Abstract
All scientific approaches to the origin of life share a common problem: a chemical path to lipids as main constituents of extant cellular enclosures. Here we show by isotope-controlled experiments that unsaturated C3,5,7,9-monocarboxylic acids form by one-pot reaction of acetylene (C2H2) and carbon monoxide (CO) in contact with nickel sulfide (NiS) in hot aqueous medium. The primary products are toto-olefinic monocarboxylic acids with CO-derived COOH groups undergoing subsequent stepwise hydrogenation with CO as reductant. In the resulting unsaturated monocarboxylic acids the double bonds are mainly centrally located with mainly trans-configuration. The reaction conditions are compatible with an origin of life in volcanic-hydrothermal sub-seafloor flow ducts.
Carbonaceous meteorites have been found to contain saturated, aliphatic C2–12-monocarboxylic acids with an excess of branched structures and with abundancies that decrease with increasing carbon atom numbers1,2,3. The relevance of these findings for the origin of life came into its own when hydrophobic residues of chloroform extracts of carbonaceous meteorites were shown to form membranous structures in aqueous media4. Meteoritic sources would, however, have been slow at releasing their lipids into the primitive ocean with the result of a highly diluted lipid solution, and possible concentration processes would have been countermanded by massive sedimentation, notably of adsorptive volcanic ashes5. A promising alternative emerged with the finding that monocarboxylic acids are formed at 400 °C by contacting metallic iron from certain meteorites with CO and H2 (1:1)6. The characteristics, however, of an absence of water (except for reaction water) and of a presence of anhydrous (hygroscopic) K2CO36 speak against hydrothermal vent scenarios for these Fischer-Tropsch reactions. Subsequent reports of a Fischer-Tropsch formation of monocarboxylic acids under water-saturated hydrothermal vent conditions turned out to be problematic because the monocarboxylic acids appear to be formed by gas-solid reactions at catalytic steel surfaces of the reactor wall7,8. Bearing in mind that the biosynthesis of fatty acids from acetyl-CoA proceeds in C2-increments we probed acetylene as simple, yet highly reactive primordial C2-precursor.
Results and Discussion
Synthetic reactions
We reacted an aqueous suspension of freshly precipitated NiS with C2H2 and CO as gas phase at a combined gas pressure of ~1 bar at room temperature, followed by heating to 105 °C under an autogenous gas/steam pressure of ~2.5 bar for 7 days. A glass reactor was used in order to avoid artifacts by catalytic reactor walls. The aqueous medium was not buffered and its pH developed autogenously to an end-pH that was measured. After freeze-drying of the supernatant organic products in the residue were silylated and analyzed by gas chromatography-mass spectrometry (GC-MS). The analysis revealed the presence of a suite of C3,5,7,9-monocarboxylic acids, their chain length increasing by increments of two C-atoms (see Table 1 and for more detailed information Suppl. Tables S1 and S2, Fig. S4). All detected C3,5,7,9-monocarboxylic acids sum up to a concentration of up to about 20 mM, (for detailed conversion rates see Suppl. Table S3). Runs with 13CO and D2O ascertained that these products are genuine reaction products. In the absence of C2H2 and/or CO they are not formed. The yields of the monocarboxylic acids show a decrease with increasing chain length (Fig. 1).
Table 1. Monocarboxylic acid products of the NiS-catalyzed reaction of acetylene with carbon monoxide.
| run | a | b | c | d | e | f |
|---|---|---|---|---|---|---|
| mmol NiS | 1 | 1 | 0.5 | 0.5 | 0 | 0 |
| mmol Ni(OH)2 (α or β) | 0 | 0 | 0.5(α) | 0.5(β) | 1(α) | 1(β) |
| end-pH | 8.8 | 6.7 | 8.3 | 8.9 | 8.0 | 9.8 |
| C3-acids (μM) | ||||||
| C2H3-COOH | 3884 | 5822 | 3318 | 6675 | 250 | 243 |
| C2H5-COOH | 7132 | 1069 | 461 | 7391 | 510 | 171 |
| ΣC3 | 11016 | 6891 | 3779 | 14066 | 760 | 414 |
| C5-acids (μM) | ||||||
| ΣC4H5-COOH | 466 | 1597 | 970 | 1463 | 53 | 0 |
| ΣC4H7-COOH | 7498 | 2641 | 1390 | 7269 | 11 | 0 |
| C4H9-COOH | 309 | 32 | 11 | 363 | 0 | 0 |
| ΣC5 | 8273 | 4270 | 2371 | 9095 | 64 | 0 |
| C7-acids (μM) | ||||||
| ΣC6H5,7-COOH | 63 | 30 | 31 | 61 | 0 | 0 |
| ΣC6H9-COOH | 320 | 148 | 69 | 246 | 0 | 0 |
| ΣC6H11-COOH | 52 | 42 | 6 | 96 | 0 | 0 |
| ΣC7 | 435 | 220 | 106 | 403 | 0 | 0 |
| C9-acids (μM) | ||||||
| ΣC6H5-C2H2,4-COOH | 1.8 | 4.4 | 3.1 | 1.5 | 0 | 0 |
| C8H11-COOH | 0.6 | 0.8 | 0.1 | 0 | 0 | 0 |
| ΣC8H13-COOH | 4.6 | 1.3 | 3.6 | 5.9 | 0 | 0 |
| C8H15-COOH | 3.9 | 0.4 | 0.4 | 3.7 | 0 | 0 |
| ΣC9 | 10.9 | 6.9 | 7.2 | 11.1 | 0 | 0 |
| ΣC3–C9 | 19735 | 11388 | 6263 | 23575 | 824 | 414 |
Reactions were carried out in 125 ml serum bottles with 5 ml aqueous liquid phase for 7 days at 105 °C; Products were identified by GC-MS as tert-butyldimethylsilyl derivatives.
