Abstract
Luminescent lanthanide chelates have been used to label antibodies in time-gated luminescence (TGL) bioimaging. However, it is a challenging task to label directly an antibody with lanthanide-binding ligands and achieve control of the target ligand/protein ratios whilst ensuring that affinity and avidity of the antibody remain uncompromised. We report the development of a new indirect detection reagent to label antibodies with detectable luminescence that circumvents this problem by labelling available lysine residues in the linker portion of the recombinant fusion protein Linker-Protein G (LPG). Succinimide-activated lanthanide chelating ligands were attached to lysine residues in LPG and Protein G (without Linker) and the resulting Luminescence-Activating (LA-) conjugates were compared for total incorporation and conjugation efficiency. A higher and more efficient incorporation of ligands at three different molar ratios was observed for LPG and this effect was attributed to the presence of eight readily available lysine residues in the linker region of LPG. These Luminescence-Activating (LA-) complexes were subsequently shown to impart luminescence (upon formation of europium(III) complexes) to cell-specific antibodies within seconds and without the need for any complicated bioconjugation procedures. The potential of this technology was demonstrated by direct labelling of Giardia cysts and Cryptosporidium oocysts in TGL bioimaging.
Lanthanide (e.g., Eu3+, Tb3+) ions are of growing interest as luminescent probes for time-gated luminescence (TGL) bioimaging1. The outstanding luminescent properties of lanthanide ions are characterised by their sharp emission profiles (<10 nm width), large Stokes shifts (>150 nm) and long (millisecond) excited-state lifetimes. These features, in conjunction with pulsed excitation and time-gated measurements, allow temporal discrimination against fast decaying (nanosecond) autofluorescence and scattered excitation light2. TGL microscopy has been used successfully to visualise biomolecules and cells in autofluorescent environments3 by exploiting the long luminescent lifetimes of lanthanide ions.
Trivalent lanthanide ions (Ln3+) have intrinsically low absorption cross-sections, thus direct excitation yields only low levels of luminescence. As a result, lanthanide ions need to be excited indirectly through a method known as sensitisation, where a lanthanide ion is chelated by an organic ligand containing a chromophore that acts as an antenna to sensitise the absorption of light and transfer of excitation energy to the chelated ion, resulting in higher luminescence and extended emission lifetimes4. This construction is referred to as a lanthanide chelate. Ligands capable of lanthanide binding can be attached to a biomolecule (e.g., antibodies and nucleic acids) via a cross-linking group.
A number of highly luminescent tetradentate bis β-diketonate-Eu3+ chelates have been used to label antibodies in TGL bioimaging (e.g., 4,4′-bis(1″,1″,1″,2″,2″,3″,3″-heptafluoro-4″,6″-hexanedion-6″-yl)-chlorosulfo-o-terphenyl (BHHCT)5 and 4,4-bis-(1″,1″,1″,2″,2″,3″,3″-heptafluoro-4″,6″-hexanedion-6″-yl) sulfonylaminopropyl-ester-N-succinimide-ester-o-terphenyl (BHHST)6, but the difficulties associated with the direct incorporation of lanthanide chelates onto antibodies (via the initial attachment of the lanthanide ion-binding ligands) are well documented. For example, BHHCT is known to cause antibody inactivation or precipitation due to poor aqueous solubility, over-conjugation of ligand to the antibody, and variations in antibody reactivity and sensitivity6,7. Hence, direct antibody modification is often inefficient and requires time-consuming optimisation, a process unique to a given antibody. Indirect methods that deliver sufficient luminescent signal with retention of antibody function have been established. For example, Connally et al.6 applied BHHST-labelled secondary antibodies for the indirect detection of Giardia with TGL microscopy but direct modification of a secondary antibody makes it susceptible to inactivation in the same fashion as directly labelling a primary antibody. Alternatively, lanthanide-labelled streptavidin has been used as an indirect detection reagent to label biotinylated secondary antibodies with detectable luminescence for TGL bioimaging8. Even so, this method requires the specific modification of antibodies with biotin and relies on the biotin-streptavidin binding interaction, which can be difficult to control. Streptavidin conjugated proteins have also the tendency of binding non-specifically to biotinylated proteins in mammalian cells resulting in unpredictable background problems9,10,11. Additional chelates can be loaded onto a carrier molecule that tolerates a high degree of labelling and can be chemically cross-linked to a detection reagent to maximise luminescence further. For example, streptavidin typically is conjugated to bovine serum albumin (BSA), and then the conjugate is labelled with lanthanide chelates prior to use as an indirect detection reagent in TGL bioimaging1,12,13,14.
