Abstract
Although melanoma is an aggressive cancer, the understanding of the virulence-conferring pathways involved remains incomplete. We have demonstrated that loss of ten-eleven translocation methylcytosine dioxygenase (TET2)-mediated 5-hydroxymethylcytosine (5-hmC) is an epigenetic driver of melanoma growth and a biomarker of clinical virulence. We also have determined that the intermediate filament protein nestin correlates with tumorigenic and invasive melanoma growth. Here we examine the relationships between these two biomarkers. Immunohistochemistry staining of nestin and 5-hmC in 53 clinically annotated primary and metastatic patient melanomas revealed a significant negative correlation. Restoration of 5-hmC, as assessed in a human melanoma cell line by introducing full-length TET2 and TET2-mutated constructs, decreased nestin gene and protein expression in vitro. Genome-wide mapping using hydroxymethylated DNA immunoprecipitation sequencing disclosed significantly less 5-hmC binding in the 3′ untranslated region of the nestin gene in melanoma compared to nevi, and 5-hmC binding in this region was significantly increased after TET2 overexpression in human melanoma cells in vitro. Our findings provide evidence suggesting that nestin regulation is negatively controlled epigenetically by TET2 via 5-hmC binding at the 3′ untranslated region of the nestin gene, providing one potential pathway for understanding melanoma growth characteristics. Studies are now indicated to further define the interplay between 5-hmC, nestin expression, and melanoma virulence.
Although human melanoma is a paradigm for cancer virulence, biomarkers that portend biological outcome are scarce, and the understanding of the underlying pathogenic mechanisms of tumorigenesis, invasion, and metastasis is incomplete. Recently, we defined two potential pathways and associated biomarkers that correlate with tumorigenic potential in melanoma. The first is global loss of 5-hydroxymethylcytosine (5-hmC), an epigenetic mark regulated by the ten-eleven translocation methylcytosine dioxygenase (TET2) pathway. This event is associated with increased tumorigenesis in experimental models1 and is a biomarker of melanocytic dysplasia2 and malignant transformation.3 Also associated with experimental tumorigenesis in melanoma is the expression of nestin,4 an intermediate filament protein considered to correlate with stem-cell activity and also to represent a biomarker of cell behavior that contributes to melanoma virulence.5 Although both observations indicate participation of these two pathways in tumorigenic melanoma growth, the possibility of a mechanistic interrelationship between them has not, until now, been explored.
There are reasons to posit that epigenetic pathways may play regulatory roles in the expression of physiological and cancer stem cell–associated proteins such as nestin. For example, regulation of the nestin gene during embryonic differentiation has been shown to relate to histone acetylation patterns.6 Recently, adipose tissue–derived stem cells with the potential to become neuronal elements have been shown to be epigenetically prepatterned to differentiate toward neuronal lineage, a process that is enhanced by demethylation of nestin-enhancer elements.7 Several studies have shown that miRNA regulates nestin expression via progenitor-cell differentiation during development.8, 9, 10, 11 In addition, miR-940 inhibits nestin expression and suppresses tumorigenesis by binding nestin 3′ untranslated region (UTR) in nasopharyngeal carcinoma.12 Accordingly, based on the concordant effects of high nestin and low 5-hmC levels in melanoma tumorigenesis, and the known role of epigenetic programing in the regulation of nestin expression during development, we hypothesized a possible mechanistic link between these two virulence-conferring mediators in human melanoma.
In this study, we initially examined patient melanomas to determine whether relationships existed between nestin and 5-hmC immunohistochemistry (IHC) staining patterns. Our findings led to transfection approaches and genomic sequencing that now provide the first evidence of regulatory interactions between DNA hydroxymethylation and nestin expression in melanoma. Because epigenetic events are reversible, these data have potential translational as well as basic biological and biomarker implications.
Materials and Methods
Histopathologic Samples and Data
A total of 53 cases of primary cutaneous and metastatic melanomas were selected from a tissue microarray previously constructed from the Archives of Pathology Department, Clinical Hospital, State University of Campinas (Campinas, Brazil). These cases were intentionally selected to investigate the biomarker characteristics of both primary and metastatic melanoma. The hematoxylin and eosin–stained sections, prior diagnoses, and reported prognostic attributes were independently reviewed and confirmed by two dermatopathologists (R.S., C.G.L.). Clinical data regarding each patient's age, sex, tumor location, Breslow thickness, ulceration, mitosis, tumor stage, tumor recurrence, and follow-up including overall survival were retrieved from medical files. Specific survival time in each case was determined by the time difference between the date of diagnosis and the date of either death (92.3%) or last follow-up (7.7%). The study protocol was approved by the Committee of Ethics at the State University of Campinas and the Institutional Review Board at Brigham and Women's Hospital (Boston, MA).
