Abstract
Background:
Synovial biomarkers have recently been adopted as diagnostic tools for periprosthetic joint infection (PJI), but their utility is uncertain. The purpose of this systematic review and meta-analysis was to synthesize the evidence on the accuracy of the alpha-defensin immunoassay and leukocyte esterase colorimetric strip test for the diagnosis of PJI compared with the Musculoskeletal Infection Society diagnostic criteria.
Methods:
We performed a systematic review to identify diagnostic technique studies evaluating the accuracy of alpha-defensin or leukocyte esterase in the diagnosis of PJI. MEDLINE and Embase on Ovid, ACM, ADS, arXiv, CERN DS (Conseil Européen pour la Recherche Nucléaire Document Server), CrossRef DOI (Digital Object Identifier), DBLP (Digital Bibliography & Library Project), Espacenet, Google Scholar, Gutenberg, HighWire, IEEE Xplore (Institute of Electrical and Electronics Engineers digital library), INSPIRE, JSTOR (Journal Storage), OAlster (Open Archives Initiative Protocol for Metadata Harvesting), Open Content, Pubget, PubMed, and Web of Science were searched for appropriate studies indexed from inception until May 30, 2015, along with unpublished or gray literature. The classification of studies and data extraction were performed independently by 2 reviewers. Data extraction permitted meta-analysis of sensitivity and specificity with construction of receiver operating characteristic curves for each test.
Results:
We included 11 eligible studies. The pooled diagnostic sensitivity and specificity of alpha-defensin (6 studies) for PJI were 1.00 (95% confidence interval [CI], 0.82 to 1.00) and 0.96 (95% CI, 0.89 to 0.99), respectively. The area under the curve (AUC) for alpha-defensin and PJI was 0.99 (95% CI, 0.98 to 1.00). The pooled diagnostic sensitivity and specificity of leukocyte esterase (5 studies) for PJI were 0.81 (95% CI, 0.49 to 0.95) and 0.97 (95% CI, 0.82 to 0.99), respectively. The AUC for leukocyte esterase and PJI was 0.97 (95% CI, 0.95 to 0.98). There was substantial heterogeneity among studies for both diagnostic tests.
Conclusions:
The diagnostic accuracy for PJI was high for both tests. Given the limited number of studies and the large cost difference between the tests, more independent research on these tests is warranted.
Level of Evidence:
Diagnostic Level II. See Instructions for Authors for a complete description of levels of evidence.
Periprosthetic joint infection (PJI) is a rare complication affecting between 0.7% and 2.4% of patients receiving joint arthroplasty1-9. A PJI after hip or knee arthroplasty in particular has extremely negative effects on the physical, emotional, social, and economic aspects of a patient’s life10. Early diagnosis can lead to less radical treatment with debridement and retention of the prostheses instead of a 1-stage or 2-stage revision. Establishing a diagnosis of infection promptly11 is therefore of paramount importance, yet may be very challenging as the classic clinical features may be absent and a painful joint replacement may be caused by other pathologies. The Musculoskeletal Infection Society (MSIS) has developed diagnostic criteria to standardize and facilitate this diagnostic process (Table I)12. The search for a single diagnostic test on synovial fluid with the requisite accuracy, sensitivity, and specificity has yielded numerous biomarkers as potential candidates—with the term biomarker meaning a biologically pertinent molecule that can be evaluated objectively to indicate the presence of a disease or biological state. Alpha-defensin13 and leukocyte esterase14 are currently among the most promising.
TABLE I.
MSIS Criteria for PJI*
| One of the 3 following criteria must be met for the diagnosis of a PJI: |
| 1. A sinus tract communicates with the prosthesis. |
| 2. A pathogen is identified on culture of ≥2 separate samples of periprosthetic tissue or fluid. |
| 3. 4 of the 6 criteria below are present: |
| Serum ESR and serum CRP concentration are elevated. |
| Synovial WBC count is elevated. |
| Synovial neutrophil percentage is elevated. |
| Purulence is found in the involved joint. |
| A microorganism is isolated in 1 periprosthetic tissue or fluid culture. |
| >5 neutrophils per HPF in 5 HPFs are detected on histological analysis of periprosthetic tissue at 400× magnification. |
Modified from Parvizi et al.12. MSIS = Musculoskeletal Infection Society, PJI = periprosthetic joint infection, ESR = erythrocyte sedimentation rate, CRP = C-reactive protein, and WBC = white blood cell, and HPF = high-power field.
Alpha-defensin is an antimicrobial peptide that is released naturally from activated neutrophils. The peptide then integrates into, and destroys, the pathogens’ cell membranes15. The alpha-defensin immunoassay (CD Diagnostics) was developed from both genomic and proteomic studies and provides a qualitative result specific for synovial fluid. The advantages of this test include its simplicity and standardization, while a disadvantage is its relatively high cost of £500 (US$760) per test.
