Abstract
Epidermal cysts are common, benign, intradermal or subcutaneous, typically asymptomatic masses, ranging from 1 to 4 cm in size. They may occur anywhere in the body, with a predilection for the face, neck, and trunk. Transformation to squamous-cell carcinoma is rare. We present a case of a 61-year-old male patient with a large, growing mass in his posterior left gluteal region. Given the concern for a malignancy, he was referred to a surgical oncologist. Magnetic resonance imaging (MRI) without contrast was performed due to poor renal function and revealed a large cystic mass in the left gluteal subcutaneous soft tissues that was subsequently excised. Pathological examination revealed an epidermal inclusion cyst that measured 17.8 × 13.18 × 5.8 cm. To our knowledge, this is the largest epidermal inclusion cyst reported in the English literature.
Abbreviations: MRI, magnetic resonance imaging
Introduction
Epidermal inclusion cysts occur as a result of the migration of epidermal cells into the dermis. They are lined with stratified squamous epithelium. These lesions are described in the literature as typically being small, solitary, and slow-growing, located on the trunk, face, scalp and neck, with uncommon cases of larger masses reported on the extremities (1, 2, 3, 4, 5). Less commonly, epidermal inclusion cysts may occur in the bones (6) or breast (1). The overlying skin almost always shows a surface punctum (7).
At the present time, MRI with and without contrast is the preferred imaging modality for evaluation of the soft-tissue epidermal inclusion cysts. On MRI, noncomplicated lesions have been reported to have high signal intensity on T2-weighted/fluid-sensitive sequences, with no enhancement or thin-rim enhancement following intravenous administration of gadolinium-based contrast media (5, 8). Most of the complicated, ruptured, epidermal inclusion cysts have septa, show irregular thick enhancement, and are associated with fuzzy enhancement of the surrounding subcutaneous soft tissues (8). The differential diagnosis includes an epidermal inclusion cyst, ganglion cyst, neurogenic tumor, myxoid tumor, nodular fasciitis, and dermatofibrosarcoma protuberans (8). Malignant degeneration to squamous-cell carcinoma is rare, reported to be 2.2% (8, 9, 10).
Clinically, it may be difficult to differentiate benign and malignant soft-tissue masses (10). Once the diagnosis of an epidermal inclusion cyst is confirmed on histology, the lesion should be widely excised with the free margin. The outcome is typically excellent.
Case report
A 61-year-old male was referred to our tertiary-care medical center by his primary care physician with a complaint of a left posterior upper-thigh mass. The patient stated that the mass had been growing over the past year, making it uncomfortable to sit for a prolonged period, and interfering with his ability to lie down. The patient thought he had a smaller mass in the same area about 15 years before, which had quickly resolved. He denied prior trauma to the area, or history of malignancy. His medical history was significant for type 2 diabetes mellitus, hypertension, and end-stage renal disease. The patient did not complain of fevers, chills, weight loss, or drainage from the area. On physical exam, there was a large protuberant mobile soft-tissue mass in the inferior left gluteal region that was soft to palpation and did not communicate with the rectum. The mass was noted to be approximately the size of a large grapefruit. Differential diagnosis included both benign and malignant soft-tissue tumors, which prompted an MRI examination for further characterization of the lesion.