IHC and Immunofluorescence Analysis
IHC and immunofluorescence staining was performed on sections (5 μm) of paraffin-embedded tissue. Sections were treated with heat-induced antigen retrieval using target retrieval solution (Dako, Carpinteria, CA) and heated in a Pascal-pressurized heating chamber (Dako; 125°C for 30 seconds, 90°C for 10 seconds). After incubation at 4°C overnight either with human nestin antibody (Millipore, Billerica, MA; 1:200 dilution) or rabbit–anti-5-hmC (Active Motif, Carlsbad, CA; 1:2000 dilution), sections were incubated with horseradish peroxidase–conjugated secondary antibodies for 30 minutes at room temperature, and signals were visualized with NovaRED horseradish peroxidase substrate (Vector Laboratories, Burlingame, CA), followed by a hematoxylin counterstain. In addition, dual-labeling immunofluorescence was performed to complement IHC analysis as a means of two-channel identification of epitopes, with cytoplasm staining for nestin and nuclear staining for 5-hmC. Instead of incubation with the secondary antibodies, these sections were incubated with a mixture of goat anti-rabbit IgG (Alexa Fluor 594-red; InvitroGen, Grand Island, NY) and goat anti-mouse IgG (Alexa Fluor 488-green; InvitroGen). Appropriate isotype-matched antibody controls and tissue controls were used for all experiments. All specimens were evaluated separately according to the 0-to-4+ grading criteria based on the percentage of positive cell counts. Intensity of staining was determined on a semiquantitative scale of 0 to 3+: no staining (0), weakly positive staining (1+), moderately positive staining (2+), and strongly positive staining (3+). The entire area of two replicate 2-mm tissue microarray cores (6.28 mm2) was assessed. An immunoreactive score was derived by multiplying the percentage of positive cells by the staining intensity.2
Cell Lines and Quantitative RT-PCR
Human melanoma cell lines A2058 and Meljuso were originally obtained from ATCC (Manassas, VA). Cells were recently confirmed to have no Mycoplasma contamination by PCR. All cells were grown in Dulbecco's modified Eagle's medium (Lonza, Hopkinton, MA). Culture media were supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT), 200 mmol/L l-glutamine, 100 IU/mL penicillin, and 100 mg/mL streptomycin (Life Technologies, Carlsbad, CA) and maintained at 37°C, 5% CO2. If not otherwise stated, subconfluent cell culture was treated with 0.25% trypsin/EDTA solution (HyClone) at 37°C for 1 to 2 minutes and then collected for RNA extraction. Total RNA of melanoma cells was extracted using the RNAeasy kit (Qiagen, Valencia, CA), and the cDNAs were synthesized using the SuperScript III First-Strand kit (InvitroGen). Relative gene expression was normalized to hypoxanthine-guanine phosphoribosyltransferase. Melanoma stable cell lines with overexpressing flag-tagged full-length wild-type TET2 (TET2-OE) or the iron-binding site (H1382RD1384) disrupted catalytically inactive TET2-mutant (TET2-M) control cell line was established as described.1 The overexpression of full-length TET2 and TET2-M control was verified by Western blot and quantitative RT-PCR, according to a standard protocol described by Lian et al.1
Hydroxymethylated DNA Immunoprecipitation Sequencing
Hydroxymethylated DNA immunoprecipitation sequencing (hMeDIP-seq) experiment was performed on human nevi, melanomas, and cultured human melanoma cell lines as previously described by Lian et al.1 Barcode adapters (Illumina, San Diego, CA) were ligated before hMeDIP-seq. Genomic DNA from human melanomas and nevi (n = 5) was purified and sonicated. Illumina barcode adapters were ligated before hMeDIP-seq. Adaptor-ligated DNA (5 mg) was denatured and incubated with 3 mL of 5-hmC antibody (Active Motif, Carlsbad, CA) at 4°C overnight. Antibody–DNA complexes were captured by protein A/G beads. The immunoprecipitated DNA was purified and sequenced followed by standard Illumina protocols. Read sequences were mapped to the human genome (hg) 19 using Eland software version 2 in the Secondary Analysis Package Casava software package version 1.6 (Illumina). Significantly enriched regions were determined using the Model-Based Analysis of ChIP-Seq data software package version 1.4.2 (MACS, http://liulab.dfci.harvard.edu/MACS, last accessed December 31, 2015). Gene Ontology term and Kyoto Encyclopedia of Genes and Genomes pathway analyses were performed by the Database for Annotation, Visualization, and Integrated Discovery (Laboratory of Immunopathogenesis and Bioinformatics, Frederick, MD).
Statistical Analysis
The U-test was used for verifying the association between continuous data and categorical variables with two categories, and for variables with three categories (T stage) the Kruskal-Wallis test was adopted. To verify normal distribution of continuous variables, the Shapiro-Wilk test was conducted. The Spearman correlation was applied to verify the correlation between continuous variables. The Kaplan-Meier curve was used for estimating the overall survival probability, and the log-rank test was used for comparing survival curves. The follow-up time was considered the period (in months) between the date of surgery or treatment until the death date or the last date with information for censored cases (survivors or those lost to follow-up). The level of 5% was considered significant in all statistical tests. The univariate Cox regression model was used for estimating hazard ratios and respective 95% CIs. Statistical computer Stata software version 10.0 (StataCorp, College Station, TX) was used for conducting all statistical analysis.
Results
5-hmC Levels and Nestin Expression in Human Primary Melanomas
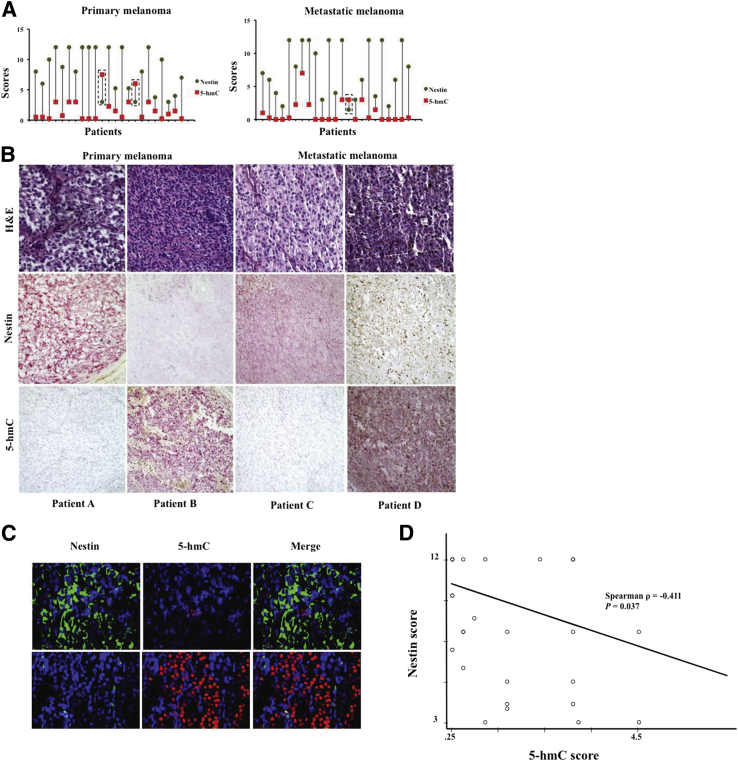
Fifty-three representative cases from primary cutaneous (n = 26) and metastatic (n = 27) melanomas were included in this study. The relevant clinical and pathologic parameters, including follow-up data, are summarized in Table 1 for primary cutaneous melanoma and metastatic melanoma. Nestin and 5-hmC immunostaining was scored semiquantitatively and assigned by percentage and intensity,1, 13 with nestin reactivity typically identified as diffuse or peripheral cytoplasmic staining, and 5-hmC reactivity identified as nuclear staining. Immunoreactivity for nestin scored between 3 and 12 (mean, 8); 5-hmC, between 0.25 and 4.5 (mean, 1.25) (Supplemental Table S1). Notably, in individual tumors, nestin scores exceeded 5-hmC scores in 90.6% of samples; the remaining five samples showed either similar scores (n = 2) or reciprocal scores (higher 5-hmC and lower nestin; n = 3). Figure 1A provides plots of the relationship of nestin expression to 5-hmC levels using this approach, as well as examples of high nestin/low 5-hmC, and high 5-hmC/low nestin patient tumors (Figure 1B). Dual labeling confirmed the reciprocal relationship of nestin expression to 5-hmC levels that was evident in the majority of the tumors studied (Figure 1C). Spearman correlation of the immunoreactivity of nestin expression with 5-hmC levels indicated a statistically significant negative correlation between nestin and 5-hmC levels in PCM cases (P = 0.037) (Figure 1D), suggesting a possible regulatory interaction [a nonsignificant negative correlation was also noted in metastatic melanoma (P = 0.234)].