Leukocyte esterase is an enzyme secreted by activated neutrophils recruited to areas of infection. Detection of leukocyte esterase has been used for many years in urinalysis to diagnose urinary tract infection16. The leukocyte esterase colorimetric strip test is performed by applying fluid to a reagent test strip. A detergent on the strip lyses the neutrophils within the fluid, and this releases esterase, which catalyzes a reaction leading to formation of a violet dye. Advantages of this test are that it is quick, easy, and inexpensive at £0.11 (US$0.17) per test (Chemstrip 7; Roche Diagnostics). A potential disadvantage is the invalidation of the result by blood contamination17, although this has been addressed by centrifugation prior to application of the fluid18.
The purpose of this systematic review and meta-analysis was to synthesize the available evidence on the accuracy of alpha-defensin and leukocyte esterase in the diagnosis of PJI.
Materials and Methods
We used a rigorous and systematic approach conforming to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines19 (see Appendix) and the critical evaluation of studies relating to the diagnosis of PJI20.
Protocol
A protocol was registered online with PROSPERO (International prospective register of systematic reviews; CRD42015023704) before commencing the review, as recommended by PRISMA.
Search Strategy
We searched all studies indexed in MEDLINE and Embase on Ovid, ACM, ADS, arXiv, CERN DS (Conseil Européen pour la Recherche Nucléaire Document Server), CrossRef DOI (Digital Object Identifier), DBLP (Digital Bibliography & Library Project), Espacenet, Google Scholar, Gutenberg, HighWire, IEEE Xplore (Institute of Electrical and Electronics Engineers digital library), INSPIRE, JSTOR (Journal Storage), OAlster (Open Archives Initiative Protocol for Metadata Harvesting), Open Content, Pubget, PubMed, and Web of Science from inception until May 30, 2015, using the search strategy shown, as applied in MEDLINE and Embase, in Table II. We also evaluated the unpublished or gray literature, with hand searches of 6 major orthopaedic journals over the last 5 years. The bibliographies of the relevant articles were then cross-checked for articles not identified in the search. Studies of patients in all age groups were included. No language restrictions were applied, which is an important consideration with the perceived international interest in the treatment of infections around hip prostheses. The screening of studies was performed by 2 independent assessors, with any disagreements resolved by a third reviewer. A spreadsheet was constructed to summarize the findings of relevant studies.
TABLE II.
Search Strategy
| Defensin/Leukocyte Esterase Search* |
| MEDLINE 13 July 2015 |
| 1. neutrophil antimicrobial peptide.mp. or alpha-defensins/ |
| 2. alpha defensin.mp. or alpha-defensins/ |
| 3. alpha-defensins/or peptide neutrophil antimicrobial.mp. |
| 4. beta-defensins/or defensin.mp. or alpha-defensins/or defensins/ |
| 5. Arthroplasty, replacement/or knee joint/or arthroplasty, replacement, knee/or arthroplasty, replacement, hip/or hip prosthesis/or joint replacement.mp. or joint prosthesis/ |
| 6. Arthroplasty, replacement, knee/or arthroplasty, replacement, elbow/or arthroplasty, subchondral/or arthroplasty, replacement, ankle/or arthroplasty.mp. or arthroplasty, replacement, finger/or arthroplasty, replacement/or arthroplasty/or arthroplasty, replacement, hip/ |
| 7. Bacterial infections/or prosthesis-related infections/or prosthetic joint infection.mp. or surgical wound infection/ |
| 8. 5 or 6 or 7 |
| 9. 1 or 2 or 3 or 4 |
| 10. 8 and 9 |
| 11. leukocyte esterase.mp. |
| 12. leucocyte esterase.mp. |
| 13. 11 or 12 |
| 14. 8 and 13 |
| 15. 10 or 14 |
| Embase 13 July 2015 |
| 1. alpha defensins.mp. or alpha defensin/ |
| 2. neutrophil antimicrobial peptide.mp. or defensin/ |
| 3. beta-defensin/or defensin.mp. or alpha-defensin/or defensin/ |
| 4. joint replacement.mp. or joint prosthesis/ |
| 5. Shoulder arthroplasty/or knee arthroplasty/or finger arthroplasty/or ankle arthroplasty/or elbow arthroplasty/or reverse shoulder arthroplasty/or arthroplasty.mp. or arthroplasty/or hip arthroplasty/ |
| 6. Prosthesis infection/or prosthetic joint infection.mp. |
| 7. 4 or 5 or 6 |
| 8. 1 or 2 or 3 |
| 9. 7 and 8 |
| 10. leukocyte esterase.mp. |
| 11. leucocyte esterase.mp. |
| 12. 10 or 11 |
| 13. 7 and 12 |
| 14. 9 or 13 |
Each part was specifically adapted for searching alternative databases.
Eligibility Criteria
We included all diagnostic studies that enrolled patients with true diagnostic uncertainty in the setting of PJI. Tests of interest were the alpha-defensin assay and the leukocyte esterase test scoring positive (++). Eligible studies had a reference standard for diagnosing PJI using the MSIS diagnostic criteria.
