Abstract
Purpose
To present a detailed phenotypic and molecular study of two families with autosomal dominant RPE65-related retinal dystrophy.
Methods
Five patients from two families were ascertained from the retinal clinics of a tertiary referral center. Phenotyping included retinal imaging and electrophysiological testing. Bidirectional Sanger sequencing of exon 13 of RPE65 and its intron–exon boundaries was performed on all reported patients and segregation confirmed in available relatives. The main outcome measures were the results of an ophthalmic examination and investigation and molecular genetic analysis.
Results
Four affected patients from two families presented with nyctalopia and central visual disturbance in adulthood progressing to severe visual loss by the fifth to eighth decades. The patients had extensive chorioretinal atrophy with a relatively preserved anterior retina. In the second family, one patient had bilateral, vitelliform-like foveal lesions consistent with adult onset vitelliform macular dystrophy and no peripheral retinal changes. These unrelated families were both heterozygous for c.1430A>G (p.Asp477Gly). One unaffected family member also tested positive for this mutation but had good vision at age 80 years.
Conclusions
Autosomal dominant retinal dystrophy resembling choroideremia can arise from a heterozygous mutation in RPE65. It may manifest with mild disease or be non-penetrant. Awareness of these unusual presentations can facilitate targeted molecular investigation.
Introduction
Retinal pigment epithelium-specific protein 65 kDa (RPE65; OMIM 180069), located on 1p31.3-p31.2, encodes a retina-specific, 65 kDa visual cycle protein, retinol isomerase, a vital component of the visual cycle [1-3]. Recessive mutations in RPE65 can cause severe early onset retinal dystrophy, including Leber congenital amaurosis (LCA) [4,5]. Patients usually present in infancy or early childhood with reduced vision and nyctalopia. The fundus appearance tends to be normal in infancy, but small subretinal white dots may appear later in childhood that may represent abnormal accumulation of retinal esters [6]. Retinal imaging reveals a variable thinning of the outer nuclear layer on optical coherence tomography (OCT) and a low signal on fundus autofluorescence (FAF) imaging, in keeping with reduced lipofuscin accumulation in the RPE [7]. Gene replacement therapy trials using subretinal administration of recombinant adeno-associated viral vectors expressing RPE65 cDNA have had promising but unsustained results [8-10].
There was one previous report in 2011 of autosomal dominant disease due to a heterozygous mutation in RPE65 with variable penetrance in two families [11]. The present study expands on this report and describes a potential new association with adult onset vitelliform macular dystrophy (AVMD).
Methods
The study protocol adhered to the tenets of the Declaration of Helsinki and the ARVO guidelines on human subjects in research and received approval from the Research Management Committee at Moorfields Eye Hospital. Written, informed consent was obtained from all participants before they were included in the study.
Two families with autosomal dominant retinal disease and a fundus appearance compatible with that previously reported by Bowne et al. were screened specifically for the dominant RPE65 allele [11]. Each patient underwent a full clinical examination, including visual acuity and dilated fundus examination. When possible, retinal fundus imaging was obtained with conventional 35° fundus color photographs (Topcon Great Britain Ltd, Berkshire, UK), 30° or 55° fundus autofluorescence (FAF) imaging (Spectralis, Heidelberg Engineering Ltd, Heidelberg, Germany), and Spectralis optical coherence tomography (OCT, Heidelberg Engineering Ltd, Heidelberg, Germany). Fundus fluorescein angiographic images for patient 1.2 were acquired using the Topcon digital retinal camera system. Full-field electroretinography (ERG) and pattern electroretinography (PERG) were performed in two patients using gold foil electrodes to incorporate the International Society for Clinical Electrophysiology of Vision (ISCEV) standards with electrooculogram (EOG) additionally performed in patient 1.2 [12-14].
