Abstract
Pyruvate dehydrogenase complex (PDC) deficiency is a major inborn error of oxidative metabolism of pyruvate in the mitochondria causing congenital lactic acidosis and primarily structural and functional abnormalities of the central nervous system. To provide an alternate source of acetyl-CoA derived from ketone bodies to the developing brain, a formula high in fat content is widely employed as a treatment. In the present study we investigated efficacy of a high-fat diet given to mothers during pregnancy and lactation on lessening of the impact of PDC deficiency on brain development in PDC-deficient female progeny.
Methods
A murine model of systemic PDC deficiency by interrupting the X-linked Pdha1 gene was employed in this study.
Results
Maternal consumption of a high-fat diet during pregnancy and lactation had no effect on number of live-birth, body growth, tissue PDC activity levels, as well as the in vitro rates of glucose oxidation and fatty acid biosynthesis by the developing brain of PDC-deficient female offspring during the postnatal age 35 days, as compared to the PDC-deficient progeny born to dams on a chow diet. Interestingly, brain weight was normalized in PDC-deficient progeny of high fat-fed mothers with improvement in impairment in brain structure deficit whereas brain weight was significantly decreased and was associated with greater cerebral structural defects in progeny of chow-fed mothers as compared to control progeny of mothers fed either a chow or high fat diet.
Conclusion
The findings provide for the first time experimental support for beneficial effects of a ketogenic diet during the prenatal and early postnatal periods on the brain development of PDC-deficient mammalian progeny.
Abbreviations: PDC, pyruvate dehydrogenase complex; PDH, pyruvate dehydrogenase; PDHA1, human gene that encodes α subunit of PDH; Pdha1, murine orthologue of PDHA1; wt, wild-type Pdha1 allele; flox8, Pdha1 floxed allele; Δex8, Pdha1 null allele; E18, embryonic day 18; P15, postnatal day 15; HF, high fat; LC, laboratory chow
Keywords: Pyruvate dehydrogenase complex deficiency, Mouse model, High fat diet, Brain development, Glucose metabolism, Prenatal treatment
1. Introduction
The pyruvate dehydrogenase complex (PDC) is one of the key enzymes in pathways of energy production and biosynthesis of lipids from glucose-derived carbons. Mammalian PDC is composed of multiple copies of three catalytic components, pyruvate dehydrogenase (PDH), dihydrolipoamide acetyltransferase and dihydrolipoamide dehydrogenase, a non-catalytic dihydrolipoamide dehydrogenase-binding protein, and two regulatory enzymes, PDH kinase (a family of four isozymes) and PDH phosphatase (a family of two isozymes) [1], [2]. PDC is regulated post-translationally by phosphorylation (inactivation) and dephosphorylation (reactivation) of the α subunit of the α2β2 heterotetrameric PDH component by PDH kinases and PDH phosphatases, respectively [1], [2]. Two genes have been found encoding the α subunit of PDH in most mammals [3], [4]. In the human, the PDHA1 gene functions in somatic tissues and maps on the chromosome X [3].
PDC deficiency is one of major inborn errors of oxidative metabolism, causing congenital lactic acidosis and heterogeneous clinical manifestations primarily related to malfunctioning of the central nervous system [5], [6], [7], [8], [9], [10]. The severity of the neurological manifestations ranges from minimal impairment (mild ataxia) to profound intellectual disability with abnormalities of motor functions. Among the large number of reported PDC deficiency cases resulting from mutations in the genes encoding PDC component proteins, the vast majority of the cases have been found to have mutations in the X-linked PDHA1 gene. Because of the X chromosomal localization of the somatic PDHA1, affected males and females manifest the disease differently, with males less likely to survive infancy and surviving females more likely to have severe neurological consequences [8], [11], [12], [13].
In the majority of reported cases of PDC deficiency, pathologic evaluation of the brain by autopsy has not been performed but abnormal brain imaging findings have been found in about one half of the reported PDC-deficient patients [5], [8], [9]. The most often reported observations of cerebral pathology for PDC deficiency include dilation of the lateral cerebral ventricles, underdevelopment of large white matter structures such as the corpus callosum, pons and pyramids, atrophy or neuronal loss combined with gliosis in the cortex and less often in basal ganglia, thalamus, hypothalamus and cerebellum and heterotopias in all brain region [5], [8], [9], [10], [14], [15], [16]. Prenatal and early imaging in a few cases have suggested that the onset of some of these neurologic deficits occurs prenatally, however, the timing and pathologic progression are not well characterized at present [5], [8], [9], [10], [12], [17].
Several strategies have been employed to treat PDC-deficient patients, with variable and often limited success [18], [19], [20], [21]. They include three major therapies (and often in some combination) [5], [8]: (i) the use of a ketogenic diet to provide ketone bodies as an alternate fuel for brain metabolism (by-passing PDC reaction) [18], [20], [21], [22], [23], [24], [25] (ii) supplementation of high doses of thiamine, presumably to meet the increased Km requirement for thiamine pyrophosphate associated with some PDHA1 mutations and/or for enzyme stability [26], [27], [28], [29], [30], [31], and (iii) administration of dichloroacetate which is known to inhibit PDH kinases decreased blood cerebrospinal fluid lactate concentrations in a large number of PDC-deficient children [19], [32]. Beneficial effect of phenylbutyrate on residual ‘active’ PDC activity was shown in skin fibroblast cell lines from PDC-deficient patients caring PDHA1 missense mutations [33], [34]. Furthermore, pyruvate therapy decrease lactate levels better clinical response on development and epilepsy in a PDC-deficient patient with a single base substitution in the PDHA1 gene [35]. Unfortunately, none of these approaches have been evaluated in a controlled manner with a sufficient number of subjects or duration to establish their efficacy. Several studies suggest that an early postnatal implementation of a ketogenic diet severely restricted in its carbohydrate content may be beneficial; however, this remains to be further evaluated [18], [20], [21], [22], [36]. The efficacy of ketogenic substrates was tested using a zebrafish model for PDC deficiency (due to deletion of the dihydrolipoamide acetyltransferase gene, Dlat) [36]. This dietary treatment promoted feeding behavior and increased survival of mutant larvae with improvement in visual response, indicating beneficial effects of the dietary treatment [36].
We have developed a murine model of PDC deficiency with a conditional mutation [37] introduced into the mouse orthologue of PDHA1, referred to as Pdha1, which is localized on chromosome X [38]. Using the Cre-loxP system, in vivo deletion of exon 8 of the Pdha1 by expressed Cre recombinase activity altered the downstream reading frame of this gene [37]. We showed that PDC-deficient female mice developed brain structural abnormalities somewhat similar to those observed in female PDC-deficient patients [39], [40], [41]. The availability of this murine PDC-deficiency model presented an opportunity to investigate the efficacy of a ketogenic diet (high-fat diet) on abnormal brain development. Considering that substantial neurologic damage can develop in utero in cases of PDC deficiency [7], [10], [12], [17], [42], [43], [44], [45], it was of interest to determine if altering the maternal diet would be beneficial to early brain development.