Another potential universal detection reagent is the recombinant fusion protein, Linker-Protein G (LPG), the subject of this report. LPG contains two functionally distinct regions; (a) a peptide linker sequence which has specific binding affinity towards silica-containing materials, and (b) Streptococcus Protein G′ which has specific binding affinity towards antibodies15. LPG has been used as an anchor point for the oriented immobilisation of antibodies onto silica-containing materials without the need for complex surface chemical modification15,16,17,18,19. In addition, the linker region of LPG presents itself as a prospective lanthanide carrier. It contains a number of accessible lysine residues whose terminal amino groups provide binding sites for succinimide-activated ligands capable of binding europium(III), ultimately allowing the addition of multiple luminophores with minimal effect on the antibody-binding ability of the Protein G region.
4,4′-Bis(1″,1″,1″,2″,2″,3″,3″-heptafluoro-4″,6″-hexanedion-6″-yl)sulfo-o-terphenyl-tetraethylene glycol-N-hydroxysuccinimide (BHHTEGST)20 is a newly developed tetradentate bis β-diketone-ligand derived from BHHST6 and BHHCT21, capable of binding europium(III). It has good aqueous solubility, excellent luminescent output (when complexed with europium(III)) and a mild succinimide reactive group that facilitates covalent attachment to biomolecules that contain lysine residues. BHHTEGST also possesses an extended tetraethylene glycol tether that projects its hydrophobic europium(III) binding moiety (BHHCT) away from the modified protein to prevent adverse interactions and enhance conjugate solubility.
In this communication, we show that LPG labelled with BHHTEGST serves as an effective indirect detection reagent to impart luminescence rapidly to antibodies for TGL bioimaging. We demonstrate also that the presence of the linker region significantly increases luminescent output, enabling rapid and simple visualisation of Giardia cysts and Cryptosporidium oocysts under time-gated conditions.
Results
Conjugation of PG (without Linker) and LPG with BHHTEGST
PG and LPG must be able to withstand extensive conjugation with the BHHTEGST ligand and retain their capacity to bind antibodies for use as universal detection reagents in TGL bioimaging. Thus, each protein underwent conjugation reactions at different BHHTEGST:Lysine ratios, yielding a total of three Luminescence-Activating (LA-) conjugates (Table 1), which will be referred to in this manuscript as LA-PGHIGH, MID or LOW and LA-LPGHIGH, MID or LOW. The average number of ligands attached to LA-PG or LA-LPG at each conjugation ratio was then semi-quantified as described in the Materials and Methods section and the results are summarised in Table 1.
Table 1. Semi-quantification of the number of BHHTEGST ligands attached to PG and LPG after conjugation reactions were performed in triplicate with BHHTEGST:Lysine ratios between 1.6-0.4.
| Conjugation ratio | Relative number of ligands |
BHHTEGST:Lysine ratioa |
||||
|---|---|---|---|---|---|---|
| LA-PG | CE (%)b | LA-LPG | CE (%) | LA-PG | LA-LPG | |
| High | 15.5 ± 1.4 | 81.6 ± 7.3 | 18.0 ± 0.7 | 66.7 ± 0.7 | 1.5 | 1.6 |
| Mid | 9.7 ± 2.8 | 51.2 ± 14.8 | 16.8 ± 0.6 | 62.1 ± 0.6 | 0.7 | 0.8 |
| Low | 4.3 ± 0.6 | 22.8 ± 3.1 | 12.3 ± 0.2 | 45.7 ± 0.2 | 0.4 | 0.4 |
aBHHTEGST:Lysine ratio is the number of moles of BHHTEGST added per lysine residue in each protein. bCE = Conjugation Efficiency (%) of total lysine residues modified with BHHTEGST).
