Abstract
Aims/hypothesis
Women with gestational diabetes mellitus (GDM) are at risk of developing type 2 diabetes, but individualised risk estimates are unknown. We conducted a meta-analysis to quantify the risk of progression to type 2 diabetes for women with GDM.
Methods
We systematically searched the major electronic databases with no language restrictions. Two reviewers independently extracted 2 × 2 tables for dichotomous data and the means plus SEs for continuous data. Risk ratios were calculated and pooled using a random effects model.
Results
There were 39 relevant studies (including 95,750 women) BMI (RR 1.95 [95% CI 1.60, 2.31]), family history of diabetes (RR 1.70 [95% CI 1.47, 1.97]), non-white ethnicity (RR 1.49 [95% CI 1.14, 1.94]) and advanced maternal age (RR 1.20 [95% CI 1.09, 1.34]) were associated with future risk of type 2 diabetes. There was an increase in risk with early diagnosis of GDM (RR 2.13 [95% CI 1.52, 3.56]), raised fasting glucose (RR 3.57 [95% CI 2.98, 4.04]), increased HbA1c (RR 2.56 [95% CI 2.00, 3.17]) and use of insulin (RR 3.66 [95% CI 2.78, 4.82]). Multiparity (RR 1.23 [95% CI 1.01, 1.50]), hypertensive disorders in pregnancy (RR 1.38 [95% CI 1.32, 1.45]) and preterm delivery (RR 1.81 [95% CI 1.35, 2.43]) were associated with future diabetes. Gestational weight gain, macrosomia in the offspring or breastfeeding did not increase the risk.
Conclusions/interpretation
Personalised risk of progression to type 2 diabetes should be communicated to mothers with GDM.
Systematic review registration:
www.crd.york.ac.uk/PROSPERO CRD42014013597
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-016-3927-2) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Keywords: Gestational diabetes, Meta-analysis, Postpartum, Predictors, Pregnancy, Risk factors, Systematic review, Type 2 diabetes
Introduction
Gestational diabetes mellitus (GDM), defined as glucose intolerance that is first diagnosed in pregnancy, is on the increase worldwide [1]. Up to half of all women with this condition progress to develop type 2 diabetes in later life, with the highest occurrence rate in the first 5 years after pregnancy [2]. Increasing numbers of women are presenting with previously undiagnosed diabetes and related complications, leading to substantial healthcare costs [3].
Current guidelines recommend following up women with GDM to identify type 2 diabetes at an early stage [4]. Ensuring compliance with this strategy is a significant global problem [5–7]. Despite evidence that the future risk of type 2 diabetes can be reduced by diet and lifestyle interventions and treatment with drugs such as metformin, [8, 9], less than a fifth of mothers with GDM undergo postpartum glucose screening [10]. Personalised risk communication with quantitative estimates can increase the number of individuals that make informed choices in screening programmes [11]. However, few prediction models for type 2 diabetes include GDM, and none of them account for pregnancy-specific characteristics [12].
Gestation-specific factors such as glycaemic status in pregnancy, gestational weight gain and obstetric complications are known to influence the future risk of diabetes [13–17]. Health-care professionals involved in the management of women with GDM do not provide individual risk estimates because of the lack of data; this greatly hinders counselling. Existing reviews have not quantified the risk of progression to type 2 diabetes [16, 18], and primary studies provide imprecise estimates because of their relatively small sample size [19–21]. We therefore undertook a systematic review to assess the strength of association of various maternal and pregnancy-related factors with GDM with the future risk of type 2 diabetes.
Methods
We conducted a systematic review following a prospective protocol in line with current recommendations, and complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting (see electronic supplementary material [ESM] PRISMA checklist) [22]. Ethical approval was not required.
Data sources and searches
We searched the MEDLINE and EMBASE databases (from inception until July 2015) for studies that assessed the risk factors in women with GDM and progression to type 2 diabetes. We used keywords, Medical Subject Headings and word variants for GDM such as ‘diabetes, gestational’, ‘GDM’, and ‘pregnancy induced diabetes’, and combined these with terms for type 2 diabetes such as ‘diabetes mellitus, type 2’, ‘NIDDM’ (i.e. non-insulin-dependent diabetes mellitus), ‘adult-onset diabetes mellitus’ and ‘ketosis-resistant diabetes’ (See ESM Search strategy). We searched the reference lists of identified papers for other relevant studies. Authors of eligible studies were contacted for further details if necessary. We did not apply any language restrictions.
Study selection
Two independent reviewers (GR and AAH) selected the studies. First, we reviewed the abstracts for potentially relevant studies. Second, we obtained full copies of all possibly eligible papers for detailed evaluation. We included studies on women with GDM that assessed at least one of the following factors: maternal characteristics such as age, BMI, ethnicity, parity and family history of type 2 diabetes; factors specific to pregnancy such as gestational age at GDM diagnosis, antenatal OGTT, HbA1c, insulin use in pregnancy, gestational weight gain, pregnancy-induced hypertension and preterm delivery; and postpartum factors such as the baby’s birthweight and breastfeeding. We excluded studies with no relevant data, an inappropriate outcome, no original data (e.g. meeting abstract, editorial, commentary or letter) or duplicate data. We accepted the authors’ definitions of GDM and type 2 diabetes. Any discrepancies in selection were resolved by discussion with a third reviewer (ST).
Data extraction and quality assessment
Two reviewers (GR and AAH) independently undertook study quality assessment and data extraction using predesigned forms. We used the Newcastle–Ottawa scale [23], which evaluates the representativeness and selection of studies, comparability among cohorts, ascertains the exposure and outcome, and evaluates the length and adequacy of follow-up. The risk of bias was considered to be low if a study obtained four stars for selection, two for comparability and three for follow-up; and medium if a study scored two or three stars for selection, one for comparability and two for follow-up. Studies scoring one or zero stars for selection and follow-up, and with no star for comparability, were deemed to have a high risk of bias [24]. Data were extracted as 2 × 2 tables for dichotomous outcomes, and as means and SEs for continuous outcomes.