2. Materials and methods
2.1. Generation of PDC-deficient mice, dietary treatments and genotyping
All procedures performed on mice were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by Institutional Animal Care and Use Committee of University at Buffalo (protocol # BCH11064N). A line of mice carrying the Pdha1flox8 alleles, consisting of two loxP sites inserted into intronic sequences flanking exon 8 was generated as reported previously [37] and maintained on a standard rodent laboratory chow (LC) (percent calorie distribution: 65% carbohydrates, 15.9% fat and 18.4% protein; Harlan Teklad, Indianapolis, IN) and water ad libitum. The EIIa-Cre transgenic line (referred to as Creall + in this study) carrying an autosomally integrated Cre transgene [46], was maintained on LC and water ad libitum. In this transgenic mouse line, expression of Cre recombinase in all tissues was under the control of adenovirus EIIa promoter and initiated in early embryonic life [46]. To initiate deletion of exon 8 in vivo in all tissues, homozygous Pdha1-floxed female mice (genotype: Pdha1flox8/Pdha1flox8) were bred with the homozygous EIIa-Cre transgenic males to obtain heterozygous female progeny (genotype: Pdha1wt/Pdha1∆ ex8, Creall +) and referred to as PDC-deficient females. To generate control female mice with the same genetic background, wild-type males (not harboring the Cre transgene) which were purchased from the Jackson Laboratory, were bred with homozygous Pdha1-floxed females, and the heterozygous female progeny (genotype: Pdha1wt/Pdha1flox8) was referred to as control females. Both progeny were nursed by their natural dams and weaned on postnatal day 24 (P24) onto a LC and water ad libitum unless otherwise indicated.
To investigate the effect of maternal consumption of a high-fat (HF) diet on progeny brain development, another set of similar breeding protocols was performed in which the Pdha1-floxed females were placed on a HF diet (percent calorie composition: 18% carbohydrates, 67% fat and 15% protein; from Bio-Serv, Frenchtown, NJ) one week prior to initiation of breeding and were continued on HF diet during gestation and lactation. The female progeny of these matings (both control and PDC-deficient females) were weaned onto the same HF diet and water ad libitum until euthanized for tissue harvesting. Brain and liver from the progeny were rapidly removed and either frozen in liquid nitrogen or processed for metabolic and histological studies as described below. At the time of killing, blood was collected in tubes pre-coated with EDTA. After centrifugation, plasma samples were stored frozen. Plasma levels of lactate [47] and d-β-hydroxybutyrate [48] were measured using spectrophotometric methods.
To detect the presence or absence of the three Pdha1 alleles [wild-type Pdha1wt (700 bp), Pdha1flox8 (800 bp) and exon8 deleted Pdha1∆ ex8 (400 bp)] and the Cre transgene (240 bp), genomic DNA from tail clips was isolated with OmniPrep kit (Geno Technology, Inc., St. Louis, MO) and subjected to polymerase chain reaction analysis using specific primers and conditions as previously described [39]. Genotypes were confirmed later using DNA isolated from tissue specimens obtained after euthanization.
2.2. PDC activity measurements
‘Active’ and ‘total’ PDC activity was measured by assessing the conversion of [1-14C]pyruvate to 14CO2 by freeze-thawed tissue homogenates from PDC-deficient and control female progeny at the indicated prenatal and postnatal ages [39]. For ‘active’ PDC activity, dichloroacetate (inhibitor of PDH kinases) and sodium fluoride (inhibitor of PDH phosphatases) were present in homogenizing buffer to preserve its in vivo phosphorylation status. For measurement of ‘total’ PDC activity, complete dephosphorylation of PDH was achieved by pretreatment of freeze-thawed homogenates with purified PDH phosphatase 1 [39]. PDC activity is expressed as munits/mg of protein.
2.3. Substrate oxidation and fatty acid synthesis
Metabolism of [U-14C]-glucose (Amersham Pharmacia Biotech, Piscataway, NJ) and [1.2-14C]-acetate (ICN Biochemicals, Inc., Aurora, OH) to 14CO2 and for 14C-fatty acid synthesis were assessed by brain and liver slices at P15 and P35 as previously described [49]. The trapping of released 14CO2 and extraction of the labeled fatty acid fraction were performed as detailed previously [49]. Radioactivity was measured using a Beckman scintillation counter (Beckman, Fullerton, CA). The results are expressed as the nanomoles of radioactive substrate oxidized to 14CO2 or incorporated into esterified fatty acids per gram of wet tissue weight per hour.
2.4. Histological analyses of the brain
Brains of control and PDC-deficient female progeny at P35 were processed by the protocol described previously [39], [40]. Briefly, following anesthesia, a subset of mice were perfused with 0.1 M phosphate buffered saline followed by 4% paraformaldehyde in phosphatase buffered saline for histological analyses. Brains were dissected, postfixed in the same solution, processed with 30% sucrose for cryoprotection and frozen with Cryo-M-Bed embedding compound (Bright Instrument Company, Huntingdon, UK). Cryosections were cut at 20 μm using Vibratome and every section was collected in a stereological fashion [40]. A set of brain sections was subjected to Nissl staining (0.2% Cresyl violet, pH 4.5) (St. Louis, MO, USA) to reveal nuclei of neuronal and glial cells. Following dehydration and mounting with Permount slides were analyzed with a Zeiss Axiovert 35 microscope. Axiovision LE Rel. 4.5 program was used for calibration and quantification of the thicknesses of the dorsomedial neocortex, cerebellar granule cell layer, corpus callosum and a number of the cerebellar Purkinje cells.
2.5. Statistical analysis
The results are expressed as means ± standard deviation (SD) of the indicated number of animals in each experiment. Whenever only two groups were being compared, Students' t-test was used for analysis. For multiple comparisons, one-way analysis of variance (ANOVA), followed by post hoc analysis using the Student-Newman-Keuls test was used. P value of < 0.05 was considered as significant.
3. Results
3.1. Litter size and genotyping
The average litter size was significantly reduced by 34% when Pdha1-floxed females consuming LC (during gestation and lactation) were bred with transgenic males (Creall +) as compared to breeding with wild-type males (Creall-) (Table 1). This is because of embryonic lethality of male embryos carrying Pdha1∆ ex8 allele [37]. A similar outcome for litter size reduction (35%) was observed when Pdha1-floxed females consuming the HF (ketogenic) diet during gestation and lactation (Table 1). No male offspring carrying Creall + were born from mating of the Pdha1-floxed females on HF diet with Creall + transgenic males, resulting in the observed reduction in average litter size. Hence, feeding of a HF diet had no beneficial effect on progeny litter size outcome at birth. Genotype analysis using tail DNA from PDC-deficient female progeny of mothers fed HF diet showed, as expected [39], the presence of Pdha1∆ ex8 (400 bp), Pdha1wt (700 bp) and the Cre allele (240 bp) (results not shown). As expected, heterozygous control female progeny generated using wild-type control males showed Pdha1wt (700 bp) and Pdha1flox8 (800 bp) alleles (results not shown; see previously reported data on genotyping of these animals) [41].
Table 1.
Breeding strategy of floxed-female on different dietary regimens with wild-type and Cre transgenic males and progeny outcomes at birth.
| Parameter | Breeding with Creall − males |
Breeding with Creall + males |
||||||
|---|---|---|---|---|---|---|---|---|
| LC |
HF |
LC |
HF |
|||||
| (n = 21) |
(n = 7) |
(n = 7) |
(n = 5) |
|||||
| M | F | M | F | M | F | M | F | |
| Number of pups | 89 | 94 | 22 | 24 | 0 | 35 | 0 | 25 |
| Total pups | 183 | 54 | 35 | 25 | ||||
| Pups/liter | 8.7 ± 1.8 | 7.7 ± 0.6 | 5.0 ± 0.5⁎ | 5.0 ± 1.2⁎⁎ | ||||
Homozygous Pdha1-floxed females (genotype: Pdha1flox8/Pdha1flox) were maintained either on a LC or HF diet during pre-pregnancy and pregnancy. These females were bred with wild-type (Creall-) males or transgenic (Creall +) males. Abbreviations: LC: Lab Chow diet; HF: High fat diet; M: males; F: females. Data on average pups/liter are means ± SD of ‘n’ observations as indicated.
Significantly different (P < 0.05) compared LC-fed control mothers.
Significantly different (P < 0.05) compared with HF-fed control mothers.