Table 1.
Clinicopathologic Characteristics of Primary Cutaneous Melanomas and Metastatic Melanomas, Respectively
| Variable | Category | Value |
|---|---|---|
| Primary cutaneous melanomas (n = 26) | ||
| Age, years | Range | 36–81 |
| Median | 58.0 | |
| Mean (SD) | 59.4 (11.9) | |
| Sex, n (%) | Female | 7 (26.9) |
| Male | 19 (73.1) | |
| Histologic subtype, n (%) | SSM | 12 (46.2) |
| LMM | 3 (11.5) | |
| ALM | 3 (11.5) | |
| NM | 8 (30.8) | |
| Ulceration, n (%) | Absent | 4 (16.0) |
| Present | 21 (84.0) | |
| Breslow's thickness, mm | Range | 2–16 |
| Median | 3.9 | |
| Mean (SD) | 4.6 (3.1) | |
| Mitotic index, mm2 | Range | 1–20 |
| Median | 5.0 | |
| Mean (SD) | 6.4 (5.5) | |
| T stage, n (%) | II | 5 (19.2) |
| III | 16 (61.5) | |
| IV | 5 (19.2) | |
| Site, n (%) | Head and neck | 6 (23.1) |
| Upper limbs | 6 (23.1) | |
| Lower limbs | 6 (23.1) | |
| Trunk | 8 (30.7) | |
| Follow-up time, months | Range | 4.0–188.5 |
| Median | 24.6 | |
| Mean (SD) | 41.3 (43.9) | |
| Follow-up status, n (%) | Survivor (NSR) | 9 (34.6) |
| Survivor with melanoma | 4 (15.4) | |
| Nonsurvivor (melanoma) | 6 (23.1) | |
| Nonsurvivor (other causes) | 5 (19.2) | |
| Lost to follow-up | 2 (7.7) | |
| Relapse, n (%) | No | 15 (57.7) |
| Yes | 11 (42.3) | |
| Time to relapse, months | Range | 0.33–92.5 |
| Median | 22.7 | |
| Mean (SD) | 27.4 (29.0) | |
| Metastatic melanomas (n = 27) | ||
| Age, years | Range | 30–88 |
| Median | 64.0 | |
| Mean (SD) | 63.2 (17.1) | |
| Sex, n (%) | Female | 16 (59.3) |
| Male | 11 (40.7) | |
| Metastases site, n (%) | Lymph node | 14 (51.9) |
| S.c. | 8 (29.6) | |
| Brain | 1 (3.7) | |
| Bone | 1 (3.7) | |
| Other visceral | 3 (11.1) | |
| Follow-up time, months | Range | 0.033–109.2 |
| Median | 13.1 | |
| Mean (SD) | 24.4 (26.2) | |
| Follow-up status, n (%) | Survivor | 11 (40.7) |
| Nonsurvivor | 13 (48.2) | |
| Lost to follow-up | 3 (11.1) |
ALM, acral lentiginous melanoma; LMM, lentigo malign melanoma; NM, nodular melanoma; NSR, no sign of recurrence; SSM, superficial spread melanoma.
Figure 1.
Relationship of 5-hydroxymethylcytosine (5-hmC) and nestin reactivity in primary and metastatic human melanomas. A: Line diagrams represent relative biomarker scores; the majority cases show patterns of higher nestin and lower 5-hmC. Three cases show inversion of these patterns (dashed boxes). B: Representative IHC staining for 5-hmC and nestin in primary and metastatic human melanomas; the patients in A and C show the dominant pattern of disparity between high nestin and low 5-hmC, and the patients in B and D represent the reciprocal pattern. C: Representative immunofluorescence staining demonstrating the trend for higher nestin and lower 5-hmC levels in primary and metastatic human melanomas. Red indicates nuclear 5-hmC; green, cytoplasmic nestin in green; blue nuclear stain, DAPI. D: Spearman correlation of the immunoreactivity of 5-hmC and nestin expression. P = 0.037; ρ = −0.411 in primary melanoma cases (D). Original magnification, ×40. H&E, hematoxylin and eosin.