Data synthesis and analysis
For various risk factors, we calculated the RRs for dichotomous variables, and plotted point estimates and 95% CIs for progression to type 2 diabetes for women with GDM associated with various risk factors. For continuous variables, we plotted pooled mean differences with 95% CIs to assess differences between women with and without type 2 diabetes. We assessed the heterogeneity of association graphically with forest plots and statistically with χ2 tests and the I2 statistic. We pooled results using random effects models. To facilitate comparison between the strength of association of various risk factors with type 2 diabetes, we transformed the pooled standardised mean differences of continuous outcomes into RRs, assuming a 20% baseline risk of type 2 diabetes [25].
We specified the following subgroup analyses a priori: length of follow-up (≤1 year vs >1 year), ethnicity (white vs non-white), use of strict criteria to exclude possible pre-existing type 2 diabetes (such as GDM before 20 weeks of pregnancy or type 2 diabetes diagnosis in the first year after delivery; yes vs no). Additionally, we investigated differences between subgroups based on the levels of fasting glucose criteria for diagnosing GDM (<5.8 mmol/l vs ≥5.8 mmol/l). All analyses were performed using the Review Manager (RevMan Computer program, Version 5.2; The Nordic Cochrane Centre, Copenhagen, Denmark) [26].
Results
Study identification
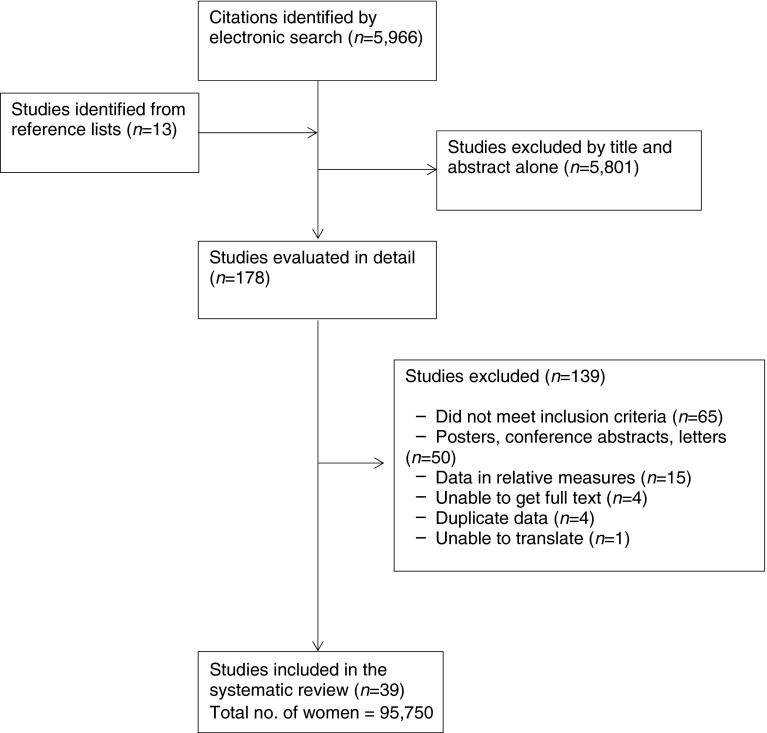
From 5,966 citations, we selected 178 studies for further evaluation (Fig. 1). After a detailed assessment, we included 39 studies (95,750 women) [13–15, 17, 19–21, 27–58]. We excluded 139 studies for the following reasons: inclusion criteria were not met (n = 65); presented as posters, conference abstracts or letters (n = 50); inappropriate data format (n = 15); only abstracts were available (n = 4); duplicate data (n = 4); and could not be translated from the language of publication (n = 1).
Fig. 1.
Flow chart for study selection for the systematic review of the predictors of progression to type 2 diabetes in women with GDM
Characteristics and quality of the included studies
In all, there were 22 prospective cohort studies (56%) [13, 14, 17, 19–21, 29, 32, 35–37, 39–41, 43, 44, 46–48, 50, 52, 55] and 17 retrospective cohort studies (44%) [15, 27, 28, 30, 31, 33, 34, 38, 42, 45, 49, 51, 53, 54, 56–58]. The studies evaluated the association of maternal characteristics, pregnancy-specific factors and postpartum characteristics with progression to type 2 diabetes in women with GDM. The studies varied in their diagnostic criteria for both GDM and type 2 diabetes. Maternal characteristics including BMI, ethnicity and family history of diabetes were evaluated, as were risk factors specific to pregnancy such as maternal age at diagnosis of GDM, parity, gestational age at diagnosis, weight gain in pregnancy, hypertensive diseases in pregnancy and preterm birth, and levels of fasting, post-load blood glucose levels during OGTT, HbA1c in pregnancy and use of insulin for managing GDM.
