Abstract
The pathogenesis of hypertension-related cognitive impairment has not been sufficiently clarified, new molecular targets are needed. p38 MAPK pathway plays an important role in hypertensive target organ damage. Activated p38 MAPK was seen in AD brain tissue. In this study, we found that long-term potentiation (LTP) of hippocampal CA1 was decreased, the density of the dendritic spines on the CA1 pyramidal cells was reduced, the p-p38 protein expression in hippocampus was elevated, and cognitive function was impaired in angiotensin II-dependent hypertensive C57BL/6 mice. In vivo, using a p38 heterozygous knockdown mice (p38KI/+) model, we showed that knockdown of p38 MAPK in hippocampus leads to the improvement of cognitive function and hippocampal synaptic plasticity in angiotensin II-dependent p38KI/+ hypertensive mice. In vitro, LTP was improved in hippocampal slices from C57BL/6 hypertensive mice by treatment with p38MAPK inhibitor SKF86002. Our data demonstrated that p38 MAPK may be a potential therapeutic target for hypertension-related cognitive dysfunction.
Cognitive decline is rapidly becoming an important cause of disability worldwide and contributes significantly to increased mortality. Cardiovascular risk factors, particularly hypertension, play an important role in the development of cognitive dysfunction, including Alzheimer’s disease (AD), leading to increased prevalence of dementia in the aging population1,2,3. Heart–brain interaction is one of the 10 most-promising advances in cardiovascular therapies and interventions for the next decade4. However, the relationship between hypertension and cognitive function is complex and not completely understood, the results of clinical trials of anti-hypertension therapies on cognitive function are not all consistent and limited by the short-term follow up1,5,6.
The hippocampus is thought to be an important region of the brain for learning and memory. It plays important roles in the consolidation of information from short-term memory to long-term memory and spatial navigation. Synaptic plasticity is the neurobiological basis of learning and memory, which includes functional plasticity of synaptic transmission and structural plasticity of dendritic spine morphology and number7,8. Long-term potentiation (LTP) is an important manifestation of functional plasticity of synaptic transmission, mostly intensely studies in the hippocampus, and is a widely studied as a cellular model of learning and memory9.
In recent decades, many reports have shown that p38 MAPK signalling pathway plays important roles in hypertension, cognitive dysfunction, and synaptic plasticity10,11,12,13. p38 MAPK inhibition can ameliorated angiotensin II–induced target organ damage and aortic stiffening14,15. Activated p38 MAPK was seen in human AD brain tissue and in AD-relevant animal models11,16,17,18. The block of LTP induced by synthetic Abeta was prevented by the p38 MAPK inhibitor19.
In this study, we investigated whether p38 MAPK mediate the deleterious effects of hypertension on cognitive function. Using angiotensin II-dependent hypertensive mice, we found that activation of p38 MAPK, synaptic plasticity impairment and memory disorder. In vivo, using a p38 heterozygous knockdown mice (p38KI/+) model, we found that synaptic plasticity and memory were improved in angiotensin II-dependent p38KI/+ hypertensive mice. In vitro, application of p38MAPK inhibitor SKF86002, we found that impaired LTP was improved in hippocampal slices from angiotensin II-dependent hypertensive mice. These data provide novel insights into the mechanisms of hypertension-related cognitive dysfunction and suggest new treatment strategies for counteracting the cognitive impairment of hypertension.
Results
Angiotensin II induces hypertension in both C57BL/6 and p38kI/+ Mice
Angiotensin II treatment was started at the age of 9 weeks and was continued for 4 weeks. Schematic depiction of experimental design in Fig. 1. In vivo experiment, at 9 weeks, there was no significant difference of systolic blood pressure between control C57BL/6 and p38KI/+ mice. Angiotensin II treatment resulted in a rapid (1 weeks after implantation), significant, and sustained raise in systolic blood pressure. Although blood pressure in p38KI/+ &HT mice slightly lower than HT mice (p < 0.001), it remains at its higher level in both HT and p38KI/+ &HT mice compared to control (Fig. 2A). In vitro experiment, angiotensin II significantly raised the systolic blood pressure (p < 0.001), and there is no difference between hypertension + vehicle group and hypertension + SKF86002 group (Fig. 2B).
Figure 1. Schematic depiction of experimental design.
(A) experimental design in vivo. (B) experimental design in vitro.
Figure 2. Mean systolic blood pressure during chronic angiotensin II or saline (Ctrl) perfusions.