The BHHTEGST ligand is amine reactive and attaches covalently to lysine residues in target proteins. PG contains 19 lysine residues whereas LPG has 27 due to the presence of an additional 8 lysines located in the linker region. Therefore it was expected that LPG would be conjugated with a higher number of ligands at each BHHTEGST:Lysine ratio tested. As shown in Table 1, in conjugations performed at the lowest BHHTEGST:Lysine ratio, PG was modified with only an average of 4.3 ligands whereas LPG had 12.3. This difference (8 ligands) correlates with the additional eight ligands present on the linker region of LPG. A similar difference (7.1 ligands) in the number of attached ligands was observed at the mid BHHTEGST:Lysine ratio, in which PG and LPG contained on average 9.7 and 16.8 ligands, respectively. However, this difference was considerably smaller (2.5 ligands) at the highest BHHTEGST:Lysine ratio tested, which resulted in PG and LPG complexes that contained on average 15.5 and 18.0 BHHTEGST ligands, respectively. These results show that better and more consistent conjugation efficiencies were achieved with LPG (45.7–66.7%) compared to PG (22.8–81.6%) across the range of BHHTEGST:Lysine ratios tested. More importantly, the results of the conjugation studies at low and medium levels suggest that the incorporation of the additional BHHTEGST ligands (8 and 7.1, respectively) takes place preferentially on the linker region of LPG, remote from the antibody-binding region.
Using lanthanide-activated complexes to label Giardia and Cryptosporidium
We investigated whether the lanthanide-activated complexes could act as universal detection reagents to impart detectable luminescence to primary antibodies without compromising their function and which conjugation ratio was the most effective. Using the strategy illustrated in Fig. 1, each of the modified conjugates from the previous section was coupled to cell-specific antibodies for the luminescent labelling of Giardia cysts (Fig. 2A) and Cryptosporidium oocysts (Fig. 2B), following exposure of slides to a europium(III) solution. The labelled cells were visualised by TGL microscopy using a Gated Auto-synchronous Luminescence Detector (GALD)3 and the SNRs from the raw digital images were calculated using ImageJ software (Table 2).
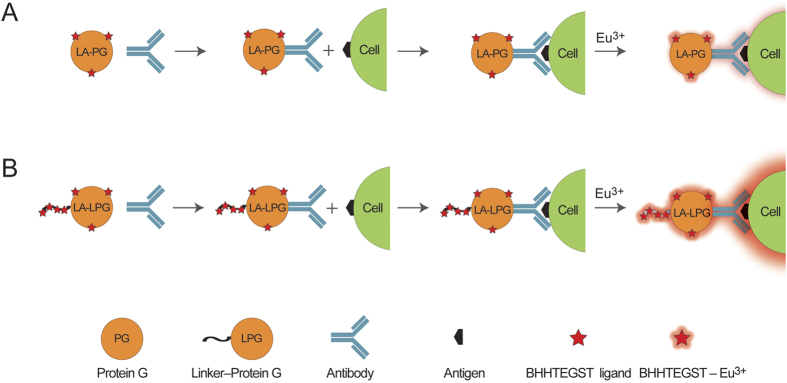
Figure 1. Illustration showing the luminescent labelling of cells using LA-PG- and LA-LPG-coupled antibodies.
Coupling of LA-PG (A) or LA-LPG (B) to an antibody results in a “LA-PG or LA-LPG–Antibody” conjugate that can be used to label a target cell with luminescence upon exposure to Eu3+. The red stars represent an unspecific number of BHHTEGST ligands incorporated onto the LA-PG (A) or LA-LPG (B) biomolecule.