Figure 1. Combined fatty acid concentrations.
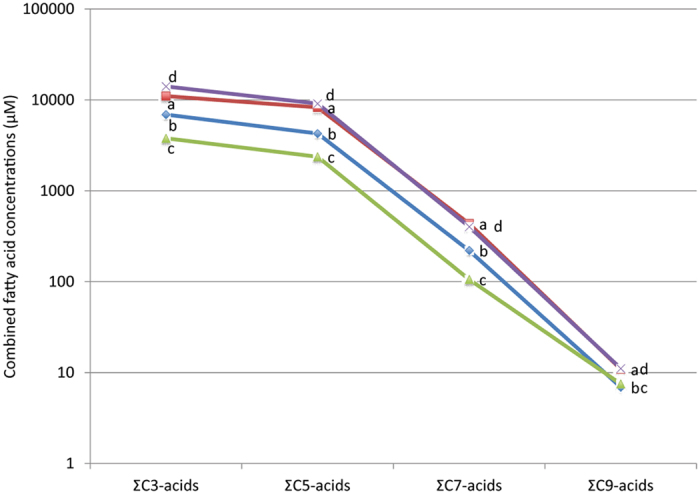
Total yields of C3,5,7,9-monocarboxylic acids of runs a to d are plotted on a logarithmic scale: a: NiS, pH8.8; b: NiS, pH6.7; c: coprecipitated NiS and α-Ni(OH)2, pH8.3; d: NiS, precipitated onto β-Ni(OH)2, pH8.9; for detailed information see Suppl. Table S1.
Ancillary investigations
For safety reasons (danger of explosion) and for technical reasons the reactions had to be carried out at low partial pressure (<1 bar) of C2H2. At a high sub-seafloor pressure (>1000 bar) yields and chain lengths would be increased, because of negative volumes of reaction and activation9,10. Productivity of the reaction is pH-sensitive with highest yields in the near neutral pH range of about 6.5 to 9, increasing from pH 6.7 (run b) to pH 8.8 (run a). Much stronger alkaline conditions disfavor reaction yields as well as chain elongation. With freshly precipitated α-Ni(OH)2 (run e) only C3,5-acids were formed, and in lower yields than with freshly precipitated NiS. With coprecipitated NiS and α-Ni(OH)2 in a molar ratio of 1:1 (run c) yields were diminished compared to NiS alone. Apparently, α-Ni(OH)2 acts as antagonist. With aged β-Ni(OH)2 alone (run f) only C3-acids were formed, and in lower yields than with α-Ni(OH)2. However, with β-Ni(OH)2 as carrier for freshly precipitated NiS the yields were slightly increased (run d compared to run a). These comparisons point to a wide range of compositions for the exploration of more effective catalysts. Against the backdrop of previous production of organics by reaction of C2H2/H2O/CO with non-sulfidic Ni-catalysts in organic reaction media11,12, it is surprising that heterogeneous catalysis by NiS in hot water generates elongated, unsaturated monocarboxlic acids, which undergo in situ hydrogenation with the same catalyst and with CO as reductant.