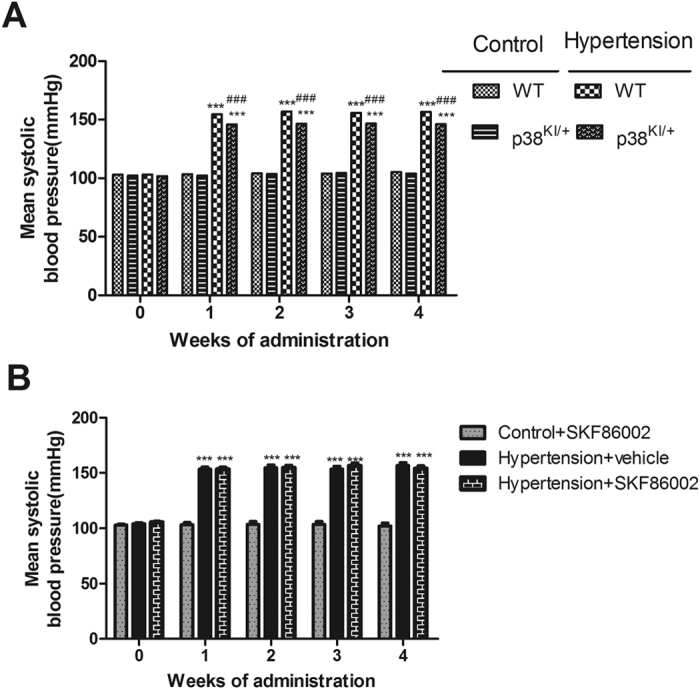
(A) in vivo experiment (n = 17–20, ***p < 0.001 vs control WT and p38KI/+ group, ###p < 0.001 vs HT group). (B) in vitro experiment, (n = 5, ***p < 0.001 vs control + vehicle group).
p38 MAPK is inhibited in p38KI/+ &HT mice hippocampus
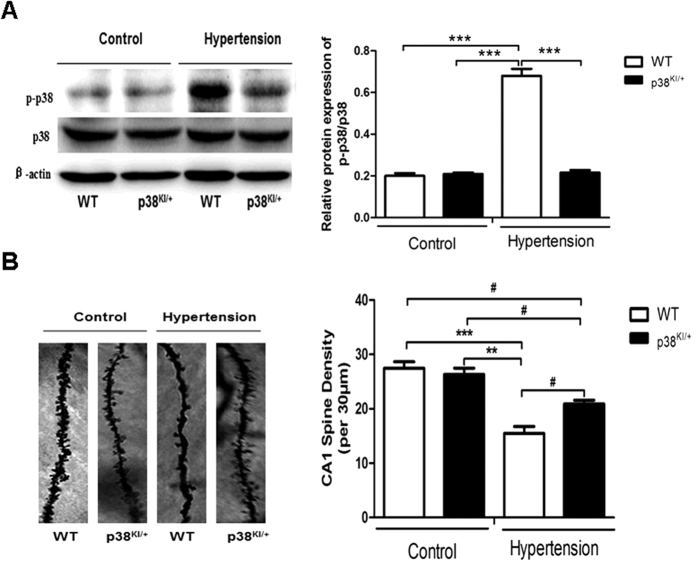
To test our hypothesis that p38 MAPK critically regulates hypertension-related cognitive dysfunction, we examined the level of p38 MAPK activity (phosphorylation) in the hippocampus. Nine weeks old WT and p38KI/+ mice were subjected to hypertension surgery and hippocampal lysates were analyzed for p38 MAPK phosphorylation at four weeks post-surgery. As shown in Fig. 3A, the phosphorylation of p38 MAPK was significantly increased in the HT mice hippocampus compared to control hippocampus (p < 0.001), which was decreased in p38KI/+ &HT mice comparison to HT mice (p < 0.001), and there was no significant difference between WT mice and p38KI/+ mice.
Figure 3. p38 MAPK knockdown increases dendritic spine number on hippocampal CA1 pyramidal cells.
(A) WT and p38KI/+ mice were subjected to hypertension surgery at 9 weeks of age. Four wks post-surgery, western blotting was performed on the hippocampal lysates.Right bar graph shows quantification of p38 MAPK phosphorylation in hippocampus, n = 3 mice per experimental group. (B) Representative Golgi stain image. Right bar graph shows quantification of dendritic spine density, n = 3, and five neurons per mouse were analyzed. Data are presented as mean ± SEM. ***p < 0.001, **p < 0.01, #p < 0.05 by one-way ANOVA with Bonferroni post-test.
Dendritic spine number is increased in p38KI/+ &HT mice
To determine the effect of p38 MAPK knockdown on dendritic spine number, WT and p38KI/+ mice were subjected to hypertension at 9 weeks of age. Dendritic spine number on hippocampal CA1 pyramidal cells, was determined on week 4 post-surgery by Golgi analysis. As shown in Fig. 3B, the density of dendritic spines (number/30 μm) was decreased in HT mice comparison to control mice (p < 0.01), but which was increased in p38KI/+ &HT mice compared to HT mice (p < 0.05).
