting parathyroid adenomas; it has a sensitivity of approximately 90% or even higher with the addition of SPECT imaging (15, 16). In-111 pentetreotide is extremely beneficial in detecting neuroendocrine tumors because it acts on somatostatin receptors, making it highly tumor-specific. In contrast, FDG accumulates in any region with increased glucose metabolism, such as areas of inflammation, therefore making it less tumor-specific (17). Another drawback of using FDG for neuroendocrine imaging is that it classically causes false negatives for carcinoid tumors and tumors less than 1 cm (18). However, one advantage of FDG is its ability to detect dedifferentiated neuroendocrine tumors that no longer concentrate other tracers. One tracer that is extremely helpful for detecting adrenal tumors such as pheochromocytoma and neuroblastoma is I-131 MIBG (15). Ga-67 is another tracer that is taken up by a variety of tumors; however, it does not seem to be as reliable as other tracers for consistently detecting neuroendocrine tumors (19).
Some newer tracers that may be used for neuroendocrine tumor imaging include Gallium-68-labeled somatostatin analogues such as Ga-68-DOTA-NOC, which may be used in PET-CT. Ga-68-DOTA-NOC is an excellent tracer for imaging somatostatin-receptor-positive tumors, and provides a high target-to-nontarget ratio that allows for the detection of very small lesions, especially of lymph node and bone metastases (20). 18-F-DOPA PET has also proven useful for detection and staging of neuroendocrine tumors (21). Other tracers being studied include gastrin-receptor analogs that may provide additional information when somatostatin-receptor-positive scintigraphy is negative (22).
Because of the efficacy of therapy for hyperparathyroidism and pituitary tumors, malignant pancreatic-islet-cell and gastrointestinal tumors are the primary life-threatening components of MEN 1. Therefore, diagnosis and treatment of these tumors should be the primary concern (5, 6, 7, 8). The presence of a high density of somatostatin receptors in numerous neuroendocrine and some nonneuroendocrine tumors allows radiolabeled somatostatin analogs to be used to image a variety of tumors and detect both primary and metastatic foci. Indium-111 pentetreotide is primarily useful in evaluating carcinoids (sensitivity 85% to 95%) and gastrinomas; however, additional tumors such as pancreatic-islet-cell neoplasms, pituitary adenomas, pheochromocytomas, neuroblastomas, paragangliomas, medullary carcinoma of the thyroid, small-cell lung cancer, and meningiomas may also be assessed with this radiopharmaceutical (15).
Most of the tumors listed in the previous paragraph are successfully imaged using radiolabeled somatostain analogs, with sensitivities ranging from 80% to 100% (15). Exceptions are insulinomas (50% to 60% sensitivity), and medullary thyroid carcinoma (65% to 70% sensitivity) (15). Indium-111 pentetroeotide is not useful in pancreatic carcinomas of exocrine origin because they do not express somatostatin receptors (15). Another important point is that because somatostatin receptors are expressed in some nonneoplastic, chronic inflammatory processes, such as granulomatous lesions (sarcoidosis, tuberculosis), Crohn’s disease, ulcerative colitis, and rheumatoid arthritis, these entities may serve as possible sources of false-positive results (15).
One topic that we would like to emphasize is the benefit of whole-body imaging in the workup of MEN 1. Whole-body gamma-camera imaging allows for cost-effective screening of patients with suspected or known endocrine tumors. Once injected with radionuclide, the patient has already received the radiation dose, and therefore whole-body scanning does not expose the patient to more radiation than a limited scan. Information acquired from whole-body imaging may confirm or reveal the presence of a lesion, detect metastases from a primary tumor, or diagnose neuroendocrine conditions in which multiple lesions exist. In addition, because somatostatin-receptor expression is seen more frequently in well-differentiated tumors, visualization may suggest a more favorable prognosis. Patients with positive indium-111 pentetreotide images are often eligible for octreotide treatment because the documentation of somatostatin receptors provides a higher likelihood of controlling hormonal hypersecretion (15).
In addition to planar whole-body imaging, SPECT may increase the sensitivity of the exam, especially in the upper abdomen where kidney, spleen, and often gallbladder activity may obscure imaging of the pancreas and duodenum. SPECT may also be useful in better evaluating suspected liver metastases. Lastly, SPECT can aid in preoperative planning and provide the surgeon with additional information about patients who are surgical candidates (15). SPECT-CT, which provides simultaneous functional and anatomic information, further increases sensitivity and specificity and thus results in improved diagnosis and patient management (15).
In conclusion, the diagnosis of MEN 1 ideally involves intravenous injection of a single tracer followed by whole-body imaging in which all endocrine tumors would be visualized. Because of the various types of endocrine tumors and different stages of patient presentation, this is not currently possible. Imaging studies and tracers must be selected on a patient-to-patient basis with regard to the individual patient’s history, physical examination, and serum biochemical results. Use of the appropriate tracers and imaging techniques including nuclear tests can benefit both patients and their families.