Screening
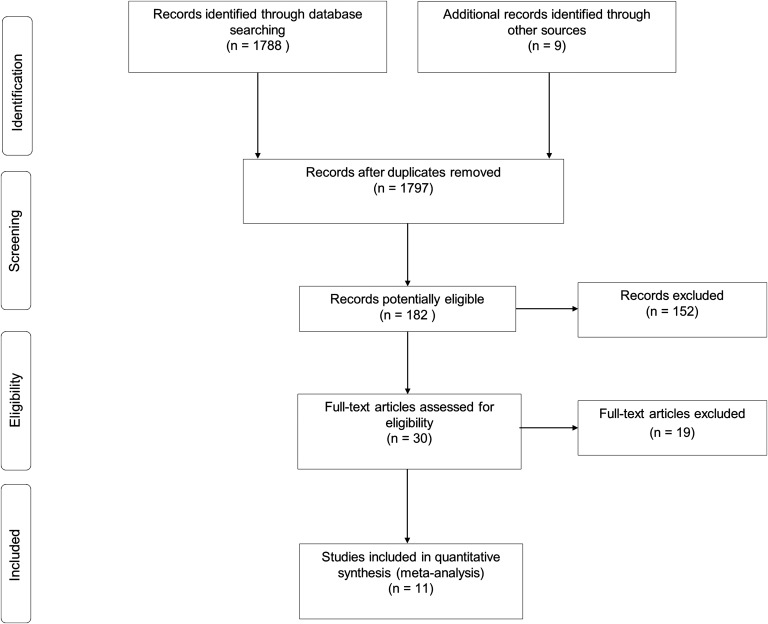
A total of 1797 records were identified from searching the literature. The titles and abstracts were screened to identify potentially useful articles for inclusion in this systematic review. After initial screening, 30 full-text articles were assessed in detail for eligibility against criteria. A PRISMA flow diagram of the progression of studies through this systematic review is provided in Figure 1.
Fig. 1.
PRISMA flow diagram.
Data Extraction
Two of the authors (M.C.W. and S.K.K.) worked independently to classify the studies and to extract the data using standardized forms. We extracted data on sensitivity, specificity, likelihood ratios, participants, joint involved, diagnostic test performed, cutoff or range definitions of the tests, whether the cutoffs were derived with the use of receiver operating characteristic (ROC) curves or predetermined by the study authors, and the nature and characteristics of the reference standard test. Quality assessment of each study was also performed using the QUADAS (Quality Assessment of Diagnostic Accuracy Studies)-2 tool21.
Statistical Analyses
Overall sensitivity and specificity values for the diagnosis of PJI were pooled using the bivariate meta-analysis framework22. The bivariate model is an improvement and an extension of the traditional summary ROC (sROC) curve and jointly models sensitivity and specificity as the starting point of the analysis; thus, it may be more reliable for estimating diagnostic accuracy23,24. The sROC curve shows the consistency of results across studies and therefore whether there was a uniform ROC curve over all studies. The sROC curve data points come from regression analysis of each study, while the ROC curve data points come from each threshold. In addition, the AUC depicts the accuracy of the test. The bivariate model employs a random effects approach, which takes into account the heterogeneity beyond chance between studies. In addition, it also accounts for between-study correlation between underlying sensitivity and specificity, caused by the use of different thresholds across studies. An I2 statistic was used to assess inconsistency among studies. An I2 statistic is the proportion of variability across studies due to patient population variability rather than to sampling error. I2 lies between 0% and 100%. A value of 0% indicates no observed heterogeneity, and values of >50% may be considered to indicate substantial heterogeneity. A priori hypotheses to explain potential heterogeneity included the site of the prosthesis and the diagnosis of infection occurring at different time points.
Pooled positive and negative likelihood ratios were calculated using the summary estimates of sensitivity and specificity. Potential sources of heterogeneity across studies could not be investigated because of the limited number of studies. In addition, tests for publication bias (e.g., the Egger test) require at least 10 studies and lower heterogeneity to be useful and valid25; therefore, we were unable to investigate for publication bias. All analyses were conducted using Stata version 14 (StataCorp), and the midas and metandi commands were used for all analyses.
Results
There were 10 articles reporting 11 evaluations13,14,17,18,26-31 that contributed to our estimates of diagnostic accuracy for both tests. Six studies explored the diagnostic accuracy of alpha-defensin for PJI13,17,26-28,31, while the remaining 5 studies explored the diagnostic accuracy of leukocyte esterase for PJI14,17,18,29,30. Study characteristics are summarized in Table III. All of the included studies were published within the last 5 years and originated from the U.S. The largest contribution for the alpha-defensin test was from Deirmengian and colleagues13,17,27,31; there was no overlap of patients within these studies.
TABLE III.