p38 MAPK inhibition rescues hippocampal LTP deficit
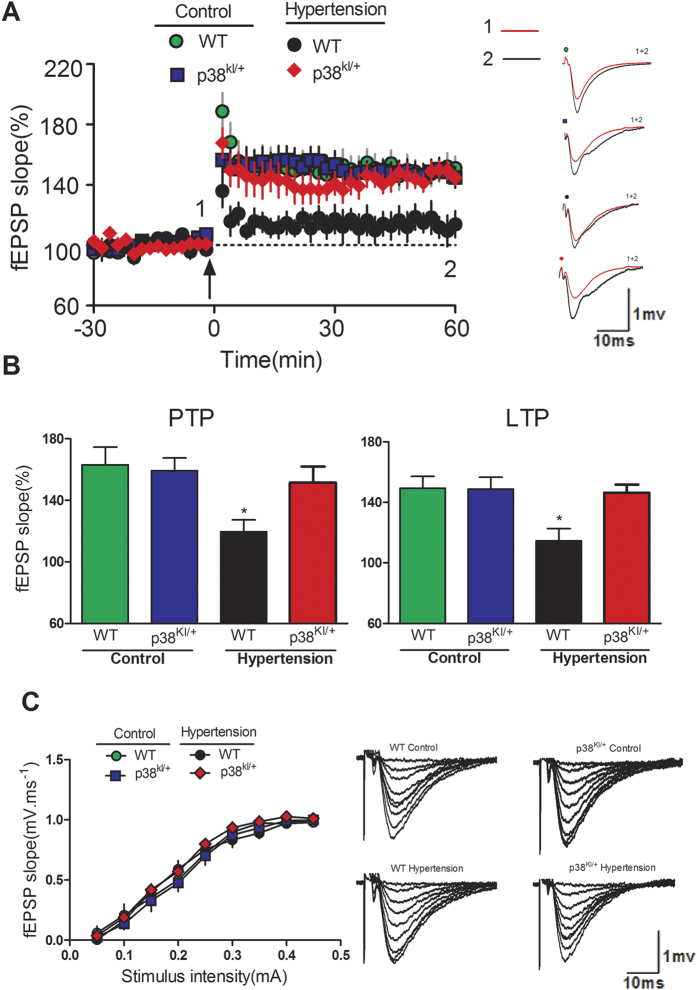
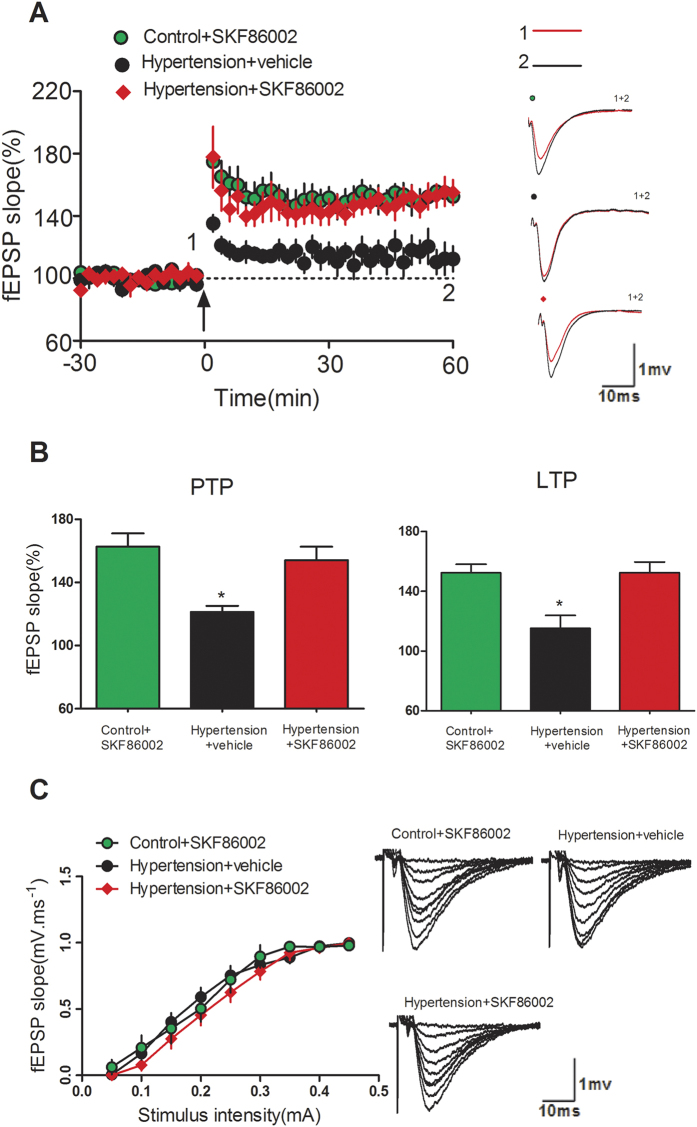
To investigate synaptic functional changes, we performed extracellular electrophysiological recordings on hippocampal slices at four weeks post-surgery. Hippocampal slices were incubated with artificial cerebrospinal fluid (ACSF) for 1 h and then LTP was detected after theta burst stimuli (TBS) delivery. Data of fEPSP slopes during 0–10 min (i.e. post-tetanic potentiation, PTP) and 50–60 min (i.e. LTP) after TBS application were summarized and compared. As shown in Fig. 4A,B, LTP induction was significantly impaired in slices of HT mice when compared with that in controls, while in slices of p38KI/+ &HT mice significantly rescued LTP induction compared to HT mice (p < 0.05). Furthermore, no unequivocal change was found for the I/O curves of the various treatment conditions. (all P > 0.05; Fig. 4C), indicating that these treatments did not alter basal synaptic transmission. In vitro experiment, p38MAPK inhibitor SKF86002 incubation significantly improved LTP induction in slices from hypertensive mice (p < 0.05)(Fig. 5A,B), and no unequivocal change was found for the I/O curves after the treatments of SKF86002 when compared with that after the vehicle treatment (all P > 0.05; Fig. 5C).
Figure 4. p38 MAPK knockdown rescues hippocampal LTP impairment.