Proposed mechanism
We suggest an organo-metal reaction mechanism (Fig. 2a), wherein CO acts as carbon source for the carboxyl group and also as reducing agent via hydride transfer. When run a was repeated with the addition of H-(CH=CH)2-COOH, but without C2H2, the same suite of C5-monocarboxylic acids was formed with the same quantitative relationships as in run a with C2H2 (Δ3 > Δ4 > Δ2). Therefore, we suggest toto-olefinic H-(CH=CH)n-CO-[Ni] as early intermediate, which is hydrolyzed to the corresponding free acid. Subsequently, the free toto-olefinic monocarboxylic acids undergo stepwise hydrogenation. The toto-olefinic monocarboxylic acids are highly reactive and have a propensity to undergo self-condensation and resinification as established for 2,4,6-heptatrienoic acid and its derivatives13,14. Therefore, the yield of long-chain monocarboxylic acids is expected to increase with increasing rates of hydrogenation. For n = 1–3 the free toto-olefinic mono-carboxylic acids have been detected. A mechanism of electrocyclization and aromatization of toto-olefinic monocarboxylic acids with the penultimate double bond in cis-configuration explains the formation of benzoic acid (n = 3)13 as well as cinnamic acid and hydrocinnamic acid (n = 4).
Figure 2. Evolution of monocarboxylic acid biosynthesis.
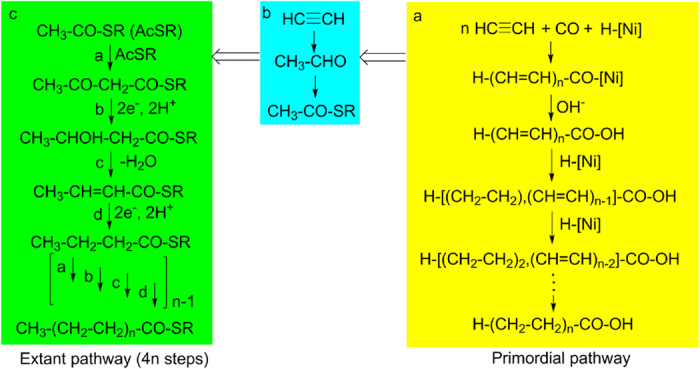
(a) Primordial one-pot pathway; (b) Conversion of acetylene to acetyl-thioester; (c) Extant biosynthesis dependent on acetyl-thioester condensation. [Ni] signifies a catalytic nickel complex (unknown nuclearity, ligand sphere, oxidation state and relationship to mineral surfaces) that converts to H-[Ni] (with hydride ligand) by oxidation of CO to CO2; ⇒ signifies an evolutionary transformation.
The mechanism explains the results with the additional proposition that hydrogenation rates follow three rules. Rule 1: The rate is higher if hydrogenation involves the α-carbon atom rather than only more distal carbon atoms. Rule 2: Hydrogenation of conjugated double bonds is favored over hydrogenation of isolated double bonds. Rule 3: Conjugated double bonds undergo preferably end-to-end hydrogenation. These rules jointly explain why the yields of the mono-unsaturated C5-acids (Suppl. Table S1) follow the order Δ3 > Δ4 > Δ2. Moreover, they show that with increasing chain length the proportion of saturated fatty acids decreases sharply and that regioisomers with double bonds in the middle of the carbon chain are favored. Remarkably, double bonds with trans-configuration are also favored. Finally, these rules mean also that multiple double bonds are mainly conjugated.
Primordial Sources of Starting Materials
We now turn to the possible sources for reactants and catalysts. In the extant atmosphere of the Earth acetylene occurs only in trace amounts close to the detection limit, but much higher levels of atmospheric acetylene have been suggested as contained in a presumptive methane-rich primordial atmosphere due to photolysis of methane15. Acetylene has also been detected in extant fumarolic gases16, in volcanic glasses17, and as products of volcanic simulation experiments18. Acetylene has been suggested as product of hydrolysis of calcium carbide (CaC2) in the context of the Archaean eon15 and in the context of the Hadean eon19,20. CaC2 may be formed by the following transformations in the hot Hadean mantle21:
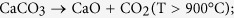 |
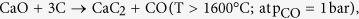 |
CaC2 would have become subsequently emplaced in the Hadean crust to later come in contact with the aqueous vent fluid. The stoichiometrically formed CO would have been continuously removed from the sites of CaC2 formation by diffusion and volcanic exhalation. CaC2 is a member of the class of acetylenic carbides, i.e. the calcium salt of acetylene, consisting of a lattice of Ca2+ cations and C22− anions. It undergoes facile hydrolysis with cold neutral water to form exclusively acetylene21. CaC2 is distinguished from iron carbides. The latter have been hydrolyzed with concentrated DCl in D2O and fully deuterated, saturated C3–7-hydrocarbons have been reported as products22.
In the presence of graphite the molar ratio of CO:CO2 increases with increasing temperature and decreasing pressure. It assumes for example a value of 1:1 at 1200 °C and 2 kbar, or at 900 °C and 0.1 kbar23. The Hadean volcanic exhalations containing a high ratio of CO:CO2 would eventually have been mixed with relatively cold cycling water in volcanic-hydrothermal flow ducts. The resulting quenching effect would have prevented equilibration of the CO:CO2 ratio to low-temperature values24.
