Abstract
Pneumocystis pneumonia (PCP) caused by Pneumocystis jirovecii is one of the most common opportunistic infections in immunosuppressed patients, particularly in patients with acquired immunodeficiency syndrome (AIDS). (1 → 3)-β-D-glucan is a component of the cell wall of P. jirovecii and other fungi such as Candida sp., Aspergillus sp. and Histoplasma sp. The measurement of serum (1 → 3)-β-D-glucan has been reported to be a highly sensitive test for PCP related to human immunodeficiency virus (HIV-PCP). We report a case of HIV-PCP not associated with elevated serum (1 → 3)-β-D glucan and highlight how HIV-PCP cannot be completely ruled out if (1 → 3)-β-D glucan is negative.
Keywords: Acquired immunodeficiency syndrome, Human immunodeficiency virus, Pneumocystis pneumonia, (1 → 3)-β-D-glucan
1. Introduction
Pneumocystis pneumonia (PCP) caused by Pneumocystis jirovecii is one of the most common opportunistic infections in immunosuppressed patients, particularly in those with acquired immunodeficiency syndrome (AIDS). (1 → 3)-β-D-glucan is a component of the cell wall of P. jirovecii and other fungi such as Candida sp., Aspergillus sp. and Histoplasma sp. The bacterial burden of P. jirovecii in PCP related to the human immunodeficiency virus (HIV-PCP) is greater than in non-HIV-PCP disease. The sensitivity of serum (1 → 3)-β-D glucan as a diagnostic test for HIV-PCP has been reported to be high enough to reduce the number of cases treated empirically and the need for a bronchoscopy, and be sufficient for ruling out PCP [1], [2]. However, a small number of cases of HIV-PCP exist not associated with elevated serum (1 → 3)-β-D glucan. To our knowledge, a case report of HIV-PCP without an elevated serum (1 → 3)-β-D glucan level has not previously been published. We herein report a case of HIV-PCP, confirmed by the identification of P. jirovecci cysts in bronchoalveolar lavage fluid (BALF), not associated with an elevation of serum (1 → 3)-β-D glucan.
2. Case report
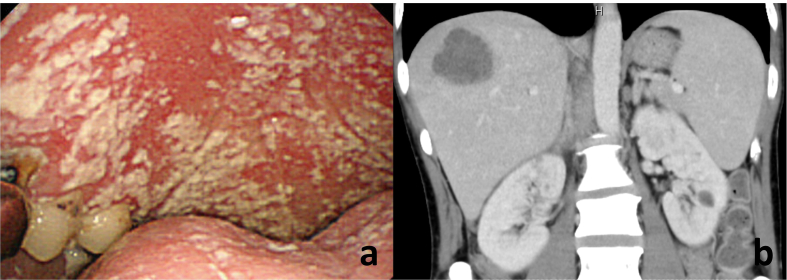
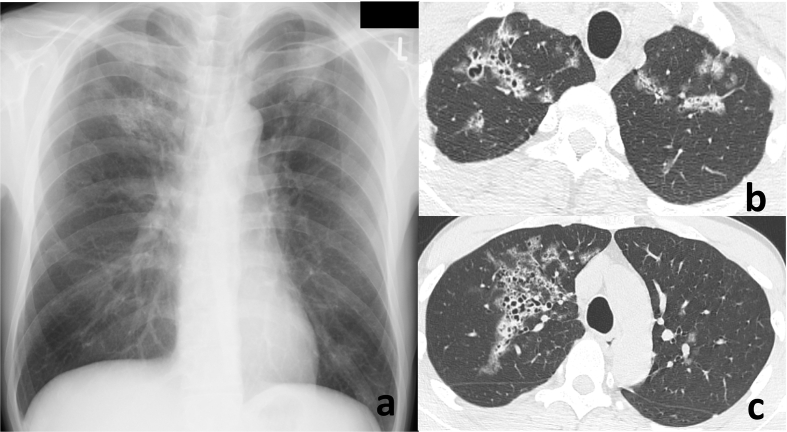
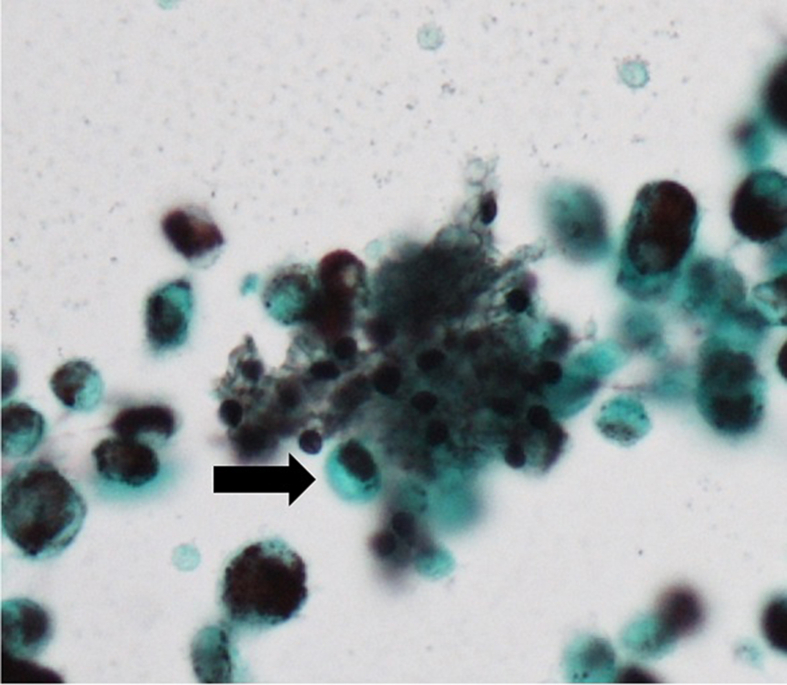
A 40-year-old Japanese man complained to his primary care physician of a 7 kg weight loss over a period of three months and two days of fever with chills. The man was subsequently referred to our hospital. He did not complain of any headaches, nausea, vomiting or diarrhea and did not have a history of recent travel or close contact with a sick person. He also lacked any relevant medical or family history. He was a non-drinker and had a 10 pack years of smoking history. The patient had a history of homosexual activity and had experienced repeated, unprotected sex with unspecified partners. His vital signs were as follows: blood pressure 132/83 mmHg, pulse rate 122 beats/min and regular, body temperature 