Molecular biology
Bidirectional Sanger sequencing of the coding region of exon 13 of RPE65 including the intron–exon boundaries was performed on affected patients, and segregation was confirmed in available relatives. Genomic DNA was isolated from peripheral blood lymphocytes using the Puregene kit (Gentra Puregene Blood Extraction Kit, Qiagen, Manchester, UK). DNA was amplified using specifically designed primers by polymerase chain reaction with BIOTAQ™ DNA polymerase (Bioline, London, UK) at an annealing temperature of 65 °C. The resulting fragments were sequenced using standard protocols (further details available on request). Mutation nomenclature was assigned in accordance with GenBank Accession number NM_000329.2 with nucleotide position 1 corresponding to the A of the ATG translation initiation codon.
Results
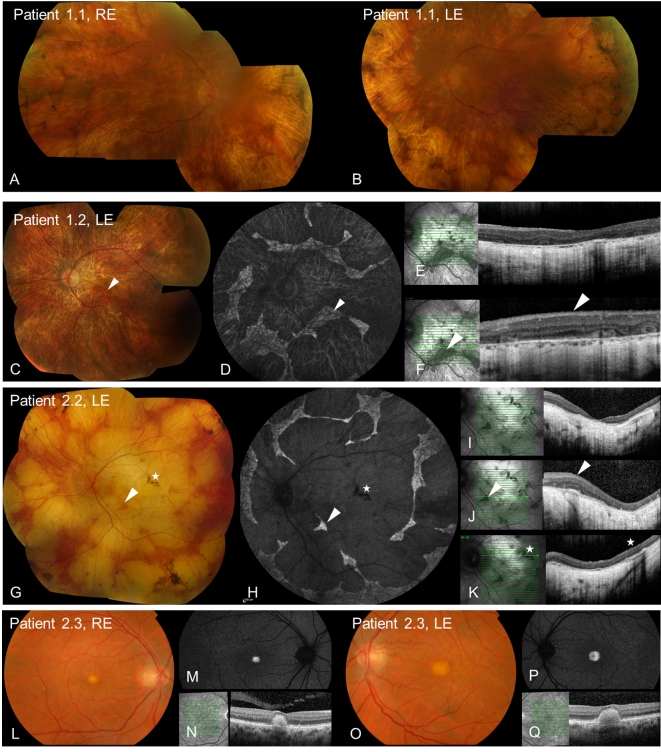
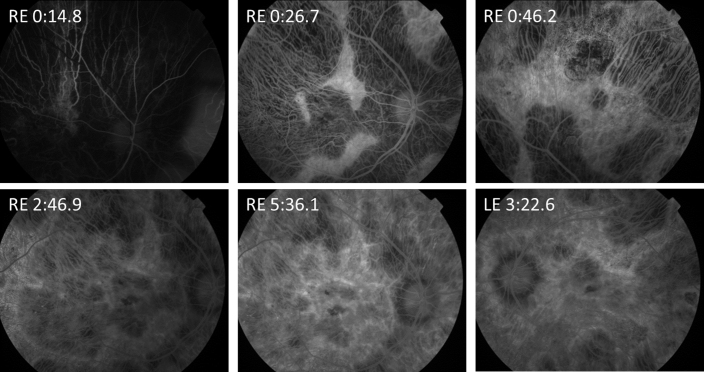
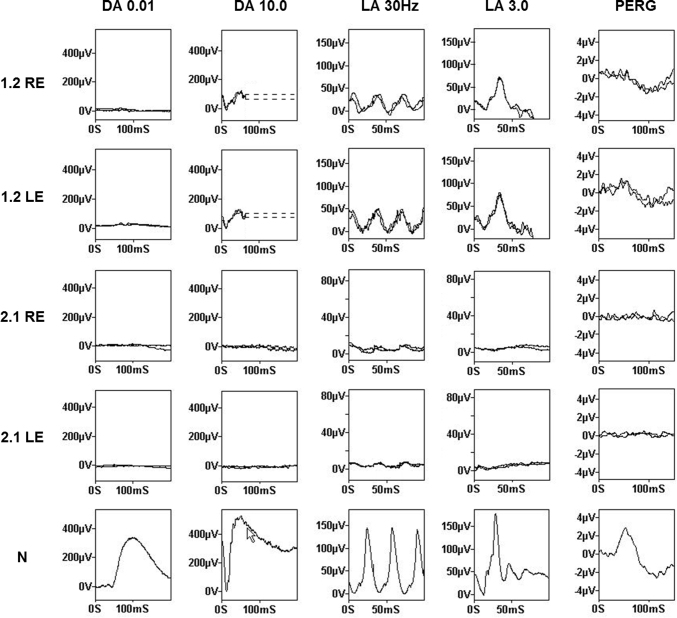
Five patients from two families were investigated. Clinical data are summarized in the Table 1 and Figure 1. Two families, both of Irish ancestry, manifested a dominant retinal dystrophy. The first family had a choroideremia-like phenotype. Patient 1.1 presented with decreased central vision and later reported loss of peripheral visual field. At the age of 67 years, she had visual acuity of 20/60 in the right eye and 20/40 in the left. By age 74, acuity had deteriorated to 20/200 in the right eye and 20/80 in the left. In contrast, her son, patient 1.2, had more severe disease at an earlier age, developing nyctalopia at age 18 years and central visual disturbance at age 33 years with deterioration to hand movement vision in each eye by age 48 years. Peripheral fields to confrontation were still preserved in patches at this age. Fundus examination in both patients showed extensive chorioretinal atrophy with more severe atrophy apparent in the periphery in patient 1.1 with peripheral increased pigment clumps (Figure 1). The OCT for patient 1.2 demonstrated extensive atrophy of the outer nuclear and photoreceptor layers, RPE, and choroid (Figure 1). In the regions of the preserved retina and RPE, outer retinal tubulation was frequently found. Autofluorescence imaging showed a generalized loss of autofluorescence except sparse, scalloped areas of the preserved retina and RPE (Figure 1). Fundus fluorescein angiography in patient 1.2 demonstrated relatively well-preserved retinal vasculature with readily visible choroidal vasculature and no abnormal leakage (Figure 2). The ERGs for patient 1.2 at age 33 years showed a rod-cone pattern of dysfunction of moderate severity with macular involvement (Figure 3). There was marked involvement at the level of the RPE as demonstrated by an abolished light rise on EOG. No other family members were available for examination.