Nickel is the second-most abundant transition metal (after iron) in the Solar System25 and in the crust of the Earth26. Iron-nickel sulfides are among the earliest stages of mineral evolution27. Therefore, nickel sulfides would have been abundant in the Hadean crust to come in contact with volcanic-hydrothermal vent fluids. Nickel ions have been found to leach out of crustal minerals into hydrothermal vent fluids28 and it has been proposed that under the very hot conditions of the Hadean Earth nickel transport in hydrothermal fluids was much more intense than today29. Upon contact with H2S nanoparticulate nickel sulfide precipitates30.
Evolutionary Considerations
Acetylene utilization has been detected in extant microbial phyla (for review see ref. 15). It shows a characteristic kinetic isotope effect31. Pelobacter acetylenicus utilizes acetylene as sole carbon and energy source by means of a tungstopterin enzyme that hydrates acetylene to acetaldehyde, which in turn converts to acetic acid plus ethanol. This enzyme still bears the mark of an original redox function32. A possibly related relic is provided by the [FeS]-enzyme IspH for the last step in the non-mevalonate pathway to isoprenoid lipids33. While it is today clearly a redox enzyme catalyzing the conversion of 1-hydroxy-2-methyl-2(E)-butenyl 4-phosphate into a mixture of isopentenyl diphosphate and dimethylallyl diphosphate, it also has an ability to hydrate acetylene34. These two enzymes may reflect a deep history of enzyme recruitments and functional recruitments.
Based on the above considerations we project that acetylene utilization may have been a prominent feature of the early metabolism. Hadean volcanic-hydrothermal vent fluids, laden with CO and C2H2, would have passed through myriads of flow zones. Thereby they would have come in contact with NiS under conditions suitable for the formation of the type of monocarboxylic acids here reported, but perhaps of greater lengths due to high reaction pressure (>1000 bar). These then would have been carried along by the fluid flow in the style of reaction chromatography, the short ones travelling faster than the longer ones35.
Let us consider now the possible function of the here reported monocarboxylic acids in the course of the cellularization of life. At the outset we note that the experimentally detected monocarboxylic acids are short (C3–9) and that under the chosen experimental conditions of a low partial pressure of acetylene (~0.6 bar at 105 °C) the productivity for the C9-monocarboxylic acids is low. It is not unrealistic, however, to expect that future explorations for more effective NiS catalysts may be successful and that higher productivities and greater chain lengths may result at a higher partial pressure of C2H2 and at a high total pressure (>1000 bar). With these provisos in mind we distinguish here two alternative scenarios for the origin of life.
Heterotrophic origin in a prebiotic broth
In this scenario it is assumed that lipid membrane vesicles form ab initio. Therefore, the concentration of dissolved monocarboxylic acids in the prebiotic broth must be high enough and their chain length great enough to be able to exceed the critical vesicle concentration (CVC). It has been found that decanoic acid has a CVC at pH 7.2 of ~20 mM36, while the CVCs are still higher for nonanoic acid and octanoic acid37. The CVC has been shown to be lowered by the inclusion of hydrocarbons4, which are known to form by Fischer-Tropsch reactions under hydrothermal conditions7 or of the alcohol equivalent of the monocarboxylic acid37. Further, the CVC is lowered by the presence of short monocarboxylic acids (e.g. those with 3 to 7 carbon atoms that are reported here) in the solution36, which may contribute to reach the saturation level of the disturbance of the structure of liquid water that is required for vesicle formation. With the concentrations of monocarboxylic acids as reported here ab initio vesicle formation from solution is questionable.
Aside from the above physico-chemical restriction of heterotrophic ab initio vesicle formation from the kind of short monocarboxylic acids here reported, we should note a more principle topological shortcoming of this approach. By the logic of this approach lipid production and supply is located external to the vesicles in question. Growth and reproduction of early vesicles would therefore require lipid nutrients to enter the membrane from the outside with the need for a flipping of lipid molecules from the outer leaflet to the inner leaflet. This outside-in process is the topological opposite of extant cell membrane growth of non-parasitic microbes, which occurs exlusively inside-out, i.e. by the internal synthesis of the lipid molecules followed by their entry into the inner leaflet with subsequent flipping from the inner leaflet to the outer leaflet. This topological difference correlates immediately with a thermodynamic difference. Membrane growth by lipid molecule insertion requires a driving force, which can only be provided by a sufficient lipid concentration. Given the restricted vesicle volume the inside-out process will easily satisfy the concentration requirement, ultimately driven by the synthetic chemical potential of the metabolism. In case of outside-in growth the outside volume is in principle an unrestricted diffusion space with the consequence of unending dilution.