Footnotes
Published: November 19, 2010
References
- 1.Brandi M.L. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86(12):5658–5671. doi: 10.1210/jcem.86.12.8070. PubMed) [DOI] [PubMed] [Google Scholar]
- 2.Triponez F. Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg. 2006;243(2):265–272. doi: 10.1097/01.sla.0000197715.96762.68. PubMed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darling T.N. Multiple facial angiofibromas and collagenomas in patients with multiple endocrine neoplasia type 1. Arch Dermatol. 1997;133(7):853–857. PubMed) [PubMed] [Google Scholar]
- 4.Larsson C. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988;332(6159):85–87. doi: 10.1038/332085a0. PubMed) [DOI] [PubMed] [Google Scholar]
- 5.Akerstrom G., Johansson H., Grama D. Surgical treatment of endocrine pancreatic lesions in MEN-1. Acta Oncol. 1991;30(4):541–545. doi: 10.3109/02841869109092415. PubMed) [DOI] [PubMed] [Google Scholar]
- 6.Sheppard B.C. Management of islet cell tumors in patients with multiple endocrine neoplasia: a prospective study. Surgery. 1989;106(6):1108–1117. PubMed) discussion 1117-8. [PubMed] [Google Scholar]
- 7.Pipeleers-Marichal M. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med. 1990;322(11):723–727. doi: 10.1056/NEJM199003153221103. PubMed) [DOI] [PubMed] [Google Scholar]
- 8.Gibril F., Jensen R.T. Advances in evaluation and management of gastrinoma in patients with Zollinger-Ellison syndrome. Curr Gastroenterol Rep. 2005;7(2):114–121. doi: 10.1007/s11894-005-0049-2. PubMed) [DOI] [PubMed] [Google Scholar]
- 9.Berna M.J. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore) 2006;85(6):295–330. doi: 10.1097/01.md.0000236956.74128.76. PubMed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess J.R. Spectrum of pituitary disease in multiple endocrine neoplasia type 1 (MEN 1): clinical, biochemical, and radiological features of pituitary disease in a large MEN 1 kindred. J Clin Endocrinol Metab. 1996;81(7):2642–2646. doi: 10.1210/jcem.81.7.8675591. PubMed) [DOI] [PubMed] [Google Scholar]
- 11.Rizzoli R., Green J., 3rd, Marx S.J. Primary hyperparathyroidism in familial multiple endocrine neoplasia type I. Long-term follow-up of serum calcium levels after parathyroidectomy. Am J Med. 1985;78(3):467–474. doi: 10.1016/0002-9343(85)90340-7. PubMed) [DOI] [PubMed] [Google Scholar]
- 12.Berna M.J. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature. evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine (Baltimore) 2006;85(6):331–364. doi: 10.1097/MD.0b013e31802b518c. PubMed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien T. Results of treatment of pituitary disease in multiple endocrine neoplasia, type I. Neurosurgery. 1996;39(2):273–278. doi: 10.1097/00006123-199608000-00008. PubMed) discussion 278-9. [DOI] [PubMed] [Google Scholar]
- 14.Burgess J.R. Prolactinomas in a large kindred with multiple endocrine neoplasia type 1: clinical features and inheritance pattern. J Clin Endocrinol Metab. 1996;81(5):1841–1845. doi: 10.1210/jcem.81.5.8626844. PubMed) [DOI] [PubMed] [Google Scholar]
- 15.Mettler J.F. Essentials of Nuclear Medicine Imaging. Fifth ed. Saunders; Philadelphia: 2006. [Google Scholar]
- 16.Palestro C.J., Tomas M.B., Tronco G.G. Radionuclide imaging of the parathyroid glands. Semin Nucl Med. 2005;35(4):266–276. doi: 10.1053/j.semnuclmed.2005.06.001. PubMed) [DOI] [PubMed] [Google Scholar]
- 17.Groshar D. PET/CT Enterography in Crohn Disease: Correlation of Disease Activity on CT Enterography with 18F-FDG Uptake. J Nucl Med. 2010 doi: 10.2967/jnumed.109.073130. PubMed) [DOI] [PubMed] [Google Scholar]
- 18.Groheux D. (PET-CT for evaluation of the solitary pulmonary nodule: an update) Rev Mal Respir. 2009;26(10):1041–1055. doi: 10.1016/s0761-8425(09)73531-4. PubMed) [DOI] [PubMed] [Google Scholar]
- 19.Watanabe R. Intense accumulation of gallium-67 citrate in pancreatic endocrine tumor. Radiat Med. 2006;24(6):456–458. doi: 10.1007/s11604-006-0043-0. PubMed) [DOI] [PubMed] [Google Scholar]
- 20.Prasad V., Baum R.P. Biodistribution of the Ga-68 labeled somatostatin analogue DOTA-NOC in patients with neuroendocrine tumors: characterization of uptake in normal organs and tumor lesions. Q J Nucl Med Mol Imaging. 2010;54(1):61–67. PubMed) [PubMed] [Google Scholar]
- 21.Haug A. Intraindividual comparison of 68Ga-DOTA-TATE and 18F-DOPA PET in patients with well-differentiated metastatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2009;36(5):765–770. doi: 10.1007/s00259-008-1030-8. PubMed) [DOI] [PubMed] [Google Scholar]
- 22.Mettler F.A., Guiberteau M.J. Essentials of nuclear medicine imaging. 5th ed. Saunders/Elsevier; Philadelphia, Pa.: 2006. p. 577. xi. [Google Scholar]