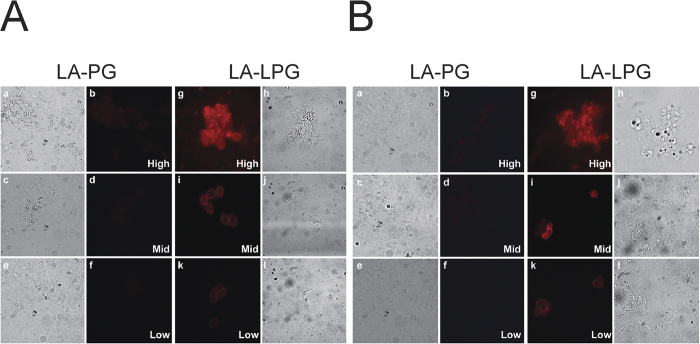
Figure 2.
Luminescent labelling of (A) Giardia cysts and (B) Cryptosporidium oocysts using (a–f) LA-PGHIGH, MID, or LOW or (g–l) LA-LPGHIGH, MID, or LOW -coupled antibodies. (a, c, e and h, j, l) Bright-field microscopy. (b, d, f and g, i, k) TGL microscopy. Exposure time for TGL microscopy was identical for all samples; as a result the brightness of the images are relative to each other.
Table 2. Signal to noise ratios (SNRs) for Giardia cysts (Fig. 2A) and Cryptosporidium oocysts (Fig. 2B) labelled with each of the LA-PG and LA-LPG complexes.
| Conjugation ratio | Signal-to-Noise ratios |
|||||
|---|---|---|---|---|---|---|
|
Giardia cysts |
Cryptosporidium oocysts |
|||||
| LA-PG | LA-LPG | Fold diff.a | LA-PG | LA-LPG | Fold diff. | |
| High | 16 | 72 | 4.5 | 21 | 76 | 3.6 |
| Mid | 7 | 76 | 10.9 | 16 | 71 | 4.4 |
| Low | 6 | 20 | 3.3 | 10 | 25 | 2.5 |
SNR analysis of raw image data was performed using ImageJ software. aFold diff. = Fold difference.
Of all the complexes studied (Fig. 2), LA-LPGMID images showed high definition and SNRs of 76 for Giardia and 71 for Cryptosporidium (Table 2). In contrast, the cells labelled with the LA-PGMID complex exhibited low luminescence and poor resolution. Accordingly, the SNRs in these images were significantly lower for both cell-types (Giardia = 7; Cryptosporidium = 16). The LA-LPGMID complex provided more than a 10-fold enhancement in the SNRs of labelled Giardia luminescence and more than a 4-fold enhancement for Cryptosporidium when compared to LA-PGMID. Cells labelled with LA-LPGHIGH displayed similar SNRs (Giardia = 72; Cryptosporidium = 76) to those shown by LA-LPGMID. However, the cells appeared oversaturated with poorly-defined boundaries. The LA-PGHIGH control generated low SNRs (Giardia = 16; Cryptosporidium = 21) and produced images that showed weak luminescence and low resolution. In comparison to LA-PGHIGH, the LA-LPGHIGH complex improved the SNRs of the labelled Giardia and Cryptosporidium by more than 4-fold and 3-fold, respectively. Low SNRs were obtained from cells labelled with the LA-LPGLOW complex (Giardia = 20; Cryptosporidium = 25) and the cells showed very weak luminescent output and poor definition with the weakest SNRs produced by cells labelled with LA-PGLOW (Giardia = 6; Cryptosporidium = 10). In these images the cells were difficult to detect and identify. When compared to LA-PGLOW, the LA-LPGLOW complex amplified SNRs by more than 3-fold and 2-fold for labelled Giardia and Cryptosporidium, respectively.