38.2 °C, respiratory rate 14 breaths/min and oxygen saturation 97% with ambient air. On physical examination, a furry coating was visible on his tongue, inner cheeks and on the roof of the mouth (Fig. 1a). Auscultation of the lungs did not reveal crackles. His cardiac sounds were regular and murmurs were not detected. His abdomen was soft and flat; however a right hypochondrium tenderness was detected. He did not have a perianal lesion. Laboratory findings were as follows: white blood cell count (WBC) was 7240/mm3, with 77.5% neutrophils, 14.0% lymphocytes, and 8.5% monocytes. The results of routine blood chemistry tests were within normal range, except for lactate dehydrogenase (LDH) levels of 257 IU/L. The C-reactive protein (CRP) serum level was elevated to 16.8 mg/dL. Serum levels of (1 → 3)-β-D glucan (β-D glucan Wako kit: Wako Pure Chemical Industries; Tokyo, Japan) were below the assay's sensitivity (<5 pg/mL). Serum levels of Krebs von den Lungen-6 (KL-6) and surfactant protein-D (SP-D) were within normal range. Abdominal CT revealed a space-occupying lesion on his liver (Fig. 1b). Chest radiography on the first visit revealed infiltrative shadows in the bilateral upper lung field (Fig. 2a). Chest high-resolution computed tomography (CT) revealed a ground-glass opacity with multiple cysts on the bilateral upper lobes (Fig. 2b, c). Upon further investigation, the patient was found to be HIV-antibody positive, and a HIV RNA quantitation test revealed a titer of 4.9 × 105 copies/mL, with a CD4 cell count of 48/μL. Induced sputum smear tests were negative for bacteria, acid-fast bacilli and P. jirovecci cysts. Cytomegalovirus (CMV) IgM antibodies were not detected, although the patient's serum tested positive for CMV IgG antibody. Blood cultures did not reveal bacterial growth. A CMV antigenemia assay was negative. Antibodies to Entamoeba histolytica were detected. Cells (4.8 × 105/mL) recovered from BALF comprised 85% macrophages and 15% lymphocytes with a CD4/CD8 ratio of 0.03. Although the BALF smear was negative for bacteria and acid-fast bacilli, a Grocott's stain demonstrated the presence of P. jirovecci cysts (Fig. 3). Polymerase chain reaction testing of P. jirovecci in BALF was also positive. We therefore diagnosed HIV-PCP complicated by an amebic liver abscess and oral thrush. Oral trimethoprim/sulfamethoxazole (960 mg per day), fluconazole (100 mg per day) and metronidazole (1500 mg per day) were started on the first day of treatment. By day 9, the patient's body temperature had normalized, and the WBC and CRP had decreased to 2280/mm3 (with 42.0% lymphocytes) and 1.1 mg/dL, respectively. Serum (1–3) β-D glucan was still negative. The patient was then transferred to a hospital specialized in the treatment of HIV infections to receive antiretroviral therapy. (556words).