3.2. Body and brain weights
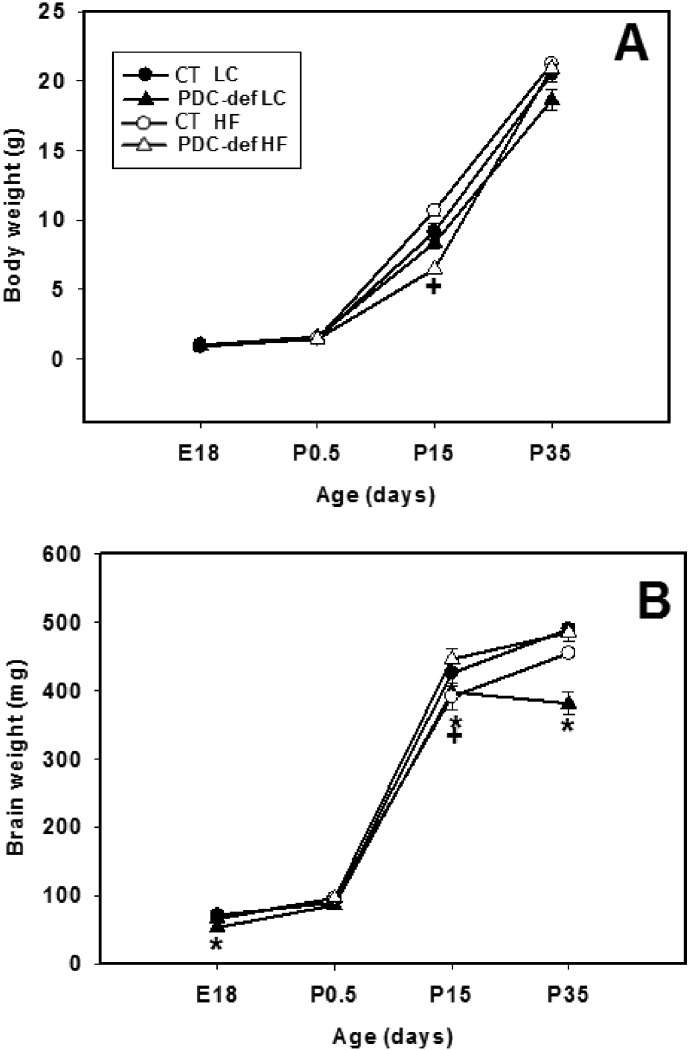
Body weights did not differ significantly, except as indicated, between control and PDC-deficient female progeny at age E18, P0.5, P15 and P35 when their dams consumed either a HF diet or LC diet (Fig. 1A). There was a significant reduction in body weights of P15 progeny of HF-fed mothers compared to control progeny of HF-fed mothers. This difference, however, was not seen at age P35 (Fig. 1A). Brain weights of PDC-deficient females born to dams consuming LC were significantly reduced at ages E18, P15 and P35 (Fig. 1B). In contrast, brain weights of PDC-deficient females born to dams on HF diet were significantly reduced on P15 only. It should be noted that there was a significant reduction (P < 0.05) in brain weights of P35 PDC-deficient progeny of LC-fed mothers whereas this reduction in brain weights was not observed in P35 PDC-deficient progeny of HF-fed mothers (Fig. 1B).
Fig. 1.
Body weights (A) and brain weights (B) of control and PDC-deficient progeny whose mothers were fed either a LC or HF diet during gestation and lactation. The results are recorded for progeny at ages E18, P0.5, P15 and P35. The results are means ± SD (n = 5–6/genotype/dietary treatment). * indicates significant difference (P < 0.05) between control and PDC-deficient progeny of mothers fed LC diet. + indicates significant difference (P < 0.05) between control and PDC-deficient progeny of mothers fed HF diet.
3.3. PDC activity
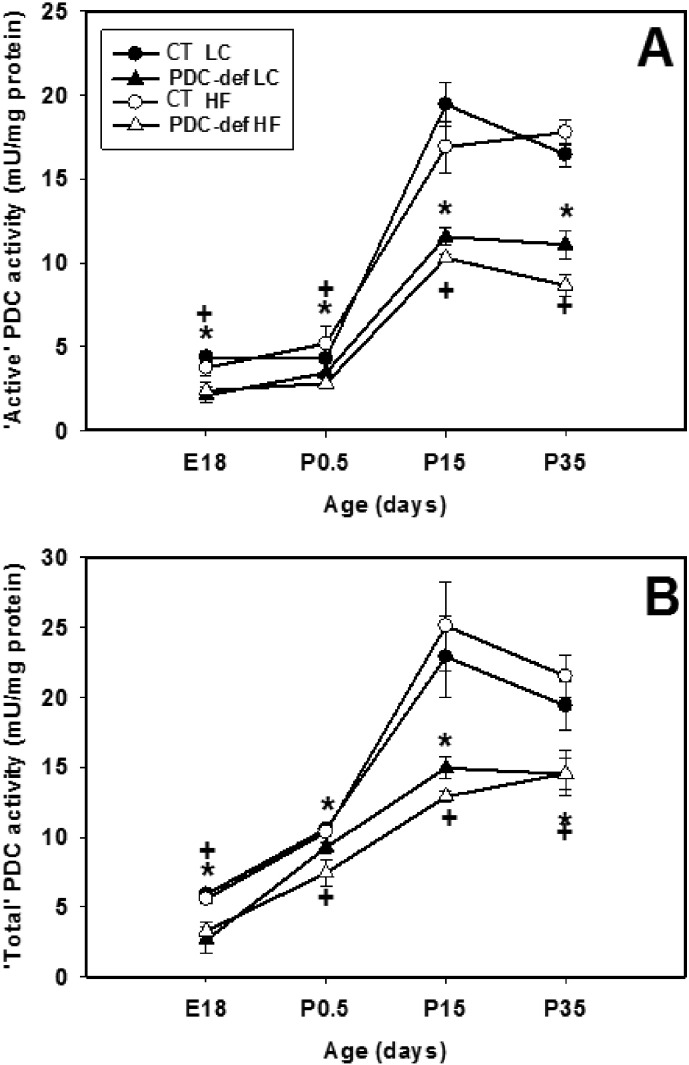
‘Total’ PDC activity was significantly decreased (about 25–45%) in PDC-deficient progeny of lab chow-fed mothers compared to control progeny at 4 different ages (Fig. 2B). As expected, maternal HF diet consumption had no effect on the ‘total’ PDC activity in the brains of control and PDC-deficient progeny at 4 different ages examined (Fig. 2B). A similar degree of reduction in ‘active’ PDC activity was also seen between the control and PDC-deficient progeny (irrespective of maternal dietary treatment) (Fig. 2A). Because ‘active’ PDC activity was significantly reduced in the brains of PDC-deficient females at 4 different ages (Fig. 2A), it is concluded that no compensatory increase in brain ‘active’ PDC activity occurred to restore the enzymatic defect in PDC-deficient female progeny. As expected based on genotype, maternal diet had no effect on reductions in ‘active’ and ‘total’ PDC activities in the brains of PDC-deficient females compared to their corresponding control females. As expected, significant reductions in both ‘active’ and ‘total’ PDC activity in liver were found in P15 and P35 PDC-deficient females (Supplemental Fig. 1).
Fig. 2.
‘Active’ PDC (A) and ‘total PDC’ (B) activities in the brain of control and PDC-deficient progeny whose mothers were fed either a LC or HF diet during gestation and lactation. ‘Active’ PDC activity represents in vivo dephosphorylated state of PDC. ‘Total’ PDC activity was measured following in vitro dephosphorylation as indicated in the Materials and methods section. The results are recorded for progeny at ages E18, P0.5, P15 and P35. The results are means ± SD (n = 4/genotype/dietary treatment). * indicates significant difference (P < 0.05) between control and PDC-deficient progeny of mothers fed LC diet. + indicates significant difference (P < 0.05) between control and PDC-deficient progeny of mothers fed HF diet.
