Abstract
Respiratory challenge MRI is the modification of arterial oxygen (PaO2) and/or carbon dioxide (PaCO2) concentration to induce a change in cerebral function or metabolism which is then measured by MRI. Alterations in arterial gas concentrations can lead to profound changes in cerebral haemodynamics which can be studied using a variety of MRI sequences. Whilst such experiments may provide a wealth of information, conducting them can be complex and challenging. In this paper we review the rationale for respiratory challenge MRI including the effects of oxygen and carbon dioxide on the cerebral circulation. We also discuss the planning, equipment, monitoring and techniques that have been used to undertake these experiments. We finally propose some recommendations in this evolving area for conducting these experiments to enhance data quality and comparison between techniques.
Keywords: Cerebral blood flow, Cerebrovascular reactivity, Magnetic resonance imaging, Respiratory challenge, Review
Highlights
-
•
Oxygen and carbon dioxide affect cerebral blood flow and metabolism.
-
•
This can be imaged with various MRI sequences.
-
•
The practicalities of these techniques are reviewed.
-
•
Examples of how this has been used to understand disease mechanisms.
1. Introduction
Alterations in the arterial partial pressure of oxygen (O2) and carbon dioxide (CO2) lead to changes in cerebral blood flow and vasculature (Kety and Schmidt, 1948a), and this response, when used in combination with a variety of imaging techniques, has been used to study brain physiology and disease for many years (Aaslid et al., 1989, Battisti-Charbonney et al., 2011, Novack et al., 1953). Magnetic resonance imaging (MRI) is a safe, non-invasive, repeatable technique with high spatial resolution, which can provide detailed structural and functional information about the brain. In this paper, we define respiratory challenge MRI as the modification of arterial oxygen (PaO2) and/or carbon dioxide (PaCO2) concentration to induce a change in cerebral function or metabolism which is then measured by MRI. This approach has been used for some time for optimization and calibration of fMRI sequences (Hoge, 2012), but there is increasing interest in the use of functional and/or perfusion MRI to examine brain pathophysiology. In particular, cerebral blood flow, oxygenation, metabolic rate and microvascular function in diseases such as stroke (Dani et al., 2010), dementia (Cantin et al., 2011), epilepsy (Kalamangalam et al., 2012) and brain neoplasm (Hsu et al., 2010, Yetkin and Mendelsohn, 2002).
A number of approaches have been explored. These can range from simple modification of respiratory rate, including breath hold (Hsu et al., 2010) and hyperventilation, to complex modelling of both respiratory parameters and brain signal change (Mutch et al., 2012, Shen et al., 2011). Whilst excellent articles reviewing the rationale and uses of these procedures are available (see Krainik for a recent review of functional imaging of brain perfusion (Krainik et al., 2013)), there are significant practical challenges in undertaking these methods. The aim of this review is to [1] review the rationale for respiratory challenge MRI in brain disease, [2] discuss techniques, equipment, monitoring and planning such experiments, and [3] propose some recommendations for optimization of these studies.
2. Rationale
The human brain employs an elegant system of regulation of cerebral blood flow (CBF) to ensure adequate delivery of O2 and nutrients to brain tissue, according to need and regardless of changes in blood pressure, oxygenation or other factors. CBF is determined by the following equation:
Normal global CBF is around 50 mL/100 g/min (Kety and Schmidt, 1948b) with higher values in grey compared to white matter (Leenders et al., 1990) (see Table 1 for further definitions). However, CBF varies according to age, time of day, anatomical area and neuronal activity in order to maintain adequate nutrient delivery. The principle mechanism by which CBF is adjusted according to demand is by changing cerebrovascular resistance. This is governed by small cerebral vessels, particularly pre-capillary arterioles (< 100 μm) (Wei et al., 1980), which are able to change calibre in response to a number of stimuli, a process known as cerebrovascular reactivity (CVR). Capillaries may also have an important role in vasoreactivity through the action of pericytes (Hall et al., 2014). If CVR is impaired, then increased CBF will not occur when required by brain activity.
Table 1.
Definitions and relevant normal values.
| Parameter | Abbreviation | Definition | Normal values |
|---|---|---|---|
| Cerebral blood flow | CBF | The volume of blood passing through the brain parenchyma in a defined time i.e. rate. This is usually defined in units of millilitres per 100 grams per minute. | ~ 50 mL/100 g/min |
| Cerebral blood volume | CBV | The fraction of a tissue volume occupied by blood | 4–6 mL/100 g |
| Cerebral metabolic rate for oxygen | CMRO2 | The amount of oxygen consumed by 100 g of brain in 1 min. | ~ 3.5 mL/100 g/min |
| Cerebrovascular reactivity | CVR | Cerebral blood flow, or BOLD signal changes in response to stimuli usually measured as a percentage change in signal per change in CO2/O2 | |
| Arterial gas concentration | PaO2 PaCO2 | Partial pressure of oxygen or carbon dioxide in arterial blood i.e. gas molecules dissolved in plasma. | PaO2: 11–13 kPa PaCO2: 4.7–6 kPa |
| End-tidal gas tension | EtO2 EtCO2 | The partial pressure or maximum concentration of oxygen or carbon dioxide at the end of an exhaled breath. | EtO2: 16–17% EtCO2: 5% (4.6–5.6 kPa) |
| Fraction of inspired gas | FiO2 FiCO2 | The fraction or percentage of oxygen or carbon dioxide in the air that is breathed by the subject. Normal air has an FiO2 of 0.21 | FiO2: 0.21 (21%) FiCO2: 0.0004 (0.04%) |
| Oxygen saturation | SaO2 | The percentage of haemoglobin molecules which are oxygenated in arterial blood. | 95–100% |
| SvO2 | |||
| Oxygen content | CaO2 | The amount of oxygen in the blood and therefore available for tissues. | 20 mL O2/dL |
| Cerebrovascular resistance | The resistance to the passage of blood created by arterioles and capillaries. | ||
| Autoregulation | Cerebral vascular bed alters vascular resistance to maintain blood flow in the face of changes in systemic blood pressure to match metabolic needs. | ||
| Vascular steal | A stimulus results in the redistribution of blood flow from regions of exhausted cerebrovascular reactivity (maximally dilated vessels) to areas with preserved vasodilatory capacity. |
Whilst a variety of methods exist for measuring CBF, there are difficulties in obtaining accurate, quantifiable CBF measurements, including interindividual variability (Leenders et al., 1990), external factors (Laurent et al., 2006), and inaccuracies in modelling methods (Eskey and Sanelli, 2005). Large patient groups or large disease-related effects may be needed to detect differences in baseline CBF in disease states.
Measuring CVR, or the response of vessels, is an alternative technique. Vasoreactivity may be measured by applying a “challenge” such as modification of arterial gas concentration or the vasodilator acetazolamide (Vagal et al., 2009b). Acetazolamide is thought to work by causing inhibition of erythrocyte carbonic anhydrase resulting in impaired clearance of CO2 and acidosis which causes vascular smooth muscle relaxation. This causes an increase in CBF, without affecting CMRO2 (Vorstrup et al., 1984). Usually administered with a standard dose of 1000 mg, it is safe and usually well tolerated, without changes in systemic parameters (Vagal et al., 2009a). There are a number of disadvantages including side effects such as paraesthesia and headaches, along with reports of reversible neurological deficts thought to be secondary to ischaemia (Komiyama et al., 1997). It has a number of drug interactions and must also be used with caution in subjects with hepatic or renal dysfunction or electrolyte disturbances, limiting its use in some patient groups (Eskey and Sanelli, 2005). Its lack of reversibility means it can only be used once in an MRI session whereas modification of arterial gas concentration, or “respiratory challenge” is reversible and more versatile.
The use of a challenge uses the individual as their own control and negates some of the problems of direct measurement (Eskey and Sanelli, 2005). CBF, and hence CVR, can be measured by MRI sequences including arterial spin labelling (ASL) or dynamic susceptibility contrast perfusion MRI (Wintermark et al., 2005).
Changes in vessel calibre in response to a challenge can lead to changes in other parameters such as oxygenation and cerebral blood volume, measurable by other MRI techniques (see below). Therefore the percentage change seen in respiratory challenge MRI is defined as:
For standardization of signal change, it should be corrected for the change in gas concentration delivered to, or expired by, the subject. Therefore the general formula for defining CVR in respiratory challenge MRI is:
2.1. Gases
O2 and CO2 have well described effects on cerebral vessel calibre and blood flow (Kety and Schmidt, 1948a). Effects are rapid and reversible, with minimal side effects. Rapid initiation and cessation of a gas challenge allows repeated measurements during MRI (unlike acetazolamide, whose effects are long-lasting). Thus the ability to interrogate vasoreactivity by measuring vascular response to changes in O2 and CO2 may be useful to investigate cerebral pathophysiology.
2.1.1. The physiology of gas transport
Air, comprising 21% O2 and 0.04% CO2, is breathed in through the mouth and nose, and conducted to the lungs by the trachea and bronchi, which form anatomic dead space (i.e. they do not take part in gas exchange). Inspired air mixes with gas in the conducting airways. Within the alveoli, O2 diffuses into capillaries and is transported to the heart. Most O2 (> 98%) is carried bound to haemoglobin within red blood cells, as oxyhaemoglobin, but the remaining amount is transported in arterial blood as a dissolved gas (referred to as the PaO2). The CaO2 is the total oxygen content of the arterial blood, including both dissolved and bound O2. It is calculated by the O2 content equation (Chambers et al., 2015):
Where:
CaO2 = arterial oxygen content (mL/dL)
Hb = Haemoglobin (g/dL)
SaO2 = arterial oxygen saturation (%)
PaO2 = arterial partial pressure of oxygen (kPa).
Once arterial blood arrives in tissues, the acidic and hypercarbic environment along with reduced PaO2 causes O2 to be released from haemoglobin. Metabolizing tissues release CO2 which diffuses from capillaries into the venous circulation. CO2 is transported as either as a dissolved gas, bicarbonate (HCO3–), or bound to proteins. In the lungs, CO2 diffuses into the alveoli, and is expired via the airways (Fig. 1A). End-tidal CO2 is the partial pressure of CO2 at the end of an exhaled breath. Normal EtCO2 is 4.0–5.7 kPa, and is 0.3–0.7 kPa lower than PaCO2 due to the mixture of gas in alveolar dead space. In normal healthy individuals, the difference is negligible but this becomes more marked in cardiac and pulmonary disease, as well as with increased age and smoking, due to increased dead space. This is also the case for differences in EtO2 and PaO2 (Bengtsson et al., 2001).