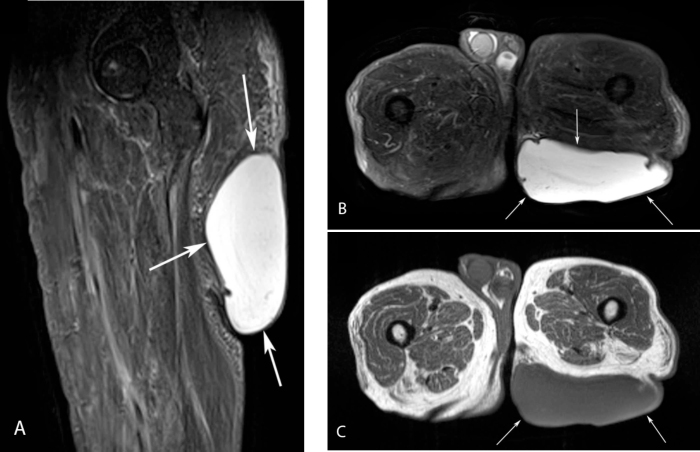
MRI of the bilateral gluteal/upper-thigh regions was performed without intravenous contrast, due to the history of end-stage renal disease. Coronal and sagittal inversion recovery (STIR), axial T2W fat-suppressed, and axial T1W MR images were acquired. The images revealed a large, exophytic, well-defined cystic mass in the left inferior gluteal subcutaneous soft tissue; it demonstrated relatively homogeneous high signal intensity on the fluid-sensitive sequences and intermediate decreased signal on the T1W images, with several small incomplete marginal septa (Fig. 1). The lesion measured 17.8 cm transverse, 13.8 cm craniocaudal, and 5.8 cm anteroposterior. The lack of contrast-enhanced sequences precluded further imaging characterization of the lesion. In addition to benign subcutaneous cystic lesions such as giant epidermoid inclusion and sebaceous cysts, the differential diagnosis included soft-tissue sarcoma with an associated large cystic component.
Figure 1.
61-year-old male with large left gluteal epidermal inclusion cyst. Sagittal STIR MR image of the proximal left thigh (A) and axial STIR MR image of the bilateral proximal thighs (B) show a large, exophytic, well-defined cystic sof- tissue mass of high signal intensity in the subcutaneous left gluteal region, with several small peripheral incomplete septa (arrows). C. The lesion shows intermediate decreased signal intensity on the axial T1W MR image of the bilateral proximal thighs (arrows).
Due to the size and location of the mass that was causing the patient’s discomfort, significant temporal enlargement of the lesion over the past year, and clinical concern for possible underlying malignancy/soft-tissue sarcoma with cystic degeneration, surgical excision of the lesion was chosen. During surgery, the mass was noted to be just below the surface of the dermis. The dermis was elevated from the mass, and the lesion was excised. The surrounding subcutaneous soft tissues were unremarkable. The mass was then incised and an odorless, purulent, white creamy fluid was expressed.
Histopathology revealed a large cystic/cavitary mass lined by stratified squamous epithelia with a granular cell layer. The central portion of the cyst was filled with keratinaceous material. These findings are diagnostic of an epidermal inclusion cyst (Fig. 2).
Figure 2.
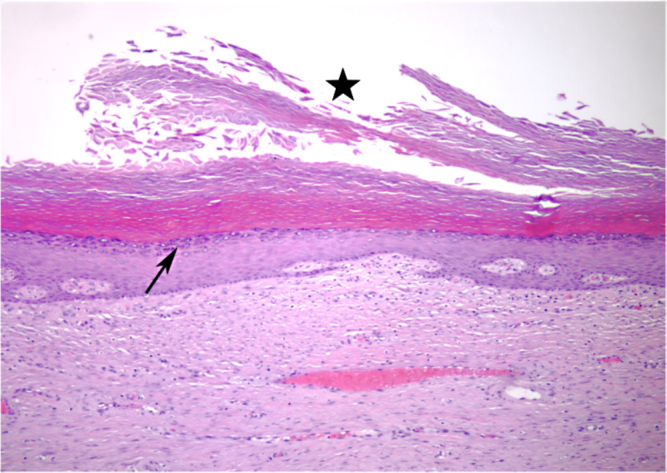
61-year-old male with large left gluteal epidermal inclusion cyst. Representative hematoxalin- and eosin-stained histopathology slide showing the cyst wall lined by stratified squamous epithelium with a granular cell layer (arrow). The central portion of the cyst is filled with keratinaceous debris (star).
Three weeks following the excision of the mass, the patient was doing well, his incisions were healing well, and there were no signs of recurrence. He was discharged from the clinic and instructed to follow up on an as-needed basis. At this time, the patient is 12 months post surgery, asymptomatic and without evidence of local recurrence.
Discussion
Inherent to all soft-tissue epidermal inclusion cysts is the migration of epidermal cells into the dermis (3, 4, 9, 11). In the dermis, the epidermal cells proliferate, collecting debris and keratin, leading to the formation of the cystic space (3, 4). These cysts have a tendency to rupture before the patient seeks medical care, and can lead to a foreign-body giant-cell reaction, potentially causing the formation of a granuloma (11).