3.4. Substrate oxidation and fatty acid synthesis
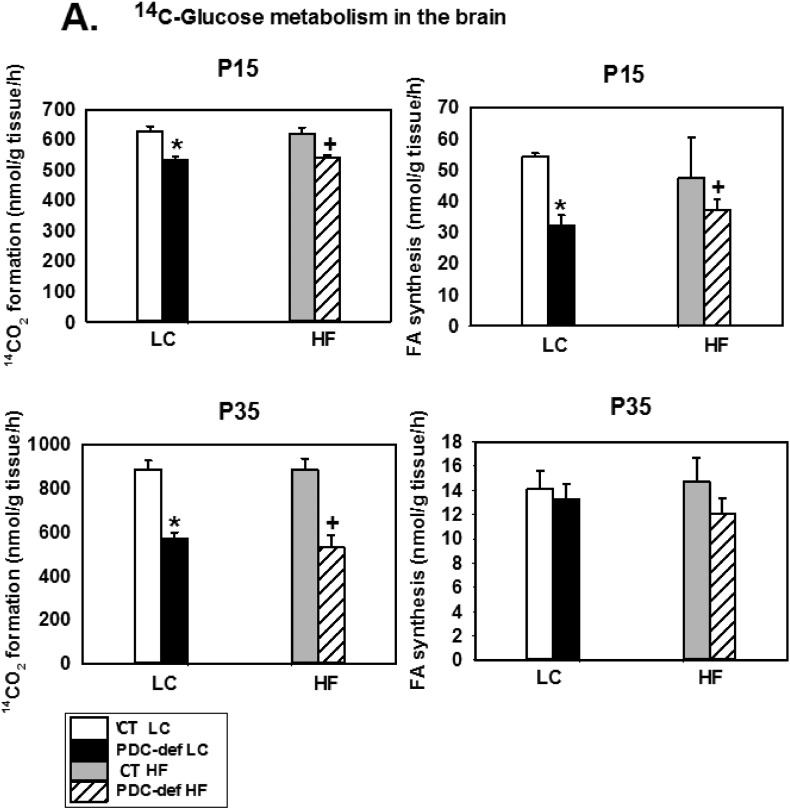
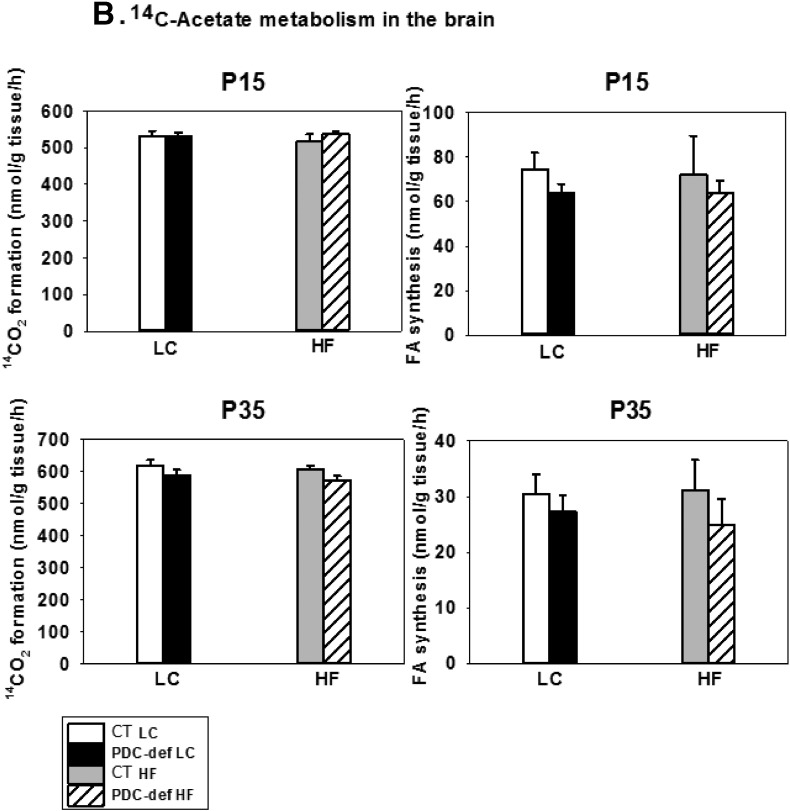
To assess oxidation of the substrates and incorporation of their carbons into lipids, brain slices were incubated with [U-14C]-glucose or [1.2-14C]-acetate plus non-radioactive glucose (Fig. 3). Using [U-14C]-glucose as a substrate, oxidation to 14CO2 was significantly decreased in the brains from PDC-deficient female progeny from both LC-fed and HF-fed mothers) compared to their corresponding diet- and age-matched control females (at both P15 and P35) (Fig. 3A). The incorporation of 14C from glucose into total fatty acids by brain slices from PDC-deficient progeny of dams either on LC or HF diet was significantly decreased compared to their corresponding dietary controls at age P15, but not at P35 (Fig. 3A). The latter finding may be due to lower rates of fatty acid biosynthesis in rodent brain in the post-weaning period at P35 [50]. Since HF diet had no effect on [U-14C]-glucose metabolism in the control progeny, the data were combined for the controls on LC and HF diet for HF analysis. As expected, when [1.2-14C]-acetate was used as a substrate, no difference in substrate oxidation and incorporation into fatty acids was seen in brain slices from P15 and P35 PDC-deficient and control females, either on LC or HF diet (Fig. 3B).
Fig. 3.
In vitro oxidation to 14CO2 and incorporation into fatty acids of [U-14C]-glucose (A) and [1.2-14C]-acetate (B) by brain slices from P15 and P35 control and PDC-deficient progeny whose mothers were fed either a LC or HF diet during gestation and lactation. The results are means ± SD (n = 5–6/genotype/dietary treatments except for CT on HF n = 2–3). * indicates significant difference (P < 0.05) between control and PDC-deficient progeny of mothers fed LC diet. + indicates significant difference (P < 0.05) between control and PDC-deficient progeny of mothers fed HF diet.
The oxidation of [U-14C]-glucose to 14CO2 and incorporation of 14C into fatty acids by liver slices from P35 PDC-deficient females were significantly decreased compared to control females (independent of their dietary treatment) (Supplemental Fig. 2A). As expected, the metabolism of [1.2-14C)-acetate to 14CO2 and fatty acid synthesis was not different between PDC-deficient and control females with the same dietary treatment (Supplemental Fig. 2B).
3.5. Plasma substrate levels
As expected, administration of a HF diet to the mothers (and weaning on a HF diet) significantly increased plasma levels of β-hydroxybutyrate in control and PDC-deficient progeny on P35 (0.68 ± 0.22 and 0.70 ± 0.14 μmol/ml, respectively), compared to progeny from LC-fed mothers were significantly lower (0.21 ± 0.06 and 0.22 ± 0.16 μmol/ml for control and PDC-deficient, respectively; P < 0.05). Plasma lactate levels did not differ between control and PDC-deficient progeny of mothers on the HF diet (2.9 ± 0.7 mM vs. 3.1 ± 0.6 mM, respectively). Plasma lactate levels also did not differ between 35 day-old control and PDC-deficient progeny of mothers fed LC diet (2.6 ± 0.4 and 2.8 ± 0.1, respectively).