Table 1. Clinical summary.
| Patient (gender) Family number | Age of onset | Age last review | Latest VA, (Snellen) | Fundus features | Age at last electrophysiology, key findings |
|---|---|---|---|---|---|
| 1.1 (f) GC16236 |
<65 years |
74 years |
R 20/200
L 20/80 |
Extensive areas chorioretinal atrophy anteriorly more than posteriorly, peripheral RPE hypertrophy |
Not performed |
| 1.2 (m) GC16236 |
18 years |
46 years |
R HM
L HM |
Extensive chorioretinal atrophy, some preserved anterior retina and well demarcated patches of posterior retina |
33 years: rod cone dystrophy of moderate severity with marked involvement at the level of the RPE |
| 2.1 (f) GC11527 |
45 years |
87 years |
R HM
L PL |
Extensive chorioretinal atrophy with well demarcated areas of preserved anterior retina, peripheral RPE hypertrophy |
86 years: very severe generalized retinal dysfunction of rods (undetectable) and cones |
| 2.2 (m) GC11527 |
35 years |
71 years |
R HM
L 20/160 |
Extensive chorioretinal atrophy sharply demarcated from preserved anterior retina, RPE hypertrophy |
Not performed |
| 2.3 (m) GC11527 | 40 years | 48 years | R 20/40 L 20/30 | Foveal vitelliform lesions | Not performed |
VA, visual acuity; RPE, retinal pigment epithelium; HM, hand movements vision; PL, perception of light
Figure 1.
Retinal imaging in dominant RPE65 disease. A, B, C, G, L, O: Color fundus photographs demonstrate extensive chorioretinal atrophy in patients 1.1, 1.2, and 2.2, with foveal yellow lesions in patient 2.3. D, H, M, P: Fundus autofluorescence (FAF) imaging demonstrates extensive loss of autofluorescence in patients 1.2 and 2.2 with scalloped regions of preserved retina and RPE, and increased autofluorescence of foveal deposits in patient 2.3. E, I, N, Q: Optical coherence tomography (OCT) through the fovea demonstrates the loss of outer nuclear and photoreceptor layers for patients 1.2 and 2.2, and dome-shaped deposits extending from the RPE to the outer retina for patient 2.3. F, J: OCT through the preserved retina and RPE in patients 1.2 and 2.2 with frequent outer retinal tubulation. K: OCT through the RPE hypertrophy in patient 2.2. Arrowheads highlight the comparative regions of the preserved retina and RPE. Stars highlight the comparative area of RPE hypertrophy.