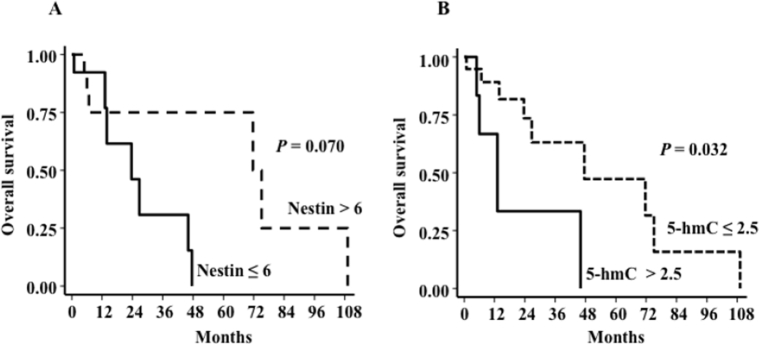
Nestin expression in primary cutaneous melanoma failed to correlate with the respective annotated prognostic attributes (Breslow depth, mitoses, ulceration, tumor stage); 5-hmC staining showed statistically significant inverse correlation with Breslow depth (P = 0.042) (Supplemental Figure S1; Supplemental Tables S2 and S3). We further analyzed by Kaplan-Meier the association between nestin and 5-hmC scores and overall survival. 5-hmC scores greater than the mean (2.5) for metastatic melanoma, but not for primary cutaneous melanoma, showed significantly greater survival (P = 0.032) (Supplemental Figure S2; Supplemental Tables S4–S6). These results suggest that the biological effects of low levels of DNA-hydroxymethylated 5-hmC, as assessed by significant correlation with prognostic and outcome attributes, may have a broader impact on melanoma virulence than does nestin expression itself, in keeping with previous findings that the extent of nestin expression alternatively regulates both virulence-conferring attributes of tumorigenic growth and invasion.4
TET2-Mediated 5-hmC Regulates Melanoma Nestin Gene and Protein Expression
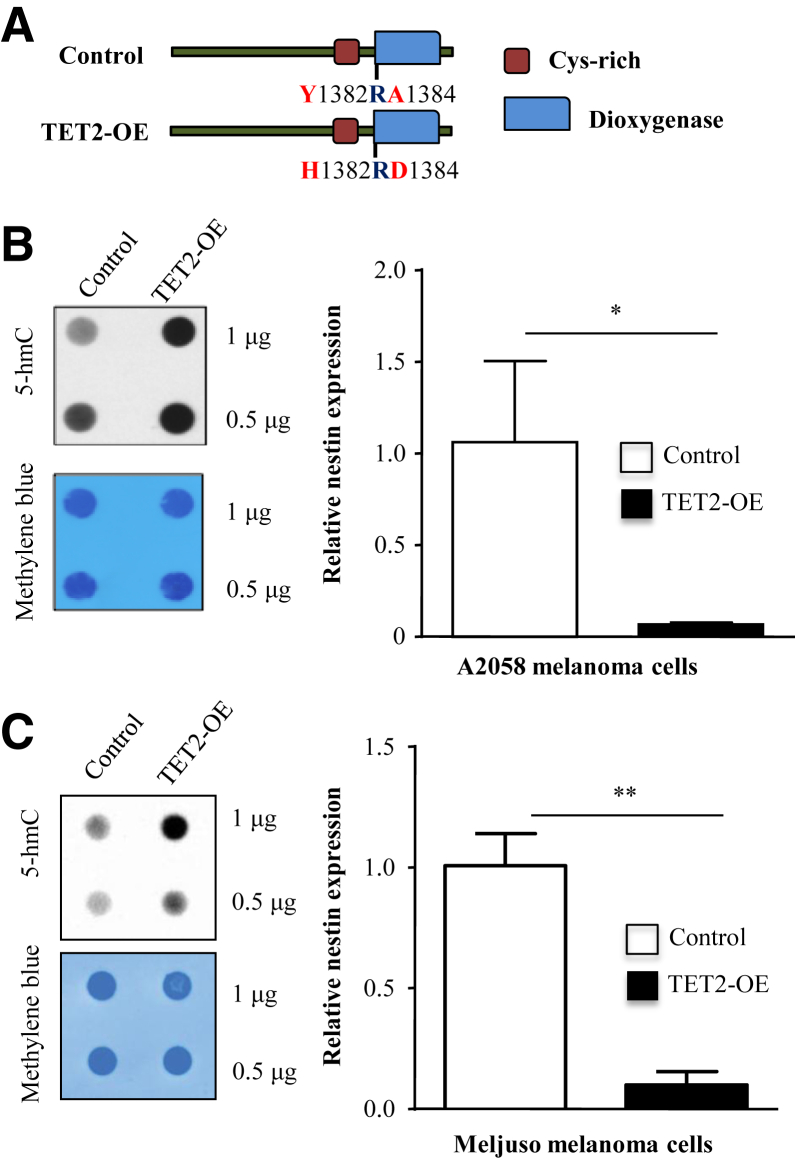
Because 5-hmC and nestin exhibited significant inversion in expression patterns, we next investigated the possible mechanistic link between these two pathways. To determine whether restoration of the 5-hmC landscape can modify nestin in human melanoma, we reintroduced TET2, the most dramatically decreased TET-family gene in human melanomas,1, 14, 15 to A2058 stable cell lines that constitutively express low 5-hmC. The selected cells were further serially diluted, and the expression of full-length TET2 (TET2-OE), or an enzymatically inactive TET2-mutant control (Figure 2A), was verified by quantitative RT-PCR. Increase in 5-hmC level by full-length TET2 was confirmed by Western blot, as previously described.1 Restoration of the 5-hmC landscape via TET2 transfection, but not by control, resulted in significantly decreased nestin mRNA expression, consistent with 5-hmC–mediated nestin regulation (Figure 2B). Nestin gene expression was significantly decreased in Meljuso TET2-OE cell lines compared to the control cells (Figure 2C).
Figure 2.
Effects of ten-eleven translocation methylcytosine dioxygenase (TET2)-mediated 5-hydroxymethylcytosine (5-hmC) on nestin gene and protein expression in cultured melanoma cells (A2058 and Meljuso). A: Schematic diagram of melanoma cell line transfected with full-length TET2 or enzymatically inactive TET2 mutant control. B: Up-regulation of 5-hmC by full-length TET2 in A2058 cells but not by mutant TET2 control was confirmed by immune dot blot. The methylene blue staining was used as a total genomic DNA loading control. RT-PCR shows a significant decrease in nestin mRNA expression as a result of TET2 overexpression. C: Significant decrease in nestin gene expression is also observed in Meljuso TET2 overexpression (OE) cell lines compared to the control cells by RT-PCR. Data are expressed as means ± SEM. n = 3 (B and C). ∗P < 0.05, ∗∗P < 0.01.