3.6. Brain structure studies
Detailed analysis and comparison of the brain structure was performed on brain sections from P35 PDC-deficient and control progeny of mothers fed HF diet and LC diets. Striking brain structure deficits were observed in the PDC-deficient progeny of LC-fed mothers in comparison to control progeny of mothers on the same diet [41]. Briefly, significant reduction in the thickness of the neocortex, visible reduction in the number of neocortical cells, and enlargement of the lateral ventricles were observed in brains of PDC-deficient progeny of LC-fed mothers during gestation and lactation (Fig. 4A). Local heterotopias were seen in the striatum, hypothalamus, thalamus and nuclei of the midbrain and brain stem (data not shown). In the cerebellum, both granule and Purkinje cell numbers were visibly reduced in brains of PDC-deficient progeny of LC-fed mothers during gestation and lactation (Fig. 4B).
Fig. 4.
Coronal sections of the (A) cerebral neocortex and (B) cerebellar cortex from P35 control and PDC-deficient female progeny whose mothers were fed either a LC or HF diet during gestation and lactation. Nissl staining with Cresyl violet. Bar is 100 μm. (A): Cerebral neocortex. Fragment of hippocampal formation (hip) is included for the orientation. Thicknesses of the neocortex (black double arrow with the actual value in mm) and of the corpus callosum (yellow double arrow with the actual value in mm) were greatly reduced in PDC-deficient female fed LC compared to control female on the same diet. Rectangles show the area enlarged for the insets (shown immediately below). Insets show cellular organization of the layers III-V of the neocortex. Cell density appeared to be lower in PDC-deficient females receiving LC or HF diet compared to control females on the same diet. (B): Cerebellar cortex. Rectangles show the area enlarged for the insets (shown immediately below). Insets provide more detailed view on the granule and Purkinje cell layers. Number of the Purkinje cells (arrows) and thickness of the granule cell layer (double arrow with the actual value in mm) were decreased in the brains of PDC-deficient female fed LC or HF diet. In PDC-deficient female fed HF diet the changes were less pronounced. Control females receiving LC diet did not show significant differences in comparison to control females fed HF diet. Each bar represents 100 μm.
In brains of PDC-deficient progeny of HF-fed mothers during gestation and lactation, these abnormalities of brain structure were less severe. Thickness of the neocortex from PDC-deficient progeny of HF diet fed-mothers (Fig. 4A, upper row far right) was 1110 compared to a value of 960 for PDC-deficient progeny of LC-fed mothers (Fig. 4A, upper row second from far left), but still was smaller than that seen in age-matched control females from either HF (value of 1185; Fig. 4A, upper row second from far right) or LC progeny (value of 1170; Fig. 4A, upper row far left). Size, number and position of cells in the neocortex appeared to be improved in PDC-deficient females from HF progeny (Fig. 4A, lower row far right) compared to PDC-deficient females from lab LC-fed mothers. Structural defects of striatum, hypothalamus and thalamus appeared to be prevented since no heterotopias were observed in brain sections from PDC-deficient females from HF progeny (data not shown). Similarly, no structural defects were seen in the nuclei of midbrain and brain stem (data not shown). Cerebellar structure improved in brain sections from PDC-deficient females from HF-fed mothers (value of 75; Fig. 4B, last row far right) compared to same genotypic group from LC-fed mothers (value of 40; Fig. 4B, last row second from far left). However, when compared to control female cerebellum from either HF-fed mothers (value of 135; Fig. 4B, last row second from far right) or LC-fed mothers (value of 115; Fig. 4B, last row far left), number of Purkinje cells and thickness of the granule cell layer were still evidently reduced in PDC-deficient female progeny of HF-fed mothers.
4. Discussion
Although the brain represents only about 2% of total body weight of an average adult, it accounts for about 20% of the resting total body oxygen consumption. In infants and children the brain's oxygen consumption accounts for even larger fraction (about 50%) during early childhood (around the age of 4–5 years). Hence the brain is most susceptible to PDC deficiency because of its dependency on glucose as the primary energy source under normal dietary conditions. PDC serves as the gatekeeper for oxidation of glucose-derived pyruvate to acetyl-CoA for further oxidation in the tricarboxylic acid cycle and also for biosynthesis of lipids in developing brain. The only other readily oxidizable substrates for the brain are ketone bodies derived from fatty acid oxidation in the liver. Hence, a ketogenic diet is a common treatment for PDC-deficient patients in the postnatal period.
Two commonly employed treatments (often in combination) in PDC deficiency are the use of a ketogenic diet and vitamin supplementation (high dose of thiamine with some other vitamins and/or compounds). The efficacy of the ketogenic diet for PDC deficiency is based on two considerations: (i) the ketogenic diet results in increased circulating plasma levels of free fatty acids and ketone bodies (β-hydroxybutyrate and acetoacetate) providing alternate sources of acetyl-CoA for energy production and biosynthetic process in the brain (ketone bodies only) and other tissues and (ii) the reduction in blood and intracellular levels of lactate and pyruvate due to the reduction or removal of dietary carbohydrates. In this regard, ketogenic diets with reduced level of carbohydrate intake or with little or no carbohydrates have been used with variable success to control lactic acidosis with little or some clinical improvement [8], [18], [20], [22], [24], [25], [51], [52]. This is due largely to several variables introduced in each case such as the variability in mutations expressed, age at the initiation of the treatment, level of fat content in the diet, quality of dietary fats, length of treatment, acceptability/adherence to the diet, etc. In two studies using magnetic resonance imaging, a ketogenic diet was associated with clinical stabilization and arrest of progressive cerebral lesions, and appearance of new lesions upon discontinuation of the diet [18], [51]. Furthermore, a nearly carbohydrate-free diet initiated shortly after birth may be useful in the treatment of PDH (and hence PDC) deficiency and recommended carnitine supplementation to maintain normal plasma free carnitine levels due to increased fatty acid oxidation in the patients [20]. Again, this treatment focuses on initiation of a ketogenic diet in the postnatal period due to either based on clinical/biochemical symptoms or on a confirmed diagnostic evidence for PDC deficiency. The correction of the biochemical parameters (such as reduction in lactate levels in the plasma and possibly in the cerebrospinal fluid) at a later stage in the postnatal period does not reverse the brain structural damage and therefore cannot promote the recovery towards normal brain functions.
The efficacy of a ketogenic diet (consisting of medium- and long-chain fatty acids in an emulsion of phosphatidylcholine) was tested using a zebrafish model for PDC deficiency due to Dlat-deficiency [36]. This dietary treatment promoted feeding behavior, reduced lactic acidosis, and increased survival of mutant larvae [34]. Additionally, this diet caused a strong to moderate improvement in visual response by PDC-deficient larvae, indicating reversal of severe neurological dysfunction such as blindness. Although these findings are of interest, this model is not directly relevant to human PDC deficiency. Our murine model of PDC deficiency is more relevant to human PDC deficiency for analyzing mammalian brain development and also for assessment of efficacy of a high fat dietary treatment.
In an earlier report we provided detailed description of structural deficits in the brains of P35 PDC-deficient females (born to mothers consuming LC during gestation and lactation) [41]. Briefly, these deficits included heterotrophic changes and reduced neuronal cell density in the neocortex, the decrease in the density of granular cells and Purkinje cells, and agenesis of white matter structures, among other abnormalities. Interestingly, many of these neuropathological alterations in PDC-deficient mice were similar to those described in PDC-deficient patients [5], [8], [40], [41]. In the present study, we found similar impairment in brain structural deficits in PDC-deficient females born to mothers fed LC during gestation and lactation (Fig. 4). Interestingly, improvements in brain structures (such as neocortex, cerebellar structure and thickness and fiber organization of white matter structures) were observed in brains of PDC-deficient female progeny of HF-fed mothers during gestation and lactation. Additionally, no structural defects in striatum, thalamus and hypothalamus as well as in midbrain and brain stem were seen in PDC-deficient female progeny of HF-fed mothers compared to the same structures from age-matched progeny of LC-fed mothers.