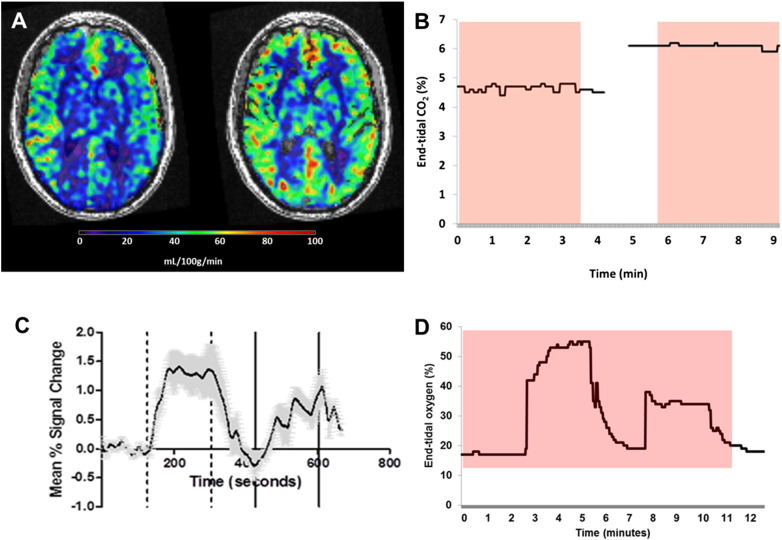
Fig. 1.
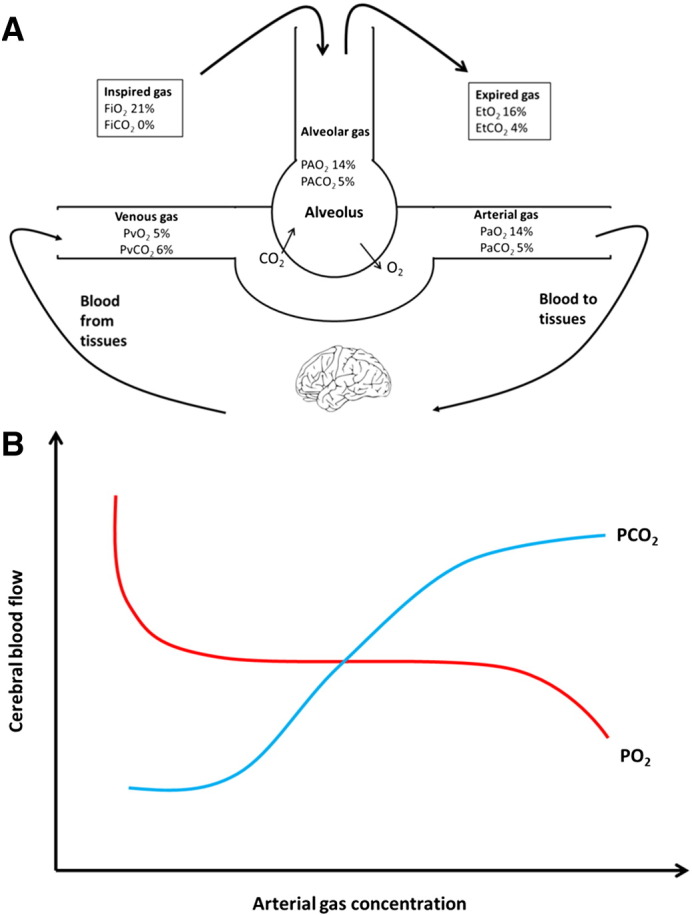
Transport of gases to the tissues and their effect on cerebral blood flow. (A) Oxygen (O2) is inspired into the alveoli and passes into the arterial blood for delivery to tissues. Carbon dioxide (CO2) produced by metabolizing cells is carried in the venous system and expelled through the lungs in expired gas. PVO2 and PVCO2 is the partial pressure of oxygen or carbon dioxide in venous blood. For other abbreviations see Table 1. (B) Cerebral blood flow (CBF) in the normal physiological range of O2 is stable, but CBF increases in response to hypoxia and decreases in the presence of hyperoxia. Elevation of CO2 causes a linear increase in CBF except at the extremes, where vasoactive properties are exhausted, producing a sigmoid curve.
2.1.2. Carbon dioxide
CO2 is one of the most important modulators of vascular tone, with increased CO2 (hypercapnia) leading to a relaxation of vascular smooth muscle and increased CBF. For each 1 mm Hg increase in PaCO2, CBF increases by 1–2 mL/100 g/min (Brian, 1998, Kety and Schmidt, 1948a). This relationship is characterized by an sigmoidal curve, with attenuated responses at the extremes (Reivich, 1964) (See Fig. 1B). Resistance arterioles (40–100 μm) are more responsive to hypercapnia than larger arterioles (up to 400 μm), but responses to hypocapnia are independent of vessel size. Recent evidence has suggested that even major vessels, such as the middle cerebral artery, demonstrate diameter change in response to altered carbon dioxide (Coverdale et al., 2014). Cerebral blood volume (CBV) has a sigmoidal relationship to changes in PaCO2 (Grubb et al., 1974).
CO2 is proposed to exert its effects after dissociating into the vasoactive agents H+ and HCO3. Increased H+ concentrations lead to activation of potassium channels and endothelial hyperpolarization (Ainslie and Duffin, 2009) which is relayed to vascular smooth muscle cells. Intracellular calcium concentration reduces and vasorelaxation occurs (Edvinsson and Krause, 2002). Alterations in CO2 have classically been thought not to lead to a change in metabolic O2 consumption (Kety and Schmidt, 1948a) but more recent evidence suggests hypercapnia may lead to altered neuronal activity (Hall et al., 2011).
Rapid CO2 diffusion across alveoli allows CBF change within 6 s of a rise in CO2 with a CBF plateau being obtained within 45 s (Poulin et al., 1996). However, the fall in CBF following restoration of normocapnia is much quicker (Poulin et al., 1996). Adaptation to sustained hypercapnia does occur, with gradual restoration of normal CBF despite sustained hypercapnia. This occurs within hours, although others have argued this can occur within 10 min (Ellingsen et al., 1987). This needs to be considered when designing respiratory challenge experiments.
Side effects such as nausea, flushing, hyperventilation, and transient neurological symptoms (Spano et al., 2013) may occur with hypercapnia along with anxiety, sensory stimulation and a panic like disorder (Colasanti et al., 2012). Hyperventilation induced by hypercapnia may increase motion artefacts (Taylor et al., 2001). Ainslie and Duffin (Ainslie and Duffin, 2009) recommended limiting delivery of FiCO2 to 8% for both subject safety and comfort. Despite side effects most subjects tolerate hypercapnia experiments. A review of 434 CVR MRI examinations using a rebreathing circuit and targeted EtCO2 of 50 mm Hg (6.7 kPa), reported transient symptoms in 11.1% of studies, no major complications, and successful CVR map generation in 83.9% of studies (Spano et al., 2013). Examined patients had a variety of diagnoses including atherosclerosis, Moyamoya vasculopathy, arteriovenous malformation, vasculitis and dissection.
Hypercapnia can increase systemic blood pressure (BP) (Kety and Schmidt, 1948a), due to activation of the sympathetic nervous system, which may affect CVR measurements (Ainslie and Duffin, 2009, Hetzel et al., 1999). The vascular response to hypercapnia is lost when vessels are maximally dilated in response to low systemic BP (e.g. hypovolaemia), in an effort to maintain CBF (Grubb et al., 1974). There is therefore a limit on the capacity to autoregulate. CVR to breath-hold and 6% CO2 breathing showed improved correlation when changes in blood flow velocity (measured by transcranial Doppler ultrasound) were corrected for changes in blood pressure, and therefore monitoring BP with compatible equipment during MRI experiments should be considered (Prakash et al., 2014).
2.1.3. Oxygen
Within normal physiological values, changes in PaO2 have little effect on vessel radius or CBF (see Fig. 1) (Watson et al., 2000). Once PaO2 drops below 6.7 kPa, metabolic signals such as adenosine, along with the direct action of hypoxia on vascular smooth muscle, results in vasodilatation and increased CBF, allowing O2 delivery to remain constant (Golanov and Reis, 1997). MRI experiments using hypoxia have been performed, usually in young healthy individuals (Noth et al., 2008). Hypoxic experiments require well controlled experimental conditions and may be inappropriate in patients.
The effects of hyperoxia are less clear but most studies have suggested it causes a reduction in CBF. In young healthy subjects blood flow, measured using phase-contrast MR angiography, decreased by up to 25% when a 100% oxygen was administered (Watson et al., 2000). Bulte and colleagues, using arterial spin labelling (ASL) MRI, proposed that CBF decreased even with minor hyperoxia, and continued to decline as FiO2 increased (Bulte et al., 2006). Hyperoxia is proposed to have a direct vasoconstrictive effect on vessels via the attenuation of the effects of nitric oxide (Demchenko et al., 2000). This is more marked with hyperbaric O2 (Omae et al., 1998). However the effect of hyperoxia is complicated by two factors. Firstly hyperoxia leads to a small but significant reduction in EtCO2 probably due to increased tidal volume. Reduced EtCO2 leads to vasoconstriction, and this may be responsible for the change in CBF seen with hyperoxia. When changes in EtCO2 were corrected for in a phase-contrast MRI experiment, changes in FiO2 did not have a significant effect on CBF, suggesting CO2 is the predominant modulator of CBF (Xu et al., 2012). The second factor is that oxygen changes the MR relaxation properties of tissue and blood, which may have profound effects on measurements, particularly when using ASL. Adjustment for this may be required for the accurate quantification of CBF (Pilkinton et al., 2012).
Studies using hyperoxia may therefore need to consider the measurement and control of CO2 and correct for changes in T1, which adds to the complexity of the experimental paradigm.
Hyperoxia can be used alone, or in combination with CO2 (carbogen) in respiratory challenge experiments. Carbogen increases both CBF and PaO2.
As well as affecting CBF, O2 has important effects on the BOLD signal in fMRI. With a FiO2 of 21% and normal atmospheric pressure, arterial haemoglobin (Hb) is almost completely saturated with O2 (creating diamagnetic oxyhaemoglobin). Elevated FiO2 increases the amount of gas dissolved in plasma in linear proportion to the partial pressure (Kalamangalam et al., 2012). This dissolved paramagnetic O2 alters the susceptibility between blood and tissue. It also has an effect via the oxygen-haemoglobin dissociation curve. Due to the increased PaO2 in the capillaries, there is a reduction in the dissociation of O2 from haemoglobin as the PaO2 does not fall sufficiently to allow its release. In conditions of hyperoxia therefore, the amount of deoxyhaemoglobin is nearly constant during capillary transit. This results in an increased T2* signal and hyperoxic BOLD contrast (Schwarzbauer and Deichmann, 2012).
Hyperoxia is associated with the release of oxygen free radicals, which may overwhelm endogenous antioxidant mechanisms, and lead to lipid peroxidation and plasma membrane breakdown particularly in the lungs (Jamieson et al., 1986). Reversible alveolar changes in human subjects after 17 h of over 95% oxygen suggested changes in alveolar-capillary barriers (Davis et al., 1983). Subjects may develop substernal pain, tracheobronchial irritation, tissue destruction and pulmonary oedema when FiO2 > 50% is given for prolonged periods (Jackson, 1985, Martin and Grocott, 2013). Hyperoxia may also lead to nitrogen washout and airway collapse (Duggan and Kavanagh, 2005). These effects occur only after several hours to days of prolonged hyperoxia, although they are enhanced by elevated atmospheric pressure (Jackson, 1985). The duration of hyperoxia given within the context of an MR experiment are not thought to have any detrimental effects, but the “safety thresholds” for oxygen administration remain unclear.