These results indicated that all of the modified complexes can bind antibodies and render them luminescent, but with greatly varied efficacy. The cells labelled with LA-LPG were significantly brighter in label intensity than LA-PG (irrespective of the conjugation concentration) an observation that can be attributed to the higher BHHTEGST content of the LA-LPG conjugates (see Table 1) and hence, europium(III) chelates. However, LA-LPGHIGH possesses the highest BHHTEGST content and produced images in which cells were highly luminescent but poorly defined. This lack of definition could possibly be due to a reduction in cell targeting efficiency. The LA-LPGMID conjugate provided clear, high-contrast and definition images, making it the best detection reagent under these conditions for the attachment to the antibodies tested. Therefore, all subsequent cell-labelling experiments were performed using LA-LPGMID-coupled antibodies.
Detection of cells in autofluorescent environments
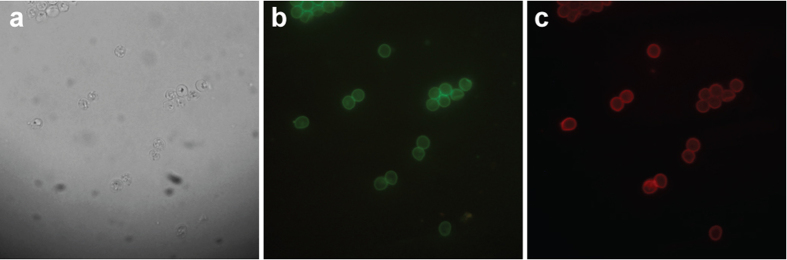
Lanthanide chelates have long luminescence lifetimes that enable temporal discrimination of shorter-lived autofluorescence, unlike conventional fluorophores. We tested whether LA-LPGMID-coupled antibodies in conjunction with TGL microscopy can be used for background-free detection of cells in autofluorescent environments (Fig. 3). In an initial control experiment, the LA-LPGMID complex was coupled to a FITC-conjugated antibody (CRY104-FITC) specific for the cell walls of Cryptosporidium oocysts. Cells were then labelled and visualised by either fluorescence microscopy or TGL microscopy. Cells observed in the fluorescent FITC channel displayed bright green fluorescence and good definition (Fig. 3b).
Figure 3. Cryptosporidium oocysts labelled with “LA-LPGMID + CRY104-FITC” complex.
(a) Bright field (b) FITC channel (c) TGL conditions.
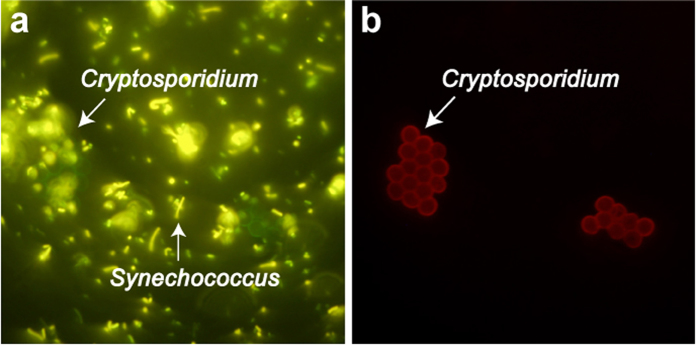
Under time-gated conditions the cells emitted red luminescence and maintained their definition (Fig. 3c). These results indicated that LA-LPGMID can be coupled easily to a different fluorophore-conjugated antibody and applied as a dual-immuno-labelling technique based on emission lifetimes. Consequently, we used this methodology to compare the efficiency of a conventional fluorophore (FITC) and our lanthanide-carrier molecule (LA-LPGMID) to label cells in an autofluorescent environment (Fig. 4). Cryptosporidium oocysts were mixed with a sample of autofluorescent Synechococcus cells and then labelled with the “LA-LPGMID + CRY104-FITC” complex (that was used to obtain the images in Fig. 3). The Cryptosporidium oocysts were fluorescent green in the FITC channel as expected, but difficult to discriminate against background autofluorescence provided by the Synechococcus cells (Fig. 4a). Switching to time-gated microscopy suppressed the background autofluorescence and generated high-contrast images in which cells exhibited red luminescence and were identified readily against a fluorescence-free background (Fig. 4b). These results show that the LA-LPGMID complex in combination with TGL microscopy can be used successfully for the sensitive detection of cell targets in the presence of background autofluorescence.