Figure 2.
Fundus fluorescein angiogram of patient 1.2 at increasing time points. Relative preservation of the retinal vasculature is apparent with the easily visible choroidal vasculature. RE, right eye. LE, left eye. Time in minutes.
Figure 3.
Electrophysiology of patients 1.2 and 2.1 with normal (N) for comparison. In both eyes of patient 1.2, there was severely subnormal rod-specific electroretinogram (ERG, DA 0.01) delayed and subnormal cone-specific ERGs (LA 30Hz and LA 3.0) and markedly subnormal pattern ERG (PERG). Findings were consistent with a rod-cone pattern of dysfunction of moderate severity with bilateral macular involvement. Both eyes of patient 2.1 demonstrated extinguished rod responses and residual cone-related activity with extinguished PERG consistent with severe generalized retinal dysfunction and severe macular involvement.
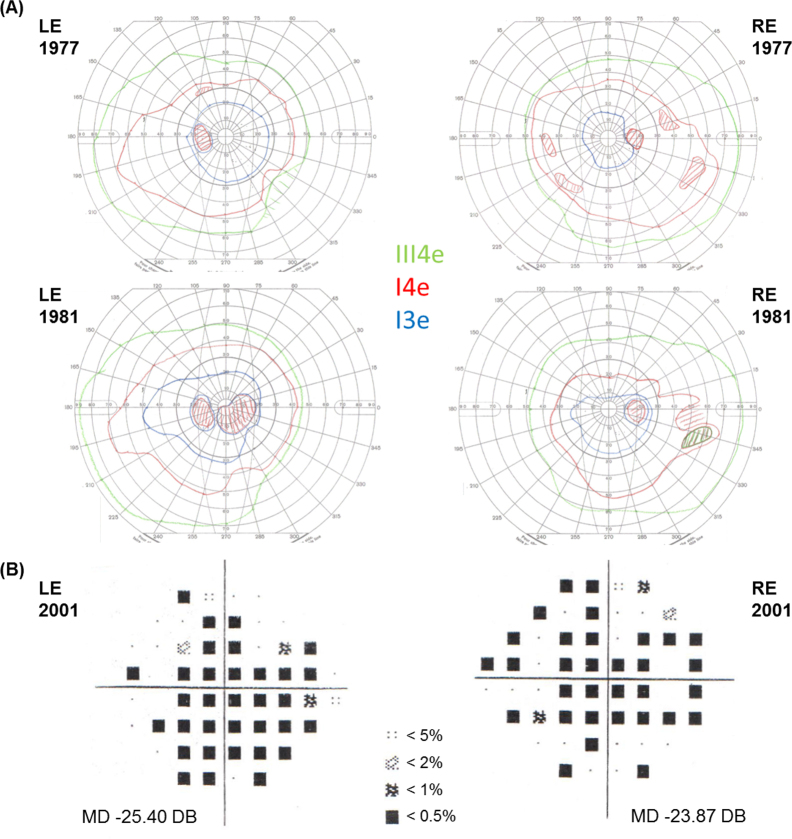
Three members were examined in the second dominant family. A mother and son, patients 2.1 and 2.2, presented at age 45 and 35 years, respectively, with central visual disturbance, which slowly progressed to severe loss of vision. Both had widespread chorioretinal degeneration on fundus examination with a well-demarcated preserved anterior retina and small clumps of increased pigment in the periphery. On the OCT of patient 2.2, extensive atrophy of the outer nuclear and photoreceptor layers, RPE, and choroid was evident with patches of preserved retina and RPE that had frequent outer retinal tubulation (Figure 1). OCT through a pigment clump in the posterior pole demonstrated a region of RPE hypertrophy, not pigment migration (Figure 1). FAF imaging highlighted the scalloped regions of the preserved retina and RPE with otherwise extensive loss of autofluorescence. ERG testing performed in patient 2.1 demonstrated severe generalized retinal dysfunction with extinguished rod responses and only residual cone related activity (Figure 3). Visual fields performed on patient 2.2 demonstrated multiple central scotomas at age 36 years on the Goldmann visual fields, with some increase in the central scotomas evident at age 40 years and extensive central field loss at age 59 years on Humphrey 24–2 (Figure 4). Patient 2.3, a cousin of patient 2.2, developed central visual disturbance at age 40 years with symptoms of difficulty reading and recognizing faces. Visual acuity at age 46 was 20/40 in the right eye and 20/30 in the left. Vitelliform-like yellow foveal deposits were present in both maculae. On OCT, the single dome-shaped deposits were demonstrated to involve the RPE and extend to the outer retina, displacing the outer plexiform layer (Figure 1). They showed increased autofluorescence on FAF imaging. Patient 2.3 declined further clinical investigation, including electrophysiology. His unaffected father was unavailable for clinical investigation.