5-hmC Binding Is Associated with the Nestin 3′ UTR
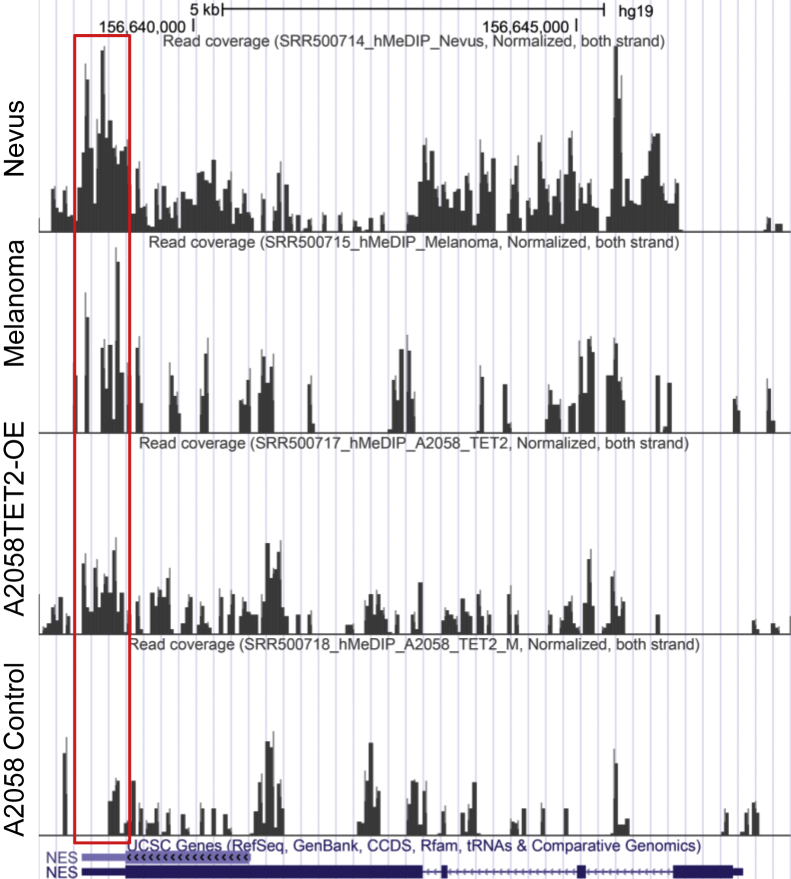
We employed genome-wide mapping of 5-hmC in human melanomas and nevi with specific reference to the nestin gene. hMeDIP-seq of pooled DNA from patient melanomas (n = 5) and melanocytic nevi (n = 5) indicated significantly less 5-hmC binding specifically at the 3′ UTR of the nestin gene of melanomas as compared to nevi (Figure 3). There was a significant increase in 5-hmC binding in nestin 3′ UTR in TET2-OE A2058 as compared to the TET2-M control melanoma cell line (Figure 3). These findings are suggestive of the potential association of DNA hydroxymethylation with nestin gene expression.
Figure 3.
Genome-wide mapping of the nestin gene using hMeDIP-seq for 5-hydroxymethylcytosine (5-hmC) in human benign nevi and melanomas, as well as human melanoma cell lines with ten-eleven translocation methylcytosine dioxygenase (TET2) overexpression (A2058 TET2-OE) and A2058 control cells. The distribution of 5-hmC revealed more abundant binding in the nestin 3′ untranslated region (red box) in nevi and A2058 TET2-OE melanoma cells compared to those of melanomas and control A2058 melanoma cells, respectively. CCDS, consensus CDS; hg, human genome; NES, nestin gene; UCSC, University of California at Santa Cruz.
Discussion
In this study, we show that there is a significant negative relationship between the expression of the intermediate-filament protein nestin and the epigenetic mark 5-hmC in human melanoma. Because high nestin expression and low 5-hmC levels are both known to be associated with tumorigenic growth and clinical virulence,1, 4, 16 a negative relationship may be anticipated and need not imply mechanistic linkage. However, the negative correlation reached statistical significance in primary cutaneous melanoma samples. Recent availability of MeDIP-seq and hMeDIP-seq has made the global mapping of DNA methylation and hydroxymethylation possible. Such studies show that the binding of 5-mC and 5-hmC are important for gene regulation not only when they bind to the promoter region, but also when they bind to the gene body as well as at 3′ UTR. In our study, we did not observe a significant difference in 5-hmC binding patterns on the promoter region of the nestin gene between nevi and melanomas. Interestingly, in the course of genomic sequencing studies, we found 5-hmC binding to be in significant abundance in the nestin 3′ UTR in benign nevi but not in melanoma. This finding prompted us to determine the effects of 5-hmC rescue on nestin expression in a relevant human melanoma line in which 5-hmC was restored by the overexpression of TET2. We found that 5-hmC restoration indeed increased 5-hmC binding in the nestin 3′ UTR region and depressed the expression of nestin mRNA, providing additional evidence that nestin expression in melanoma is under some degree of potentially reversible epigenetic control.8, 9, 10, 11, 12 Given that miRNAs are known to regulate gene expression by binding at the 3′ UTR region, the association of 5-hmC with the nestin 3′ UTR raises the possibility of miRNA involvement in this potentially complex epigenetic regulatory process. Thus, our observation raises the possibility that 5-hmC may regulate nestin gene expression via miRNAs, and studies are currently underway in our laboratory to elucidate this possibility. Others studies are now indicated to explore further this theory. The regulation of nestin by epigenetic pathways has not heretofore been identified in human cancers, although it is well-known to play a key role in embryogenesis during cellular specification.6, 7 Compared to adult tissue, embryonic cells are enriched for 5-hmC, where, at the zygote stage, genome-wide 5-mC is hydroxylated to 5-hmC, the levels of which correlate with the pluripotent cell state.17 In contrast, whereas 5-hmC is enriched in the central nervous system of adult mammals, levels are significantly lower in stem cell–rich areas.18 This finding is potentially relevant to the negative correlation between 5-hmC and the expression of nestin, itself a putative marker of neural stem cells, found in our study.
Regarding nestin expression as a biomarker, a study has revealed that nestin expression, as assessed by IHC, is associated with decreased survival time in melanoma patients with advanced disease.19 Moreover, higher nestin expression has been noted in biopsy samples of metastatic melanomas compared to primary melanomas and benign nevi.20 In contrast, The Cancer Genome Atlas analysis of a large-scale melanoma cohort (n = 478 samples from 470 patients)21, 22 failed to relate nestin expression to overall survival (Supplemental Figure S3). Herein we also did not identify the clinical implications of nestin expression, although experimental melanoma models of nestin regulation indicate that this intermediate filament protein may play a role in the reciprocal expression of two virulence-conferring attributes, namely tumorigenic versus invasive growth.4 Specifically, experimental nestin overexpression in human melanoma cell lines enhances spherogenic growth, whereas knockdown results in up-regulation of multiple matrix metalloproteinases fundamental to tumor cell invasion of stroma necessary for metastasis beyond the primary site. This coordination between tumorigenic and invasive subpopulations of melanoma cells is crucial to behavior that mimics epithelial–mesenchymal transition and that combines to mediate clinical virulence.23, 24 Thus, the epigenetic regulation of nestin expression may have implications as to how individual melanoma cells at given time points behave within tumors, while not correlating with overall clinical virulence over more prolonged periods. Of interest, a recent study showed that factors in the immediate peritumoral microenvironment of squamous cell carcinoma may mediate epigenetic modifications in DNA methylation relevant to tumor cell invasion and metastasis.25 Whether similar factors at the interface between tumorigenic, nestin-rich melanoma nodules may affect 5-hmC levels to favor a low nestin/high metalloproteinase phenotype at the tumor periphery is an area of active interest and investigation. In addition, studies have shown that melanin pigmentation correlates with melanocyte biology.26, 27 Although we did not observe a significant relationship between melanin pigmentation and nestin expression/5-hmC levels in the cohort of this study, future investigation in a larger-scale sample will be required to exclude a link between these biomarkers.