3. Technique
3.1. preparation
Subjects with brain disease may have difficulties with informed consent and comprehension of protocols. Well planned exclusion criteria accompanied by thorough and early screening for any contraindications to MRI, may prevent subject drop out, avoid scanning delays and enhance data quality.
3.1.1. Exclusions
Normal MRI exclusion criteria should be followed (Kanal et al., 2013). Subjects with claustrophobia or anxiety may struggle with MR scanning, and use of a face mask within the confines of the scanner bore may exacerbate this. Panic disorder patients may be hypersensitive to the anxiety inducing effects of CO2 (Colasanti et al., 2012). Patients with significant pulmonary or cardiac disease may need to be excluded from studies due to difficulties tolerating abnormal gas concentrations, issues with monitoring (see Monitoring below) and accurate measurement of end-tidal gases, and altered cerebral haemodynamics, such as in atrial fibrillation (Lavy et al., 1980, Petersen et al., 1989).
3.1.1.1. Oxygen
Patients requiring continuous O2 such as those with pneumonia (relevant in acute research such as stroke) may not tolerate periods of normoxia. Due to the potential risk of pulmonary atelectasis from hyperoxia, patients with bronchiectasis should be excluded. Subjects with type II respiratory failure have inadequate ventilation, causing a rise in resting PaCO2; hence the respiratory centre becomes driven by hypoxaemia. Hyperoxia can reduce ventilation leading to rises in PaCO2. Therefore patients with certain types of chronic obstructive pulmonary disease or respiratory muscle weakness (e.g. uncontrolled myasthenia gravis) should be excluded.
3.1.1.2. Carbon dioxide
Subjects with known type II respiratory failure should not be subjected to hypercapnia in order to avoid further increase in PaCO2. Hyperventilation (to induce hypocapnia) should be avoided in those with a history of epilepsy as it may induce seizures (Guaranha et al., 2005). In subjects with unruptured intracranial aneurysms, conditions that cause alterations in the aneurysms transmural pressure gradient, such as hypertension or sudden alterations in intracranial pressure (ICP) secondary to hyperventilation, are proposed to increase the risk of rupture during aneurysm surgery (Chowdhury et al., 2014). Therefore these subjects should be excluded.
3.2. Standardization of testing conditions
Factors that may influence blood flow and reactivity include time of day (Strohm et al., 2014), nicotine (Shinohara et al., 2006), food (Tsai et al., 2004), alcohol (Gundersen et al., 2013), body mass (Selim et al., 2008), haematocrit (Hudak et al., 1986) and hormonal cycles (Bartelink et al., 1990). Visual stimulation and speaking will result in increased CBF to active brain regions (Ito et al., 2001), but sleep can reduce the cerebrovascular response to CO2 (Ainslie and Duffin, 2009), so most tests may be best performed on an awake, silent patient with closed eyes. Numerous medications may influence cerebral haemodynamics including cholesterol-lowering drugs (Murakami et al., 2008), angiotensin-receptor enzyme inhibitors (Walters et al., 2004), calcium channel blockers (Kuridze et al., 2000) and hormone replacement therapy (Ohkura et al., 1995), and patients may need to be excluded or medication withheld.
Caffeine reduces cerebral blood flow (Lunt et al., 2004) and thus abstinence for a short period prior to a study may be advisable. However chronic caffeine use may lead to upregulation of vascular adenosine receptors to preserve CBF, and abstinence prior to a study could lead to a “withdrawal” rebound increase in CBF (Addicott et al., 2009). As this is only likely to occur in patients with very high caffeine use (> 600 mg/day or 4–7 cups of coffee) it has been suggested that these subjects should be excluded from perfusion studies (Addicott et al., 2009) but the exact dosage of caffeine intake can be difficult to ascertain due to the variety of sources.
Temperature is known to influence cerebral blood flow (Kuluz et al., 1993). The temperature within the bore of a magnet may increase as scanning progresses due to radiofrequency energy (Westbrook et al., 2011). For studies using repeat imaging or prolonged scanning sessions, an increase in temperature could therefore potentially influence perfusion characteristics of the subject in the bore as there may be an increase in CMRO2 (Edvinsson and Krause, 2002).
Standardised conditions developed for peripheral vascular function assessment are similarly applicable to cerebral perfusion studies, although may be difficult to implement in acute studies (Laurent et al., 2006, Van Bortel et al., 2002). Table 2 reproduces some of these standards and includes others relevant to respiratory challenge MRI experiments.
Table 2.
Standardization of confounding factors in vascular testing (adapted from (Van Bortel et al. (2002)).
| Confounding factors | In practice |
|---|---|
| Room temperature | Document. Be aware of increase in temperature with prolonged scanning and plan experiments with this in mind. |
| Time of day | Similar time of day for repeated measurements. |
| Smoking | Avoid for 3 h prior |
| Food | Avoid for 3 h prior |
| Caffeine | Avoid for 3 h prior (and exclude those with very high intake). |
| Alcohol | Avoid for 10 h prior |
| Menstrual cycle | Stage in cycle recorded Aim for similar stage in repeat measurements |
| Height, weight | Record |
| Visual stimulation Speaking | Ask patients not to speak and to keep their eyes shut during reactivity testing |
| Haematocrit | Record |
| Medication | Record Consider withholding/excluding vasoactive medication |
| Sleeping | Patient should not be allowed to sleep during the scan |
| Cardiac rhythm | Consider if this may influence data |
3.3. A trial run
A trial run of the challenge outside the MRI with close monitoring including heart rate, blood pressure, respiratory rate and inspired and expired respiratory gas concentrations allows priming of the subject to the experience of the respiratory challenge in a less claustrophobic environment. Subjects have the opportunity to ask questions and report side effects. Safety concerns may also be identified prior to the MR experiment. Compliance and experimental success is likely to be increased (Taylor et al., 2001). Local ethics committees may require trained medical personnel to be present but this is likely to vary according to protocol and patient group.
3.4. The respiratory challenge
The respiratory challenge should be easy to perform, tolerable, and give reliable gas concentrations. Unfortunately these ideal requirements are not always compatible, as highly accurate modifications in PaO2 and PaCO2 require a completely closed circuit and invasive monitoring i.e. an intubated and ventilated patient.
3.4.1. Ventilatory techniques
Ventilatory techniques, in their simplest form, do not require additional equipment, but do require patient compliance, limiting their use in cognitive impairment, sedation or confusion. Breath-holding causes hypercapnia and increased CBF, comparable to that achieved with 5% CO2 (Kastrup et al., 1999, Ratnatunga and Adiseshiah, 1990). Breath hold may occur at the end of inspiration or expiration. End-expiration breath hold leads to an immediate rise in EtCO2, but the tolerable duration is shorter (due to hypoxia) and it may be more unpleasant for the patient thus increasing motion artefacts (Ratnatunga and Adiseshiah, 1990). Measurement of EtCO2 is also challenging during an end-expiration breath hold, with lower values and a confounding inspiration before the exhalation is measured. In comparison, end-inspiration breath hold is longer and more comfortable but changes in intrathoracic pressure result in a biphasic change in BOLD signal which needs to be factored into MRI measurements (Thomason et al., 2007, Thomason and Glover, 2008). Both techniques are simple and practical for use in MRI. There are concerns about experimental repeatability due to variations in breath-hold duration (Thomason et al., 2007), although as long as EtCO2 is measured repeatable measurements can be obtained (Bright and Murphy, 2013). Additions to the technique include visual cueing, measurement of compliance with an abdominal pneumatic belt and paced breathing (Scouten and Schwarzbauer, 2008, Thomason et al., 2005), all of which add complexity and may reduce subject acceptability.
Respiratory tasks, including breath hold, and other conditions that result in a rise in hypercapnia, can cause confounding motion artefacts
Voluntary hyperventilation causes hypocapnia and reduced CBF (Raichle and Plum, 1972). A period of > 60 s can induce a fall of around 25% (Rostrup et al., 2005). Subjects are asked to modify their breathing rate and depth via visual cues, for a certain period or to a target EtCO2, which require training outside MRI (Vogt et al., 2011). Hyperventilation may increase motion artefacts (Naganawa et al., 2002).
3.4.2. Fixed inspiratory challenge
Delivery of a fixed gas concentration via a non-rebreathing mask is passive and easy to perform. It requires a constant gas supply and a delivery method. Premixed gas cylinders can be used, or gas blenders may allow more variation in gas concentrations. 5% CO2 is proposed to raise CBF by 50% (Kety and Schmidt, 1948a) and 100% O2 to reduce CBF by between 10% and 25% (Bulte et al., 2006) (Watson et al., 2000). Fixed inspiratory challenge is effective for steady-state measures, such as ASL (62), or those with a block design, where a stimulus is given for set periods on several occasions in order to allow summation of results, usually with a continuous imaging method (Kalamangalam et al., 2012) (Fig. 2).
Fig. 2.
Steady-state and dynamic respiratory challenges. (A) Arterial spin labelling (ASL) MRI performed whilst receiving air (left) and 6% CO2/air mixture (right) in a patient with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), demonstrating increased CBF in response to hypercapnia. Delivery of a 6% CO2 gas mixture caused a change in end-tidal CO2 (B) and separate scans were performed during each steady state. The red box represents the duration of the ASL scans. Continuous imaging can allow the application of repeated challenges and assess temporal resolution of signal in relation to gas concentrations. In (C) T2* signal changes vary over an 11min fMRI in 4 normal volunteers given a dual hyperoxic challenge. Changes in end-tidal oxygen concentration for one patient receiving this challenge is shown in (D) and can be correlated with signal changes. The red box represents the duration of the scan.
A fixed FiO2 or FiCO2 does not necessarily equate to a specific arterial concentration as this depends on ventilation, metabolic rate, age, lung function and the adequacy of the delivery circuit (Fierstra et al., 2013). Flow rates may depend on circuit design, including the use of reservoir bags. Circuits must be capable of supplying high enough flow rate to support the increased tidal volume seen with hypercapnia. An undershoot in EtCO2, may be seen following hypercapnia experiments as patients hyperventilate to expel excess CO2 (Wise et al., 2007).
Arterial concentrations may change even when the inspired concentration is kept constant e.g. increased ventilation in response to hypercapnia may lead to an elevated EtO2, which may influence MRI signal (Wise et al., 2007).
For these reasons, end-tidal gas concentrations should therefore be monitored to allow accurate signal quantification.
3.4.3. Rebreathing
Rebreathing of exhaled gas will result in equilibration of alveolar and arterial gases and a gradual increase in PaCO2. It is performed with a simple breathing circuit, with or without additional gases, and is generally well tolerated (Saunders, 1980). However the speed and values of PaCO2 are not predictable and a plateau is difficult to achieve. Partial rebreathing circuits with fixed inspiratory challenges are proposed to achieve more stable EtCO2, but require significant patient cooperation (Vesely et al., 2001). Rebreathing techniques allow the measurement of dynamic changes to a range of EtCO2 (Ainslie and Duffin, 2009).
Rebreathing in a closed circuit will lead to a fall in delivered FiO2. Isooxia should be maintained for CVR measurement and to prevent the patient receiving a hypoxic gas mixture. Rebreathing methods where isooxia is achieved and ETCO2 is representative of PaCO2 have been described (for a recent review see Fierstra et al. (2013))).