Figure 4.
Visual fields for patient 2.2. A: Goldmann visual fields at ages 36 and 40 years of the left eye (LE) and the right eye (RE) demonstrate central scotomas increasing over time particularly on the LE. Three different light stimulus sizes and intensities, III4e, I4e, and I3e, are indicated with colored isopters. Light stimulus size III is larger than I, and intensity level 4 is greater than 3. B: Humphrey 24–2 visual fields at age 59 years demonstrate extensive loss of the central field in both eyes. Mean deviation (MD) indicates the overall average loss of field in decibels (dB). Shaded symbols represent probability indicators of the statistical likelihood of the field being normal at that location. The darker the symbol, the less likely it is that the field is normal at that tested location.
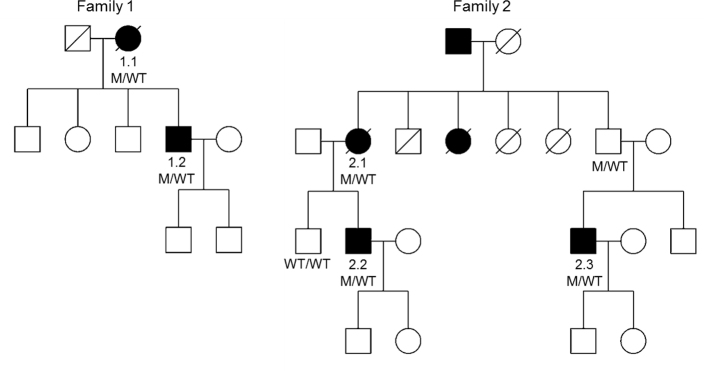
Both families had screened negative for mutations in REP1 and PRPH2, and in addition, the c.489C>G (p.Ser163Arg) mutation in CIQTNF5 screened negative in patient 2.2. Following the report of c.1430 A>G (p.Asp477Gly) causing dominantly inherited RPE65-related disease, exon 13 of the RPE65 gene was screened. In family 1, this identified the same previously reported mutation in patients 1.1 and 1.2 (Figure 5) [11]. In family 2, the same mutation was found in all three affected patients and, following segregation in available family members, was also identified in the asymptomatic father of patient 2.3 (with reported normal visual acuity at age 80 years) and was absent in the unaffected brother of patient 2.2 (Figure 5).
Figure 5.
Pedigrees of two families with mutation segregation.
Discussion
This study presents data from five patients from two families with a dominant mutation in RPE65. Four affected patients presented in adulthood with central visual disturbance that slowly progressed and was associated with extensive chorioretinal atrophy. One member of family 2 who carried the causative mutation presented with an AVMD phenotype.