Autotrophic origin in a volcanic-hydrothermal fluid flow
In this scenario it is assumed that cellularization is preceded by a surface metabolism with a cascade of intervening functional steps as precursors of the eventual lipid function. The first step in this hypothetical cascade consists of a lipophilization of the catalytic mineral surface by the accumulation of surface-bonded monocarboxylic acids that operate like a two-dimensional hydrophobic solvent38. All here reported monocarboxylic acids, even the short ones, are capable of contributing to such surface lipophilization. As a consequence the activity of H2O (and of H3O+ or OH−) is lower at the mineral-water interface than in bulk water, thereby protecting hydrolytically sensitive constituents, notably organo-metal intermediates and condensation products. By this collectively autocatalytic effect ever-longer monocarboxylic acids would form in ever-greater proportions. Eventually, a state would be reached, wherein monocarboxylic acids with lipid function form surface-bonded bilayer membranes38. At this stage the above-mentioned saturation effects36 may come into play. Eventually, semi-cellular structures would form, with a cytosol bounded partly by a lipid membrane and partly by a mineral surface. Still later, evolutionary precursors of true cellular entities with internal catalytic NiS would emerge38. Throughout this evolutionary cascade the principle of continuity (topological, structural, nutritional, catalytic and biochemical) would be maintained. Monocarboxylic acids would form first on the open NiS surfaces, later on the NiS surfaces inside the semi-cellular structures, still later on NiS particles inside membrane vesicles, and finally by intracellular enzyme catalysis. Throughout this cascade membrane growth would have proceeded as today — inside-out.
With the cooling of the Earth the acetylene nutrient would have vanished in most habitats of life and the biosynthesis of lipids by organo-metal C2-incremental acetylene fixation would have been replaced by enzymatic C2-incremental acetyl-CoA condensation (Fig. 2c). This gradual transformation would have been mediated by hydration of acetylene to acetaldehyde, followed by oxidative reaction with a mercaptan to form an acetyl-thioester (Fig. 2b). Moreover, we note that extant membranes of the domains Bacteria and Archaea typically comprise a mixture of fatty acid lipids and that a mixture of an even-numbered monocarboxylic acid and an uneven-numbered monocarboxylic acid shows a lowered CVC compared to the single even-numbered case36. Therefore, a gradual transition from uneven-numbered to even-numbered carbon chains would not have violated the principle of evolutionary continuity.
Monocarboxylic acids previously invoked in the context of the cellularization of life have been exclusively saturated. By contrast, the here reported synthetic pathways begin by the formation of toto-olefinic monocarboxylic acids that undergo subsequent incremental hydrogenation of their double bonds. With the C3,5-monocarboxylic acids the fully hydrogenated, saturated state was reached. But with the higher C7–9-monocarboxylic acids the saturated states are not reached and the products consist of mixtures of unsaturated monocarboxylic acids with one, two or three double bonds. The double bonds have mainly trans-configuration and multiple double bonds are mainly conjugated. These insights open hitherto unexplored avenues of research into cellularization.
Extant cell membranes of the domains Bacteria and Eukarya are characterized by the presence of natural unsaturated fatty-acyl lipids within membranes of saturated lipids. The unsaturated monocarboxylic acids reported here agree with natural unsaturated lipids with regard to the location of the double bonds in the middle of the hydrophobic tail. While in the here reported synthetic pathway the unsaturated C>3-monocarboxylic acids are the exclusive reaction products, the natural unsaturated fatty acids are produced in anaerobic bacteria as minor components by special variants of the fatty acid synthesis machinery.
The natural unsaturated fatty acids have mainly cis-configuration that causes a kinked structure with the effect of a decrease of the membrane packing density. This means an increase of membrane fluidity, as it is required by a mesophilic lifestyle. The trans-unsaturated monocarboxylic acids are actually more similar to their saturated monocarboxylic acid counterparts in terms of their molecular structure and in terms of their effect on membrane properties39. With the presence of multiple trans-unsaturations, in their carbon chain the molecules become overall more rigid and less bulky. Now, when the multiple trans-unsaturations are conjugated, as projected for the type of reactions here reported, the carbon chains are even more rigid and rather flat with the result of greater membrane compactness. This provides an outlook to primordial membranes of monocarboxylic acids with conjugated polyunsaturations that may well have properties that are more in tune with the requirements of a (hyper)thermophilic lifestyle. These considerations are speculative, but suitable for empirical verification or falsification.