3.4.4. Dynamic end-tidal forcing
More precise control of EtCO2 and EtO2 has been advocated to permit more accurate adjustment of changes in MRI signal responses. Dynamic end-tidal forcing uses breath-by-breath computer control of inspired gas to achieve target EtCO2 and EtO2. This may increase repeatability both between subjects and sessions, and allows more complex variations of respiratory challenges to be performed (Wise et al., 2007). It is more expensive and complex to run than fixed inspiratory challenges, and breath-by-breath analysis can be hampered by the need for long gas sampling lines in MRI. It requires a high flow rate and there is the potential for the delivery of an anoxic mixture although this is avoided with use of the correct circuit (Wise et al., 2007). Issues with the accuracy of using EtCO2 to predict PaCO2 remain (Fierstra et al., 2013).
3.4.5. Prospective end-tidal targeting
Prospective end-tidal targeting has been a developed as a method to permit more accurate correlation of changes in EtCO2 with MRI signal responses (Ito et al., 2008). A tight-fitting mask is attached to a 3-valve circuit with inspiratory and expiratory reservoirs. Gas flow to the mask moves through a computer-controlled gas blender (Respiract; Thornhill Research, Toronto, Canada) which supplies O2, CO2 and nitrogen to achieve target EtCO2 and EtO2 independent of breathing pattern. Its advantages are that it allows rapid changes in gas concentration and that the end-tidal gases are equivalent to that of alveolar ventilation, and thus more accurately reflect tissue concentrations (Fierstra et al., 2013). A more detailed explanation of the method is available in Fierstra et al. (2013). It has been used to study cerebral physiology in a number of different disease states (Fierstra et al., 2011, Heyn et al., 2010, Mikulis et al., 2005). This method in theory could allow the inclusion of patients with pulmonary disease as the EtCO2 will continue to accurately reflect PaCO2 (Ito et al., 2008), although the danger of exposure to hypercarbia or hyperoxia in these patients remains.
3.4.6. Motion artefacts
Respiratory challenges that result in hypercapnia or require the patient to make extreme ventilatory effort, such as breath hold, can result in motion artefacts which may result in a measured signal difference. This may confound the interpretation of results, particularly when a “baseline” versus “challenge” imaging design is used. It is important to ensure the patient is securely strapped into the head coil. Motion correction software or exclusion of patients with excessive head motion may also be needed(Bulte et al., 2009).
3.5. The environment and equipment
Use of respiratory challenge adds complexities to the already difficult MR environment. As subject safety is paramount, all staff should undergo MRI safety induction training as per local guidelines (Farling et al., 2002).
3.5.1. Monitoring
Monitoring of gas concentrations is important for calculation of CVR, but may also be required for subject safety. Access to subjects is limited as the head is usually placed in a tight-fitting receiver coil (to enhance signal), padded and secured to limit movement. This complicates the delivery of gas, monitoring of subjects and verbal communication. Additional equipment must be MR safe or MR conditional and effective, and close liaison with anesthetists is recommended. Standard MR monitoring equipment must be correctly positioned to avoid the formation of inductive loops which may cause burns (Dempsey and Condon, 2001). ECG leads are prone to interference (Farling et al., 2002) and fibre optic MR connections preferred. Anaesthetic guidelines recommend that remote MR monitoring in the control room is available to reduce occupational exposure to magnetic fields and hearing damage (Farling et al., 2002, Farling et al., 2010).
Whilst respiratory challenge MRI aims to modify cerebral tissue O2 and CO2, this is difficult to measure directly, and whilst arterial concentrations are representative they also require invasive monitoring. Non-invasive measurement of end-tidal gases is a more straightforward, if indirect measurement. Sampling should be performed as close to the subject's expired air flow as possible. The long sample lines necessitated by MRI will increase the time delay between sampling and recording (up to 20 s) (Farling et al., 2002). Secretions may block lines, and water traps dry out the sampled air, giving a dry value for expired gas. In normal atmospheric pressure a wet gas correction value of 0.94 can be applied to dry gas measurements (Bengtsson et al., 2001). Capnography involves both the measurement and display of the EtCO2 value and waveform. It offers the advantage of assessment of circuit integrity (i.e. a reasonable seal) and allows identification of leaks or unplanned buildup of CO2.
There does however remain some question as to the accuracy of end-tidal measurements. Use of EtCO2 may only be appropriate with use of regression equations to adjust for changes in tidal volume, as ventilation will increase with a rise in delivered carbon dioxide (Ainslie and Duffin, 2009). Use of prospective end-tidal targeting may offer advantages over unadjusted end-tidal gas readings (Ito et al., 2008, Slessarev et al., 2007).
3.5.2. Gas delivery
3.5.2.1. Nasal cannula
Nasal cannulas are simple, cheap and well tolerated, allowing unimpeded verbal communication. Tolerability is reduced with high flow rates as this can cause painful drying of the nasal mucosa. The main disadvantage is that patients can still mouth breath, although explanation may reduce this. It is possible to deliver 1–6 L/min of O2 (24–44%) and fixed concentrations of CO2 (Cantin et al., 2011). There is greatly reduced control over inspired fractions, even with exclusive nasal breathing, as each patient entrails a different fraction of room air depending on their tidal volume. Normal nasal cannulas do not allow the monitoring of end-tidal gases but nasal cannulas with O2/CO2 combination monitoring are available. An alternative is the use of a mouth piece with nasal clip (Yezhuvath et al., 2012). However communication is hampered and patients may still be able to entrain room air from around the mouth piece.
3.5.2.2. Face mask
Standard vented face masks are available which can deliver FiO2 up to 60% using 5-10L/min (Marino, 2013). Normal air can be breathed in through the vents, diluting the delivered gas concentrations. Most subjects will fit these masks and the seal can be improved by the use of tape. They are generally well tolerated but when used in a narrow head coil, they may be uncomfortable and result in subject drop out (Cantin et al., 2011).
3.5.2.3. Non-vented face mask
A non-rebreather mask or anaesthetic mask (non-vented), offers a much improved seal and reduced mask volume resulting in more controlled gas delivery and sampling. However the tight fit may make them less tolerable to patients and their structure is less malleable than standard face masks making them more difficult to fit into MRI head coils. Modified masks have been used to allow fit (Shen et al., 2011). As these masks are non-vented they create the risk of suffocation by rebreathing expired gas, and thus must be used with an appropriate breathing circuit such as a uni-directional valved circuit with a gas reservoir for safety (Shen et al., 2011, Tancredi et al., 2014). These may improve the reliability of CBF experiments (Cain et al., 2013).
4. MRI sequences and examples of use in brain disease
4.1. BOLD signal: fMRI and SWI
Functional MRI using gradient-echo echo-planar imaging with strong T2* weighting measures the blood-oxygenation level dependent (BOLD) signal. Oxyhaemoglobin is diamagnetic and has limited effect on T2*-weighted signal. Deoxyhaemoglobin is paramagnetic and leads to a reduction in T2*-weighted signal. The BOLD signal depends on CBF, CBV, cerebral metabolic rate of oxygen (CMRO2), haematocrit and PaO2,although in healthy subjects it is dominated by CBF (Shiino et al., 2003).
Hypercapnia-induced CBF increase “washes out” deoxyhaemoglobin causing an increase in T2* weighted signal (Shiino et al., 2003). The signal difference between normocapnia and hypercapnia can be used to measure CVR. Validation studies using 15O PET have been used to compare BOLD signal change to relative CBF change in healthy volunteers(Rostrup et al., 2000) In 25 patients with arterial steno-occlusive disease there was a strong correlation between hemispheric CVR using BOLD MRI and ASL MRI (Mandell et al., 2008). In Moyamoya disease, CVR correlated with severity and the presence of collaterals (Heyn et al., 2010). CVR mapping performed pre and post-operatively showed areas of vascular steal correlating with severe stenosis, which was resolved following successful revascularization, suggesting CVR mapping could be used for pre-operative planning (Mikulis et al., 2005) (Han et al., 2011). CO2 challenge also demonstrated impaired CVR around arteriovenous malformations in those patients prone to seizures compared to those without a history of seizure (Fierstra et al., 2011).
In hyperoxic states, the extra dissolved O2 means oxygen remains bound to haemoglobin and results in a reduced concentration of deoxyhaemoglobin in tissues thereby increasing T2* signal. We have investigated O2 challenge T2*-weighted MRI in subjects with stroke within 24h of onset. The putative infarct core showed diminished T2*-weighted signal increase compared to normal tissue, suggesting this technique may be able to tease out changes in metabolic activity (Dani et al., 2010). The technique has recently been validated in rodent models using T2* oxygen challenge with measurements of CBF (Robertson et al., 2015). O2 challenge T2*-weighted MRI has also been investigated in temporal lobe epilepsy, with 7/10 patients showing changes in T2* responses identifying seizure lateralization including two patients with normal MRI scans (Kalamangalam et al., 2012).
BOLD imaging allows the use of continuous measurements and assessment of dynamic responses to altered gas concentrations. In patients with Alzheimer's disease, maximal BOLD signal took longer to achieve than in normal controls suggesting some vasomotor impairment (Cantin et al., 2011).
BOLD imaging with a respiratory challenge has therefore been used to: demonstrate physiological impairments such as reduced vascular reserve and vascular steal phenomenon; confirm disease laterality; correlate with clinical outcome measures; and to plan and predict surgical outcomes of revascularization.
Susceptibility-weighted imaging (SWI) is an alternative sequence sensitive to BOLD signal, and shows cerebral venous architecture and iron-containing tissues with high-spatial resolution. Expected BOLD responses to carbogen (95% O2, 5% CO2) and hyperoxia challenges have been detected in normal volunteers using SWI (Rauscher et al., 2005). Heterogeneous tumour response to carbogen and O2 was demonstrated in two patients with glioma, indicating tissue heterogeneity caused by necrotic, oedematous tissue and abnormal neovascularization (Rauscher et al., 2005). As tumour responsiveness to radiotherapy may be increased with increased tumour O2 levels (Gray et al., 1953), investigation of oxygenation and flow within brain tumours may aid disease management. SWI offers a way of imaging regional BOLD changes with high spatial resolution (Rauscher et al., 2005). However SWI is susceptible to motion artefacts which may be more likely to occur with higher FiCO2 (Sedlacik et al., 2008).
CVR assessment may also be used to exclude patients with impaired CVR from fMRI studies, due to the risk of abolished BOLD responses in these patients (Krainik et al., 2005).
4.2. Perfusion
Arterial spin labelling (ASL) imaging involves magnetically labelling protons in the arterial blood supply. As this blood passes into the brain, it causes a small signal loss in brain tissue compared to non-labelled images, which allows measurement of CBF. When used with a challenge, paired blood flow measurements, i.e. a baseline and then a stimulus, can be obtained allowing calculation of CVR. ASL offers the advantages of being non-invasive, repeatable, and quantifiable, but has the disadvantage of low signal-to-noise ratio in comparison to dynamic susceptibility weighted contrast methods for investigating perfusion (Wintermark et al., 2005). Arterial occlusion can lead to vessel-based artefacts in collateral circulation. These problems can be improved with high-field MRI and/or non-continuous ASL sequences.