Figure 4. A sample of Cryptosporidium oocysts was spiked with autofluorescent Synechococcus cells and then labelled with “LA-LPGMID + CRY104-FITC” complex.
(a) Bright field (b) FITC channel (c) TGL conditions.
Discussion
We labelled the lysine residues within PG and LPG with three different molar ratios of BHHTEGST and observed different levels of incorporation (Table 1). Overall, LA-LPG had a greater number of attached BHHTEGST ligands than LA-PG at each ratio tested. The conjugation efficiency of LA-PG decreased almost proportionality to the molar ratio of BHHTEGST, whereas LA-LPG underwent less obvious reductions in conjugation efficiency as the molar ratio of BHHTEGST was lowered. Such differences may be explained by variations in lysine content and accessibility of lysine residues on the linker and Protein G regions. The linker accounts for ~30% of the lysine residues on LPG and can be modified with up to 8 amine-reactive ligands. The intrinsic structural disorder of the linker19 may allow it to undergo conformational changes that enable interaction between its lysine residues and BHHTEGST, such that ligand attachment in this linker region is favoured at lower molarities in comparison with attachment at lysines residues on the Protein G component. In contrast, PG has a highly stable and ordered structure. PG also retains its activity at extreme temperatures and across a wide range of pH values. High concentrations of denaturant are required to unfold the protein22. The IgG-binding domains of PG have structural homology with the rigid and inflexible protein, ubiquitin23. Thus, unlike the linker, the PG region cannot extensively alter its conformation to interact readily with BHHTEGST, preventing high levels of incorporation at lower molarities. Further analysis of the amino acid sequence of LPG using a neural network predictor of natural disordered regions (PONDR, http://pondr.com) was performed (results not shown). It identified the ordered structure of the Fc-binding domains within PG and confirmed the disordered structure of the linker region. We compared the relative SNRs of Giardia cysts and Cryptosporidium oocysts that were labelled with either LA-PG or LA-LPG complexes (Table 2) to determine which LA-complexes were the most compatible for TGL bioimaging. We found that cells labelled with LA-PG complexes exhibited weak luminescence that was unacceptable for TGL microscopy. Conversely, cells labelled with LA-LPG complexes displayed up to 10-fold higher SNRs. This result shows that the linker region can act as an effective lanthanide-carrier portion of the LPG molecule that is capable of bearing additional chelates to increase the luminescent output of LPG for TGL bioimaging applications.
LA-LPGMID provided bright, high-contrast and high-definition images, making it the best LA-complex for indirect detection in TGL bioimaging. It contains approximately seventeen BHHTEGST ligands (Table 1), if the eight lysine residues on the linker are preferentially modified (as discussed above) then the remaining nine BHHTEGST ligands are likely to be located on the PG region. Markela et al.24 reported that Protein G retained optimal affinity and binding specificity for IgGs when labelled with up to nine europium chelates. The results with the LA-LPGMID complex highlight the linker’s ability to impart high luminescence to a detection reagent without affecting its binding avidity and specificity.
A loss in binding specificity generally occurs when the detection reagent is over-modified1. In this study, the LA-LPGHIGH complex had the highest BHHTEGST ligand content and produced cellular images with high SNRs, but the labelled cells were poorly defined and a large amount of non-specific binding was observed. We propose that this lack of specificity is due to the over-modification of LA-LPGHIGH reducing its affinity towards antibodies. The over-modification of antibody-binding proteins (ABPs) with lanthanide chelates has been observed to reduce their antibody-binding affinity24,25,26,27. For example, the attachment of chelates to just 15% of the available lysine residues on Protein A results in ~50% loss in its binding affinity25. Furthermore, the attachment of different chelate types can vary the affinity of ABPs towards antibodies from different species27.