Characteristics of Included Studies
| Study* | Dates of Study† | Site of Arthroplasty | Inclusion Criteria | No. of Patients‡ | Age§ and Sex | Method |
| Alpha-defensin | ||||||
| Deirmengian et al.13 (2014) | NA | Hip or knee | Pain at site of total hip or knee arthroplasty | 37/149 | 65 (41-89) and 47% male | Citrano Medical Laboratories (CD Diagnostics) |
| Deirmengian et al.31 (2015) | 2012-2014 | Hip, knee, or shoulder | Samples from patients in setting of a workup for PJI | 244/1937 | NA | Synovasure (Citrano Medical Laboratories of CD Diagnostics) |
| Bingham et al.26 (2014) | 2013 | Hip or knee | Failed or painful hip or knee arthroplasty, or undergoing second-stage reimplantation | 19/61# | NA | Synovasure (Citrano Medical Laboratories of CD Diagnostics) |
| Deirmengian et al.27 (2014) | 2009-2014 | Hip or knee | Patients evaluated for possible infection of a hip or knee arthroplasty | 29/95 | 67 (41-86) and 48% male | CD Diagnostics |
| Frangiamore et al.28 (2015) | 2012-2013 | Shoulder | All patients evaluated for pain at site of shoulder arthroplasty by 2 surgeons | 11/33 | 61.7 (SD 12.4) and 43% male | Synovasure (Citrano Medical Laboratories of CD Diagnostics) |
| Deirmengian et al.17 (2015) | 2012 | Hip or knee | Patients evaluated for possible infection of at site of hip or knee arthroplasty | 23/46 | 65 and 43% male | CD Diagnostics |
| Leukocyte esterase | ||||||
| Tischler et al.18 (2014) | 2009-2013 | Hip or knee | Patients with revision total knee or hip arthroplasty for either aseptic failure or PJI | 52/189 | 63 (21-90) and 48% male | Chemstrip 7 urine test strip (Roche) |
| Colvin et al.29 (2015) | 2013-2014 | Hip, knee, elbow, or ankle | Unexplained painful hip, knee, elbow, or ankle arthroplasty, routine implant testing, or clinical suspicion of PJI or septic arthritis | 19/52 | 69.1 (31-91) and 47% male | Chemstrip 10 UA or Chemstrip 7 urine test strips (Roche) |
| Parvizi et al.14 (2011) | 2007-2010 | Knee | Patients undergoing revision knee arthroplasty or workup for possible PJI at knee | 30/108 | 64 (28-89) and 44% male | Chemstrip 7 urine test strip (Roche) |
| Wetters et al.30 (2012) | NA | Hip or knee | Patients with suspicion of PJI after hip or knee arthroplasty | 39/158 | 63.3 (33-88) and 45.5% male | Chemstrip 7 urine test strip (Roche) |
| Deirmengian et al.17 (2015) | 2012 | Hip or knee | Patients evaluated for possible PJI at site of hip or knee arthroplasty | 11/38** | 65 and 43% male | Chemstrip 7 urine test strip (Roche) |
All studies were performed in the U.S.
NA = not available.
The values are given as the number of patients with an infection/total number of patients in study.
The values are given as the mean age in years, with the range or standard deviation (SD) given in parentheses. NA = not available.
Aspirations.
Eight of 46 patients had samples excluded because of blood interference.
Five studies on alpha-defensin included patients who had hip or knee arthroplasty13,17,26,27,31 and 2 studies included patients who had shoulder arthroplasty28,31. When details were provided, the mean ages of patients in these studies ranged from 61 to 67 years and 43% to 48% were male. In the studies of leukocyte esterase, all investigations included patients who had knee arthroplasty14,17,18,29,30, 4 studies included patients who had hip arthroplasty17,18,29,30, and 1 study also included patients who had elbow or ankle arthroplasty29. The mean ages of the patients ranged from 63 to 69 years, and 43% to 48% were male. Only 1 study noted the time since the index arthroplasty, which was a mean of 42.7 months (range, 7 days to 458 months)30. The total numbers of patients contributing to the meta-analyses of alpha-defensin and leukocyte esterase were 2,321 and 545, respectively, with 363 and 151 patients with PJI.
Quality Assessment
In Table IV, the QUADAS-2 assessments for each study are listed. Using the QUADAS-2 tool, which has a maximum score of 14, the studies had a mean score of 13 (range, 11 to 14). This indicates that the studies used in this meta-analysis were of good quality.
TABLE IV.
QUADAS-2 Evaluation
| QUADAS Score21* |
|||||||||||||||
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Total |
| Alpha-defensin | |||||||||||||||
| Bingham et al.26 (2014) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 13 |
| Deirmengian et al.27 (2014) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 |
| Deirmengian et al.13 (2014) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 13 |
| Frangiamore et al.28 (2015) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 |
| Deirmengian et al.31 (2015) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 12 |
| Deirmengian et al.17 (2015) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 12 |
| Leukocyte esterase | |||||||||||||||
| Tischler et al.18 (2014) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 |
| Wetters et al.30 (2012) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 |
| Parvizi et al.14 (2011) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 |
| Colvin et al.29 (2015) | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 11 |
| Deirmengian et al.17 (2015) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 12 |
A number 1 indicates “yes,” and 0 indicates “no.” The numbers in the top row correspond to the following questions: 1. Was the spectrum of patients representative of the patients who will receive the test in practice? 2. Were selection criteria clearly described? 3. Is the reference standard likely to correctly classify the target condition? 4. Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the 2 tests? 5. Did the whole sample or a random selection of the sample, receive verification using a reference standard of diagnosis? 6. Did patients receive the same reference standard regardless of the index text result? 7. Was the reference standard independent of the index test (i.e., the index test did not form part of the reference standard)? 8. Was the execution of the index test described in sufficient detail to permit replication of the test? 9. Was the execution of the reference standard described in sufficient detail to permit its replication? 10. Were the index test results interpreted without knowledge of the results of the reference standard? 11. Were the reference standard results interpreted without knowledge of the results of the index test? 12. Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? 13. Were uninterpretable/intermediate test results reported? 14. Were withdrawals from the study explained?