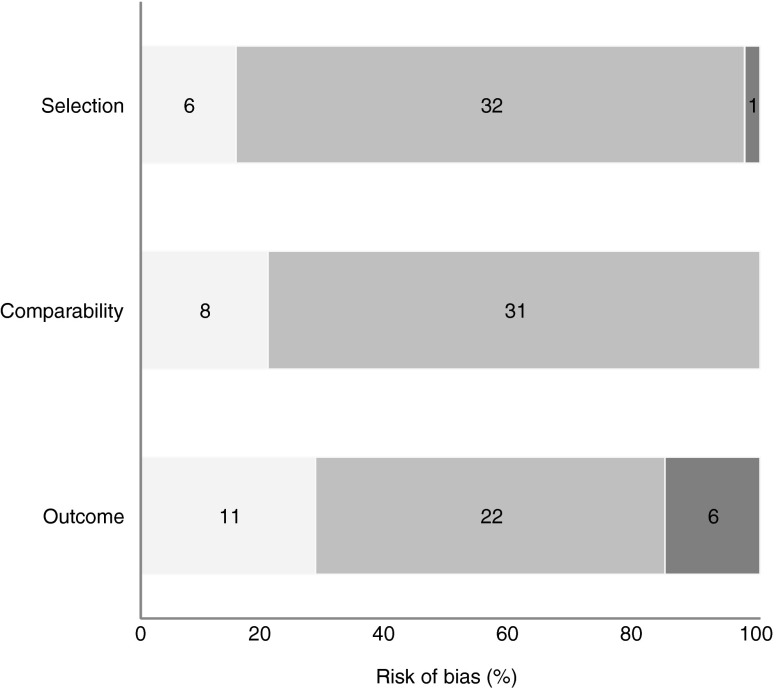
The duration of follow-up varied from 6 weeks to 20 years after birth. A total of 29 studies (74%) evaluated the long-term risk of type 2 diabetes (>1 year after delivery) and 10 (26%) evaluated the risk of developing type 2 diabetes in the first year after childbirth. Detailed characteristics of the included studies are provided in ESM Table 1. In all, six studies (15%) had a low risk of selection bias, 32 (82%) had a medium risk and one (3%) had a high risk. Eight studies (21%) had a low risk of bias for comparability of cohorts, and 31 (79%) had a medium risk of bias. For outcome assessment, 11 studies (28%) had a low risk of bias, 22 (56%) had a medium risk and 6 (15%) had a high risk (see Fig. 2 and ESM Table 2).
Fig. 2.
Risk of bias assessment on the Newcastle–Ottawa Scale for studies included in the systematic review of type 2 diabetes prediction in women with GDM. Light grey bars, low risk; mid grey bars, medium risk; dark grey bars, high risk. The numbers of studies are shown
Maternal characteristics and progression to type 2 diabetes
A high BMI doubled the risk of future type 2 diabetes (RR 1.95 [95% CI 1.60, 2.31]; I2 = 65%), and the risk was increased in obese and overweight women for BMI thresholds of 25 kg/m2 (RR 3.18 [95% CI 1.96, 5.16]; I2 = 77%), 27 kg/m2 (RR 2.52 [95% CI 1.69, 3.74]; I2 = 23%) and 30 kg/m2 (RR 2.85 [95% CI 2.21, 3.69]; I2 = 45%). A family history of diabetes (RR 1.70 [95% CI 1.47, 1.97]; I2 = 13%), non-white ethnicity (RR 1.49 [95% CI 1.14, 1.94]; I2 = 88%) and older age (RR 1.20 [95% CI 1.09, 1.34]; I2 = 0%) were found to be significant risk factors for progression to diabetes after GDM. No increased risk was observed for individual age cut-offs of 30 and 35 years (Fig. 3).
Fig. 3.
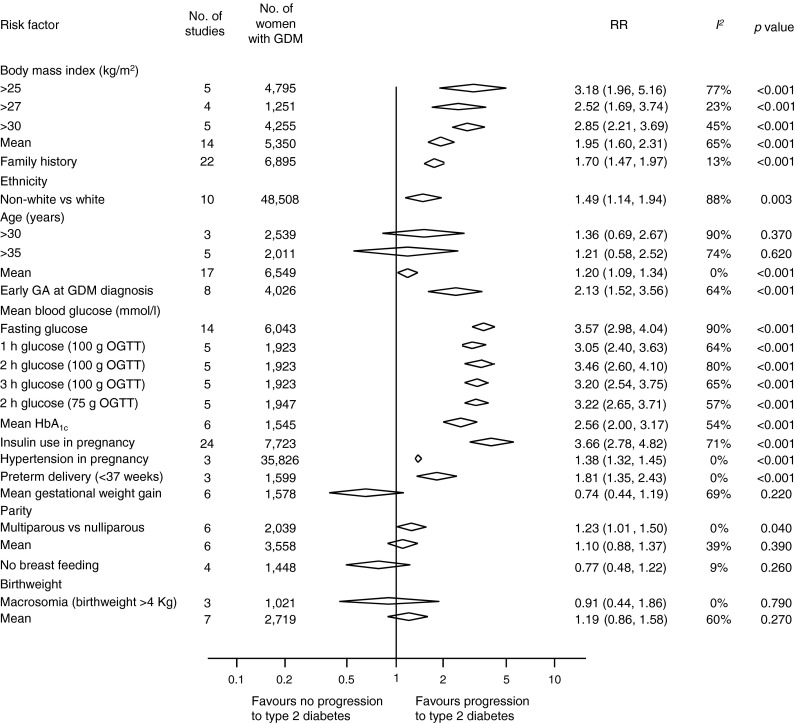
Summary estimates for the association of maternal risk factors with progression to type 2 diabetes in women with GDM. ‘Exclusively’ and ‘mostly’ breastfed were combined into a single breastfeeding category. ‘Mixed or inconsistent’ breastfeeding and ‘exclusively or mostly formula fed’ were combined into a single ‘no breastfeeding’ category [17]. Similarly, data reported for age <34 years in one study [37] was categorised as age <35 years, and data reported as BMI of <28 kg/m2 for one study [50] was classified as a BMI of <27 kg/m2 because all other studies used this cut-off value. GA, gestational age
Pregnancy-specific factors and risk of future diabetes
Increased levels of fasting (RR 3.57; 95% CI 2.98, 4.04; I2 = 90%), 1 h (RR 3.05; 95% CI 2.40, 3.63; I2 = 64%), 2 h (RR 3.46; 95% CI 2.60, 4.10; I2 = 80%) and 3 h (RR 3.2; 95% CI 2.54, 3.75; I2 = 65%) blood glucose levels after OGTT and high HbA1c were associated with an increased risk of future diabetes (RR 2.56; 95% CI 2.00, 3.17; I2 = 54%). Women who required insulin to manage GDM were more likely to develop type 2 diabetes (RR 3.66 [95% CI 2.78, 4.82]; I2 = 71%) compared with those managed without insulin (Fig. 3).