(A) LTP induced by TBS in four group mice. Insert is representative waves before and after TBS application. Right graph shows the traces in a represented fEPSPs recorded in a hippocampal slice from four group mice. (B) PTP and LTP data from four group were summarized and compared together, n = 8 slices from five animals for each group, *p < 0.05, HT group vs WT group or p38KI/+ group or p38KI/+ &HT group. (C) I/O curves are shown (all P > 0.05).
Figure 5. SKF86002 rescued hippocampal LTP deficit in hypertensive mice.
(A) LTP induced by TBS in three group mice. Insert is representative waves before and after TBS application. Right graph shows the traces in a represented fEPSPs recorded in a hippocampal slice from three group. (B) PTP and LTP data from three group were summarized and compared together, n = 8 slices from five animals for each group, *p < 0.05, hypertension + vehicle group vs control + SKF86002 group or hypertension + SKF86002 group. (C) I/O curves are shown (all P > 0.05).
p38 MAPK knockdown protects against memory deficit in p38KI/+ &HT mice
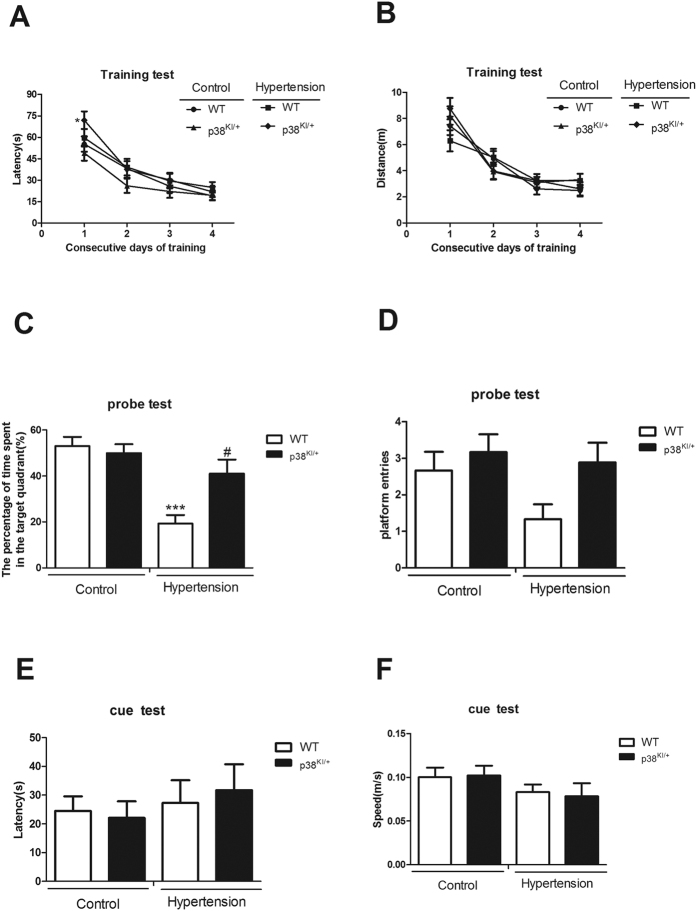
To investigate the influence of p38 MAPK knockdown on cognitive function, four group mice were subjected to the Morris water maze test at three weeks post-surgery. During the training days, the latency and distance for finding the escape platform were measured to assess spatial learning abilities. There were no differences in learning abilities among different groups, even though the latency in p38KI/+ &HT mice was longer than p38KI/+ mice in first training day (p < 0.05). (Fig. 6A,B). Because, the distance and other 3 days latency were no differences.
Figure 6. p38 MAPK knockdown prevents memory deficit, n = 9–12.
(A) Latency taken to escape from the water in the platform trials. (B) Distance taken to escape from the water in the platform trials. (C) Percentage of time spent in the quadrant area relative to the total time spent in the pool in the probe trial. (D) The number of crosses over the exact location of the hidden platform in the probe trial. (E) Latency taken to escape from the water when the platform is visible in the cue test. (F) Swimming speed in the cue test. *p < 0.05, p38KI/+ &HT group vs p38KI/+ group; ***p < 0.001, HT group vs WT group or p38KI/+ group; #p < 0.05, p38KI/+ &HT group vs HT group.
Memory was assessed by the probe test. The time spent in the target quadrant of HT mice was less compared to the control mice (p < 0.001), while p38KI/+ &HT mice spent more time in the target quadrant area than HT mice (p < 0.05). (Fig. 6C). p38KI/+ &HT mice entered the platform area more frequently than HT mice, but the difference between the two groups did not reach the statistical significance (Fig. 6D).
The cue test, where the latency is used to measure sight and speed to evaluate motricity, showed no significant difference among different groups (Fig. 6E,F).
Discussion
In this study, we demonstrated that hippocampal p38 MAPK activation, synaptic plasticity impairment and memory disorder in angiotensin II-dependent hypertensive mice. Furthermore, we showed that impaired synaptic plasticity and memory were rescued in transgenic p38 MAPK knockdown hypertensive mice, and p38 MAPK inhibitor SKF86002 rescued hippocampal LTP deficit in slices from hypertensive C57BL/6 mice. This study provided evidence for p38 MAPK may be a potential therapeutic target for the treatment of hypertension-related cognitive dysfunction.