ASL has been validated in conditions of rest, activity and hypercapnia challenge against 15O PET (Chen et al., 2008). Brain perfusion in dementia has been investigated using ASL and a 5% CO2 challenge in 49 patients. Regional CBF (rCBF) was lower and CVR impaired in frontal and temporal cortices of Alzheimer's disease patients, compared patients with in vascular dementia (Gao et al., 2013). Transcranial Doppler ultrasound (TCD) failed to demonstrate any difference in blood velocity or CVR. If regional patterns distinguish different types of dementia, this may offer an advantage over global measures of flow such as TCD. A rebreathing challenge was used to demonstrate an association between high cardiovascular risk profile and impaired hippocampal vasoreactivity in mild cognitive impairment (Glodzik et al., 2011), which was more sensitive than baseline CBF or brain volume. ASL performed with hypocapnic hyperventilation and CO2 rebreathing (95% O2, 5% CO2) challenges in 39 chronic large territory stroke patients, demonstrated reduced ipsilateral CBF but widespread impaired vasodilatory capacity with preserved vasoconstriction. This demonstrated that impaired vasoreactivity extends beyond the infarcted region. The continuous ASL technique has been shown to be quantifiable, although the long transit times seen in stroke may lead to underestimation of blood flow in stroke patients (Zhao et al., 2009). This is because the flow is so slow, that the signal decays by the time it has reached the tissue being imaged.
Perfusion imaging with gadolinium bolus tracking is less commonly used in respiratory challenge experiments due to it being less easily repeated, requiring IV access, and gadolinium contrast having safety concerns in the face of extravasation or impaired renal function. MRI bolus tracking and acetazolamide challenge was used in 15 patients with CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) to show impaired perfusion and reactivity in white matter hyperintensities (Chabriat et al., 2000) and we are currently undertaking both ASL and gadolinium bolus tracking with CO2 challenge in 22 CADASIL patients.
4.3. Dual echo imaging (BOLD and perfusion)
BOLD and ASL can be used in sequence to provide information about the cerebrovascular system. Using both methods can permit the assessment of multiple measures of cerebral haemodynamics including CBF, CVR, BOLD CVR, oxygen extraction fraction and CMRO2(Bulte et al., 2012, Gauthier et al., 2012, Wise et al., 2013). In a study of patients with internal carotid artery occlusion, BOLD CVR signal was lower in the affected middle cerebral artery territory (De Vis et al., 2015).
Vessel-specific labelling of blood, known as vessel encoded ASL (VE-ASL) has also been used in combination with BOLD in patients with cerebrovascular disease. BOLD signal change and CBF reactivity correlated significantly in response to carbogen, and was able to identify disease lateralisation in steno-occlusive carotid disease, although its application remains complex (Faraco et al., 2015). The use of BOLD and carbogen results in an increase in BOLD signal both due to increased flow and increased partial pressure of oxygen, and therefore its use for calculation of CVR is complex and can lead to misleading results (Hare et al., 2013).
4.4. Cerebral blood volume: VASO
Cerebral blood volume (CBV) has a linear relationship to changes in PaCO2 but a non-linear relationship to changes in CBF (Grubb et al., 1974). The vascular space occupancy (VASO) technique is a CBV-weighted imaging technique which measures change in extravascular parenchymal signal. It has better spatial resolution than BOLD, but poor signal-to-noise ratio. It has been used to demonstrate signal decreases (suggesting increased intravascular blood volume) in grey matter in response to short periods of breath-hold in normal volunteers and patients with a meningioma (Hsu et al., 2012). More negative VASO reactivity in response to breath-hold challenge was seen in patients with ICA stenosis compared to controls, proposed to represent haemodynamic impairment(Donahue et al., 2009). Using VASO with BOLD techniques may help extract the influence of CBV on the BOLD signal in disease states.
4.5. Other
Other MRI sequences have also been used with respiratory challenge. Hyperventilation is known to provoke epileptiform activity, and was used to compare changes in diffusion (ADC) of temporal lobe epilepsy (TLE) patients with controls. TLE with hippocampal sclerosis patients showed higher baseline ADC in the abnormal hippocampus and a significant decrease in ADC compared to controls. Altered ADC at baseline is thought to represent altered cellular organization with the decrease in ADC with hyperventilation (and hypocapnia) caused by altered cellular permeability, cellular swelling and reduced extracellular space (Leonhardt et al., 2002). Gadolinium-DTPA infusion with gradient-echo imaging, accompanied by T2* weighted imaging, has been used to study contrast uptake in meningiomas. A hyperoxic hypercapnic challenge improved tumour oxygenation and increased contrast uptake rate in 6 out of 10 patients, who the authors proposed may be more responsive to radiotherapy with hyperoxygenation (Rijpkema et al., 2004).
5. Recommendations
Despite the variety of techniques available, application of respiratory challenge MRI may benefit from some standardization. Subject withdrawal may be reduced by ensuring the recruitment of subjects without MR or gas exclusions and undertaking a trial run of the procedure outside the MR scanner (Taylor et al., 2001). Whilst centres will vary in the availability of equipment or expertise to use certain challenges or MR sequences, recording subject parameters and standardizing testing conditions may reduce testing variability and allow studies to be compared more readily. Some proposed recommendations for the conduct of respiratory challenge MRI studies are outlined in Table 3.
Table 3.
Proposed recommendations for conducting a respiratory challenge MRI experiment.
| Perform a trial run of the gas challenge with the subject outside the scanner to assess tolerability and optimize subject compliance. |
| Standardize testing conditions by following published guidelines for performing vascular tests including those outlined in Table 2. |
| Record delivered and end-tidal gases, along with respiratory rate and heart rate to ensure patient safety and to allow correlation with signal change. These values can then be used for quantification of the change in MR parameter. |
| If examining steady-state effects of hypercarbia or hyperoxia, allow sufficient time for steady-state to be obtained. |
| Liaise with anesthetists and MR physics department to ensure breathing apparatus and monitoring equipment is safe and MR appropriate. |
| Exclude patients with significant pulmonary or cardiac disease. |
| Ensure the gas supply is sufficient to support increases in minute ventilation. |
| Use a maximum FiCO2 of 8% to avoid subject discomfort unless specifically indicated (Ainslie and Duffin, 2009). |
6. Conclusions
Respiratory challenge MRI has the potential to be widely used in the assessment of brain diseases due to its safety, tolerability and repeatability. Whilst problems remain with reliable gas administration, it has been used to provide valuable insights into brain pathophysiology. For such techniques to function as biomarkers to assess disease progression or treatment response, standardization of testing is important.
Author contribution statement
FM reviewed the literature and wrote the first draft of the manuscript, and modified all subsequent drafts. KD planned the review and contributed to all drafts of the manuscript. CG, and KOH contributed to design of the review and contributed to all drafts of the manuscript. KWM contributed to design of the review and made modifications to all drafts of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
Respiratory challenge research undertaken in the Queen Elizabeth University Hospital has been supported by two project grants from the Chief Scientists Office of Scotland (ETM/244 and ETM/104).
References
- Aaslid R., Lindegaard K.F., Sorteberg W., Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- Addicott M.A., Yang L.L., Peiffer A.M., Burnett L.R., Burdette J.H., Chen M.Y., Hayasaka S., Kraft R.A., Maldjian J.A., Laurienti P.J. The effect of daily caffeine use on cerebral blood flow: how much caffeine can we tolerate? Hum. Brain Mapp. 2009;30:3102–3114. doi: 10.1002/hbm.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie P.N., Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R1473–R1495. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- Bartelink M.L., Wollersheim H., Theeuwes A., van Duren D., Thien T. Changes in skin blood flow during the menstrual cycle: the influence of the menstrual cycle on the peripheral circulation in healthy female volunteers. Clin. Sci. 1990;78:527–532. doi: 10.1042/cs0780527. [DOI] [PubMed] [Google Scholar]
- Battisti-Charbonney A., Fisher J., Duffin J. The cerebrovascular response to carbon dioxide in humans. J. Physiol. 2011;589:3039–3048. doi: 10.1113/jphysiol.2011.206052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson J., Bake B., Johansson Å., Bengtson J.P. End-tidal to arterial oxygen tension difference as an oxygenation index. Acta Anaesthesiol. Scand. 2001;45:357–363. doi: 10.1034/j.1399-6576.2001.045003357.x. [DOI] [PubMed] [Google Scholar]
- Brian J.E.J. Carbon dioxide and the cerebral circulation. Anesthesiology. 1998;88:1365–1386. doi: 10.1097/00000542-199805000-00029. [DOI] [PubMed] [Google Scholar]
- Bright M.G., Murphy K. Reliable quantification of BOLD fMRI cerebrovascular reactivity despite poor breath-hold performance. NeuroImage. 2013;83:559–568. doi: 10.1016/j.neuroimage.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulte D.P., Chiarelli P.A., Wise R.G., Jezzard P. Cerebral perfusion response to hyperoxia. J. Cereb. Blood Flow Metab. 2006;27:69–75. doi: 10.1038/sj.jcbfm.9600319. [DOI] [PubMed] [Google Scholar]