The chemical reaction here reported is by itself not restricted to any particular scenario as long as the required materials and conditions are present. When viewed, however, in the Iron-Sulfur World context of a volcanic-hydrothermal flow scenario for a chemo-autotrophic origin of life24,35,38,40, the here presented reactions show a considerable coherence with previously reported synthetic reactions (nutrients in volcanic-hydrothermal fluid flows and catalytic minerals from crustal flow ducts): Lower alkyl mercaptans form from CO2 and H2S with FeS41; NH3 forms from N2 with FeS/H2S42; CH3-CO-SCH3 forms from CO/CH3SH with (Fe,Ni)S43; amino acids form from HCN with NiS44; amino acids are activated by CO/H2S/NiS to form peptides that engage in a peptide cycle by undergoing N-terminal degradation by CO/H2S/NiS45. This degree coherence may be viewed as a road sign to the origin of life in an open flow system: chemically singular and chemically predetermined.
Methods
In a typical run a 125 ml glass serum bottle was charged with 0.5 or 1.0 mmol NiSO4 • 6H2O and closed with a silicon stopper. Additionally or alternatively, β-Ni(OH)2 was charged in runs d and f, respectively. For achieving a constant ion strength run d and f were supplemented with 0.5 mmol and 1 mmol Na2SO4, respectively. Three times the bottle was evacuated and filled with argon, finally ending in a deaerated state. Subsequently the bottle was charged with argon-saturated water (calculated for the end volume of 5 ml), with 0.5 or 1.0 mL argon-saturated 1M Na2S solution, with 0.5 (run a und b), 1.0 (run c) or 2.0 ml (run e) 1M NaOH solution and finally with 60 ml CO and 60 ml acetylene, using for the injections gas-tight syringes. To confirm the authenticity of the products, 13CO or D2O were used in representative experiments. Variations in the initial pH of the reaction batches were induced through the addition of 1M NaOH or 1M H2SO4. Reactions were carried out at 105 °C.
After 7 days the reaction mixture was allowed to cool down and was centrifuged at 10,000 rpm for 5 minutes. The pH was measured by a glass electrode and 1 ml of the supernatant was freeze-dried. For analysis by GC-MS, the residue was dissolved in 250 μl anhydrous acetonitrile and derivatized with 250 μl N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide for 30 minutes at 70 °C.
Analysis was performed with GC-MS, using GC-2010 coupled with MS-QP2010 Plus (Shimadzu GmbH, D-Duisburg) with a 30 m × 0.25 mm × 0.25 μm fused silica capillary column (Equity TM5, Supelco, Bellefonte, PA, USA) and AOC-20i auto injector. Temperature program and settings:
Program 1: 0–6 min at 60 °C; 6–25 min at 60–280 °C, 10 °C/min; 25–28 min at 280 °C; injector temperature: 260 °C; detector temperature: 260 °C; column flow rate: 1 mL/min; scan interval: 0.5 sec; injection volume 0.2 μl.
Program 2 (used for analysis of C9 acids): 0–6 min at 90 °C; 6–25 min at 90–280 °C, 10 °C/min; 25–28 min at 280 °C;
Otherwise identical to program 1; injection volume 1 μl.
Peak assignment was achieved by comparison of retention times and mass spectra of purchased reference compounds, synthesized products and data from NIST spectra library; for details see Footnotes of Supplemental Table S1.
Quantification was performed by external calibration using known concentrations of commercially available reference compounds (for details see Footnotes of Supplemental Table S1).
All chemicals were purchased from Sigma Aldrich GmbH (D-Steinheim) in the highest purity available. Acetylene was purchased from Linde AG (D-Pullach). Carbon monoxide, Argon 4.6 from Westfalen AG (D-Münster) and 13CO from Cambridge Isotopes Laboratories Inc. (USA-MA-Tewksbury).
Heptatrienoic acid was not commercially available and was synthesized by reacting 2 mmol trans-glutaconic acid with 2.2 mmol acrolein in tetrahydrofuran (THF) in the presence of 8 mmol 4-dimethylaminopyridine (DMAP) at 70 °C for 24 h. The product was isolated from the reaction mixture and confirmed by NMR-spectroscopy (AV500 Bruker, Rheinstetten) and GC-MS.
Additional Information
How to cite this article: Scheidler, C. et al. Unsaturated C3,5,7,9-Monocarboxylic Acids by Aqueous, One-Pot Carbon Fixation: Possible Relevance for the Origin of Life. Sci. Rep. 6, 27595; doi: 10.1038/srep27595 (2016).
Supplementary Material
Acknowledgments
This work was funded by Hans-Fischer Gesellschaft. Development of the experimental techniques was funded by Deutsche Forschungsgemeinschaft (EI-384 3-1). We thank A. Bacher and M. Groll for encouragement and support. We thank A. Bacher and M. Groll for encouragement and support and German Research Foundation (DFG) and Technical University of Munich (TUM) in the framework of the Open Access Publishing Program. This work is dedicated to the memory of Prof. Dr. Helmut Simon.