Fig. 1.
A furry coating was visible on his tongue, inner cheeks and on the roof of the mouth (a). Abdominal CT revealed a space-occupying lesion on his liver (b).
Fig. 2.
Chest radiography on the first visit revealed infiltrative shadows in the bilateral upper lung field (a). Chest high-resolution computed tomography (CT) revealed a ground-glass opacity with multiple cysts on the bilateral upper lobes (b, c).
Fig. 3.
BALF smear stained with a Grocott's stain demonstrated the presence of P. jirovecci cysts (arrow). (Grocott's stein; × 40).
3. Discussion
In this case, although a diagnosis of HIV-PCP was confirmed by the identification of P. jirovecci cysts in BALF, the serum (1–3)-β-D-glucan level proved negative. PCP is a potentially life-threatening infection that occurs in immunocompromised hosts. HIV-infected patients with a CD4 count below 200/μL tend to suffer from PCP [3]. In a diagnosis for PCP, the most reliable finding is histological or cytological evidence of P. jirovecii in bronchoalveolar lavage, lung biopsy, or sputum specimens.
The assay for serum (1 → 3)-β-D-glucan has been reported to be highly sensitive for detecting this in HIV-PCP patient sera. Previous studies, primarily retrospective case series in patients with HIV-PCP using the Fungitell assay (East Falmouth, MA, USA), had reported a sensitivity for serum (1 → 3)-β-D glucan ranging from 90% to 100% [4], [5], [6]. The β-D glucan assay by Wako used in our case has also been reported to be highly sensitive, with a sensitivity of 92.3% [7]. The high sensitivity of the (1 → 3)-β-D glucan assay is noteworthy, so a negative value strongly supports alternative diagnoses [1]. However, it has previously been reported that even in a group with high pre-test probability of PCP, the negative likelihood ratio of the (1 → 3)-β-D glucan test is only 0.1, leading to a post-test probability of 40% [8]. This report indicated that a negative (1 → 3)-β-D glucan does not indicate empiric PCP treatment should be stopped, assuming there is high clinical concern for PCP [8]. Furthermore, it should be remembered that there are rare cases of HIV-PCP that test negative for serum (1 → 3)-β-D glucan. For example, in 69 patients with HIV-PCP, three patients (4.3%) tested negative for (1 → 3)-β-D glucan, although P. jirovecii was detected in their BALF of these three patients [6]. Of these three patients, two presented with a low P. jirovecii burden, as estimated by a semi-quantitative immunofluorescence method, which may account for the low serum (1 → 3)-β-D glucan level measured [6]. The authors of this study claimed the measured serum (1 → 3)-β-D glucan level was negative due to a very recent infection in which the P. jirovecii load was still small, so that the infection had not yet caused elevated levels of serum (1 → 3)-β-D glucan [6]. In our case, it may be that the PCP was still at an early stage of infection because the lung lesion was limited to the upper lobe, and respiratory symptoms had not yet appeared. In 173 HIV-PCP patients enrolled in the AIDS Clinical Trials Group (ACTG) A5164 (cases without an identification of P. jirovecii were also included in this study), three patients did not show respiratory symptoms (dyspnea or shortness of breath, coughing or production of sputum, or pleuritic chest pain) and tested negative for serum (1 → 3)-β-D glucan [9]. However, in this study ten patients who were negative for serum (1 → 3)-β-D glucan showed respiratory symptoms [9]. Additionally, no correlation may exist between the level of (1 → 3)-β-D glucan and the severity of respiratory symptoms [5]. Therefore, we should pay attention to respiratory symptoms and any diagnosis of HIV-PCP, even if the (1 → 3)-β-D glucan level is negative.