Multiparity was a significant risk factor compared with nulliparity (RR 1.23 [95% CI 1.01, 1.50]; I2 = 0%). Women with pregnancy complications such as hypertensive disease (RR 1.38 [95% CI 1.32, 1.45]; I2 = 0%) and preterm delivery (<37 weeks) (RR 1.81 [95% CI 1.35, 2.43]; I2 = 0%) were more likely to develop type 2 diabetes in the future. There were no differences in gestational weight gain (mean difference −0.83 kg [95% CI −2.18, 0.51]; I2 = 65%) between the two groups (ESM Fig. 1).
Delivery and postpartum factors
The risk of developing type 2 diabetes was not associated with birthweight (RR 1.19 [95% CI 0.86, 1.58]; I2 = 60%), fetal macrosomia (RR 0.91 [95% CI 0.44, 1.86]) or breastfeeding (RR 0.77 [95% CI 0.48, 1.22]; I2 = 9%).
Subgroup analysis
There were significant between-group differences based on follow-up time (<1 year vs >1 year) for risk factors such as fasting glucose (p = 0.04), BMI (p = 0.03) and insulin use (p = 0.006) and type 2 diabetes. We did not observe any between-group differences based on ethnicity and GDM diagnostic criteria (fasting glucose level of <5.8 mmol/l or ≥5.8 mmol/l) for predictors such as maternal age, BMI, family history of diabetes, need for insulin in pregnancy and the risk of type 2 diabetes. There was a significant difference between subgroups based on strict criteria for excluding possible pre-existing type 2 diabetes for fasting glucose as a predictor of type 2 diabetes (p = 0.02), but no differences were observed for associations with other predictors (Table 1).
Table 1.
Subgroup analysis for progression to type 2 diabetes after GDM by follow-up time, ethnicity, timing of diagnosis and fasting glucose criteria for GDM diagnosis
| Risk factor | Follow-up | Ethnicity | Strict criteria used to exclude pre-existing type 2 diabetes?a | Fasting glucose for GDM diagnosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 year | ≥1 year | p value | White | Non-white | Mixed | p value | Yes | No | p value | <5.8 mmol/l | ≥5.8 mmol/l | p value | |
| Age | 1.27 [1.11, 1.45] | 1.14 [0.97, 1.32] | 0.29 | 1.29 [0.99, 1.64] | 1.19 [0.97, 1.41] | 1.20 [1.03, 1.39] | 0.85 | 1.11 [0.81, 1.45] | 1.15 [0.96, 1.38] | 0.79 | 1.47 [0.92, 2.17] | 1.15 [1.01, 1.29] | 0.32 |
| Fasting glucose | 4.13 [3.32, 4.59] | 3.13 [2.54, 3.65] | 0.04 | 4.13 [1.43, 4.91] | 3.34 [2.78, 3.83] | 3.38 [2.54, 4.03] | 0.80 | 2.40 [1.89, 2.94] | 3.48 [2.76, 4.04] | 0.02 | – | – | – |
| BMI | 1.56 [1.15, 2.04] | 2.20 [1.89, 2.51] | 0.03 | 2.11 [1.29, 3.05] | 2.06 [1.66, 2.51] | 1.74 [1.24, 2.33] | 0.63 | 1.95 [1.47, 2.47] | 2.38 [2.04, 2.74] | 0.18 | 1.98 [1.39, 2.60] | 1.87 [1.41, 2.40] | 0.81 |
| Family history | 1.63 [1.19, 2.24] | 1.74 [1.46, 2.07] | 0.72 | 1.49 [1.20, 1.85] | 1.78 [1.35, 2.35] | 1.95 [1.45, 2.62] | 0.31 | 1.80 [1.27, 2.54] | 1.73 [1.39, 2.15] | 0.86 | 1.83 [1.25, 2.66] | 1.72 [1.42, 2.09] | 0.78 |
| Insulin | 7.19 [4.14, 12.48] | 3.06 [2.39, 3.92] | 0.006 | 3.49 [2.25, 5.40] | 4.09 [2.27, 7.34] | 3.37 [2.43, 4.67] | 0.85 | 2.62 [1.70, 4.02] | 3.43 [2.62, 4.49] | 0.29 | 4.37 [2.46, 7.76] | 4.46 [3.63, 5.49] | 0.95 |
Data are RR (95% CIs)
aDiagnosis of GDM before 20 weeks of gestation or type 2 diabetes less than 1 year after delivery
Discussion
Summary of findings
The future risk of diabetes appears to be mainly influenced by the gestational glycaemic status, and not by the mother’s gestational weight gain or baby’s birthweight. We found that both hypertensive disorders in pregnancy and preterm delivery in GDM pregnancies were associated with future onset of type 2 diabetes, which was previously unknown. Factors specific to pregnancy such as gestational age at onset of GDM and general maternal characteristics such as BMI, ethnicity and family history were also associated with future onset of type 2 diabetes. This systematic review has thus collated the information necessary for postnatal counselling of women with GDM.