Diagnostic Value of Alpha-Defensin for PJI
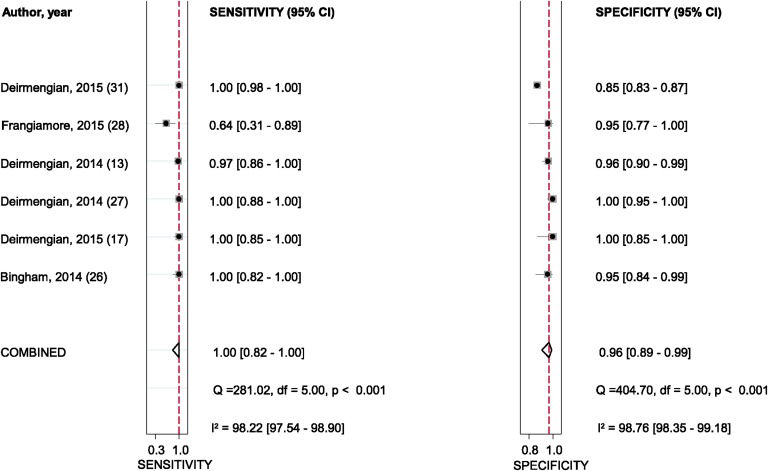
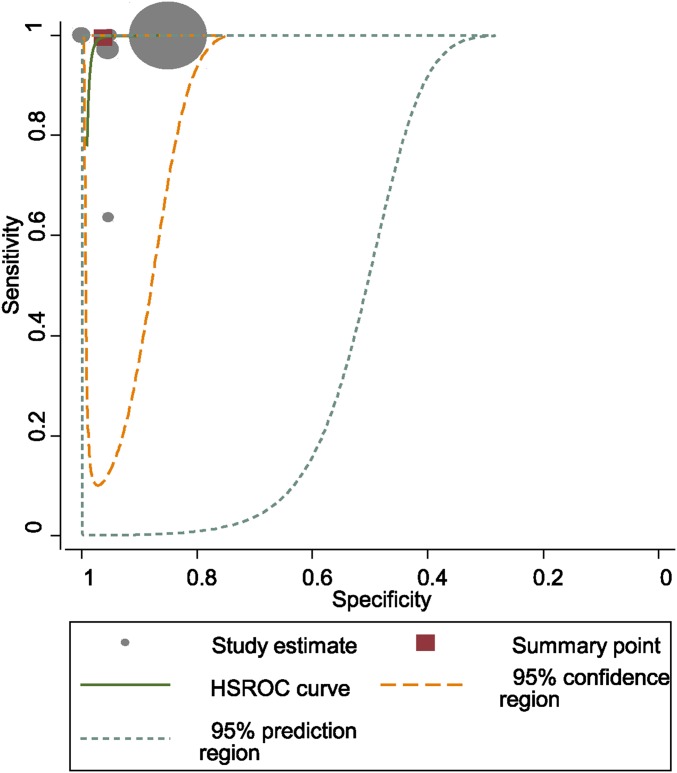
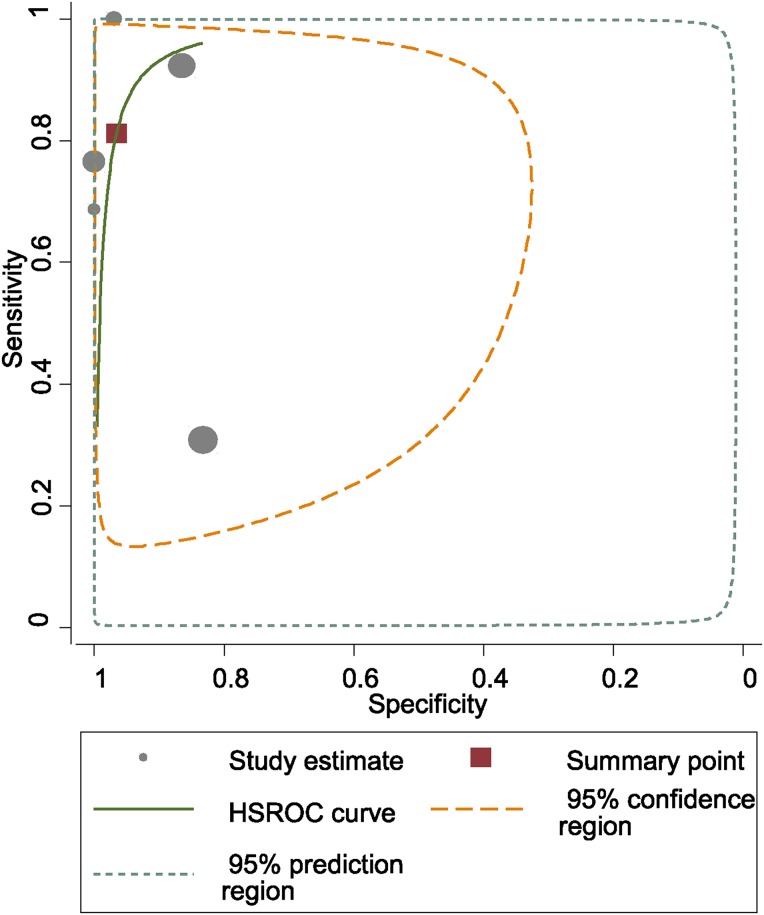
The pooled diagnostic sensitivity and specificity of alpha-defensin for PJI were 1.00 (95% confidence interval [CI], 0.82 to 1.00) and 0.96 (95% CI, 0.89 to 0.99), respectively (Fig. 2). There was substantial heterogeneity among studies; the I2 statistics for sensitivity and specificity values were 98.2% (95% CI, 97.5% to 98.9%) and 98.8% (95% CI, 98.4% to 99.2%), respectively. The pooled positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic score were 27.0 (95% CI, 9.0 to 80.6), 0.00 (95% CI, 0.00 to 0.22), and 8.94 (95% CI, 4.73 to 13.15), respectively. The AUC for alpha-defensin and PJI was 0.99 (95% CI, 0.98 to 1.00) (Fig. 3).