Feeding a HF-diet to pregnant mice are known to cause increased plasma levels of ketone bodies that would be transported across the placenta to the fetal circulation. Both rodent and human fetal brains are capable of metabolizing ketone bodies [40], [49], [50], [53], [54]. Hence feeding a HF diet to pregnant mice provided ketone bodies as an alternate fuel for fetal brain. Availability of ketone bodies as an alternate fuel reduced the impact of PDC-deficiency by generating ATP via the oxidation of acetyl-CoA formed from ketone bodies. Acetyl-CoA generated from ketone bodies can also be utilized for lipid biosynthesis in the fetal brain hence reducing the ill effect of PDC deficiency on lipid synthesis, as evident from increased brain weights of PDC-deficient progeny of HF-fed mothers. Since the availability of ketone bodies to the fetal brain were initiated from fetal life, there was less impairment in fetal brain development due to PDC deficiency. Furthermore, there was some protection from deleterious effects of PDC deficiency during the suckling period because mouse milk is rich in its fat content, resulting in postnatal ketosis in the suckling pups. We have shown that rodent brains can oxidize ketone bodies to CO2 and can incorporate ketone body-carbons in brain lipids in the immediate postnatal period [50], [54]. Furthermore, we also demonstrated that acetyl-CoA generated from acetoacetate in the cytosol was readily utilized for lipid synthesis by brains from 1-week-old rats [54]. Hence, the availability of ketone bodies during both prenatal and early postnatal periods in PDC–deficient mice resulted in the reduction in the severity of impairments caused by PDC deficiency. Since the majority of mutations in the PDHA1 gene (as well as in the other PDC genes) in children are sporadic and diagnosis of PDC deficiency is commonly made after birth, it is not commonly possible to institute a ketogenic dietary treatment during pregnancy.
5. Conclusions
The results presented here show that early prenatal treatment with a ketogenic diet might be very beneficial during pregnancy with a family history of PDC deficiency (with a previously affected sibling) or with a positive prenatal diagnosis of PDC deficiency) [43], [44], [45], because this dietary treatment has the potential to reduce irreversible cerebral damage occurring during the prenatal period. The findings presented here also provide for the first time experimental support for initiation of a high fat (ketogenic) dietary treatment as early as possible after birth to diminish further deterioration of brain development and function.
The following are the supplementary data related to this article.
‘Active’ PDC and ‘total’ PDC activities in the liver from P15 and P35 control and PDC-deficient progeny whose mothers were fed either a LC or HF diet during gestation and lactation. ‘Active’ PDC activity represents in vivo dephosphorylated state of PDC. ‘Total’ PDC activity was measured following in vitro dephosphorylation as indicated in the Materials and methods section. The results are means ± SD (n = 4/genotype/dietary treatment). * indicates significant difference (P < 0.05) between control and PDC-deficient progeny of mothers fed LC diet. + indicates significant difference (P < 0.05) between control and PDC-deficient progeny of mothers fed HF diet.
In vitro oxidation to 14CO2 and incorporation into fatty acids of [U-14C]-glucose (A) and [1.2-14C]-acetate (B) by liver slices form P35 control and PDC-deficient progeny whose mothers were fed either a LC or a HF diet during gestation and lactation. The results are means ± SD (n = 5–6/genotype/dietary treatment except for CT on HF diet, n = 2–3). * indicates significant difference (P < 0.05) between control and PDC-deficient progeny of mothers fed LC diet. + indicates significant difference (P < 0.05) between control and PDC-deficient progeny of mothers fed HF diet.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgments
This study was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grant DK20478. We thank Dr. Murray Ettinger of University at Buffalo, and Dr. Douglas Kerr and Dr. Suzanne DeBrosse of Case Western Reserve University, Cleveland for critical reading of this manuscript.
References
- 1.Harris R.A., Bowker-Kinley M.M., Huang B., Wu P. Regulation of the activity of the pyruvate dehydrogenase complex. Adv. Enzym. Regul. 2002;42:249–259. doi: 10.1016/s0065-2571(01)00061-9. [DOI] [PubMed] [Google Scholar]
- 2.Patel M.S., Korotchkina L.G. The biochemistry of the pyruvate dehydrogenase complex. Biochem. Mol. Biol. Educ. 2003;31:5–15. [Google Scholar]
- 3.Maragos C., Hutchison W.M., Hayasaka K., Brown G.K., Dahl H.H. Structural organization of the gene for the E1 alpha subunit of the human pyruvate dehydrogenase complex. J. Biol. Chem. 1989;264:12294–12298. [PubMed] [Google Scholar]
- 4.Dahl H.H., Brown R.M., Hutchison W.M., Maragos C., Brown G.K. A testis-specific form of the human pyruvate dehydrogenase E1 alpha subunit is coded for by an intronless gene on chromosome 4. Genomics. 1990;8:225–232. doi: 10.1016/0888-7543(90)90275-y. [DOI] [PubMed] [Google Scholar]
- 5.Debrosse S.D., Okajima K., Zhang S., Nakouzi G., Schmotzer C.L., Lusk-Kopp M., Frohnapfel M.B., Grahame G., Kerr D.S. Spectrum of neurological and survival outcomes in pyruvate dehydrogenase complex (PDC) deficiency: lack of correlation with genotype. Mol. Genet. Metab. 2012;107:394–402. doi: 10.1016/j.ymgme.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Imbard A., Boutron A., Vequaud C., Zater M., de Lonlay P., de Baulny H.O., Barnerias C., Mine M., Marsac C., Saudubray J.M., Brivet M. Molecular characterization of 82 patients with pyruvate dehydrogenase complex deficiency. Structural implications of novel amino acid substitutions in E1 protein. Mol. Genet. Metab. 2011;104:507–516. doi: 10.1016/j.ymgme.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Kerr D.S., Wexler I.D., Tripatara A., Patel M.S. Human Defects of the Pyruvate Dehydrogenase Complex. In: Patel M.S., Roche T.E., Harris R.A., editors. Alpha-Keto Acid Dehydrogenase Complexes. Birkhauser; Switzerland: 1996. [Google Scholar]
- 8.Patel K.P., O'Brien T.W., Subramony S.H., Shuster J., Stacpoole P.W. The spectrum of pyruvate dehydrogenase complex deficiency: clinical, biochemical and genetic features in 371 patients. Mol. Genet. Metab. 2012;105:34–43. doi: 10.1016/j.ymgme.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quintana E., Gort L., Busquets C., Navarro-Sastre A., Lissens W., Moliner S., Lluch M., Vilaseca M.A., De Meirleir L., Ribes A., Briones P. Mutational study in the PDHA1 gene of 40 patients suspected of pyruvate dehydrogenase complex deficiency. Clin. Genet. 2010;77:474–482. doi: 10.1111/j.1399-0004.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- 10.Robinson B.H. Lactic acidemia (disorders of pyruvate carboxylase, pyruvate dehydrogenase) In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. The Metabolic and Molecular Basis of Inherited Disease. McgGraw-Hill; New York: 2001. pp. 2275–2295. [Google Scholar]