Strengths and limitations
To our knowledge, this is the first review to quantify the links between clinical characteristics (especially those relevant to pregnancy) and the future onset of diabetes in women with GDM. This systematic review used a prospective protocol and a comprehensive search without any language restrictions. Our meta-analysis included a large number of studies, and we were able to study most of the potentially relevant risk factors. Previous reviews were limited in their findings by the small number of studies included and the absence of summary estimates [2, 16, 18, 59]. We have provided clinically relevant estimates as RRs for use in counselling of women.
The studies included had varying definitions of population, risk factors and methods of ascertaining the outcome, which included different lengths of follow-up. Subgroup analysis was planned a priori to identify any sources of heterogeneity. It is possible that some studies could have misclassified pre-existing type 2 diabetes as GDM, especially when the diagnosis was made as early as 6 weeks after delivery. However, we did not observe significant between-group differences based on strict criteria for excluding pre-existing type 2 diabetes (studies that excluded women with GDM diagnosed within 20 weeks’ gestation or diabetes in the first year after delivery vs studies that did not). Some studies included only diet-controlled GDM, and this may have underestimated the risk of progression to type 2 diabetes.
The studies varied in the criteria used for diagnosing GDM and in the thresholds for commencing insulin in pregnancy; a third used Carpenter and Coustan criteria for diagnosing GDM. Our subgroup analysis was based on a fasting glucose cut-off value of 5.8 mmol/l: 69% of studies under this cut-off used Carpenter and Coustan criteria, and showed no significant differences in estimates for progression based on the criteria used for GDM diagnosis. Despite these variations, we consistently observed an increased risk of future diabetes in women with gestational hyperglycaemia.
We evaluated associations of individual predictors with outcomes, but could not adjust for confounding variables such as BMI because of the lack of access to individual data. The lack of detail in reported data made it difficult to evaluate the simultaneous influence of multiple factors on outcome. However, we could provide robust, precise estimates for individual risk factors that are relevant for providing postnatal information to women with GDM.
Comparison with existing evidence
In the non-pregnant state, BMI, family history of diabetes and ethnicity are associated with the risk of type 2 diabetes [18, 60–63]. We observed the same in mothers with GDM, and there was a greater risk of progression in non-white than in white women. Individual studies have shown that particular ethnic groups such as blacks and South Asians are at an increased risk [15, 34]. Owing to the various classifications of ethnicity used in primary studies, we were unable to provide progression rates for specific ethnic subgroups, which may have contributed to the heterogeneity.
We did not identify an increased risk for women with high weight gain during pregnancy. However, it is likely that women with severe GDM undergo intensive monitoring and receive significant input regarding their diet and lifestyles, thereby restricting their weight gain in pregnancy [50].
Although previous studies have shown an improvement in glucose homeostasis with breastfeeding [17, 64], we found no significant association between the absence of breast-feeding and progression to diabetes. This could be attributed to the imprecise estimates obtained owing to the small number of studies and individuals. Previous studies have shown improved glucose values on OGTTs, especially in the short term [64]. Our review measured the impact on type 2 diabetes and not on lesser degrees of glucose intolerance. Furthermore, the beneficial effect on glucose levels may not have been sustained in the long term. The rigour with which breast-feeding information was collected also varied by study; most data were self-reported by the women.
In current practice, the criteria used for diagnosing GDM are not applicable in many centres. However, given the long-term outcomes evaluated in our review, we were unable to assess for heterogeneity in estimates based on the WHO 2013 criteria for diagnosing GDM [65], which are recommended in many existing national and international guidelines.
Implications for clinical practice
Pregnancy is an important point in the life of a woman when she has regular contact with the healthcare system, thus providing opportunities to influence the future health of both mother and child. One of the major factors responsible for poor postnatal screening for diabetes and subsequent follow-up has been the lack of clear communication between secondary and primary care providers [10, 66]. Pregnancy-specific findings such as glycaemic control, despite being associated with a fourfold increase in the risk of future diabetes, are not taken into count during counselling.
Postnatal advice to women with GDM should incorporate information on their individual risk factors. Communication between hospitals and general practitioners on the mother’s risk of future diabetes could be improved by providing discharge summaries with pregnancy-specific details such as OGTT results, gestational age at GDM diagnosis, use of insulin, and complications such as pre-eclampsia and preterm birth. An efficient system of data linkage and communication between secondary and primary care providers will enable general practitioners to incorporate this information into their management of mothers; in particular, the management of women at a high-risk of developing type 2 diabetes could involve measures such as reminder systems [67]. Women are more likely to comply with diet and lifestyle changes if they know their individual risks of future diabetes [68].
Research recommendations
Current prediction models for type 2 diabetes should be updated and validated by including the factors identified in this review. There is a need for well-designed, prospective long-term studies to explore the association between breastfeeding and future type 2 diabetes [64]. The meta-analysis of individual participant data (IPD) could overcome many of the limitations of our meta-analysis [69]. A large-scale IPD meta-analysis could enable us to predefine the desired clinically relevant endpoints (e.g. timing of diabetes onset) and cut-off values for clinical variables, standardise the definitions of predictors and outcomes, take into account the performance of many candidate prognostic variables, directly handle missing data on both predictors and outcomes, and account for heterogeneity in baseline risks.
Conclusion
Postnatal counselling of women with GDM should be individualised for the risk of future diabetes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 149 kb)
(PDF 182 kb)
(PDF 181 kb)
(PDF 206 kb)
(PDF 51 kb)
Abbreviations
- GDM
Gestational diabetes mellitus
- IPD
Individual participant data
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Funding
This independent research was funded by European Union’s Seventh Framework Programme under EC-GA No. 278917 (GIFTS ‘Genomic and lifestyle predictors of fetal outcome relevant to diabetes and obesity and their relevance to prevention strategies in South Asian peoples’). The funding body played no role in designing or conducting the study or preparing the report.