To our knowledge, the 17.8 × 13.18 × 5.8-cm epidermal inclusion cyst encountered in our patient is the largest reported in the English literature, with the previous largest one reported measuring 14 × 8 × 8 cm (2). Typically, epidermal inclusion cysts are small and slow-growing, frequently referred to sebaceous, epithelial, or keratin cysts (11). They are the most frequently encountered epithelial cysts found on clinical examination (1). These lesions are commonly found on the face, scalp, neck, and trunk, with only 10% of the cases involving the extremities (3, 4, 9). In patients with multiple epidermal inclusion cysts, the physician should be keen to exclude a gastrointestinal neoplasm, due to the association with Gardner syndrome (11). Up to 53% of patients with Gardner syndrome have epidermal inclusion cysts, in addition to intestinal polyposis, osteomas, and thyroid nodules (11).
Currently, there is no definitive understanding of how epidermal inclusion cysts actually develop; however, a few theories are documented in the literature (3, 4, 9). The first theory is based on aberrant embryogenesis, with ectodermal cells misplaced during cellular differentiation (3, 4, 11). Another theory suggests that epidermal cells are transplanted into the dermis following trauma to the area, such as an injection (3, 4, 11). This theory is frequently used to explain cysts on the extremities (4). In another common theory, pilosebaceous structures become inflamed, leading to a cystic reaction in the dermis (3, 4, 11). This theory is typically used to explain the presence of cysts on the face, neck, and trunk (4). The final theory links lesions that appear only on the palms of the hands and soles of the feet to an infection of eccrine ducts with Human Papilloma Virus 60 (3, 4, 7).
There have been rare cases of epidermal inclusion cysts undergoing malignant degeneration (9). In a study of 3,300 cases of epidermal inclusion cysts by Bauer, there was a 2.2% rate of malignant degeneration into squamous-cell carcinoma, with a majority of the carcinomas being well differentiated (9). Some authors suggest that the rate of malignant transformation may be higher, owing to the fact that a number of these lesions are removed in the physician’s office and are never examined microscopically (10).
Because of concern about possible malignancy, MRI is frequently used to characterize larger masses. On MRI, unruptured epidermal inclusion cysts are well-defined oval or round lesions with intermediate to slightly increased signal intensity on T1-weighted images and high signal intensity on T2-weighted/fluid-sensitive sequences (5, 8). Epidermal inclusion cysts may also have a combination of a mixed high and low signal intensity on T2-weighted images, accounting for the keratin debris layers that are frequently found in the cysts (5, 8). This heterogeneity helps differentiate epidermal inclusion cysts from other fluid-filled masses such as ganglion cysts, which would typically have more homogeneous signal intensity on T2-weighted images (5, 8). With the addition of intravenous, gadolinium-based, contrast-enhanced sequences, noncomplicated lesions show no enhancement or thin-rim enhancement (5, 8). Ruptured epidermal inclusion cysts may have similar signal characteristics as unruptured cysts in regard to signal intensity on T1 and T2-weighted/fluid-sensitive sequences (5). Ruptured cysts, however, frequently show septation and a thick, irregular rim of enhancement on the postcontrast images (5). These signal characteristics are similar to abscesses and soft-tissue malignancies, with central areas of necrosis, making a biopsy of the mass imperative (5). The thickened peripheral ring of enhancement is due to an inflammatory reaction that occurs when the content of the cyst ruptures into the dermis, inciting an intense soft-tissue reaction along with a giant-cell mediated immune response (11).
Because intravenous contrast was not administered, the diagnosis of an epidermal inclusion cyst was difficult to make based on MRI. Myxoid tumors, neurogenic tumors, and dermatofibrosarcoma protuberans can have high signal intensity on T1 and T2-weighted/fluid-sensitive sequences; however, these masses frequently show central contrast enhancement, separating them from epidermal inclusion cysts (5).