Autosomal dominant RPE65 disease has been reported previously in two Irish families [11]. Initially, it was identified in a large pedigree in whom linkage mapping identified an 8.8 Mb region on chromosome 1, and then, with candidate gene sequencing in parallel with whole exome sequencing, the heterozygous p.Asp477Gly mutation was identified. The same mutation was also then identified in a second Irish family who had been diagnosed with choroideremia but who were negative for mutations in REP1 [11]. Intrafamilial phenotypic variability was reported with mild disease in some affected individuals manifesting as peripheral retinal pigment migration only and more severe disease in others with extensive chorioretinal atrophy. There were three unaffected at-risk family members and one obligate carrier also seemingly unaffected. In the present series, two additional families were identified with the same allele, supporting its association with retinal dystrophy. The father of patient 2.3 in this series carries the mutation but is asymptomatic at age 80 years, confirming that some gene carriers can be non-penetrant. As he was unavailable for clinical examination, sub-clinical retinal changes could have been present.
Both families in this report have Irish ancestry. They are not known to be related to each other or to the originally reported families, but as the families have the same rare allele this raises the likely possibility of a common ancestor. Four of the five affected patients presented with severe disease that resembles choroideremia with extensive atrophy of the outer retina, RPE, and choroid on OCT and scalloped patches of the surviving retina. Outer retinal tubulation was frequently found in the regions of surviving retina and RPE that are thought to indicate invagination of photoreceptors at the border of outer retinal atrophy [15]. Choroideremia has a similar end stage fundus appearance as dominant RPE65 disease but typically has an earlier, childhood onset of night blindness [16,17]. In addition, the patients in this report further differ from choroideremia with prominent early central visual disturbance and a relatively preserved anterior retina. Choroideremia is an X-linked recessive disorder due to hemizygous REP1 mutations and thus predominantly affects men with no reported non-penetrance. In female carriers, a typical mottled appearance of the RPE most apparent on FAF imaging can be detected, but severity varies from asymptomatic to severely affected due to random X-inactivation [17]. However, it would be unusual for a female carrier to manifest as severe disease as observed in patients 1.1 and 2.1.
REP1 is an ubiquitously expressed Rab escort protein important in post-translational prenylation of Rab proteins, with mutations leading to an accumulation of toxic unprenylated Rab27A and independent degeneration of the retina and the RPE followed by the choroid [18,19]. RPE65, expressed in the RPE, is the key visual cycle isomerase, with mutations leading to photoreceptor dysfunction and degeneration followed by RPE degeneration [8]. In dominant RPE65 disease, the photoreceptor dysfunction has a rod-cone characteristic on ERG, similar to the recessive disease, but in the families reported to date, there is much more extensive RPE and choroidal degeneration compared to retinal degeneration. The cause of disease in the dominant families is unlikely to be due to haploinsufficiency of RPE65 as the parents of patients with RPE65-related LCA have normal retinal function [20]. The Asp477Gly mutation is predicted to destabilize folding of the tertiary RPE65 structure [11]. One attractive hypothesis is that the mutant protein in some, but not all, gene carriers has a toxic effect on the RPE, similar to unprenylated Rab27A, giving rise to the atrophy of the RPE and the inner choroid in these patients. In support of this, the EOG in patient 1.2 identified an abolished light rise indicating severe RPE dysfunction.
The fifth case presented with AVMD. AVMD has previously been associated with mutations in peripherin 2 (PRPH2; OMIM 179605), bestrophin 1 (BEST1, OMIM 607854), and interphotoreceptor matrix proteoglycan 1 (IMPG1, OMIM 602870) but not RPE65 [21-23]. The case in this series was phenotypically distinct from BEST1 and was negative for mutations on sequencing of PRPH2. He and his unexamined father suggest that heterozygotes for this RPE65 allele can be mildly affected, but it remains possible that the cause of his AVMD is unrelated. Unfortunately, neither one was available for electrophysiology or psychophysical testing. In dominant retinal disease due to a PRPH2 mutation, retinitis pigmentosa and vitelliform macular dystrophy have also been shown to cosegregate in the same family [24]. In this context, it is possible that the mutant allele has two or more distinct toxic effects on the retina that may be modified independently by environmental or other genetic factors. In conclusion, RPE65-related retinal dystrophy may manifest atypically and should be considered in patients presenting with chorioretinal atrophy or AVMD in whom targeted molecular screening has been negative.