Fig. 2.
Sensitivity and specificity of alpha-defensin in the diagnosis of PJI, according to Deirmengian et al.17, Deirmengian et al.27, Bingham et al.26, and Frangiamore et al.28. The dashed red lines indicate the pooled sensitivity or specificity estimate. df = degrees of freedom.
Fig. 3.
HSROC (hierarchical summary receiver-operating characteristic) curves of alpha-defensin in the diagnosis of PJI.
Diagnostic Value of Leukocyte Esterase for PJI
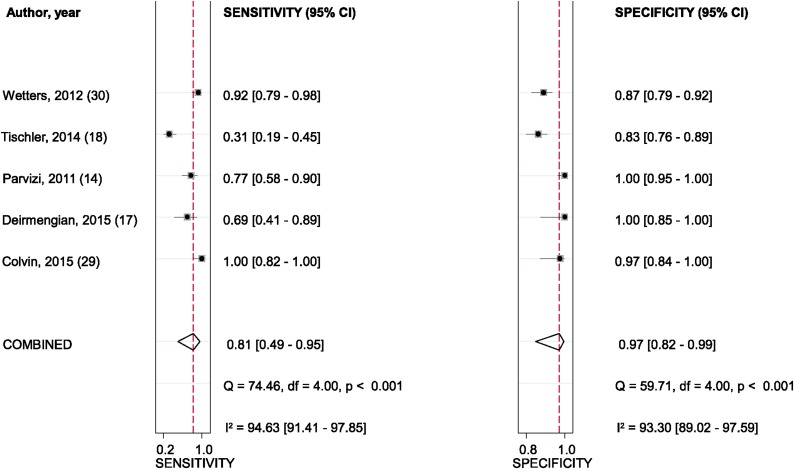
The pooled diagnostic sensitivity and specificity of leukocyte esterase for PJI were 0.81 (95% CI, 0.49 to 0.95) and 0.97 (95% CI, 0.82 to 0.99), respectively (Fig. 4). There was substantial heterogeneity among studies, the I2 statistic for sensitivity and specificity values were 94.6% (95% CI, 91.4% to 97.9%) and 93.3% (95% CI, 89.0% to 97.6%), respectively. The pooled PLR, NLR, and diagnostic score were 23.9 (95% CI, 3.8 to 152.1), 0.19 (95% CI, 0.06 to 0.66), and 4.82 (95% CI, 2.27 to 7.36), respectively. The AUC for leukocyte esterase and PJI was 0.97 (95% CI, 0.95 to 0.98) (Fig. 5).
Fig. 4.
Sensitivity and specificity of leukocyte esterase in the diagnosis of PJI, according to Wetters et al.30, Tischler et al.18, Parvizi et al.14, Deirmengian et al.17, and Colvin et al.29. The dashed red lines indicate pooled sensitivity or specificity estimate. df = degrees of freedom.
Fig. 5.
HSROC (hierarchical summary receiver-operating characteristic) curves of leukocyte esterase in the diagnosis of PJI.
Discussion
The prompt diagnosis of PJI remains a clinical challenge because of the diverse clinical presentations of patients having this complication and the overlap of some of these features with other diagnoses and causes of failure of a joint replacement. The distinction between PJI and other causes of failure is important as the surgical management, and the chance of a successful outcome, differs according to the mode of failure and thus the treatment strategy employed.
This systematic review has shown that alpha-defensin is extremely sensitive and specific in identifying PJI and that leukocyte esterase is slightly less sensitive but also extremely specific. Both are therefore good candidates for diagnostic biomarkers. Traditional tests to diagnose PJI are less effective. In a study examining diagnostic accuracy for inflammatory serological markers in PJI32, the pooled sensitivity and specificity for the erythrocyte sedimentation rate (ESR) were 88% (95% CI, 86% to 90%) and 70% (95% CI, 68% to 72%), respectively. The pooled sensitivity and specificity for C-reactive protein were 97% (95% CI, 93% to 99%) and 91% (95% CI, 87% to 94%), respectively. However, that study did not use the MSIS criteria as its gold standard.