Cases with a continued elevated serum (1 → 3)-β-D glucan level after the initiation of treatment for PCP have been reported [5]. However, in our case, the (1 → 3)-β-D glucan level was still negative after one week of treatment.
In conclusion, we report a case of HIV-PCP without any associated elevation of serum (1 → 3)-β-D glucan. Testing for serum (1 → 3)-β-D glucan is an attractive option for a diagnosis of, or to rule out PCP because this is an easier test than other testing modalities such as bronchoscopy. However, we have to recognize that HIV-PCP cannot be completely ruled out when the serum (1 → 3)-β-D glucan level is negative. (608words).
Conflict of interest
None.
Acknowledgements
None.
Contributor Information
Takahiro Kamada, Email: takahiro00920619@gmail.com.
Kenjiro Furuta, Email: k.furuta0113@gmail.com.
Hiromi Tomioka, Email: htomy@kobe-nishishimin-hospi.jp.
References
- 1.Sax Paul E., Komarow Lauren, Finkelman Malcolm A. Blood (1→3)-β-D-Glucan as a diagnostic test for HIV-related Pneumocystis jirovecii Pneumonia. Clin. Infect. Dis. 2011;53:197–202. doi: 10.1093/cid/cir335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Wei-Jie, Guo Ya-Ling, Liu Tang-Juan. Diagnosis of pneumocystis pneumonia using serum (1-3)-β-D-Glucan: a bivariate meta-analysis and systematic review. J. Thorac. Dis. 2015;7(12):2214–2225. doi: 10.3978/j.issn.2072-1439.2015.12.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang L., Crothers K. HIV-associated opportunistic pneumonias. Respirology. 2009;14:474–485. doi: 10.1111/j.1440-1843.2009.01534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desmet S., Van Wijngaerden E., Maertens J. Serum (1-3)-beta-D-glucan as a tool for diagnosis of Pneumocystis jirovecii pneumonia in patients with human immunodeficiency virus infection or hematological malignancy. J. Clin. Microbiol. 2009;47:3871–3874. doi: 10.1128/JCM.01756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe T., Yasuoka A., Tanuma J. Serum (1-->3) beta-D-glucan as a noninvasive adjunct marker for the diagnosis of Pneumocystis pneumonia in patients with AIDS. Clin. Infect. Dis. 2009;49:1128–1131. doi: 10.1086/605579. [DOI] [PubMed] [Google Scholar]
- 6.Esteves F., Lee C.H., de Sousa B. (1-3)-beta-D-glucan in association with lactate dehydrogenase as biomarkers of Pneumocystis pneumonia (PcP) in HIV-infected patients. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1173–1180. doi: 10.1007/s10096-014-2054-6. [DOI] [PubMed] [Google Scholar]
- 7.Tasaka S., Hasegawa N., Kobayashi S. Serum indicators for the diagnosis of pneumocystis pneumonia. Chest. 2007;131:1173–1180. doi: 10.1378/chest.06-1467. [DOI] [PubMed] [Google Scholar]
- 8.Wood B.R., Komarow L., Zolopa A.R. Beta-glucan for Pneumocystis pneumonia diagnosis in persons with AIDS: authors' reply. AIDS. 2013;28(27):2967–2968. doi: 10.1097/QAD.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 9.Wood B.R., Komarow L., Zolopa A.R. Test performance of blood beta-glucan for Pneumocystis jirovecii pneumonia in patients with AIDS and respiratory symptoms. AIDS. 2013;27(27):967–972. doi: 10.1097/QAD.0b013e32835cb646. [DOI] [PMC free article] [PubMed] [Google Scholar]