More than 2000 genes appear to be affected by a loss of 5-hmC,1 and accordingly it is likely that the loss of 5-hmC in melanoma drives numerous other pathways, in addition to nestin, that mediate tumor virulence. This concept is consistent with the ability to correlate low 5-hmC levels, but not nestin expression, with several parameters of clinical aggressiveness in this study. The ability to regulate 5-hmC expression therapeutically via epigenetic reprogramming is an intriguing prospect for the design of novel therapies to influence melanoma. Epigenetic agents, such as the DNA methyltransferase inhibitor 5-azacitidine, have been shown to block proliferation, impede invasion, and enhance chemosensitivity in solid tumors and have provided promising results in clinical trials.28, 29, 30 In addition, other compounds (eg, ascorbic acid) have been shown to inhibit proliferation, prevent invasiveness, and enhance chemosensitivity in melanoma.31, 32 These findings implicate the reestablishment of the TET2-mediated 5-hmC landscape via epigenetic modulators as a novel therapeutic approach to melanoma management by regulating tumor virulence–associated target genes. Future studies designed to address the biological effects of nestin-regulated loss of 5-hmC expression in normal and sun-damaged melanocytes and nevus cells, as well as experimental approaches to achieving complete loss of 5-hmC in melanoma cell lines through triple-knockdown approaches (eg, TET1, -2, and -3) would be expected to provide additional insight into this epigenetic pathway in melanomagenesis. In summary, this study documents a relationship between two biomarkers recently shown to be of biological significance in melanoma. Because nestin plays a biological role in a variety of human neoplasms, the documentation of the epigenetic regulation of nestin in melanoma through the TET2/5-hmC pathway may have implications that transcend this tumor type. Moreover, it is now possible to consider epigenetic strategies to influence nestin-mediated tumorigenic and invasive properties that may synergize to enhance melanoma virulence.
Footnotes
Supported by NIH grant R01 CA158467-01A1 (Brigham and Women's Hospital); the Harvard Medical School Eleanor and Miles Shore Fellowship Program (C.G.L.); the Karin Grunebaum Cancer Research Foundation (C.G.L.); Fundação de Amparo a Pesquisa do Estado de São Paulo-FAPESP grants 2012/13834-4 and 2012/14008-0; and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES grant AUX-PDSE-456512014-02.
Disclosures: G.F.M. receives funding from Bristol-Myers-Squibb for work on melanoma biomarkers unrelated to the current report.
Current address of G.Q., Shanghai, China.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.01.020.
Contributor Information
George F. Murphy, Email: gmurphy@rics.bwh.harvard.edu.
Christine G. Lian, Email: cglian@bwh.harvard.edu.
Supplemental Data
Supplemental Figure S1.
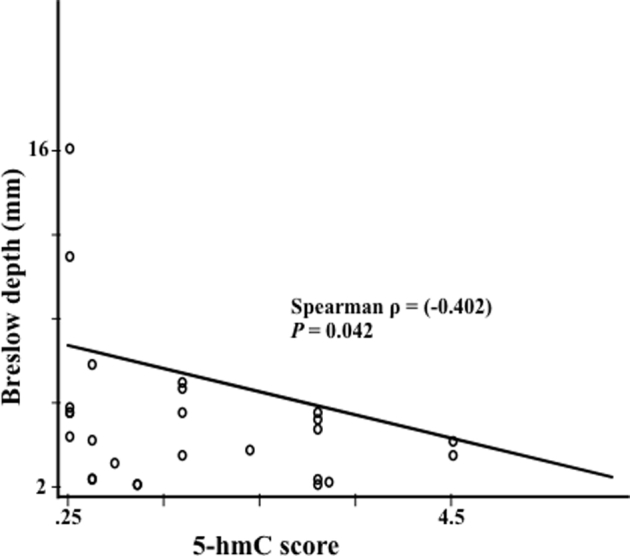
Spearman correlation of the immunoreactivity of 5-hydroxymethylcytosine (hmC) levels and Breslow depth in primary human melanomas. P = 0.042; ρ = −0.402.
Supplemental Figure S2.
Kaplan-Meier overall survival (OS) in metastatic human melanomas. A: There is no significant correlation between nestin expression and OS, although patients with greater nestin expression (mean, 6) have a greater likelihood of survival. B: There is a statistically significant difference in OS between metastatic melanoma patients with and without low levels of 5-hydroxymethylcytosine (hmC).
Supplemental Figure S3.