Duality of interest statement
All authors completed the International Committee of Medical Journal Editors (ICMJE) uniform disclosure form (www.icmje.org/coi_disclosure.pdf) and declare that they have received no support from any organisation for the submitted work, have had no financial relationship over the last 3 years with any organisation that might have an interest in the submitted work, and have no other relationships or activities that could influence the submitted work.
Contribution statement
GAH and KSK contributed to the study design; GR and ST designed the protocol; AAH and GR selected eligible studies; GR developed the data extraction form; AAH and GR extracted the data; ST resolved discrepancies between reviewers on text selection and data extraction; GR, JZ and ST wrote the statistical analysis plan; GR cleaned and analysed the data; GR drafted the paper, AAH, GAH, JZ, KSK and ST revised the manuscript; and ST oversaw the conduct of study at all stages. All authors read and approved the final draft before submission. GR is the guarantor of this work.
References
- 1.Galtier F. Definition, epidemiology, risk factors. Diabetes Metab. 2010;36:628–651. doi: 10.1016/j.diabet.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 3.Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med. 2012;29:855–862. doi: 10.1111/j.1464-5491.2012.03698.x. [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence (2008) Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. Clinical Guideline 63. National Institute for Health and Care Excellence, London [PubMed]
- 5.Almario CV, Ecker T, Moroz LA, Bucovetsky L, Berghella V, Baxter JK. Obstetricians seldom provide postpartum diabetes screening for women with gestational diabetes. Am J Obstet Gynecol. 2008;198(528):e521–e525. doi: 10.1016/j.ajog.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara A, Peng T, Kim C. Trends in postpartum diabetes screening and subsequent diabetes and impaired fasting glucose among women with histories of gestational diabetes mellitus: a report from the Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care. 2009;32:269–274. doi: 10.2337/dc08-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker AM, Brody SC, Salisbury K, Schectman R, Hartmann KE. Postpartum glucose tolerance screening in women with gestational diabetes in the state of North Carolina. N C Med J. 2009;70:14–19. [PubMed] [Google Scholar]
- 8.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratner RE, Christophi CA, Metzger BE, et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774–4779. doi: 10.1210/jc.2008-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGovern A, Butler L, Jones S, et al. Diabetes screening after gestational diabetes in England: a quantitative retrospective cohort study. Br J Gen Pract. 2014;64:e17–e23. doi: 10.3399/bjgp14X676410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards AG, Naik G, Ahmed H, et al (2013) Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev (2):CD001865. doi:10.1002/14651858.CD001865.pub3 [DOI] [PMC free article] [PubMed]
- 12.Kengne AP, Beulens JW, Peelen LM, et al. Non-invasive risk scores for prediction of type 2 diabetes (EPIC-InterAct): a validation of existing models. Lancet Diabetes Endocrinol. 2014;2:19–29. doi: 10.1016/S2213-8587(13)70103-7. [DOI] [PubMed] [Google Scholar]
- 13.Kwak SH, Choi SH, Jung HS, et al. Clinical and genetic risk factors for type 2 diabetes at early or late post partum after gestational diabetes mellitus. J Clin Endocrinol Metab. 2013;98:E744–E752. doi: 10.1210/jc.2012-3324. [DOI] [PubMed] [Google Scholar]
- 14.Gobl CS, Bozkurt L, Prikoszovich T, Winzer C, Pacini G, Kautzky-Willer A. Early possible risk factors for overt diabetes after gestational diabetes mellitus. Obstet Gynecol. 2011;118:71–78. doi: 10.1097/AOG.0b013e318220e18f. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Chen L, Horswell R, et al. Racial differences in the association between gestational diabetes mellitus and risk of type 2 diabetes. J Womens Health (Larchmt) 2012;21:628–633. doi: 10.1089/jwh.2011.3318. [DOI] [PubMed] [Google Scholar]
- 16.Golden SH, Bennett WL, Baptist-Roberts K, et al. Antepartum glucose tolerance test results as predictors of type 2 diabetes mellitus in women with a history of gestational diabetes mellitus: a systematic review. Gend Med. 2009;6(Suppl 1):109–122. doi: 10.1016/j.genm.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Gunderson EP, Hedderson MM, Chiang V, et al. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: the SWIFT cohort. Diabetes Care. 2012;35:50–56. doi: 10.2337/dc11-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baptiste-Roberts K, Barone BB, Gary TL, et al. Risk factors for type 2 diabetes among women with gestational diabetes: a systematic review. Am J Med. 2009;122:207–214.e204. doi: 10.1016/j.amjmed.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung NW, Helmink D. Gestational diabetes: the significance of persistent fasting hyperglycemia for the subsequent development of diabetes mellitus. J Diabetes Complicat. 2006;20:21–25. doi: 10.1016/j.jdiacomp.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Oldfield MD, Donley P, Walwyn L, Scudamore I, Gregory R. Long term prognosis of women with gestational diabetes in a multiethnic population. Postgrad Med J. 2007;83:426–430. doi: 10.1136/pgmj.2006.056267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tura A, Grassi A, Winhofer Y, et al. Progression to type 2 diabetes in women with former gestational diabetes: time trajectories of metabolic parameters. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clin Res Ed) 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells G, Shea B, O’Connell D et al The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Available from www.ohri.ca/programs/clinical_epidemiology/oxford.asp, accessed 20 Sept 2014