Hypertension is a risk factor for senile dementia. Many observational studies have shown that hypertension is a risk factor for cognitive impairment or dementia in the elderly20,21,22. In the largest and longest prospective study, involving 3,381 adults (aged 18–30 years at baseline) in four cities in the USA and with 25 years of follow-up, cognitive function was assessed at year 25 with the most-sophisticated technology, which showed that cumulative levels of blood pressure were associated with impaired cognition2. In hypertensive animal models, behavioural studies revealed cognitive impairment23,24,25,26,27. In our study, we found that learning ability was not impaired and that memory storage was affected in HT mice, this may be explained by the period of hypertension is not too long, and memory impairment is the earliest and most prominent clinical features28.
Although the relationship between hypertension and dementia is well established, the impact of antihypertensive treatment and management of other risk factors on cognition is less clear. Several studies have investigated the effect of antihypertensive treatment on the development of dementia in the hypertensive patients29,30,31,32,33. However, the results were markedly different among the studies, and meta-analyses have not reached a conclusion34,35. Furthermore, high SBP was associated with better cognitive performance in centenarians36,37. Deposition of β-amyloid in brain is enhanced in mouse models of arterial hypertension38,39. Howere, bapineuzumab and solanezumab, 2 Aβ targeting monoclonal antibodies, have failed to meet trial cognitive end points in mild to moderate AD patients40,41.
The hippocampus is essential for consolidation of declarative information and spatial navigation. Synaptic plasticity is an important cellular mechanism that regulates memory formation. LTP in the CA1 region of the hippocampus has been the primary model to study the cellular and molecular basis of synaptic plasticity. Alterations of dendritic spine morphology, changes of spine type ratios or density have consequently been found in paradigms of learning and memory, and accompany many neurodegenerative disease. A significant amount of evidence suggests that the p38 MAPK signalling cascade plays a crucial role in synaptic plasticity and in neurodegenerative diseases. p38 MAPK as a possible therapeutic target for Alzheimer’s disease42. At the same time, p38 MAPK inhibition also plays protective effect in the cardiovascular disease43,44. We first found that increased hippocampal p-p38 MAPK expression in HT mice, which was significantly reduced in p38KI/+ &HT mice, furthermore, impaired hippocampal synaptic plasticity and memory were improved in p38KI/+ &HT mice, and LTP deficit in HT mice was rescued by treatment with p38MAPK inhibitor.
We also found that blood pressure was partially reduced in p38KI/+ &HT mice compared with untreated HT mice. This result was similar to previous study which used p38 MAPK inhibitor in diet14. While the lower blood pressure may reflect a milder cerebral damage, we think that p38 MAPK knockdown was a major cause for the improvement of synaptic plasticity and cognitive function in p38KI/+ &HT mice. There are several points to support our idea. Firstly, hypertension persisted at a level of >140 mmHg. Secondly, the impact of antihypertensive treatment on cognition is less clear. Last but not least, impaired hippocampal CA1 LTP in hippocampal slices from hypertensive mice can be rescued by p38 MAPK inhibitor treatment in vitro.
In conclusion, we reported that hippocampal p38 MAPK activation, synaptic plasticity impairment and memory disorder in angiotensin II-dependent hypertensive mice. In vivo, we found that synaptic plasticity and memory are improved in angiotensin II-dependent p38KI/+ hypertensive mice. In vitro, impaired LTP was improved in hippocampal slices from hypertensive mice by p38MAPK inhibitor treatment. Those data indicate that p38MAPK inhibition effectively improves synaptic plasticity and memory in mouse model of hypertension. This study provides novel insights into the mechanisms of hypertension-related cognitive dysfunction and suggests new treatment strategies for counteracting the cognitive impairment of hypertension.
Methods
Study protocol was approved by the Ethics Committee of Kunming Medical University. All procedures were performed in accordance with our Institutional Guidelines for Animal Research and the investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 2011).
Experimental design
In vivo experiment, four experimental groups were analyzed: (1) 0.9% saline solution control C57BL/6 mice (wild-type [WT] group); (2) angiotensin II-treated C57BL/6 mice (hypertensive [HT] group); (3) 0.9% saline solution p38KI/+ mice (p38KI/+ group); and (4) angiotensin II-treated p38KI/+ mice (p38KI/+ &HT group). In vitro experiment, three experimental C57BL/6 groups were analyzed: (1) control + SKF86002 group; (2) hypertension + vehicle group; (3) hypertension + SKF86002 group.
Animals
Eight week-old C57BL/6 wild-type male mice and p38KI/+ male mice (22–26 g) were used for this study. p38KI/+ mice in C57BL/6 background have been described previously (in which activating phosphorylation sites Thr180 and Tyr182 are mutated, leading to an inactivation of phosphorylation of these sites.)45, which were obtained from Institute of Molecular and Cell Biology (Biopolis Drive, Proteos, Singapore). Animals were kept under a 12 h/12 h light/dark cycle with the light on at 07:00 AM. They were fed with standard chow and water ad libitum. Ambient temperature and relative humidity were maintained at 22 ± 2 °C and 55 ± 5%, respectively.