Several other synovial biomarkers have been investigated in relation to PJI. The synovial white blood-cell count typically shows sensitivity of 84% to 93% and specificity of 51% to 100%33-36. The proportion of synovial white blood cells that are polymorphonuclear, and thus concerned with fighting infection, typically shows sensitivity of 81% to 93% and specificity of 69% to 83%33-36. While C-reactive protein in serum may have high sensitivity and poor specificity for PJI, measurement in synovial fluid shows sensitivity of 96% to 97% and specificity of 90% to 93%27,37. In one study included in our review, a broad range of synovial biomarkers were investigated and several in particular, including neutrophil elastase, bactericidal/permeability-increasing protein, neutrophil gelatinase-associated lipocalin, and lactoferrin, merit further study27.
The strengths and potential limitations of this analysis deserve mention. Our meta-analysis is, to our knowledge, the first of its kind, and it examined data on nearly 2,000 patients in studies that were performed to a very high standard. All studies included in the review used the MSIS criteria, thereby minimizing classification bias by using a common and widely accepted reference standard12. The studies in our meta-analysis mainly included patients who had hip or knee arthroplasty, although some studies included patients who had shoulder, ankle, or elbow arthroplasty. The age ranges and proportions of male patients were similar among studies, but information on the time since arthroplasty was highly limited. This may be important as some biomarkers may have different diagnostic accuracy in the early postoperative period36. Future studies should aim to assess differences in diagnostic accuracy relating to potential sources of heterogeneity, including arthroplasty site, time since index surgery, and patient characteristics.
A number of the studies of alpha-defensin came from the same research group, which could hamper the generalization of our findings; however, these findings were replicated by other groups, yet there was no identifiable source of selection or detection bias. The limited number of studies precluded the ability to explore for publication bias, an important issue in diagnostic accuracy studies, in which results of new tests with poor sensitivity and specificity may remain unpublished. None of the studies noted blinding, which potentially may have introduced selection bias. Further large-scale, independent, and rigorous studies are warranted to evaluate the use of these synovial markers as diagnostic tools for PJI.
Alpha-defensin is substantially more expensive (£500 [US$760] per test) than leukocyte esterase (£0.11 [US$0.17] per test), although the latter may require the initial capital cost of a centrifuge and the costs associated with its use. However, alpha-defensin may be more specific in diagnosing PJI, and both tests may have clinical roles as biomarkers. We recommend that further comparisons be made between these 2 promising biomarkers and traditional diagnostic tests to assess their relative clinical effectiveness and cost-effectiveness.
Appendix
A table showing the PRISMA checklist is available with the online version of this article as a data supplement at jbjs.org.
Footnotes
Investigation performed at the Princess Elizabeth Orthopaedic Centre, Royal Devon and Exeter NHS Foundation Trust, Exeter, and the University of Bristol, Bristol, United Kingdom
A commentary by Emily Ruth Dodwell, MD, MPH, is linked to the online version of this article at jbjs.org.
Disclosure: This article presents independent research funded by the National Institute for Health Research under its Programme Grants for Applied Research programme (RP-PG-1210-12005). On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work.
Disclaimer: The views expressed in this article are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
References
- 1.Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004. July;86(5):688-91. [DOI] [PubMed] [Google Scholar]
- 2.Blom AW, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total hip arthroplasty. The Avon experience. J Bone Joint Surg Br. 2003. September;85(7):956-9. [DOI] [PubMed] [Google Scholar]
- 3.Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty. 2009. September;24(6)(Suppl):105-9. Epub 2009 Jun 2. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012. September;27(8)(Suppl):61-5. e1. Epub 2012 May 2. [DOI] [PubMed] [Google Scholar]
- 5.Huotari K, Peltola M, Jämsen E. The incidence of late prosthetic joint infections: a registry-based study of 112,708 primary hip and knee replacements. Acta Orthop. 2015. June;86(3):321-5. Epub 2015 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dale H, Fenstad AM, Hallan G, Havelin LI, Furnes O, Overgaard S, Pedersen AB, Kärrholm J, Garellick G, Pulkkinen P, Eskelinen A, Mäkelä K, Engesæter LB. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop. 2012. October;83(5):449-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achermann Y, Vogt M, Spormann C, Kolling C, Remschmidt C, Wüst J, Simmen B, Trampuz A. Characteristics and outcome of 27 elbow periprosthetic joint infections: results from a 14-year cohort study of 358 elbow prostheses. Clin Microbiol Infect. 2011. March;17(3):432-8. [DOI] [PubMed] [Google Scholar]