- 24.Wilson A, Lissauer D, Thangaratinam S, Khan KS, MacArthur C, Coomarasamy A. A comparison of clinical officers with medical doctors on outcomes of caesarean section in the developing world: meta-analysis of controlled studies. BMJ (Clin Res Ed) 2011;342:d2600. doi: 10.1136/bmj.d2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19:3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::AID-SIM784>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 26.Cochrane (2012) Review Manager (RevMan) version 5.2. The Nordic Cochrane Centre, Copenhagen
- 27.Lin PC, Hung CH, Huang RD, Chan TF. Predictors of type 2 diabetes among Taiwanese women with prior gestational diabetes mellitus. Jpn J Nurs Sci. 2015;13:3–9. doi: 10.1111/jjns.12077. [DOI] [PubMed] [Google Scholar]
- 28.Eades CE, Styles M, Leese GP, Cheyne H, Evans JM. Progression from gestational diabetes to type 2 diabetes in one region of Scotland: an observational follow-up study. BMC Pregnancy Childbirth. 2015;15:11. doi: 10.1186/s12884-015-0457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao W, Yeung E, Tobias DK, et al. Long-term risk of type 2 diabetes mellitus in relation to BMI and weight change among women with a history of gestational diabetes mellitus: a prospective cohort study. Diabetologia. 2015;58:1212–1219. doi: 10.1007/s00125-015-3537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carvalho Ribeiro AM, Nogueira-Silva C, Melo-Rocha G, Pereira ML, Rocha A. Gestational diabetes: determination of risk factors to diabetes mellitus. Rev Port Endocrinol Diabetes Metab. 2015;10:8–13. [Google Scholar]
- 31.Feig DS, Shah BR, Lipscombe LL, et al. Preeclampsia as a risk factor for diabetes: a population-based cohort study. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chew WF, Rokiah P, Chan SP, Chee WSS, Lee LF, Chan YM. Prevalence of glucose intolerance, and associated antenatal and historical risk factors among Malaysian women with a history of gestational diabetes mellitus. Singap Med J. 2012;53:814–820. [PubMed] [Google Scholar]
- 33.Mukerji G, Chiu M, Shah BR. Impact of gestational diabetes on the risk of diabetes following pregnancy among Chinese and South Asian women. Diabetologia. 2012;55:2148–2153. doi: 10.1007/s00125-012-2549-6. [DOI] [PubMed] [Google Scholar]
- 34.Xiang AH, Li BH, Black MH, et al. Racial and ethnic disparities in diabetes risk after gestational diabetes mellitus. Diabetologia. 2011;54:3016–3021. doi: 10.1007/s00125-011-2330-2. [DOI] [PubMed] [Google Scholar]
- 35.Ekelund M, Shaat N, Almgren P, Groop L, Berntorp K. Prediction of postpartum diabetes in women with gestational diabetes mellitus. Diabetologia. 2010;53:452–457. doi: 10.1007/s00125-009-1621-3. [DOI] [PubMed] [Google Scholar]
- 36.Ogonowski J, Miazgowski T. The prevalence of 6 weeks postpartum abnormal glucose tolerance in Caucasian women with gestational diabetes. Diabetes Res Clin Pract. 2009;84:239–244. doi: 10.1016/j.diabres.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Hossein-nezhad A, Mirzaei K, Maghbooli Z, Larijani B. Maternal glycemic status in GDM patients after delivery. Iran J Diabetes Lipid Disord. 2009;8:95–104. [Google Scholar]
- 38.Russell C, Dodds L, Armson BA, Kephart G, Joseph KS. Diabetes mellitus following gestational diabetes: role of subsequent pregnancy. BJOG. 2008;115:253–259. doi: 10.1111/j.1471-0528.2007.01459.x. [DOI] [PubMed] [Google Scholar]
- 39.Rivero K, Portal VL, Vieira M, Behle I. Prevalence of the impaired glucose metabolism and its association with risk factors for coronary artery disease in women with gestational diabetes. Diabetes Res Clin Pract. 2008;79:433–437. doi: 10.1016/j.diabres.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Krishnaveni GV, Hill JC, Veena SR, et al. Gestational diabetes and the incidence of diabetes in the 5 years following the index pregnancy in South Indian women. Diabetes Res Clin Pract. 2007;78:398–404. doi: 10.1016/j.diabres.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zonenberg A, Telejko B, Topolska J, et al. Factors predisposing to disturbed carbohydrate tolerance in patients with previous gestational diabetes mellitus. Diabetol Dosw Klin. 2006;6:143–150. [Google Scholar]
- 42.Weijers RNM, Bekedam DJ, Goldschmidt HMJ, Smulders YM. The clinical usefulness of glucose tolerance testing in gestational diabetes to predict early postpartum diabetes mellitus. Clin Chem Lab Med. 2006;44:99–104. doi: 10.1515/CCLM.2006.019. [DOI] [PubMed] [Google Scholar]
- 43.Kousta E, Efstathiadou Z, Lawrence NJ, et al. The impact of ethnicity on glucose regulation and the metabolic syndrome following gestational diabetes. Diabetologia. 2006;49:36–40. doi: 10.1007/s00125-005-0058-6. [DOI] [PubMed] [Google Scholar]
- 44.Kim SH, Kim MY, Yang JH, et al. Nutritional risk factors of early development of postpartum prediabetes and diabetes in women with gestational diabetes mellitus. Nutrition. 2011;27:782–788. doi: 10.1016/j.nut.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Jarvela IY, Juutinen J, Koskela P, et al. Gestational diabetes identifies women at risk for permanent type 1 and type 2 diabetes in fertile age: predictive role of autoantibodies. Diabetes Care. 2006;29:607–612. doi: 10.2337/diacare.29.03.06.dc05-1118. [DOI] [PubMed] [Google Scholar]