- 11.Brown G.K., Haan E.A., Kirby D.M., Scholem R.D., Wraith J.E., Rogers J.G., Danks D.M. “Cerebral” lactic acidosis: defects in pyruvate metabolism with profound brain damage and minimal systemic acidosis. Eur. J. Pediatr. 1988;147:10–14. doi: 10.1007/BF00442603. [DOI] [PubMed] [Google Scholar]
- 12.Brown G.K., Otero L.J., LeGris M., Brown R.M. Pyruvate dehydrogenase deficiency. J. Med. Genet. 1994;31:875–879. doi: 10.1136/jmg.31.11.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Meirleir L. Defects of pyruvate metabolism and the Krebs cycle. J. Child Neurol. 2002;17(Suppl. 3) (3S26-33; discussion 23S33-24) [PubMed] [Google Scholar]
- 14.Dahl H.H., Hansen L.L., Brown R.M., Danks D.M., Rogers J.G., Brown G.K. X-linked pyruvate dehydrogenase E1 alpha subunit deficiency in heterozygous females: variable manifestation of the same mutation. J. Inherit. Metab. Dis. 1992;15:835–847. doi: 10.1007/BF01800219. [DOI] [PubMed] [Google Scholar]
- 15.Matthews P.M., Brown R.M., Otero L., Marchington D., Leonard J.V., Brown G.K. Neurodevelopmental abnormalities and lactic acidosis in a girl with a 20-bp deletion in the X-linked pyruvate dehydrogenase E1 alpha subunit gene. Neurology. 1993;43:2025–2030. doi: 10.1212/wnl.43.10.2025. [DOI] [PubMed] [Google Scholar]
- 16.Michotte A., De Meirleir L., Lissens W., Denis R., Wayenberg J.L., Liebaers I., Brucher J.M. Neuropathological findings of a patient with pyruvate dehydrogenase E1 alpha deficiency presenting as a cerebral lactic acidosis. Acta Neuropathol. 1993;85:674–678. doi: 10.1007/BF00334680. [DOI] [PubMed] [Google Scholar]
- 17.Cross J.H., Connelly A., Gadian D.G., Kendall B.E., Brown G.K., Brown R.M., Leonard J.V. Clinical diversity of pyruvate dehydrogenase deficiency. Pediatr. Neurol. 1994;10:276–283. doi: 10.1016/0887-8994(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 18.Wijburg F.A., Barth P.G., Bindoff L.A., Birch-Machin M.A., van der Blij J.F., Ruitenbeek W., Turnbull D.M., Schutgens R.B. Leigh syndrome associated with a deficiency of the pyruvate dehydrogenase complex: results of treatment with a ketogenic diet. Neuropediatrics. 1992;23:147–152. doi: 10.1055/s-2008-1071331. [DOI] [PubMed] [Google Scholar]
- 19.Stacpoole P.W., Barnes C.L., Hurbanis M.D., Cannon S.L., Kerr D.S. Treatment of congenital lactic acidosis with dichloroacetate. Arch. Dis. Child. 1997;77:535–541. doi: 10.1136/adc.77.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wexler I.D., Hemalatha S.G., McConnell J., Buist N.R., Dahl H.H., Berry S.A., Cederbaum S.D., Patel M.S., Kerr D.S. Outcome of pyruvate dehydrogenase deficiency treated with ketogenic diets. Studies in patients with identical mutations. Neurology. 1997;49:1655–1661. doi: 10.1212/wnl.49.6.1655. [DOI] [PubMed] [Google Scholar]
- 21.Klepper J., Leiendecker B., Riemann E., Baumeister F.A. The ketogenic diet in German-speaking countries: update 2003. Klin. Padiatr. 2004;216:277–285. doi: 10.1055/s-2004-44906. [DOI] [PubMed] [Google Scholar]
- 22.Di Pisa V., Cecconi I., Gentile V., Di Pietro E., Marchiani V., Verrotti A., Franzoni E. Case report of pyruvate dehydrogenase deficiency with unusual increase of fats during ketogenic diet treatment. J. Child Neurol. 2012;27:1593–1596. doi: 10.1177/0883073812436424. [DOI] [PubMed] [Google Scholar]
- 23.El-Gharbawy A.H., Boney A., Young S.P., Kishnani P.S. Follow-up of a child with pyruvate dehydrogenase deficiency on a less restrictive ketogenic diet. Mol. Genet. Metab. 2011;102:214–215. doi: 10.1016/j.ymgme.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Scholl-Burgi S., Holler A., Pichler K., Michel M., Haberlandt E., Karall D. Ketogenic diets in patients with inherited metabolic disorders. J. Inherit. Metab. Dis. 2015;38:765–773. doi: 10.1007/s10545-015-9872-2. [DOI] [PubMed] [Google Scholar]
- 25.Stenlid M.H., Ahlsson F., Forslund A., von Dobeln U., Gustafsson J. Energy substrate metabolism in pyruvate dehydrogenase complex deficiency. J. Pediatr. Endocrinol. Metab. 2014;27:1059–1064. doi: 10.1515/jpem-2013-0423. [DOI] [PubMed] [Google Scholar]
- 26.Byrd D.J., Krohn H.P., Winkler L., Steinborn C., Hadam M., Brodehl J., Hunneman D.H. Neonatal pyruvate dehydrogenase deficiency with lipoate responsive lactic acidaemia and hyperammonaemia. Eur. J. Pediatr. 1989;148:543–547. doi: 10.1007/BF00441554. [DOI] [PubMed] [Google Scholar]
- 27.Joao Silva M., Pinheiro A., Eusebio F., Gaspar A., Tavares de Almeida I., Rivera I. Pyruvate dehydrogenase deficiency: identification of a novel mutation in the PDHA1 gene which responds to amino acid supplementation. Eur. J. Pediatr. 2009;168:17–22. doi: 10.1007/s00431-008-0700-7. [DOI] [PubMed] [Google Scholar]
- 28.Naito E., Ito M., Yokota I., Saijo T., Matsuda J., Ogawa Y., Kitamura S., Takada E., Horii Y., Kuroda Y. Thiamine-responsive pyruvate dehydrogenase deficiency in two patients caused by a point mutation (F205L and L216F) within the thiamine pyrophosphate binding region. Biochim. Biophys. Acta. 2002;1588:79–84. doi: 10.1016/s0925-4439(02)00142-4. [DOI] [PubMed] [Google Scholar]
- 29.Pastoris O., Savasta S., Foppa P., Catapano M., Dossena M. Pyruvate dehydrogenase deficiency in a child responsive to thiamine treatment. Acta Paediatr. 1996;85:625–628. doi: 10.1111/j.1651-2227.1996.tb14104.x. [DOI] [PubMed] [Google Scholar]
- 30.Rubio-Gozalbo M.E., Heerschap A., Trijbels J.M., De Meirleir L., Thijssen H.O., Smeitink J.A. Proton MR spectroscopy in a child with pyruvate dehydrogenase complex deficiency. Magn. Reson. Imaging. 1999;17:939–944. doi: 10.1016/s0730-725x(99)00002-8. [DOI] [PubMed] [Google Scholar]
- 31.Sedel F., Challe G., Mayer J.M., Boutron A., Fontaine B., Saudubray J.M., Brivet M. Thiamine responsive pyruvate dehydrogenase deficiency in an adult with peripheral neuropathy and optic neuropathy. J. Neurol. Neurosurg. Psychiatry. 2008;79:846–847. doi: 10.1136/jnnp.2007.136630. [DOI] [PubMed] [Google Scholar]