Footnotes
Author Contributions C.S. and J.S. jointly performed the experiments; W.E. and G.W. designed this study; C.H. developed the methodology, supervised and coordinated the experiments. All authors read, commented on and jointly approved submission of this article.
References
- Lawless J. G. & Yuen G. U. Quantification of monocarboxylic acids in the Murchison carbonaceous meteorite. Nature 282, 396–398 (1979). [Google Scholar]
- Yuen G., Blair N., Des Marais D. J. & Chang S. Carbon isotope composition of low molecular weight hydrocarbons and monocarboxylic acids from Murchison meteorite. Nature 307, 252–254 (1984). [DOI] [PubMed] [Google Scholar]
- Shimoyama A., Naraoka H., Yamamoto H. & Harada K. Carboxylic acids in the Yamato-791198 carbonaceous chondrites from Antarctica. Chem. Lett. 1561–1564 (1986). [Google Scholar]
- Deamer D. Boundary structures are formed by organic components of the Murchison carbonaceous meteorite. Nature 317, 792–794 (1985). [Google Scholar]
- Brooks J. & Shaw G. Origin and development of living systems. (Academic Press, London 1973), p. 359. [Google Scholar]
- Nooner D. W. & Oro J. Synthesis of fatty acids by a closed system Fischer-Tropsch process In Hydrocarbon synthesis from carbon monoxide and hydrogen Advances in Chemistry (eds Kugler E. F. & Steffgen F. W.). 178, 159–171, (1979). [Google Scholar]
- McCollom T. M. & Seewald J. S. Abiotic Synthesis of organic compounds in deep-sea hydrothermal environments. Chem. Rev. 107, 382–401 (2007). [DOI] [PubMed] [Google Scholar]
- McCollom T. M. Miller-Urey and beyond: What we have learned about prebiotic organic synthesis reactions in the past 60 years? Annu. Rev. Earth Planet. Sci. 41, 207–229 (2013). [Google Scholar]
- Matsumoto K., Sera A. & Uchida T. Organic Synthesis under high pressure. Synthesis 1–26 (1985). [Google Scholar]
- Klärner F.-G. & Wurche F. The effect of pressure on organic reactions. J. Prakt. Chem. 342, 609–636 (2000). [Google Scholar]
- Trotus I.-T., Zimmermann T. & Schüth F. Catalytic reactions of acetylene: A feedstock for the chemical industry revisited. Chem. Rev. 114, 1761–1782 (2014). [DOI] [PubMed] [Google Scholar]
- Lin T. J., Meng X. & Shi L. Catalytic hydrocarboxylation of acetylene to acrylic acid using Ni2O3 and cupric bromide as combined catalysts. J. Mol. Cat. A. 396, 77–83 (2015). [Google Scholar]
- Cairns T. L., Engelhardt V. A., Jackson H. L., Kalb G. H. & Sauer J. C. The reaction of acetylene with acrylic compounds. J. Am. Chem. Soc. 74, 5636–5640 (1952). [Google Scholar]
- Acker D. S. & Anderson B. C. Some reactions of methyl 2,4,6-heptatrienooate J. Org. Chem. 24, 1162–1163 (1959). [Google Scholar]
- Oremland R. S. & Voytek M. A. Acetylene as fast food: Implications for development of life on anoxic primordial Earth and in the outer Solar System. Astrobiology 8, 45–58 (2008). [DOI] [PubMed] [Google Scholar]
- Igari S., Maekawa T. & Sakata S. Light hydrocarbons in fumarolic gases: A case study in the Kakkonda geothermal area. Chikyukagau 34, 103–109 (2000). [Google Scholar]
- Muenow D. W. High temperature mass spectrometric gas-release studies of Hawaiian volcanic glass: Pele’s tears. Geochim. Cosmochim. Acta 37, 1551–1561 (1973). [Google Scholar]
- Mukhin L. M. Volcanic processes and synthesis of simple organic compounds on primitive Earth. Orig. Life 7, 355–368 (1976). [DOI] [PubMed] [Google Scholar]
- Wächtershäuser G. The place of RNA in the origin and early evolution of the genetic machinery. Life 4, 1050–1091 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B. H., Percivalle C., Ritson D. J., Duffy C. D. & Sutherland J. D. Common origins of RNA protein and lipid precursors in a cyanosulfidic protometabolism. Nature Chem. 7, 301–307 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg N. Hollemann-Wiberg, Lehrbuch der anorganischen Chemie 1243–1247 (Walter de Gruyter, 2007). [Google Scholar]
- Marquez C., Lazcano A., Miller S. L. & Oro J. Fully deuterated aliphatic hydrocarbons obtained from iron carbide treated with DCl and D2O. Orig. Life Evol. Biosph. 26, 3–5. [Google Scholar]