Acknowledgments
Funding support: The National Institute for Health Research (UK) and Biomedical Research Centre at Moorfields Eye Hospital and the UCL Institute of Ophthalmology (BRC2_003), Foundation Fighting Blindness (USA, C-CL:0710–0505-MEH10–02), Fight For Sight (1318 and 1801), Moorfields Eye Hospital Special Trustees (ST1109B), Rosetrees Trust (M184). Conflict of Interest: ATM is the Chairman of Data Safety and on the Management Board of gene therapy trials for retinal disease sponsored by Sanofi and Oxford Biomedica and has served on an Advisory Board for Sucampo Phamaceuticals and Sanofi. He has also participated in a clinical trial of oral retinoid therapy for RPE65 deficiency sponsored by QLT. No other conflicting relationships exist.
References
- 1.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci USA. 2005;102:12413–8. doi: 10.1073/pnas.0503460102. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16116091&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–59. doi: 10.1016/j.cell.2005.06.042. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16096063&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci USA. 2005;102:13658–63. doi: 10.1073/pnas.0504167102. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16150724&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet. 1997;17:139–41. doi: 10.1038/ng1097-139. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9326927&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 5.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–7. doi: 10.1038/ng1097-194. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9326941&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 6.Weleber RG, Michaelides M, Trzupek KM, Stover NB, Stone EM. The phenotype of Severe Early Childhood Onset Retinal Dystrophy (SECORD) from mutation of RPE65 and differentiation from Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2011;52:292–302. doi: 10.1167/iovs.10-6106. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20811047&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 7.Lorenz B, Wabbels B, Wegscheider E, Hamel CP, Drexler W, Preising MN. Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology. 2004;111:1585–94. doi: 10.1016/j.ophtha.2004.01.033. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15288992&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 8.Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, Roman AJ, Olivares MB, Schwartz SB, Komáromy AM, Hauswirth WW, Aguirre GD. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci USA. 2013;110:E517–25. doi: 10.1073/pnas.1218933110. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23341635&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Testa F, Maguire AM, Rossi S, Pierce EA, Melillo P, Marshall K, Banfi S, Surace EM, Sun J, Acerra C, Wright JF, Wellman J, High KA, Auricchio A, Bennett J, Simonelli F. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology. 2013;120:1283–91. doi: 10.1016/j.ophtha.2012.11.048. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23474247&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, Georgiadis A, Mowat FM, Beattie SG, Gardner PJ, Feathers KL, Luong VA, Yzer S, Balaggan K, Viswanathan A, de Ravel TJ, Casteels I, Holder GE, Tyler N, Fitzke FW, Weleber RG, Nardini M, Moore AT, Thompson DA, Petersen-Jones SM, Michaelides M, van den Born LI, Stockman A, Smith AJ, Rubin G, Ali RR. Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med. 2015;372:1887–97. doi: 10.1056/NEJMoa1414221. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25938638&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowne SJ, Humphries MM, Sullivan LS, Kenna PF, Tam LC, Kiang AS, Campbell M, Weinstock GM, Koboldt DC, Ding L, Fulton RS, Sodergren EJ, Allman D, Millington-Ward S, Palfi A, McKee A, Blanton SH, Slifer S, Konidari I, Farrar GJ, Daiger SP, Humphries P. A dominant mutation in RPE65 identified by whole-exome sequencing causes retinitis pigmentosa with choroidal involvement. Eur J Hum Genet. 2011;19:1074–81. doi: 10.1038/ejhg.2011.86. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21654732&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015;130:1–12. doi: 10.1007/s10633-014-9473-7. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25502644&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 13.Bach M, Brigell MG, Hawlina M, Holder GE, Johnson MA, McCulloch DL, Meigen T, Viswanathan S. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc Ophthalmol. 