- Bulte D.P., Drescher K., Jezzard P. Comparison of hypercapnia-based calibration techniques for measurement of cerebral oxygen metabolism with MRI. Magn. Reson. Med. 2009;61:391–398. doi: 10.1002/mrm.21862. [DOI] [PubMed] [Google Scholar]
- Bulte D.P., Kelly M., Germuska M., Xie J., Chappell M.A., Okell T.W., Bright M.G., Jezzard P. Quantitative measurement of cerebral physiology using respiratory-calibrated MRI. NeuroImage. 2012;60:582–591. doi: 10.1016/j.neuroimage.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain J.R., Parkes L.M., Eadsforth P., Beards S.C., Jackson A. Impact of gas delivery systems on imaging studies of human cerebral blood flow. Radiology Research and Practice. 2013;2013:5. doi: 10.1155/2013/694803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin S., Villien M., Moreaud O., Tropres I., Keignart S., Chipon E., Le Bas J.F., Warnking J., Krainik A. Impaired cerebral vasoreactivity to CO2 in Alzheimer's disease using BOLD fMRI. NeuroImage. 2011;58:579–587. doi: 10.1016/j.neuroimage.2011.06.070. [DOI] [PubMed] [Google Scholar]
- Chabriat H., Pappata S., Ostergaard L., Clark C.A., Pachot-Clouard M., Vahedi K., Jobert A., Le Bihan D., Bousser M.G. Cerebral hemodynamics in CADASIL before and after acetazolamide challenge assessed with MRI bolus tracking. Stroke. 2000;31:1904–1912. doi: 10.1161/01.str.31.8.1904. [DOI] [PubMed] [Google Scholar]
- Chambers D., Huang C., Matthews G. Cambridge University Press; 2015. Basic Physiology for Anaesthetists. [Google Scholar]
- Chen J.J., Wieckowska M., Meyer E., Pike G.B. Cerebral blood flow measurement using fMRI and PET: a cross-validation study. International Journal of Biomedical Imaging. 2008;2008:12. doi: 10.1155/2008/516359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury T., Petropolis A., Wilkinson M., Schaller B., Sandu N., Cappellani R.B. Controversies in the anesthetic management of intraoperative rupture of intracranial aneurysm. Anesthesiology Research and Practice. 2014;2014:10. doi: 10.1155/2014/595837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti A., Esquivel G., Schruers K., Griez E. On the psychotropic effects of carbon dioxide. Curr. Pharm. Des. 2012;18:5627–5637. doi: 10.2174/138161212803530745. [DOI] [PubMed] [Google Scholar]
- Coverdale N.S., Gati J.S., Opalevych O., Perrotta A., Shoemaker J.K. 2014. Cerebral Blood Flow Velocity Underestimates Cerebral Blood Flow During Modest Hypercapnia and Hypocapnia. [DOI] [PubMed] [Google Scholar]
- Dani K.A., Santosh C., Brennan D., McCabe C., Holmes W.M., Condon B., Hadley D.M., Macrae I.M., Shaw M., Muir K.W. T2*-weighted magnetic resonance imaging with hyperoxia in acute ischemic stroke. Ann. Neurol. 2010;68:37–47. doi: 10.1002/ana.22032. [DOI] [PubMed] [Google Scholar]
- Davis W.B., Rennard S.I., Bitterman P.B., Crystal R.G. Pulmonary oxygen toxicity. N. Engl. J. Med. 1983;309:878–883. doi: 10.1056/NEJM198310133091502. [DOI] [PubMed] [Google Scholar]
- De Vis J.B., Petersen E.T., Bhogal A., Hartkamp N.S., Klijn C.J.M., Kappelle L.J., Hendrikse J. Calibrated MRI to evaluate cerebral hemodynamics in patients with an internal carotid artery occlusion. J. Cereb. Blood Flow Metab. 2015;35:1015–1023. doi: 10.1038/jcbfm.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demchenko I.T., Boso A.E., O'Neill T.J., Bennett P.B., Piantadosi C.A. Nitric oxide and cerebral blood flow responses to hyperbaric oxygen. J. Appl. Physiol. 2000;88:1381–1389. doi: 10.1152/jappl.2000.88.4.1381. [DOI] [PubMed] [Google Scholar]
- Dempsey M.F., Condon B. Thermal injuries associated with MRI. Clin. Radiol. 2001;56:457–465. doi: 10.1053/crad.2000.0688. [DOI] [PubMed] [Google Scholar]
- Donahue M.J., van Laar P.J., van Zijl P.C., Stevens R.D., Hendrikse J. Vascular space occupancy (VASO) cerebral blood volume-weighted MRI identifies hemodynamic impairment in patients with carotid artery disease. J. Magn. Reson. Imaging. 2009;29:718–724. doi: 10.1002/jmri.21667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan M., Kavanagh B. Pulmonary atelectasis: a pathogenic perioperative entity. American Society of Anesthesiologists. 2005;102:838–854. doi: 10.1097/00000542-200504000-00021. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Krause D.N. second ed. Lippincott Williams & Wilkins; Philadelphia; London: 2002. Cerebral Blood Flow and Metabolism. [Google Scholar]
- Ellingsen I., Hauge A., Nicolaysen G., Thoresen M., Walloe L. Changes in human cerebral blood flow due to step changes in PAO2 and PACO2. Acta Physiol. Scand. 1987;129:157–163. doi: 10.1111/j.1748-1716.1987.tb08054.x. [DOI] [PubMed] [Google Scholar]
- Eskey C.J., Sanelli P.C. Perfusion imaging of cerebrovascular reserve. Neuroimaging Clin. N. Am. 2005;15:367–381. doi: 10.1016/j.nic.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Faraco C.C., Strother M.K., Dethrage L.M., Jordan L., Singer R., Clemmons P.F., Donahue M.J. Dual echo vessel-encoded ASL for simultaneous BOLD and CBF reactivity assessment in patients with ischemic cerebrovascular disease. Magn. Reson. Med. 2015;73:1579–1592. doi: 10.1002/mrm.25268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farling P., Bullen K., Britton J., Darwent G., DeWilde J., Giles S., Goddard P., King S., McBrien M., McDonald P., Menon D., Ridgway J., Sury M., Wilson S. The Association of Anaesthetists of Great Britain and Ireland; London: 2002. Provision of Anaesthetic Services in Magnetic Resonance Units. [Google Scholar]
- Farling P.A., Flynn P.A., Darwent G., De Wilde J., Grainger D., King S., McBrien M.E., Menon D.K., Ridgway J.P., Sury M., Thornton J., Wilson S.R. Safety in magnetic resonance units: an update. Anaesthesia. 2010;65:766–770. doi: 10.1111/j.1365-2044.2010.06377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierstra J., Conklin J., Krings T., Slessarev M., Han J.S., Fisher J.A., Terbrugge K., Wallace M.C., Tymianski M., Mikulis D.J. Impaired peri-nidal cerebrovascular reserve in seizure patients with brain arteriovenous malformations. Brain. 2011;134:100–109. doi: 10.1093/brain/awq286. [DOI] [PubMed] [Google Scholar]
- Fierstra J., Sobczyk O., Battisti-Charbonney A., Mandell D.M., Poublanc J., Crawley A.P., Mikulis D.J., Duffin J., Fisher J.A. Measuring cerebrovascular reactivity: what stimulus to use? J. Physiol. 2013;591:5809–5821. doi: 10.1113/jphysiol.2013.259150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.-Z., Zhang J.-J., Liu H., Wu G.-Y., Xiong L., Shu M. Regional cerebral blood flow and cerebrovascular reactivity in Alzheimer's disease and vascular dementia assessed by arterial spinlabeling magnetic resonance imaging. Current Neurovascular Research. 2013;10 doi: 10.2174/156720213804806016. February. [DOI] [PubMed] [Google Scholar]
- Gauthier C.J., Desjardins-Crépeau L., Madjar C., Bherer L., Hoge R.D. Absolute quantification of resting oxygen metabolism and metabolic reactivity during functional activation using QUO2 MRI. NeuroImage. 2012;63:1353–1363. doi: 10.1016/j.neuroimage.2012.07.065. [DOI] [PubMed] [Google Scholar]
- Glodzik, L., Rusinek, H., Brys, M., Tsui, W.H., Switalski, R., Mosconi, L., Mistur, R., Pirraglia, E., de, S.S., Li, Y., Goldowsky, A., de Leon, M.J., 2011. Framingham cardiovascular risk profile correlates with impaired hippocampal and cortical vasoreactivity to hypercapnia. J. Cereb. Blood Flow Metab. 31, 671–679. [DOI] [PMC free article] [PubMed]
- Golanov E.V., Reis D.J. Oxygen and cerebral blood flow. In: Caplan L., Reis D., Siesjo B., Weir B., Welch K.M., editors. Primer on Cerebrovascular Diseases. Academic Press; 1997. pp. 58–60. [Google Scholar]
- Gray L.H., Conger A.D., Ebert M., Hornsey S., Scott O.C.A. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- Grubb R.L., Raichle M.E., Eichling J.O., Ter-Pogossian M.M. The effects of changes in PaCO2 cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5:630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- Guaranha M.S.B., Garzon E., Buchpiguel C.A., Tazima S., Yacubian E.M.T., Sakamoto A.C. Hyperventilation revisited: physiological effects and efficacy on focal seizure activation in the era of video-EEG monitoring. Epilepsia. 2005;46:69–75. doi: 10.1111/j.0013-9580.2005.11104.x. [DOI] [PubMed] [Google Scholar]
- Gundersen H., van Wageningen H., Grüner R. Alcohol-induced changes in cerebral blood flow and cerebral blood volume in social drinkers. Alcohol Alcohol. 2013;48:160–165. doi: 10.1093/alcalc/ags121. [DOI] [PubMed] [Google Scholar]
- Hall E.L., Driver I.D., Croal P.L., Francis S.T., Gowland P.A., Morris P.G., Brookes M.J. The effect of hypercapnia on resting and stimulus induced MEG signals. NeuroImage. 2011;58:1034–1043. doi: 10.1016/j.neuroimage.2011.06.073. [DOI] [PubMed] [Google Scholar]
- Hall C.N., Reynell C., Gesslein B., Hamilton N.B., Mishra A., Sutherland B.A., O'Farrell F.M., Buchan A.M., Lauritzen M., Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.S., Abou-Hamden A., Mandell D.M., Poublanc J., Crawley A.P., Fisher J.A., Mikulis D.J., Tymianski M. Impact of extracranial-intracranial bypass on cerebrovascular reactivity and clinical outcome in patients with symptomatic moyamoya vasculopathy. Stroke. 2011;42:3047–3054. doi: 10.1161/STROKEAHA.111.615955. [DOI] [PubMed] [Google Scholar]
- Hare H.V., Germuska M., Kelly M.E., Bulte D.P. Comparison of CO2 in air versus carbogen for the measurement of cerebrovascular reactivity with magnetic resonance imaging. J. Cereb. Blood Flow Metab. 2013;33:1799–1805. doi: 10.1038/jcbfm.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzel A., Braune S., Guschlbauer B., Dohms K. CO2 reactivity testing without blood pressure monitoring? Stroke. 1999;30:398–401. doi: 10.1161/01.str.30.2.398. [DOI] [PubMed] [Google Scholar]
- Heyn C., Poublanc J., Crawley A., Mandell D., Han J.S., Tymianski M., Terbrugge K., Fisher J.A., Mikulis D.J. Quantification of cerebrovascular reactivity by blood oxygen level-dependent MR imaging and correlation with conventional angiography in patients with moyamoya disease. American Journal of Neuroradiology. 2010;31 doi: 10.3174/ajnr.A1922. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge R.D. Calibrated FMRI. [review] NeuroImage. 2012;62:930–937. doi: 10.1016/j.neuroimage.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hsu Y.Y., Chu W.C., Lim K.E., Liu H.L. Vascular space occupancy MRI during breathholding at 3 Tesla. J. Magn. Reson. Imaging. 2012;36:1179–1185. doi: 10.1002/jmri.23745. [DOI] [PubMed] [Google Scholar]