Angiotensin II-induced hypertension
Mice were anaesthetized with intraperitoneal administration of pentobarbital sodium (1.0%, 80 mg/kg). The skin was cleaned with 70% ethanol between the ears and a small skin incision was made to insert an osmotic pump under the skin in the lower back region. After surgery, stitches were used to hold the two edges of tissue together, so the wound could heal. Each osmotic pump (Alzet model 1004) delivered 0.11 μl/h for a 28 day period and was filled with either 1900 ng/kg/min of angiotensin II or a 0.9% saline solution.
Blood pressure measurement
Blood pressure was taken by non-invasive tail-cuff plethysmography (IITC Life Science, USA). The measurements were taken five days before surgery to accustom the mice to the procedure. Afterwards, blood pressure was measured once a week until the end of the experiment. The animals were displaced in the blood pressure measurement room at least one hour before measurements. All measurements were taken between 8 AM and noon. For each animal, four valid measurements for each time point. Data were not considered valid by the software in the following situations: 1) blood flow was insufficient in the tail; 2) the overall shape of the trace did not fit the expected profile; 3) the diastolic and systolic readings occurred too far apart or too close together.
Western blot analysis
Hippocampal was homogenized in RIPA buffer (150 mM NaCl, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCL, PH 8.0) containing protease inhibitor and phosphatase inhibitor (Thermo). Lysates were then dissolved in 2 X laemmli sample buffer (Biorad), and boiled at 95 °C for 5 minutes. For western blot, lysates (50 ug protein) were added in SDS-PAGE and electroblotted onto PVDF membrane. The membranes were blocked with 5% skim milk in TBS-T, and then probed with desired antibodies. Primary antibodies against p-p38 (Cell Signaling), p38(Anbo) and β-actin (Sigma) were used. Immobilon Western Chemiluminescent HRP Substrate (Merck) was used to reveal the antibody-antigen complexes.
Golgi staining
Mice were processed and stained according to the protocol provided by the manufacture (FD Neuro Technologies). In brief, after brain removal, hemispheres were immersed in Golgi-Cox staining solution for 14d at room temperature in the dark with one change of solution after 24 h. Brains were transferred to C solution at 4 °C for 72 h with one change of solution after 24 h. Coronal sections (150 μm) were mounted onto slides coated with C solution. After drying in the dark, slides were immersed into dH2O two times for 2 min and then transferred to a developing solution for 10 min. Slides were then rinsed two times for 2 min in dH2O, dehydrated through graded ethanol, immersed into dimethylbenzene two times for 4 min and then coverslipped using resinene. Golgi-stained sections were imaged with an confocal microscopy (Leica, Germany) and used the LAS AF Lite of the imaging software (Leica). Measurements of dendritic morphology were performed on neurons randomly chosen in the CA1 region of the hippocampus for analysis. Two-order branches of apical dendrites from pyramidal neurons in hippocampal CA1 were acquired. At least 5 branches from 5 different neurons were analyzed per animal and calculated the density of spines per 30 μm dendrite.
Hippocampal slice preparation
Mice were anaesthetized and decapitated. Acute hippocampal slices were cut 350 μm thick using a vibratome (WPI). The slices were gently transferred to a submersion holding chamber containing ACSF (mM: NaCl 126, KCl 2.5, NaH2PO4 1, CaCl2 2.5, MgSO4 1.5, NaHCO3 26, and glucose 10) bubbled with 95% O2 and 5% CO2. Slices were recovered at room temperature for at least 1 h before being transferred to a submersion recording chamber (PSMI; Harvard Apparatus, Holliston, MA, USA), in which the slice were continually perfused with oxygenated ACSF at a rate 1–2 ml/min at room temperature.
Electrophysiological recording
Field excitatory postsynaptic potentials (fEPSPs) were recorded in the stratum radiatum of the hippocampal CA1 region by means of a glass microelectrode filled with 3 M NaCl (resistance 1–4 MΩ). The Schaffer collateral pathway was stimulated with concentric bipolar electrodes (Frederick Haer Co, Bowdoinham, ME, USA). After an optimal fEPSP wave was found, an input–output (I/O) curve was established with stimulating pulses (0.2 ms duration) at different intensities. Baseline fEPSP was recorded at 0.033 Hz with a stimulating strength adjusted to yield about 40% of the maximal response. After baseline responses had stably lasted for at least 30 min, LTP was induced by delivering theta burst stimuli (TBS; four trains of 10 bursts of four stimuli with 20 s, 200 ms, and 10 ms intervals between trains, bursts, and stimuli, respectively). The electrophysiological data was acquired with a multiclamp 700 A amplifier (Axon instruments, Molecular Devices, USA), filtered at 0.1–5 KHz, digitized at 10 KHz, and analyzed with Clampfit version 10.0 (Axon Instruments, USA). The analyzed data was further processed with Origin 5.1 (Microcal Software Northampton, MA).
p38 MAPK inhibitor application in vitro
p38 MAPK inhibitor SKF86002 was purchased from Sigma. It was dissolved in 100% dimethylsulfoxide (DMSO) stock solution and stored at −20 °C until the day of the experiment. The final concentration of DMSO was kept at 0.02%. SKF86002 (2 μM was used at final concentration) was preperfused over the slices for 10 min before LTP induction.