Ultrasonography of the epidermal inclusion cysts is typically performed when the cysts are present in the breast. Ultrasound commonly shows a well-circumscribed, hypoechoic or inhomogeneously hypoechoic solid-appearing mass (1). In one series, three of eleven epidermal inclusion cysts in breast had multiple heterogeneous microcalcifications detected on mammography (1).
On histopathology, epidermal cysts are lined by a stratified squamous epithelium containing a granular layer, as seen in our patient (Fig. 2) (7, 11). The epithelial granular layer of epidermal inclusion cysts differentiates them from trichilemmal (sebaceous) cysts, which are keratin-containing cysts frequently found on the scalp and arising from isthmus of hair follicles (7, 11). When an epidermal inclusion cyst is found on the scalp, it is important to differentiate it from a trichilemmal cyst, because a trichilemmal cyst can resemble a proliferating trichilemmal cyst (pilar tumor), a locally aggressive mass that is considered to be a variant of squamous-cell carcinoma by some dermatopathologists (7, 11).
Epidermal inclusion cysts, especially large lesions that may cause patient discomfort, do not regress with medical treatment alone and thus require surgical excision (11). The surgeon must take care when removing the cyst in order to avoid spilling any of the contents into the soft tissue, as an inflammatory reaction is then likely to occur (11). The entire cyst wall must be removed in order to reduce the risk of recurrence; however, even if the entire wall is removed, some reports have noted a 3% recurrence rate (11).
Overall, an epidermal inclusion cyst is a frequently encountered benign, cystic mass of the dermis. Even though malignant degeneration is rare, it is important for the physician to exclude malignancy in cases of giant lesions through proper imaging with the use of gadolinium-contrast-enhanced MRI. If gadolinium-contrast-enhanced MRI cannot be obtained due to patient comorbidities, then an adequate biopsy and excision should be performed.
Footnotes
Published: December 31, 2010
References
- 1.Denison CM, Ward VL. Epidermal inclusion cysts of the breast: Three lesions with calcifications. Radiology. 1997;204:493–496. doi: 10.1148/radiology.204.2.9240542. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto T, Murakami K, Kashimada A. Large epidermoid cyst involving the ischiorectal fossa: MR Demonstration. Clin Imaging. 1993;17:146–148. doi: 10.1016/0899-7071(93)90056-s. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Handa U, Chhabra S. Epidermal inclusion cyst: Cytomorphological features and differential diagnosis. Diagn Cytopathol. 2008;36:861–863. doi: 10.1002/dc.20923. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Momeni MG, Anavim A. Giant epidermal inclusion cyst of buttock. Skeletal Radiol. 2006;35:864–866. doi: 10.1007/s00256-006-0098-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Shibata T, Hatori M. Magnetic resonance imaging features of epidermoid cyst in the extremities. Arch Orthop Trauma Surg. 2003;123:239–241. doi: 10.1007/s00402-003-0509-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Patel K, Bhuiyu T. Epidermal inclusion cyst of phalanx: A case report and review of the literature. Skeletal Radiol. 2006;35:861–863. doi: 10.1007/s00256-005-0058-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Weedon D. Weedon’s skin pathology. 3rd Edition. Elsevier; 2010. pp. 442–446. [Google Scholar]
- 8.Hong SH, Chung HW. MRI findings of subcutaneous epidermal cysts: Emphasis on the presence of rupture. Am. J. Rotengenol. 2006;186:961–966. doi: 10.2214/AJR.05.0044. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Bauer BS, Lewis VL., Jr. Carcinoma arising in sebaceous and epidermoid cysts. Ann Plast Surg. 1980;5:222–226. doi: 10.1097/00000637-198009000-00008. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Lin CY, Jwo SC. Squamous cell carcinoma arising in an epidermal inclusion cyst. Chang Gung Med J. 2002;25:279–282. [PubMed] [PubMed] [Google Scholar]
- 11.Pandya KA, Radke F. Benign skin lesions: Lipomas, epidermal inclusion cysts, muscle and nerve biopsies. Surg Clin North Am. 2009;89:677–687. doi: 10.1016/j.suc.2009.03.002. [PubMed] [DOI] [PubMed] [Google Scholar]