- Hsu Y.Y., Kuan W.C., Lim K.E., Liu H.L. Breathhold-regulated blood oxygenation level-dependent (BOLD) MRI of human brain at 3 tesla. J. Magn. Reson. Imaging. 2010;31:78–84. doi: 10.1002/jmri.22015. [DOI] [PubMed] [Google Scholar]
- Hudak M.L., Koehler R.C., Rosenberg A.A., Traystman R.J., Jones M.D. 1986. Effect of Hematocrit on Cerebral Blood Flow. [DOI] [PubMed] [Google Scholar]
- Ito S., Mardimae A., Han J., Duffin J., Wells G., Fedorko L., Minkovich L., Katznelson R., Meineri M., Arenovich T., Kessler C., Fisher J.A. Non-invasive prospective targeting of arterial PCO2 in subjects at rest. J. Physiol. 2008;586:3675–3682. doi: 10.1113/jphysiol.2008.154716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Takahashi K., Hatazawa J., Kim S.-G., Kanno I. Changes in human regional cerebral blood flow and cerebral blood volume during visual stimulation measured by positron emission tomography. J. Cereb. Blood Flow Metab. 2001;21:608–612. doi: 10.1097/00004647-200105000-00015. [DOI] [PubMed] [Google Scholar]
- Jackson R.M. Pulmonary oxygen toxicity. CHEST Journal. 1985;88:900–905. doi: 10.1378/chest.88.6.900. [DOI] [PubMed] [Google Scholar]
- Jamieson D., Chance B., Cadenas E., Boveris A. The relation of free radical production to hyperoxia. Annu. Rev. Physiol. 1986;48:703–719. doi: 10.1146/annurev.ph.48.030186.003415. [DOI] [PubMed] [Google Scholar]
- Kalamangalam G.P., Nelson J.T., Ellmore T.M., Narayana P.A. Oxygen-enhanced MRI in temporal lobe epilepsy: diagnosis and lateralization. Epilepsy Res. 2012;98:50–61. doi: 10.1016/j.eplepsyres.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Kanal E., Barkovich A.J., Bell C., Borgstede J.P., Bradley W.G., Froelich J.W., Gimbel J.R., Gosbee J.W., Kuhni-Kaminski E., Larson P.A., Lester J.W., Nyenhuis J., Schaefer D.J., Sebek E.A., Weinreb J., Wilkoff B.L., Woods T.O., Lucey L., Hernandez D. ACR guidance document on MR safe practices: 2013. J. Magn. Reson. Imaging. 2013;37:501–530. doi: 10.1002/jmri.24011. [DOI] [PubMed] [Google Scholar]
- Kastrup A., Li T.Q., Glover G.H., Moseley M.E. Cerebral blood flow-related signal changes during breath-holding. Ajnr: American Journal of Neuroradiology. 1999;20:1233–1238. [PMC free article] [PubMed] [Google Scholar]
- Kety S.S., Schmidt C.F. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J. Clin. Invest. 1948;27:484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety S.S., Schmidt C.F. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J. Clin. Invest. 1948;27:476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama M., Nishikawa M., Yasui T., Sakamoto H. Reversible pontine ischemia caused by acetazolamide challenge. Am. J. Neuroradiol. 1997;18:1782–1784. [PMC free article] [PubMed] [Google Scholar]
- Krainik, A., Hund-Georgiadis, M., Zysset, S., von Cramon, D.Y., 2005. Regional impairment of cerebrovascular reactivity and BOLD signal in adults after stroke. Stroke 36, 1146–1152. [DOI] [PubMed]
- Krainik A., Villien M., Troprès I., Attyé A., Lamalle L., Bouvier J., Pietras J., Grand S., Le Bas J.F., Warnking J. Functional imaging of cerebral perfusion. Diagnostic and Interventional Imaging. 2013;94:1259–1278. doi: 10.1016/j.diii.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Kuluz J.W., Prado R., Chang J., Ginsberg M.D., Schleien C.L., Busto R. Selective brain cooling increases cortical cerebral blood flow in rats. Am. J. Physiol. Heart Circ. Physiol. 1993;265:H824–H827. doi: 10.1152/ajpheart.1993.265.3.H824. [DOI] [PubMed] [Google Scholar]
- Kuridze N., Czernicki Z., Jarus-Dziedzic K., Jurkiewicz J., Cervos-Navarro J. Regional differences of cerebrovascular reactivity effected by calcium channel blocker – Dotarizine. J. Neurol. Sci. 2000;175:13–16. doi: 10.1016/s0022-510x(00)00275-6. [DOI] [PubMed] [Google Scholar]
- Laurent S., Cockcroft J., Van Bortel L., Boutouyrie P., Giannattasio C., Hayoz D., Pannier B., Vlachopoulos C., Wilkinson I., Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- Lavy S., Stern S., Melamed E., Cooper G., Keren A., Levy P. Effect of chronic atrial fibrillation on regional cerebral blood flow. Stroke. 1980;11:35–38. doi: 10.1161/01.str.11.1.35. [DOI] [PubMed] [Google Scholar]
- Leenders K.L., Perani D., Lammertsma A.A., Heather J.D., Buckingham P., Jones T., Healy M.J.R., Gibbs J.M., Wise R.J.S., Hatazawa J., Herold S., Beaney R.P., Brooks D.J., Spinks T., Rhodes C., Frackowiak R.S.J. Cerebral blood flow, blood volume and oxygen utilization: normal values and effect of age. Brain. 1990;113:27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- Leonhardt G., de Greiff A., Marks S., Ludwig T., Doerfler A., Forsting M., Konermann S., Hufnagel A. Brain diffusion during hyperventilation: diffusion-weighted MR-monitoring in patients with temporal lobe epilepsy and in healthy volunteers. Epilepsy Res. 2002;51:269–278. doi: 10.1016/s0920-1211(02)00154-7. [DOI] [PubMed] [Google Scholar]
- Lunt M.J., Ragab S., Birch A.A., Schley D., Jenkinson D.F. Comparison of caffeine-induced changes in cerebral blood flow and middle cerebral artery blood velocity shows that caffeine reduces middle cerebral artery diameter. Physiol. Meas. 2004;25:467–474. doi: 10.1088/0967-3334/25/2/006. [DOI] [PubMed] [Google Scholar]
- Mandell D.M., Han J.S., Poublanc J., Crawley A.P., Stainsby J.A., Fisher J.A., Mikulis D.J. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in patients with arterial steno-occlusive disease: comparison with arterial spin labeling MRI. Stroke. 2008;39:2021–2028. doi: 10.1161/STROKEAHA.107.506709. [DOI] [PubMed] [Google Scholar]
- Marino P. Lippincott Williams & Wilkins; 2013. Marino's the ICU Book. [Google Scholar]
- Martin D.S., Grocott M.P.W. Oxygen therapy in critical illness: precise control of arterial oxygenation and permissive hypoxemia. Crit. Care Med. 2013;41:423–432. doi: 10.1097/CCM.0b013e31826a44f6. [DOI] [PubMed] [Google Scholar]
- Mikulis D.J., Krolczyk G., Desal H., Logan W., Deveber G., Dirks P., Tymianski M., Crawley A., Vesely A., Kassner A., Preiss D., Somogyi R., Fisher J.A. Preoperative and postoperative mapping of cerebrovascular reactivity in moyamoya disease by using blood oxygen level-dependent magnetic resonance imaging. J. Neurosurg. 2005;103:347–355. doi: 10.3171/jns.2005.103.2.0347. [DOI] [PubMed] [Google Scholar]
- Murakami M., Fujioka S., Hirata Y., Kuratsu J.-I. Low-dose of statin treatment improves cerebrovascular reactivity in patients with ischemic stroke: single photon emission computed tomography analysis. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association. 2008;17:16–22. doi: 10.1016/j.jstrokecerebrovasdis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Mutch W.A., Mandell D.M., Fisher J.A., Mikulis D.J., Crawley A.P., Pucci O., Duffin J. Approaches to brain stress testing: BOLD magnetic resonance imaging with computer-controlled delivery of carbon dioxide. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganawa S., Norris D.G., Zysset S., Mildner T. Regional differences of fMR signal changes induced by hyperventilation: comparison between SE-EPI and GE-EPI at 3-T. J. Magn. Reson. Imaging. 2002;15:23–30. doi: 10.1002/jmri.10028. [DOI] [PubMed] [Google Scholar]
- Noth U., Kotajima F., Deichmann R., Turner R., Corfield D.R. Mapping of the cerebral vascular response to hypoxia and hypercapnia using quantitative perfusion MRI at 3 T. NMR Biomed. 2008;21:464–472. doi: 10.1002/nbm.1210. [DOI] [PubMed] [Google Scholar]
- Novack P., Shenkin H.A., Bortin L., Goluboff B., Soffe A.M., Batson P., Golden D. The effects of carbon dioxide inhalation upon the cerebral blood flow and cerebral oxygen consumption in vascular disease. J. Clin. Investig. 1953;32:696–702. doi: 10.1172/JCI102783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura T., Teshima Y., Isse K., Matsuda H., Inoue T., Sakai Y., Iwasaki N., Yaoi Y. Estrogen increases cerebral and cerebellar blood flows in postmenopausal women. Menopause. 1995;2:13–18. [Google Scholar]
- Omae T., Ibayashi S., Kusuda K., Nakamura H., Yagi H., Fujishima M. Effects of high atmospheric pressure and oxygen on middle cerebral blood flow velocity in humans measured by transcranial Doppler. Stroke. 1998;29:94–97. doi: 10.1161/01.str.29.1.94. [DOI] [PubMed] [Google Scholar]
- Petersen P., Kastrup J., Videbaek R., Boysen G. Cerebral blood flow before and after cardioversion of atrial fibrillation. J. Cereb. Blood Flow Metab. 1989;9:422–425. doi: 10.1038/jcbfm.1989.62. [DOI] [PubMed] [Google Scholar]
- Pilkinton D.T., Hiraki T., Detre J.A., Greenberg J.H., Reddy R. Absolute cerebral blood flow quantification with pulsed arterial spin labeling during hyperoxia corrected with the simultaneous measurement of the longitudinal relaxation time of arterial blood. Magn. Reson. Med. 2012;67:1556–1565. doi: 10.1002/mrm.23137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin M.J., Liang P.J., Robbins P.A. Dynamics of the cerebral blood flow response to step changes in end-tidal PCO2 and PO2 in humans. J. Appl. Physiol. 1996;81:1084–1095. doi: 10.1152/jappl.1996.81.3.1084. [DOI] [PubMed] [Google Scholar]
- Prakash K., Chandran D.S., Khadgawat R., Jaryal A.K., Deepak K.K. Correction for blood pressure improves correlation between cerebrovascular reactivity assessed by breath holding and 6% CO2 breathing. J. Stroke Cerebrovasc. Dis. 2014;23:630–635. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., Plum F. Hyperventilation and cerebral blood flow. Stroke. 1972;3:566–575. doi: 10.1161/01.str.3.5.566. [DOI] [PubMed] [Google Scholar]
- Ratnatunga C., Adiseshiah M. Increase in middle cerebral artery velocity on breath holding: a simplified test of cerebral perfusion reserve. Eur. J. Vasc. Surg. 1990;4:519–523. doi: 10.1016/s0950-821x(05)80795-9. [DOI] [PubMed] [Google Scholar]
- Rauscher A., Sedlacik J., Barth M., Haacke E.M., Reichenbach J.R. Noninvasive assessment of vascular architecture and function during modulated blood oxygenation using susceptibility weighted magnetic resonance imaging. Magn. Reson. Med. 2005;54:87–95. doi: 10.1002/mrm.20520. [DOI] [PubMed] [Google Scholar]