Morris water maze
The Morris water maze (MWM) was used for testing hippocampus-dependent spatial memory. It was conducted following the protocols reported previously with minor modification26. In brief, animal activity was measured by a video-based tracking system (ANY-maze, Stoelting). The pool was filled with opaque water (by adding washable white paint) and surrounded by extramaze cues. The escape platform (10 cm in diameter) was placed in the center of a designated quadrant with its top positioned 1 cm below the water surface. The light was dimmed and the water temperature kept at 23 ± 2 °C degree. The training phase lasted 4 days during which the mice were allowed four 90 second trials per day to find the escape platform. Each trial was 20 min apart. The probe and cue tests were administered on the 5th day. During the probe test, the hidden platform was removed from the pool, and mice were allowed to swim for 60 sec. The time spent in each quadrant, as well as the number of platform crossings were recorded. The platform was then placed, some water was removed so that the escape platform became visible. One trial was given to evaluate sight and motricity. Sight was measured by the latency finding the escape platform and motricity was assessed by the speed of the mice during the trial.
Statistical analysis
All data are expressed as mean ± SEM. The data on BPs, electrophysiology and distance and latency of MWM were analyzed by two-way repeated-measures ANOVA. One-way ANOVA was used to analyse other datas. Bonferroni multiple-comparison test was performed for comparisons between groups. Values of P < 0.05 were considered statistically significant. Statistical analysis was performed with SPSS 17.0 (SPSS Inc).
Additional Information
How to cite this article: Dai, H.-L. et al. p38 MAPK Inhibition Improves Synaptic Plasticity and Memory in Angiotensin II-dependent Hypertensive Mice. Sci. Rep. 6, 27600; doi: 10.1038/srep27600 (2016).
Acknowledgments
We would like to thank Prof. Ming Zhang, Ms. Xin Li, Mr. Lei Zhou and Ms. Yue-qin Zeng for their help in the experiment. This work was supported by grants from the National Natural Science Foundation of China (grant numbers: 81360037, 31160230), Natural Science Foundation of Yunnan (grant numbers: 2012FB009, 2013FZ284), Dr. Academic Award of Yunnan (grant numbers: 2013), the Talent Program (grant numbers: 2011HA013), Yunnan Province, China and Monash Professorial Fellowship, Monash University, Australia.
Footnotes
Author Contributions H.-L.D., X.-f.G. and Z.-c.X. designed experiments and co-wrote the paper; H.-L.D. and W.-y.H. analysed data; H.-L.D., W.-y.H., L.-h.J. and L.L. performed experiments.
References
- Shimamoto K. et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 37, 253–390 (2014). [DOI] [PubMed] [Google Scholar]
- Yaffe K. et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 129, 1560–1567 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm M. et al. Systolic blood pressure variation and mean heart rate is associated with cognitive dysfunction in patients with high cardiovascular risk. Hypertension 65, 651–661 (2015). [DOI] [PubMed] [Google Scholar]
- Fuster V. Top 10 cardiovascular therapies and interventions for the next decade. Nat Rev Cardiol 11, 671–683 (2014). [DOI] [PubMed] [Google Scholar]
- Mancia G. et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 31, 1281–1357 (2013). [DOI] [PubMed] [Google Scholar]
- Faraco G. & Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension 62, 810–817 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. C. et al. Differential modulation of drug-induced structural and functional plasticity of dendritic spines. Mol Pharmacol 82, 333–343 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchlik A. Dynamic learning and memory, synaptic plasticity and neurogenesis: an update. Front Behav Neurosci 8, 106 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchese F. et al. Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J Clin Invest 118, 2796–2807(2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto S. et al. Different effects of amlodipine and enalapril on the mitogen-activated protein kinese/extraeellular signal-regulated kinase kinase-extracellular signal-regulated kinase pathway for induction of vascular smooth muscle cell differentiation in vivo. Hypertens Res 29, 179–186 (2006). [DOI] [PubMed] [Google Scholar]
- Sun A., Liu M., Nguyen X. V. & Bing G. P38 MAP kinase is activated at early stages in Alzheimer’s disease brain. Exp Neurol 183, 394–405 (2003). [DOI] [PubMed] [Google Scholar]
- Munoz L. et al. A novel p38 alpha MAPK inhibitor suppresses brain proinflammatory cytokine up-regulation and attenuates synaptic dysfunction and behavioral deficits in an Alzheimer’s disease mouse model. J Neuroinflammation 4, 21 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa S. A. & Eales K. L. The Role of p38 MAPK and Its Substrates in Neuronal Plasticity and Neurodegenerative Disease. J Signal Transduct 2012, 649079 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. K. et al. p38 mitogen-activated protein kinase inhibition ameliorates angiotensin II-induced target organ damage. Hypertension 49, 481–489(2007). [DOI] [PubMed] [Google Scholar]
- Wu J. et al. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res 114, 616–625 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swatton J. E. et al. Increased MAP kinase activity in Alzheimer’s and Down syndrome but not in schizophrenia human brain. Eur J Neurosci 19, 2711–2719 (2014). [DOI] [PubMed] [Google Scholar]