- 8.Henricson A, Nilsson JÅ, Carlsson A. 10-year survival of total ankle arthroplasties: a report on 780 cases from the Swedish Ankle Register. Acta Orthop. 2011. December;82(6):655-9. Epub 2011 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padegimas EM, Maltenfort M, Ramsey ML, Williams GR, Parvizi J, Namdari S. Periprosthetic shoulder infection in the United States: incidence and economic burden. J Shoulder Elbow Surg. 2015. May;24(5):741-6. Epub 2015 Jan 13. [DOI] [PubMed] [Google Scholar]
- 10.Andersson AE, Bergh I, Karlsson J, Nilsson K. Patients’ experiences of acquiring a deep surgical site infection: an interview study. Am J Infect Control. 2010. November;38(9):711-7. [DOI] [PubMed] [Google Scholar]
- 11.Moojen DJ, Zwiers JH, Scholtes VA, Verheyen CC, Poolman RW. Similar success rates for single and multiple debridement surgery for acute hip arthroplasty infection. Acta Orthop. 2014. August;85(4):383-8. Epub 2014 Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011. November;469(11):2992-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014. September 3;96(17):1439-45. [DOI] [PubMed] [Google Scholar]
- 14.Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. J Bone Joint Surg Am. 2011. December 21;93(24):2242-8. [DOI] [PubMed] [Google Scholar]
- 15.Lehrer RI, Ganz T. Defensins: endogenous antibiotic peptides from human leukocytes. Ciba Found Symp. 1992;171:276-90; discussion 290–3. [DOI] [PubMed] [Google Scholar]
- 16.Leighton PM, Little JA. Leucocyte esterase determination as a secondary procedure for urine screening. J Clin Pathol. 1985. February;38(2):229-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Booth RE Jr, Parvizi J. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015. January;473(1):198-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for musculoskeletal infection society criteria. J Bone Joint Surg Am. 2014. November 19;96(22):1917-20. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009. October;62(10):1006-12. Epub 2009 Jul 23. [DOI] [PubMed] [Google Scholar]
- 20.Buntinx F, Aertgeerts B, Macaskill P. Guidelines for conducting systematic reviews of studies evaluating the accuracy of diagnostic tests. In: Knottnerus JA, Buntinx F, editors. The evidence base of clinical diagnosis: theory and methods of diagnostic research, 2nd edition London: Wiley-Blackwell; 2009. p 180-212. [Google Scholar]
- 21.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011. October 18;155(8):529-36. [DOI] [PubMed] [Google Scholar]
- 22.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005. October;58(10):982-90. [DOI] [PubMed] [Google Scholar]
- 23.Harbord RM, Whiting P, Sterne JA, Egger M, Deeks JJ, Shang A, Bachmann LM. An empirical comparison of methods for meta-analysis of diagnostic accuracy showed hierarchical models are necessary. J Clin Epidemiol. 2008. November;61(11):1095-103. [DOI] [PubMed] [Google Scholar]
- 24.Kriston L, Härter M, Hölzel L. Challenges in reporting meta-analyses of diagnostic accuracy studies. Ann Intern Med. 2009. March 17;150(6):430. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [Updated March 2011]. 2011. http://www.cochrane-handbook.org. Accessed 2016 Jan 29.
- 26.Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014. December;472(12):4006-9. Epub 2014 Sep 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014. November;472(11):3254-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frangiamore SJ, Saleh A, Grosso MJ, Kovac MF, Higuera CA, Iannotti JP, Ricchetti ET. α-defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015. July;24(7):1021-7. Epub 2015 Feb 8. [DOI] [PubMed] [Google Scholar]
- 29.Colvin OC, Kransdorf MJ, Roberts CC, Chivers FS, Lorans R, Beauchamp CP, Schwartz AJ. Leukocyte esterase analysis in the diagnosis of joint infection: can we make a diagnosis using a simple urine dipstick? Skeletal Radiol. 2015. May;44(5):673-7. Epub 2015 Jan 29. [DOI] [PubMed] [Google Scholar]
- 30.Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012. September;27(8)(Suppl):8-11. Epub 2012 May 17. [DOI] [PubMed] [Google Scholar]
- 31.Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, Booth RE Jr. The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop Relat Res. 2015. July;473(7):2229-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berbari E, Mabry T, Tsaras G, Spangehl M, Erwin PJ, Murad MH, Steckelberg J, Osmon D. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2010. September 1;92(11):2102-9. [DOI] [PubMed] [Google Scholar]
- 33.Lenski M, Scherer MA. Diagnostic potential of inflammatory markers in septic arthritis and periprosthetic joint infections: a clinical study with 719 patients. Infect Dis (Lond). 2015. June;47(6):399-409. Epub 2015 Mar 6. [DOI] [PubMed] [Google Scholar]
- 34.Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012. October;27(9):1589-93. Epub 2012 Apr 28. [DOI] [PubMed] [Google Scholar]
- 35.Bedair H, Ting N, Jacovides C, Saxena A, Moric M, Parvizi J, Della Valle CJ. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011. January;469(1):34-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yi PH, Cross MB, Moric M, Sporer SM, Berger RA, Della Valle CJ. The 2013 Frank Stinchfield Award: diagnosis of infection in the early postoperative period after total hip arthroplasty. Clin Orthop Relat Res. 2014. February;472(2):424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omar M, Ettinger M, Reichling M, Petri M, Guenther D, Gehrke T, Krettek C, Mommsen P. Synovial C-reactive protein as a marker for chronic periprosthetic infection in total hip arthroplasty. Bone Joint J. 2015. February;97(2):173-6. [DOI] [PubMed] [Google Scholar]