- Reivich M. Arterial PCO2 and cerebral hemodynamics. Am. J. Physiol. 1964;206:25–35. doi: 10.1152/ajplegacy.1964.206.1.25. [DOI] [PubMed] [Google Scholar]
- Rijpkema M., Schuuring J., Bernsen P.L., Bernsen H.J., Kaanders J.H., van der Kogel A.J., Heerschap A. BOLD MRI response to hypercapnic hyperoxia in patients with meningiomas: correlation with gadolinium-DTPA uptake rate. Magn. Reson. Imaging. 2004;22:761–767. doi: 10.1016/j.mri.2004.01.055. [DOI] [PubMed] [Google Scholar]
- Robertson C.A., McCabe C., Lopez-Gonzalez M.R., Deuchar G.A., Dani K., Holmes W.M., Muir K.W., Santosh C., Macrae I.M. Detection of ischemic penumbra using combined perfusion and T2* oxygen challenge imaging. International Journal of Stroke. 2015;10:42–50. doi: 10.1111/ijs.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostrup E., Knudsen G.M., Law I., Holm S., Larsson H.B., Paulson O.B. The relationship between cerebral blood flow and volume in humans. NeuroImage. 2005;24:1–11. doi: 10.1016/j.neuroimage.2004.09.043. [DOI] [PubMed] [Google Scholar]
- Rostrup E., Law I., Blinkenberg M., Larsson H.B.W., Born A.P., Holm S., Paulson O.B. Regional differences in the CBF and BOLD responses to hypercapnia: a combined PET and fMRI study. NeuroImage. 2000;11:87–97. doi: 10.1006/nimg.1999.0526. [DOI] [PubMed] [Google Scholar]
- Saunders K.B. Methods in the assessment of the control of breathing. Br. J. Clin. Pharmacol. 1980;9:3–9. doi: 10.1111/j.1365-2125.1980.tb04789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer C., Deichmann R. Vascular component analysis of hyperoxic and hypercapnic BOLD contrast. NeuroImage. 2012;59:2401–2412. doi: 10.1016/j.neuroimage.2011.08.110. [DOI] [PubMed] [Google Scholar]
- Scouten A., Schwarzbauer C. Paced respiration with end-expiration technique offers superior BOLD signal repeatability for breath-hold studies. NeuroImage. 2008;43:250–257. doi: 10.1016/j.neuroimage.2008.03.052. [DOI] [PubMed] [Google Scholar]
- Sedlacik J., Kutschbach C., Rauscher A., Deistung A., Reichenbach J.R. Investigation of the influence of carbon dioxide concentrations on cerebral physiology by susceptibility-weighted magnetic resonance imaging (SWI) NeuroImage. 2008;43:36–43. doi: 10.1016/j.neuroimage.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Selim M., Jones R., Novak P., Zhao P., Novak V. The effects of body mass index on cerebral blood flow velocity. Clin. Auton. Res. 2008;18:331–338. doi: 10.1007/s10286-008-0490-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Ahearn T., Clemence M., Schwarzbauer C. Magnetic resonance imaging of the mean venous vessel size in the human brain using transient hyperoxia. NeuroImage. 2011;55:1063–1067. doi: 10.1016/j.neuroimage.2010.12.084. [DOI] [PubMed] [Google Scholar]
- Shiino A., Morita Y., Tsuji A., Maeda K., Ito R., Furukawa A., Matsuda M., Inubushi T. Estimation of cerebral perfusion reserve by blood oxygenation level-dependent imaging: comparison with single-photon emission computed tomography. J. Cereb. Blood Flow Metab. 2003;23:121–135. doi: 10.1097/01.WCB.0000037546.46809.CA. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Nagata K., Yokoyama E., Sato M., Matsuoka S., Kanno I., Hatazawa J., Domino E.F. Acute effects of cigarette smoking on global cerebral blood flow in overnight abstinent tobacco smokers. Nicotine and Tobacco Research. 2006;8 doi: 10.1080/14622200500431759. February. [DOI] [PubMed] [Google Scholar]
- Slessarev M., Han J., Mardimae A., Prisman E., Preiss D., Volgyesi G., Ansel C., Duffin J., Fisher J.A. Prospective targeting and control of end-tidal CO2 and O2 concentrations. J. Physiol. 2007;581:1207–1219. doi: 10.1113/jphysiol.2007.129395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano V.R., Mandell D.M., Poublanc J., Sam K., Battisti-Charbonney A., Pucci O., Han J.S., Crawley A.P., Fisher J.A., Mikulis D.J. CO2 blood oxygen level-dependent MR mapping of cerebrovascular reserve in a clinical population: safety, tolerability, and technical feasibility. Radiology. 2013;266:592–598. doi: 10.1148/radiol.12112795. [DOI] [PubMed] [Google Scholar]
- Strohm J., Duffin J., Fisher J.A. Circadian cerebrovascular reactivity to CO2. Respiratory Physiology & Neurobiology. 2014;197:15–18. doi: 10.1016/j.resp.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Tancredi F., Lajoie I., Hoge R. A simple breathing circuit allowing precise control of inspiratory gases for experimental respiratory manipulations. BMC Research Notes. 2014;7:235. doi: 10.1186/1756-0500-7-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N.J., Baddeley H., Goodchild K.A., Powell M.E., Thoumine M., Culver L.A., Stirling J.J., Saunders M.I., Hoskin P.J., Phillips H., Padhani A.R., Griffiths J.R. BOLD MRI of human tumor oxygenation during carbogen breathing. J. Magn. Reson. Imaging. 2001;14:156–163. doi: 10.1002/jmri.1166. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Glover G.H. Controlled inspiration depth reduces variance in breath-holding-induced BOLD signal. NeuroImage. 2008;39:206–214. doi: 10.1016/j.neuroimage.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Burrows B.E., Gabrieli J.D., Glover G.H. Breath holding reveals differences in fMRI BOLD signal in children and adults. NeuroImage. 2005;25:824–837. doi: 10.1016/j.neuroimage.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Foland L.C., Glover G.H. Calibration of BOLD fMRI using breath holding reduces group variance during a cognitive task. Hum. Brain Mapp. 2007;28:59–68. doi: 10.1002/hbm.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W.-C., Li Y.-H., Lin C.-C., Chao T.-H., Chen J.-H. Effects of oxidative stress on endothelial function after a high-fat meal. Clin. Sci. 2004;106:315–319. doi: 10.1042/CS20030227. [DOI] [PubMed] [Google Scholar]
- Vagal A.S., Leach J.L., Fernandez-Ulloa M., Zuccarello M. The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. Am. J. Neuroradiol. 2009;30:876–884. doi: 10.3174/ajnr.A1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagal A.S., Leach J.L., Fernandez-Ulloa M., Zuccarello M. The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. AJNR Am. J. Neuroradiol. 2009;30:876–884. doi: 10.3174/ajnr.A1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bortel L., Duprez D., Starmans-Kool M., Safar M., Giannattasio C., Cockcroft J., Kaiser D., Thuillez C. Clinical applications of arterial stiffness, task force III: recommendations for user procedures. Am. J. Hypertens. 2002;15:445–452. doi: 10.1016/s0895-7061(01)02326-3. [DOI] [PubMed] [Google Scholar]
- Vesely A., Sasano H., Volgyesi G., Somogyi R., Tesler J., Fedorko L., Grynspan J., Crawley A., Fisher J.A., Mikulis D. MRI mapping of cerebrovascular reactivity using square wave changes in end-tidal PCO2. Magn. Reson. Med. 2001;45:1011–1013. doi: 10.1002/mrm.1134. [DOI] [PubMed] [Google Scholar]
- Vogt K.M., Ibinson J.W., Schmalbrock P., Small R.H. Comparison between end-tidal CO2 and respiration volume per time for detecting BOLD signal fluctuations during paced hyperventilation. Magn. Reson. Imaging. 2011;29:1186–1194. doi: 10.1016/j.mri.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstrup S., Henriksen L., Paulson O.B. Effect of acetazolamide on cerebral blood flow and cerebral metabolic rate for oxygen. J. Clin. Investig. 1984;74:1634–1639. doi: 10.1172/JCI111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters M., Muir S., Shah I., Lees K. Effect of perindopril on cerebral vasomotor reactivity in patients with lacunar infarction. Stroke. 2004;35:1899–1902. doi: 10.1161/01.STR.0000131748.12553.ed. [DOI] [PubMed] [Google Scholar]
- Watson N.A., Beards S.C., Altaf N., Kassner A., Jackson A. The effect of hyperoxia on cerebral blood flow: a study in healthy volunteers using magnetic resonance phase-contrast angiography. Eur. J. Anaesthesiol. 2000;17:152–159. doi: 10.1046/j.1365-2346.2000.00640.x. [DOI] [PubMed] [Google Scholar]
- Wei E.P., Kontos H.A., Patterson J.L. Dependence of pial arteriolar response to hypercapnia on vessel size. Am. J. Physiol. 1980;238:697–702. doi: 10.1152/ajpheart.1980.238.5.H697. [DOI] [PubMed] [Google Scholar]
- Westbrook C., Kaut Roth C., Talbot J. fourth ed. Blackwell Publishing Ltd; UK: 2011. MRI in Practice. [Google Scholar]
- Wintermark M., Sesay M., Barbier E., Borbély K., Dillon W.P., Eastwood J.D., Glenn T.C., Grandin C.B., Pedraza S., Soustiel J.-F., Nariai T., Zaharchuk G., Caillé J.-M., Dousset V., Yonas H. Comparative overview of brain perfusion imaging techniques. Stroke. 2005;36:e83–e99. doi: 10.1161/01.STR.0000177884.72657.8b. [DOI] [PubMed] [Google Scholar]
- Wise R.G., Harris A.D., Stone A.J., Murphy K. Measurement of OEF and absolute CMRO2: MRI-based methods using interleaved and combined hypercapnia and hyperoxia. NeuroImage. 2013;83:135–147. doi: 10.1016/j.neuroimage.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R.G., Pattinson K.T., Bulte D.P., Chiarelli P.A., Mayhew S.D., Balanos G.M., O'Connor D.F., Pragnell T.R., Robbins P.A., Tracey I., Jezzard P. Dynamic forcing of end-tidal carbon dioxide and oxygen applied to functional magnetic resonance imaging. J. Cereb. Blood Flow Metab. 2007;27:1521–1532. doi: 10.1038/sj.jcbfm.9600465. [DOI] [PubMed] [Google Scholar]
- Xu F., Liu P., Pascual J.M., Xiao G., Lu H. Effect of hypoxia and hyperoxia on cerebral blood flow, blood oxygenation, and oxidative metabolism. J. Cereb. Blood Flow Metab. 2012;32:1909–1918. doi: 10.1038/jcbfm.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetkin F.Z., Mendelsohn D. Hypoxia imaging in brain tumors. [Review] [141 refs] Neuroimaging Clin. N. Am. 2002;12:537–552. doi: 10.1016/s1052-5149(02)00029-1. [DOI] [PubMed] [Google Scholar]
- Yezhuvath, U.S., Uh, J., Cheng, Y., Martin-Cook, K., Weiner, M., Diaz-Arrastia, R., van, O.M., Lu, H., 2012. Forebrain-dominant deficit in cerebrovascular reactivity in Alzheimer's disease. Neurobiol. Aging 33, 75–82. [DOI] [PMC free article] [PubMed]
- Zhao P., Alsop D.C., Abduljalil A., Selim M., Lipsitz L., Novak P., Caplan L., Hu K., Novak V. Vasoreactivity and peri-infarct hyperintensities in stroke. Neurology. 2009;72:643–649. doi: 10.1212/01.wnl.0000342473.65373.80. [DOI] [PMC free article] [PubMed] [Google Scholar]