- Holloway J. R. & Blank J. G. Applications of experimental results to C-O-H species in natural melts. Reviews in Mineralogy. 30, 187–230 (1994). [Google Scholar]
- Wächtershäuser G. On the chemistry and evolution of the pioneer organism. Chem. Biodivers. 4, 584–602 (2007). [DOI] [PubMed] [Google Scholar]
- Lodders K. Solar System abundances and condensation temperatures of the elements. The astrophysical Journal. 591, 1220–1247 (2003). [Google Scholar]
- Cox P. A. The elements: Their origin, abundance and distribution. (Oxford University Press, 1989). [Google Scholar]
- Hazen R. Evolution of minerals. Sci. Am. 302, 58–65 (2010). [DOI] [PubMed] [Google Scholar]
- Douville E. et al. The rainbow vent fluids (36°14′N, MAR): the influence of ultramafic rocks and phase separation on trace metal content in Mid-Atlantic Ridge hydrothermal fluids. Chem. Geol. 184, 37–48 (2002). [Google Scholar]
- Kornhauser K. O. et al. Oceanic nickel depletion and a methanogen famine before the Great Oxidation Event. Nature 458, 750–753 (2009). [DOI] [PubMed] [Google Scholar]
- Huang et al., The composition of nanoparticulate nickel sulfide. Chem. Geol. 277, 207–213 (2010). [Google Scholar]
- Miller L. G., Baesman S. M. & Oremland R. S. Stable carbon isotope fractionation during bacterial acetylene fermentation: Potential for life detection in hydrocarbon-rich volatiles of icy planet(oid)s. Astrobiology 15, 977–986 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiffert G. B. et al. Structure of the non-redox-active tungsten/[4Fe:4S] enzyme acetylene hydratase. Proc. Natl. Acad. Sci. USA 104, 3073–3077 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohdich F. et al. Studies on the nonmevalonate terpene biosynthetic pathway: Metabolic role of IspH (LytB) protein. Proc. Natl. Acad. Sci. USA 99, 1158–1163 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Span I. et al. Discovery of acetylene hydratase activity of the iron-sulphur protein IspH. Nat. Commun. 3, 1042, doi: 10.1038/ncomms2052 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wächtershäuser G. From volcanic origins of chemoautotrophic origin of life to bacteria, archaea aeukarya. Phil. Trans. R. Soc. B 361, 1787–1808 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape J. L., Monnard P.-A. & Boncella J. M. Prebiotically relevant mixed fatty acid vesicles support anionic solute encapsulation and photochemically catalyzed trans-membrane charge transport. Chem. Sci. 2, 661–671 (2011). [Google Scholar]
- Apel C. L., Deamer D. W. & Mautner M. N. Self-assembled vesicles of monocarboxylic acids and alcohols: conditions for stability and for the encapsulation of biopolymers. Biochim. Biophys. Acta 1559, 1–9 (2002). [DOI] [PubMed] [Google Scholar]
- Wächtershäuser G. Before enzymes and templates: theory of surface metabolism. Microbiol. Rev. 52, 452–484 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach C. et al. Comparison of cis and trans fatty acid containing phosphatidylcholines on membrane properties. Biochem. 43, 6344–6351 (2004). [DOI] [PubMed] [Google Scholar]
- Wächtershäuser G. Groundworks for an evolutionary biochemistry:The iron-sulphur world. Prog. Biophys. molec. Biol. 58, 85–201 (1992). [DOI] [PubMed] [Google Scholar]
- Heinen W. & Lauwers A. M. Organic sulfur compounds resulting from the interaction of iron sulfide, hydrogen sulfide and carbon dioxide in an anaerobic aqueous environment. Orig. Life Evol. Biosph. 26, 131–150 (1996). [DOI] [PubMed] [Google Scholar]
- Dörr M. et al., A possible prebiotic formation of ammonia from dinitrogen on iron-sulfide surfaces. Angew. Chem. Int. Ed. 42, 1540–1543 (2003). [DOI] [PubMed] [Google Scholar]
- Huber C. & Wächtershäuser G. Activated acetic acid by carbon fixation on (Fe,Ni)S under primordial conditions. Science 276, 245–247 (1997). [DOI] [PubMed] [Google Scholar]
- Huber C. & Wächtershäuser G. α-Hydroxy and α-amino acids under possible hadean, volcanic origin-of-life conditions. Science 324, 630–632 (2006). [DOI] [PubMed] [Google Scholar]
- Huber C., Eisenreich W., Hecht S. & Wächtershäuser G. A possible primordial peptide cycle. Science 301, 938–940 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