- Savage M. J., Lin Y. G., Ciallella J. R., Flood D. G. & Scott R. W. Activation of c-Jun N-terminal kinase and p38 in an Alzheimer’s disease model is associated with amyloid deposition. J Neurosci 22, 3376–3385 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. et al. Sodium ferulate prevents amyloid-beta-induced neurotoxicity through suppression of p38 MAPK and upregulation of ERK-1/2 and Akt/protein kinase B in rat hippocampus. Acta Pharmacol Sin 26, 943–951 (2005). [DOI] [PubMed] [Google Scholar]
- Wang Q., Walsh D. M., Rowan M. J., Selkoe D. J. & Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci 24, 3370–3378 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A. et al. Day-to-day variability in home blood pressure is associated with cognitive decline: the Ohasama study. Hypertension 63, 1333–1338 (2014). [DOI] [PubMed] [Google Scholar]
- Gottesman R. F. et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol 71, 1218–1227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler S. et al. Temporal evolution of cognitive changes in incident hypertension: prospective cohort study across the adult age span. Hypertension 63, 245–251 (2014). [DOI] [PubMed] [Google Scholar]
- Hartman R. E., Kamper J. E., Goyal R., Stewart J. M. & Longo L. D. Motor and cognitive deficits in mice bred to have low or high blood pressure. Physiol Behav 105, 1092–1097 (2012). [DOI] [PubMed] [Google Scholar]
- Gentile M. T. et al. β-Amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol Aging 30, 222–228 (2009). [DOI] [PubMed] [Google Scholar]
- Carnevale D. et al. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 60, 188–197 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchemin S., Belanger E., Wu R., Ferland G. & Girouard H. Chronic perfusion of angiotensin II causes cognitive dysfunctions and anxiety in mice. Physiol Behav 109, 63–68 (2013). [DOI] [PubMed] [Google Scholar]
- Wang M. H., Chang W. J., Soung H. S. & Chang K. C. (−)-Epigallocatechin-3-gallate decreases the impairment in learning and memory in spontaneous hypertension rats. Behav Pharmacol 23, 771–780 (2012). [DOI] [PubMed] [Google Scholar]
- Mizuno K., Wakai M., Takeda A. & Sobue G. Medial temporal atrophy and memory impairment in early stage of Alzheimer’s disease: an MRI volumetric and memory assessment study. J Neurol Sci 173, 18–24 (2000). [DOI] [PubMed] [Google Scholar]
- Haag M. D., Hofman A., Koudstaal P. J., Breteler M. M. & Stricker B. H. Duration of antihypertensive drug use and risk of dementia: A prospective cohort study. Neurology. 72, 1727–1734 (2009). [DOI] [PubMed] [Google Scholar]
- Hajjar I., Brown L., Mack W. J. & Chui H. Impact of Angiotensin receptor blockers on Alzheimer disease neuropathology in a large brain autopsy series. Arch Neurol 69, 1632–1638 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N.-C. et al. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ 340, b5465 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol 10, 43–53 (2011). [DOI] [PubMed] [Google Scholar]
- Williamson J. D. et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med 174, 324–333 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Booth A. & Peters J. A systematic review of calcium channel blocker use and cognitive decline/dementia in the elderly. J Hypertens 32, 1945–1957 (2014). [DOI] [PubMed] [Google Scholar]
- Beishon L. C. et al. The evidence for treating hypertension in older people with dementia: a systematic review. J Hum Hypertens 28, 283–287 (2014). [DOI] [PubMed] [Google Scholar]
- Richmond R., Law J. & Kay-Lambkin F. Higher blood pressure associated with higher cognition and functionality among centenarians in Australia. Am J Hypertens 24, 299–303 (2011). [DOI] [PubMed] [Google Scholar]
- Szewieczek J. et al. Better cognitive and physical performance is associated with higher blood pressure in centenarians. J Nutr Health Aging 15, 618–622 (2011). [DOI] [PubMed] [Google Scholar]
- Cifuentes D. et al. Hypertension accelerates the progression of Alzheimer-like pathology in a mouse model of the disease. Hypertension 65, 218–224 (2015). [DOI] [PubMed] [Google Scholar]
- Kruyer A., Soplop N., Strickland S. & Norris E. H. Chronic Hypertension Leads to Neurodegeneration in the TgSwDI Mouse Model of Alzheimer’s Disease. Hypertension 66, 175–182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S. et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370, 322–333 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody R. S. et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 370, 311–321 (2014). [DOI] [PubMed] [Google Scholar]
- Dalrymple S. A. p38 mitogen activated protein kinase as a therapeutic target for Alzheimer’s disease. J Mol Neurosci 19, 295–299(2012). [DOI] [PubMed] [Google Scholar]
- Cheriyan J. et al. Inhibition of p38 mitogen-activated protein kinase improves nitric oxide-mediated vasodilatation and reduces inflammation in hypercholesterolemia. Circulation 123, 515–523(2011). [DOI] [PubMed] [Google Scholar]
- Newby L. K. et al. Losmapimod, a novel p38 mitogen-activated protein kinase inhibitor, in non-ST-segment elevation myocardial infarction: a randomised phase 2 trial. Lancet 384, 1187–1195 (2014). [DOI] [PubMed] [Google Scholar]
- Wong E. S. et al. p38MAPK controls expression of multiple cell cycle inhibitors and islet proliferation with advancing age. Dev Cell 17, 142–149 (2009). [DOI] [PubMed] [Google Scholar]