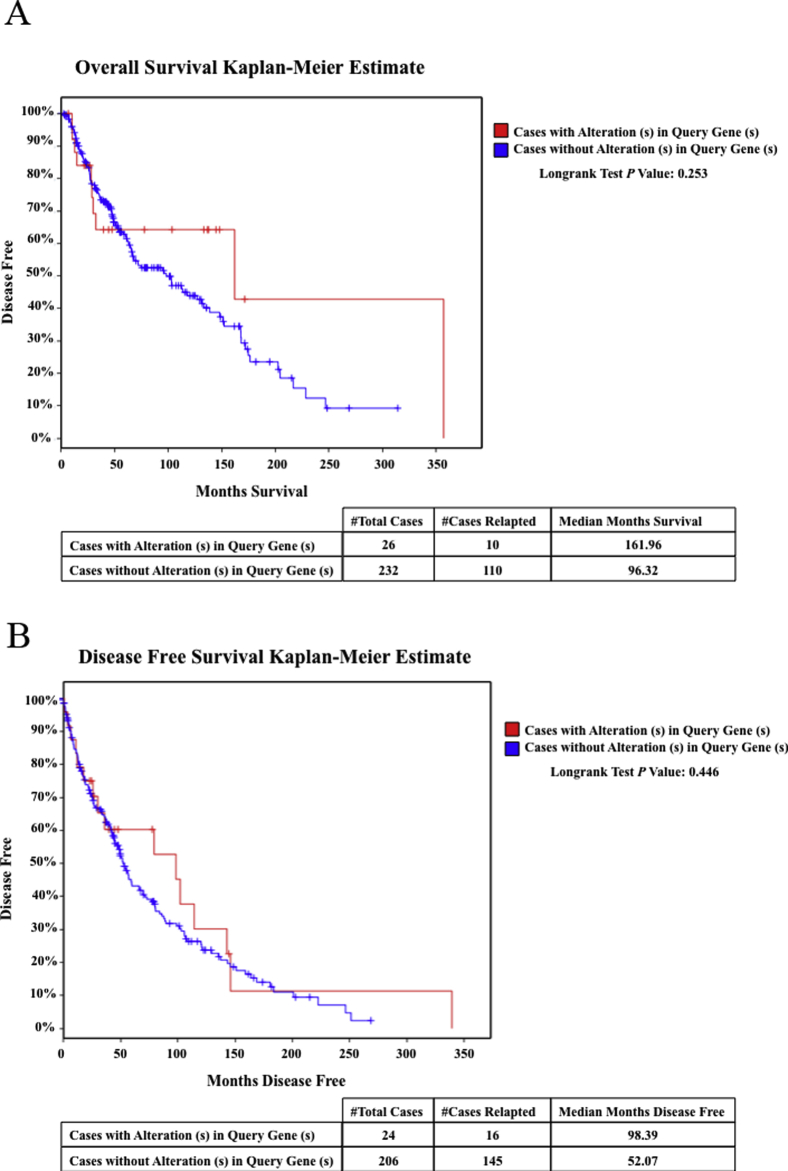
A and B: Kaplan-Meier curves of overall survival (A) and disease-free survival (B) in a large cohort of patients with The Cancer Genome Atlas melanoma and with (red lines) and without (blue lines) alteration in nestin. Data are publicly accessible via cBioPortal for Cancer Genomics (http://www.cbioportal.org, last accessed January 9, 2016) with analysis of cases of cutaneous melanoma. n = 50 (with alteration); n = 438 (without alteration).
References
- 1.Lian C.G., Xu Y., Ceol C., Wu F., Larson A., Dresser K., Xu W., Tan L., Hu Y., Zhan Q., Lee C.W., Hu D., Lian B.Q., Kleffel S., Yang Y., Neiswender J., Khorasani A.J., Fang R., Lezcano C., Duncan L.M., Scolyer R.A., Thompson J.F., Kakavand H., Houvras Y., Zon L.I., Mihm M.C., Jr., Kaiser U.B., Schatton T., Woda B.A., Murphy G.F., Shi Y.G. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell. 2012;150:1135–1146. doi: 10.1016/j.cell.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larson A.R., Dresser K.A., Zhan Q., Lezcano C., Woda B.A., Yosufi B., Thompson J.F., Scolyer R.A., Mihm M.C., Jr., Shi Y.G., Murphy G.F., Lian C.G. Loss of 5-hydroxymethylcytosine correlates with increasing morphologic dysplasia in melanocytic tumors. Mod Pathol. 2014;27:936–944. doi: 10.1038/modpathol.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J.J., Granter S.R., Laga A.C., Saavedra A.P., Zhan Q., Guo W., Xu S., Murphy G.F., Lian C.G. 5-Hydroxymethylcytosine expression in metastatic melanoma versus nodal nevus in sentinel lymph node biopsies. Mod Pathol. 2015;28:218–229. doi: 10.1038/modpathol.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee C.W., Zhan Q., Lezcano C., Frank M.H., Huang J., Larson A.R., Lin J.Y., Wan M.T., Lin P.I., Ma J., Kleffel S., Schatton T., Lian C.G., Murphy G.F. Nestin depletion induces melanoma matrix metalloproteinases and invasion. Lab Invest. 2014;94:1382–1395. doi: 10.1038/labinvest.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein W.M., Wu B.P., Zhao S., Wu H., Klein-Szanto A.J., Tahan S.R. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007;20:102–107. doi: 10.1038/modpathol.3800720. [DOI] [PubMed] [Google Scholar]
- 6.Han D.W., Do J.T., Arauzo-Bravo M.J., Lee S.H., Meissner A., Lee H.T., Jaenisch R., Scholer H.R. Epigenetic hierarchy governing Nestin expression. Stem Cells. 2009;27:1088–1097. doi: 10.1002/stem.43. [DOI] [PubMed] [Google Scholar]
- 7.Boulland J.L., Mastrangelopoulou M., Boquest A.C., Jakobsen R., Noer A., Glover J.C., Collas P. Epigenetic regulation of nestin expression during neurogenic differentiation of adipose tissue stem cells. Stem Cells Dev. 2013;22:1042–1052. doi: 10.1089/scd.2012.0560. [DOI] [PubMed] [Google Scholar]
- 8.Das E., Bhattacharyya N.P. MicroRNA-432 contributes to dopamine cocktail and retinoic acid induced differentiation of human neuroblastoma cells by targeting NESTIN and RCOR1 genes. FEBS Lett. 2014;588:1706–1714. doi: 10.1016/j.febslet.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Wu R., Tang Y., Zang W., Wang Y., Li M., Du Y., Zhao G., Xu Y. MicroRNA-128 regulates the differentiation of rat bone mesenchymal stem cells into neuron-like cells by Wnt signaling. Mol Cell Biochem. 2014;387:151–158. doi: 10.1007/s11010-013-1880-7. [DOI] [PubMed] [Google Scholar]
- 10.Thomsen R., Pallesen J., Daugaard T.F., Borglum A.D., Nielsen A.L. Genome wide assessment of mRNA in astrocyte protrusions by direct RNA sequencing reveals mRNA localization for the intermediate filament protein nestin. Glia. 2013;61:1922–1937. doi: 10.1002/glia.22569. [DOI] [PubMed] [Google Scholar]
- 11.Cui Y., Xiao Z., Han J., Sun J., Ding W., Zhao Y., Chen B., Li X., Dai J. MiR-125b orchestrates cell proliferation, differentiation and migration in neural stem/progenitor cells by targeting Nestin. BMC Neurosci. 2012;13:116. doi: 10.1186/1471-2202-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma J., Sun F., Li C., Zhang Y., Xiao W., Li Z., Pan Q., Zeng H., Xiao G., Yao K., Hong A., An J. Depletion of intermediate filament protein Nestin, a target of microRNA-940, suppresses tumorigenesis by inducing spontaneous DNA damage accumulation in human nasopharyngeal carcinoma. Cell Death Dis. 2014;5:e1377. doi: 10.1038/cddis.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remmele W., Stegner H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 14.Song F., Amos C.I., Lee J.E., Lian C.G., Fang S., Liu H., MacGregor S., Iles M.M., Law M.H., Lindeman N.I., Montgomery G.W., Duffy D.L., Cust A.E., Jenkins M.A., Whiteman D.C., Kefford R.F., Giles G.G., Armstrong B.K., Aitken J.F., Hopper J.L., Brown K.M., Martin N.G., Mann G.J., Bishop D.T., Bishop J.A., MELc Geno, Kraft P., Qureshi A.A., Kanetsky P.A., Hayward N.K., Hunter D.J., Wei Q., Han J. Identification of a melanoma susceptibility locus and somatic mutation in TET2. Carcinogenesis. 