- 32.Berendzen K., Theriaque D.W., Shuster J., Stacpoole P.W. Therapeutic potential of dichloroacetate for pyruvate dehydrogenase complex deficiency. Mitochondrion. 2006;6:126–135. doi: 10.1016/j.mito.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Ferriero R., Manco G., Lamantea E., Nusco E., Ferrante M.I., Sordino P., Stacpoole P.W., Lee B., Zeviani M., Brunetti-Pierri N. Phenylbutyrate therapy for pyruvate dehydrogenase complex deficiency and lactic acidosis. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3004986. (175ra131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferriero R., Boutron A., Brivet M., Kerr D., Morava E., Rodenburg R.J., Bonafe L., Baumgartner M.R., Anikster Y., Braverman N.E., Brunetti-Pierri N. Phenylbutyrate increases pyruvate dehydrogenase complex activity in cells harboring a variety of defects. Ann. Clin. Transl. Neurol. 2014;1:462–470. doi: 10.1002/acn3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koga Y., Povalko N., Katayama K., Kakimoto N., Matsuishi T., Naito E., Tanaka M. Beneficial effect of pyruvate therapy on Leigh syndrome due to a novel mutation in PDH E1alpha gene. Brain Dev. 2012;34:87–91. doi: 10.1016/j.braindev.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Taylor M.R., Hurley J.B., Van Epps H.A., Brockerhoff S.E. A zebrafish model for pyruvate dehydrogenase deficiency: rescue of neurological dysfunction and embryonic lethality using a ketogenic diet. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4584–4589. doi: 10.1073/pnas.0307074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson M.T., Mahmood S., Hyatt S.L., Yang H.S., Soloway P.D., Hanson R.W., Patel M.S. Inactivation of the murine pyruvate dehydrogenase (Pdha1) gene and its effect on early embryonic development. Mol. Genet. Metab. 2001;74:293–302. doi: 10.1006/mgme.2001.3249. [DOI] [PubMed] [Google Scholar]
- 38.Brown R.M., Dahl H.H., Brown G.K. Pyruvate dehydrogenase E1 alpha subunit genes in the mouse: mapping and comparison with human homologs. Somat. Cell Mol. Genet. 1990;16:487–492. doi: 10.1007/BF01233198. [DOI] [PubMed] [Google Scholar]
- 39.Pliss L., Pentney R.J., Johnson M.T., Patel M.S. Biochemical and structural brain alterations in female mice with cerebral pyruvate dehydrogenase deficiency. J. Neurochem. 2004;91:1082–1091. doi: 10.1111/j.1471-4159.2004.02790.x. [DOI] [PubMed] [Google Scholar]
- 40.Pliss L., Mazurchuk R., Spernyak J.A., Patel M.S. Brain MR imaging and proton MR spectroscopy in female mice with pyruvate dehydrogenase complex deficiency. Neurochem. Res. 2007;32:645–654. doi: 10.1007/s11064-007-9295-z. [DOI] [PubMed] [Google Scholar]
- 41.Pliss L., Hausknecht K.A., Stachowiak M.K., Dlugos C.A., Richards J.B., Patel M.S. Cerebral developmental abnormalities in a mouse with systemic pyruvate dehydrogenase deficiency. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harada M., Tanouchi M., Arai K., Nishitani H., Miyoshi H., Hashimoto T. Therapeutic efficacy of a case of pyruvate dehydrogenase complex deficiency monitored by localized proton magnetic resonance spectroscopy. Magn. Reson. Imaging. 1996;14:129–133. doi: 10.1016/0730-725x(95)02047-w. [DOI] [PubMed] [Google Scholar]
- 43.Brown R.M., Brown G.K. Prenatal diagnosis of pyruvate dehydrogenase E1 alpha subunit deficiency. Prenat. Diagn. 1994;14:435–441. doi: 10.1002/pd.1970140604. [DOI] [PubMed] [Google Scholar]
- 44.Robinson J.N., Norwitz E.R., Mulkern R., Brown S.A., Rybicki F., Tempany C.M. Prenatal diagnosis of pyruvate dehydrogenase deficiency using magnetic resonance imaging. Prenat. Diagn. 2001;21:1053–1056. doi: 10.1002/pd.187. [DOI] [PubMed] [Google Scholar]
- 45.Tamaru S., Kikuchi A., Takagi K., Okuno J., Ishikawa K., Imada S., Horikoshi T., Goto Y., Hirabayashi S. A case of pyruvate dehydrogenase E1alpha subunit deficiency with antenatal brain dysgenesis demonstrated by prenatal sonography and magnetic resonance imaging. J. Clin. Ultrasound. 2012;40:234–238. doi: 10.1002/jcu.20864. [DOI] [PubMed] [Google Scholar]
- 46.Lakso M., Pichel J.G., Gorman J.R., Sauer B., Okamoto Y., Lee E., Alt F.W., Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noll F. Methode zur quantitativen bestimmung von L-(+)-lactat-dehydrogenase und glutamat-pyruvat-transaminase. Biochem. Z. 1966;346:41–49. [Google Scholar]
- 48.Williamson D.H., Medley J. d-(-)β-Hydroxybutyrate. In: Bergmeyer H.U., editor. Methods of Enzymatic Analysis. Academic Press; New York: 1965. pp. 459–461. [Google Scholar]
- 49.Patel M.S., Tonkonow B.L. Development of lipogenesis in rat brain cortex: the differential incorporation of glucose and acetate into brain lipids in vitro. J. Neurochem. 1974;23:309–313. doi: 10.1111/j.1471-4159.1974.tb04359.x. [DOI] [PubMed] [Google Scholar]
- 50.Patel M.S., Owen O.E. Development and regulation of lipid synthesis from ketone bodies by rat brain. J. Neurochem. 1977;28:109–114. doi: 10.1111/j.1471-4159.1977.tb07715.x. [DOI] [PubMed] [Google Scholar]
- 51.Krageloh-Mann I., Grodd W., Niemann G., Haas G., Ruitenbeek W. Assessment and therapy monitoring of Leigh disease by MRI and proton spectroscopy. Pediatr. Neurol. 1992;8:60–64. doi: 10.1016/0887-8994(92)90055-4. [DOI] [PubMed] [Google Scholar]
- 52.Weber T.A., Antognetti M.R., Stacpoole P.W. Caveats when considering ketogenic diets for the treatment of pyruvate dehydrogenase complex deficiency. J. Pediatr. 2001;138:390–395. doi: 10.1067/mpd.2001.111817. [DOI] [PubMed] [Google Scholar]
- 53.Haney P.M., Patel M.S. Regulation of succinyl-CoA:3-oxoacid CoA-transferase in developing rat brain: responsiveness associated with prenatal but not postnatal hyperketonemia. Arch. Biochem. Biophys. 1985;240:426–434. doi: 10.1016/0003-9861(85)90047-5. [DOI] [PubMed] [Google Scholar]
- 54.Patel M.S., Owen O.E. Lipogenesis from ketone bodies in rat brain. Evidence for conversion of acetoacetate into acetyl-coenzyme A in the cytosol. Biochem. J. 1976;156:603–607. doi: 10.1042/bj1560603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
‘Active’ PDC and ‘total’ PDC activities in the liver from P15 and P35 control and PDC-deficient progeny whose mothers were fed either a LC or HF diet during gestation and lactation. ‘Active’ PDC activity represents in vivo dephosphorylated state of PDC. ‘Total’ PDC activity was measured following in vitro dephosphorylation as indicated in the Materials and methods section. The results are means ± SD (n = 4/genotype/dietary treatment). * indicates significant difference (P < 0.05) between control and PDC-deficient progeny of mothers fed LC diet. + indicates significant difference (P < 0.05) between control and PDC-deficient progeny of mothers fed HF diet.
In vitro oxidation to 14CO2 and incorporation into fatty acids of [U-14C]-glucose (A) and [1.2-14C]-acetate (B) by liver slices form P35 control and PDC-deficient progeny whose mothers were fed either a LC or a HF diet during gestation and lactation. The results are means ± SD (n = 5–6/genotype/dietary treatment except for CT on HF diet, n = 2–3). * indicates significant difference (P < 0.05) between control and PDC-deficient progeny of mothers fed LC diet. + indicates significant difference (P < 0.05) between control and PDC-deficient progeny of mothers fed HF diet.