2013;126:1–7. doi: 10.1007/s10633-012-9353-y. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23073702&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 14.Marmor MF, Brigell MG, McCulloch DL, Westall CA, Bach M, International Society for Clinical Electrophysiology of Vision ISCEV standard for clinical electro-oculography (2010 update). Doc Ophthalmol. 2011;122:1–7. doi: 10.1007/s10633-011-9259-0. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21298321&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 15.Goldberg NR, Greenberg JP, Laud K, Tsang S, Freund KB. Outer retinal tubulation in degenerative retinal disorders. Retina. 2013;33:1871–6. doi: 10.1097/IAE.0b013e318296b12f. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23676993&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts MF, Fishman GA, Roberts DK, Heckenlively JR, Weleber RG, Anderson RJ, Grover S. Retrospective, longitudinal, and cross sectional study of visual acuity impairment in choroideraemia. Br J Ophthalmol. 2002;86:658–62. doi: 10.1136/bjo.86.6.658. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12034689&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renner AB, Kellner U, Cropp E, Preising MN, MacDonald IM, van den Hurk JA, Cremers FP, Foerster MH. Choroideremia: variability of clinical and electrophysiological characteristics and first report of a negative electroretinogram. Ophthalmology. 2006;113:2066.e1–10. doi: 10.1016/j.ophtha.2006.05.045. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16935340&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 18.Roosing S, Collin RW, den Hollander AI, Cremers FP, Siemiatkowska AM. Prenylation defects in inherited retinal diseases. J Med Genet. 2014;51:143–51. doi: 10.1136/jmedgenet-2013-102138. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24401286&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 19.Edwards TL, Groppe M, Jolly JK, Downes SM, MacLaren RE. Correlation of retinal structure and function in choroideremia carriers. Ophthalmology. 2015;122:1274–6. doi: 10.1016/j.ophtha.2014.12.036. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25682176&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 20.Galvin JA, Fishman GA, Stone EM, Koenekoop RK. Evaluation of genotype-phenotype associations in leber congenital amaurosis. Retina. 2005;25:919–29. doi: 10.1097/00006982-200510000-00016. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16205573&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 21.Wells J, Wroblewski J, Keen J, Inglehearn C, Jubb C, Eckstein A, Jay M, Arden G, Bhattacharya S, Fitzke F. Mutations in the human retinal degeneration slow (RDS) gene can cause either retinitis pigmentosa or macular dystrophy. Nat Genet. 1993;3:213–8. doi: 10.1038/ng0393-213. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8485576&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 22.Allikmets R, Seddon JM, Bernstein PS, Hutchinson A, Atkinson A, Sharma S, Gerrard B, Li W, Metzker ML, Wadelius C, Caskey CT, Dean M, Petrukhin K. Evaluation of the Best disease gene in patients with age-related macular degeneration and other maculopathies. Hum Genet. 1999;104:449–53. doi: 10.1007/s004390050986. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10453731&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 23.Manes G, Meunier I, Avila-Fernández A, Banfi S, Le Meur G, Zanlonghi X, Corton M, Simonelli F, Brabet P, Labesse G, Audo I, Mohand-Said S, Zeitz C, Sahel JA, Weber M, Dollfus H, Dhaenens CM, Allorge D, De Baere E, Koenekoop RK, Kohl S, Cremers FP, Hollyfield JG, Sénéchal A, Hebrard M, Bocquet B, Ayuso García C, Hamel CP. Mutations in IMPG1 cause vitelliform macular dystrophies. Am J Hum Genet. 2013;93:571–8. doi: 10.1016/j.ajhg.2013.07.018. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23993198&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manes G, Guillaumie T, Vos WL, Devos A, Audo I, Zeitz C, Marquette V, Zanlonghi X, Defoort-Dhellemmes S, Puech B, Said SM, Sahel JA, Odent S, Dollfus H, Kaplan J, Dufier JL, Le Meur G, Weber M, Faivre L, Cohen FB, Béroud C, Picot MC, Verdier C, Sénéchal A, Baudoin C, Bocquet B, Findlay JB, Meunier I, Dhaenens CM, Hamel CP. High prevalence of PRPH2 in autosomal dominant retinitis pigmentosa in france and characterization of biochemical and clinical features. Am J Ophthalmol. 2015;159:302–14. doi: 10.1016/j.ajo.2014.10.033. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25447119&dopt=Abstract [DOI] [PubMed] [Google Scholar]