2014;35:2097–2101. doi: 10.1093/carcin/bgu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J.J., Sholl L.M., Lindeman N.I., Granter S.R., Laga A.C., Shivdasani P., Chin G., Luke J.J., Ott P.A., Hodi F.S., Mihm M.C., Jr., Lin J.Y., Werchniak A.E., Haynes H.A., Bailey N., Liu R., Murphy G.F., Lian C.G. Targeted next-generation sequencing reveals high frequency of mutations in epigenetic regulators across treatment-naive patient melanomas. Clin Epigenetics. 2015;7:59. doi: 10.1186/s13148-015-0091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laga A.C., Zhan Q., Weishaupt C., Ma J., Frank M.H., Murphy G.F. SOX2 and nestin expression in human melanoma: an immunohistochemical and experimental study. Exp Dermatol. 2011;20:339–345. doi: 10.1111/j.1600-0625.2011.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruzov A., Tsenkina Y., Serio A., Dudnakova T., Fletcher J., Bai Y., Chebotareva T., Pells S., Hannoun Z., Sullivan G., Chandran S., Hay D.C., Bradley M., Wilmut I., De Sousa P. Lineage-specific distribution of high levels of genomic 5-hydroxymethylcytosine in mammalian development. Cell Res. 2011;21:1332–1342. doi: 10.1038/cr.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Globisch D., Munzel M., Muller M., Michalakis S., Wagner M., Koch S., Bruckl T., Biel M., Carell T. Tissue distribution of 5-hydroxymethylcytosine and search for active demethylation intermediates. PLoS One. 2010;5:e15367. doi: 10.1371/journal.pone.0015367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piras F., Perra M.T., Murtas D., Minerba L., Floris C., Maxia C., Demurtas P., Ugalde J., Ribatti D., Sirigu P. The stem cell marker nestin predicts poor prognosis in human melanoma. Oncol Rep. 2010;23:17–24. [PubMed] [Google Scholar]
- 20.Brychtova S., Fiuraskova M., Hlobilkova A., Brychta T., Hirnak J. Nestin expression in cutaneous melanomas and melanocytic nevi. J Cutan Pathol. 2007;34:370–375. doi: 10.1111/j.1600-0560.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- 21.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E., Cerami E., Sander C., Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., Antipin Y., Reva B., Goldberg A.P., Sander C., Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moro N., Mauch C., Zigrino P. Metalloproteinases in melanoma. Eur J Cell Biol. 2014;93:23–29. doi: 10.1016/j.ejcb.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z., Zhu S., Yang Y., Ma X., Guo S. Matrix metalloproteinase-12 expression is increased in cutaneous melanoma and associated with tumor aggressiveness. Tumour Biol. 2015;36:8593–8600. doi: 10.1007/s13277-015-3622-9. [DOI] [PubMed] [Google Scholar]
- 25.DesRochers T.M., Shamis Y., Alt-Holland A., Kudo Y., Takata T., Wang G., Jackson-Grusby L., Garlick J.A. The 3D tissue microenvironment modulates DNA methylation and E-cadherin expression in squamous cell carcinoma. Epigenetics. 2012;7:34–46. doi: 10.4161/epi.7.1.18546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slominski A., Tobin D.J., Shibahara S., Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 27.Slominski A.T., Carlson J.A. Melanoma resistance: a bright future for academicians and a challenge for patient advocates. Mayo Clin Proc. 2014;89:429–433. doi: 10.1016/j.mayocp.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gustafson C.B., Yang C., Dickson K.M., Shao H., Van Booven D., Harbour J.W., Liu Z.J., Wang G. Epigenetic reprogramming of melanoma cells by vitamin C treatment. Clin Epigenetics. 2015;7:51. doi: 10.1186/s13148-015-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia C., Leon-Ferre R., Laux D., Deutsch J., Smith B.J., Frees M., Milhem M. Treatment of resistant metastatic melanoma using sequential epigenetic therapy (decitabine and panobinostat) combined with chemotherapy (temozolomide) Cancer Chemother Pharmacol. 2014;74:691–697. doi: 10.1007/s00280-014-2501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tawbi H.A., Beumer J.H., Tarhini A.A., Moschos S., Buch S.C., Egorin M.J., Lin Y., Christner S., Kirkwood J.M. Safety and efficacy of decitabine in combination with temozolomide in metastatic melanoma: a phase I/II study and pharmacokinetic analysis. Ann Oncol. 2013;24:1112–1119. doi: 10.1093/annonc/mds591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serrano O.K., Parrow N.L., Violet P.C., Yang J., Zornjak J., Basseville A., Levine M. Antitumor effect of pharmacologic ascorbate in the B16 murine melanoma model. Free Radic Biol Med. 2015;87:193–203. doi: 10.1016/j.freeradbiomed.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 32.Venturelli S., Sinnberg T.W., Berger A., Noor S., Levesque M.P., Bocker A., Niessner H., Lauer U.M., Bitzer M., Garbe C., Busch C. Epigenetic impacts of ascorbate on human metastatic melanoma cells. Front Oncol. 2014;4:227. doi: 10.3389/fonc.2014.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.