- 46.Hunger-Dathe W, Mosebach N, Samann A, Wolf G, Muller UA. Prevalence of impaired glucose tolerance 6 years after gestational diabetes. Exp Clin Endocrinol Diabetes. 2006;114:11–17. doi: 10.1055/s-2005-873015. [DOI] [PubMed] [Google Scholar]
- 47.Cho NH, Jang HC, Park HK, Cho YW. Waist circumference is the key risk factor for diabetes in Korean women with history of gestational diabetes. Diabetes Res Clin Pract. 2006;71:177–183. doi: 10.1016/j.diabres.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Lin CH, Wen SF, Wu YH, Huang YY, Huang MJ. The postpartum metabolic outcome of women with previous gestational diabetes mellitus. Chang Gung Med J. 2005;28:794–800. [PubMed] [Google Scholar]
- 49.Schaefer-Graf UM, Buchanan TA, Xiang AH, Peters RK, Kjos SL. Clinical predictors for a high risk for the development of diabetes mellitus in the early puerperium in women with recent gestational diabetes mellitus. Am J Obstet Gynecol. 2002;186:751–756. doi: 10.1067/mob.2002.121895. [DOI] [PubMed] [Google Scholar]
- 50.Dalfra MG, Lapolla A, Masin M, et al. Antepartum and early postpartum predictors of type 2 diabetes development in women with gestational diabetes mellitus. Diabetes Metab. 2001;27:675–680. [PubMed] [Google Scholar]
- 51.Pallardo F, Herranz L, Garcia-Ingelmo T, et al. Early postpartum metabolic assessment in women with prior gestational diabetes. Diabetes Care. 1999;22:1053–1058. doi: 10.2337/diacare.22.7.1053. [DOI] [PubMed] [Google Scholar]
- 52.Buchanan TA, Xiang AH, Kjos SL, Trigo E, Lee WP, Peters RK. Antepartum predictors of the development of type 2 diabetes in Latino women 11–26 months after pregnancies complicated by gestational diabetes. Diabetes. 1999;48:2430–2436. doi: 10.2337/diabetes.48.12.2430. [DOI] [PubMed] [Google Scholar]
- 53.Steinhart JR, Sugarman JR, Connell FA. Gestational diabetes is a herald of NIDDM in Navajo women. High rate of abnormal glucose tolerance after GDM. Diabetes Care. 1997;20:943–947. doi: 10.2337/diacare.20.6.943. [DOI] [PubMed] [Google Scholar]
- 54.Greenberg LR, Moore TR, Murphy H. Gestational diabetes mellitus: antenatal variables as predictors of postpartum glucose intolerance. Obstet Gynecol. 1995;86:97–101. doi: 10.1016/0029-7844(95)00103-X. [DOI] [PubMed] [Google Scholar]
- 55.Damm P, Kuhl C, Bertelsen A, Molsted-Pedersen L. Predictive factors for the development of diabetes in women with previous gestational diabetes mellitus. Am J Obstet Gynecol. 1992;167:607–616. doi: 10.1016/S0002-9378(11)91559-2. [DOI] [PubMed] [Google Scholar]
- 56.Liu H, Zhang C, Zhang S, et al. Prepregnancy body mass index and weight change on postpartum diabetes risk among gestational diabetes women. Obesity. 2014;22:1560–1567. doi: 10.1002/oby.20722. [DOI] [PubMed] [Google Scholar]
- 57.Capula C, Chiefari E, Vero A, Foti DP, Brunetti A, Vero R. Prevalence and predictors of postpartum glucose intolerance in Italian women with gestational diabetes mellitus. Diabetes Res Clin Pract. 2014;105:223–230. doi: 10.1016/j.diabres.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Bentley-Lewis R, Dawson DL, Wenger JB, Thadhani RI, Roberts DJ. Placental histomorphometry in gestational diabetes mellitus: the relationship between subsequent type 2 diabetes mellitus and race/ethnicity. Am J Clin Pathol. 2014;141:587–592. doi: 10.1309/AJCPX81AUNFPOTLL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 60.Garber AJ (2012) Obesity and type 2 diabetes: which patients are at risk? Diabetes Obes Metab14:399–408 [DOI] [PubMed]
- 61.Bakker LE, Sleddering MA, Schoones JW, Meinders AE, Jazet IM. Pathogenesis of type 2 diabetes in South Asians. Eur J Endocrinol. 2013;169:R99–R114. doi: 10.1530/EJE-13-0307. [DOI] [PubMed] [Google Scholar]
- 62.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes. 2000;49:2201–2207. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- 63.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 64.Much D, Beyerlein A, Rossbauer M, Hummel S, Ziegler AG. Beneficial effects of breastfeeding in women with gestational diabetes mellitus. Mol Metab. 2014;3:284–292. doi: 10.1016/j.molmet.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.WHO . Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 66.Pierce M, Modder J, Mortagy I, Springett A, Hughes H, Baldeweg S. Missed opportunities for diabetes prevention: post-pregnancy follow-up of women with gestational diabetes mellitus in England. Br J Gen Pract. 2011;61:e611–e619. doi: 10.3399/bjgp11X601316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Middleton P, Crowther CA (2014) Reminder systems for women with previous gestational diabetes mellitus to increase uptake of testing for type 2 diabetes or impaired glucose tolerance. Cochrane Database Syst Rev (3):CD009578. doi: 10.1002/14651858.CD009578.pub2 [DOI] [PMC free article] [PubMed]
- 68.Kim C, McEwen LN, Piette JD, Goewey J, Ferrara A, Walker EA. Risk perception for diabetes among women with histories of gestational diabetes mellitus. Diabetes Care. 2007;30:2281–2286. doi: 10.2337/dc07-0618. [DOI] [PubMed] [Google Scholar]
- 69.Lambert PC, Sutton AJ, Abrams KR, Jones DR. A comparison of summary patient-level covariates in meta-regression with individual patient data meta-analysis. J Clin Epidemiol. 2002;55:86–94. doi: 10.1016/S0895-4356(01)00414-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 149 kb)
(PDF 182 kb)
(PDF 181 kb)
(PDF 206 kb)
(PDF 51 kb)