The BHHTEGST ligand is a molecule that becomes covalently attached to proteins via lysine amino groups. The truncated variant of PG28 used here includes two domains with high affinity towards the Fc region of IgGs29. Both domains consist of a four-stranded β-sheet crossed diagonally by a single α-helix. X-ray crystallography23 and site-directed mutagenesis studies30 have identified two lysine residues at the centre of the α-helix that are integral to the IgG-binding interaction. Therefore, preservation of the four lysine residues on the α-helices of the two IgG-binding domains of PG is essential for optimum binding affinity and specificity. We used a protein model that depicts an IgG-binding domain (i.e., the C2 domain) from Protein G in complex with the Fc region of human IgG (PDB - 1FCC; Fig. 5) to investigate further the potential impact of BHHTEGST incorporation into PG or LPG. In this model, BHHTEGST-Eu3+ chelates were attached to the two lysines present on the α-helix of the IgG-binding domain that participate in antibody-binding. Both of the relatively large BHHTEGST-Eu3+ chelates were shown to project from this domain through the entire Fc region of the bound human IgG. Therefore, any modification of these residues with BHHTEGST could sterically hinder the binding interactions between PG and antibodies. With LPG the accessibility and preferential modification of lysine residues within the linker region positions the BHHTEGST ligands away from the antibody-binding region (PG), thus reducing the potential modification of residues that mediate antibody-binding.
Figure 5. Protein structure models of an IgG-binding domain (C2) from Protein G (blue) in complex with the Fc-region of human IgG (grey).
(A) The two lysine residues located on the α-helix of the IgG-binding domain (marked in red) have each been modified with the BHHTEGST (green)-Eu3+ (red ball) complex, resulting in unfavorable steric interactions in these models. (B) 90° view.
TGL bioimaging has relied on lanthanide-labelled streptavidin as an indirect detection reagent to deliver sufficient luminescence to antibodies without impeding their biological function8. This methodology requires the modification of secondary antibodies with biotin and cell-labelling procedures can take up to 24 h1,8,12,13,14. As an alternative, ABPs such as Protein G and Protein A, rapidly bind with high affinity and specificity to a broad spectrum of IgG species and subclasses without influencing their capacity to recognise and bind antigens29. ABPs labelled with lanthanide chelates have been used in the past as detection reagents for indirect time-resolved fluoroimmunoassays (TR-FIA)24,31,32. We have shown that LA-PG and LA-LPG complexes can be used as indirect detection reagents for TGL bioimaging and impart luminescence to antibodies within seconds and without the need for antibody-modification. It should be noted that Protein G labelled with lanthanide chelates are commercially available as an indirect detection reagent for TR-FIA in the dissociative enhancement format (DELFIA)24. In this method, a protein is labelled with non-photoactive chelates that bind lanthanide ions. The ions are then dissociated and released into an enhancement solution in which they become luminescent and can be detected. Unfortunately this method is incompatible with TGL microscopy because spatial resolution would be lost in a dissociation-enhanced format. For this reason, TGL microscopy requires detection reagents to contain intrinsically luminescent lanthanide chelates (e.g., tetradentate bis β-diketonate-Eu3+ chelates) to label bio-targets effectively1,5,8,12,13,14,33. Although ABPs labelled with conventional fluorophores (e.g., FITC and Alexa Fluor) are available commercially for use as indirect detection reagents in fluorescence microscopy, we are not aware of any reports in which ABPs have been labelled with intrinsically luminescent chelates for TGL microscopy.
Typically, indirect detection reagents are cross-linked to carrier molecules and the resulting conjugate (e.g., SA-BSA) is then labelled with luminescent chelates1,5,12,13,14,33 allowing higher luminescence for TGL bioimaging to be achieved. In some cases detection reagents have been protein engineered to substitute a small number of surface residues with lysines to improve chelate incorporation34. Here we investigated whether the linker region of LPG could serve as a carrier segment for BHHTEGST ligands and subsequent europium(III) chelation. Conventional carrier molecules often reduce the specificity of the detection reagent to which they are cross-linked35. For example, Ius et al.25 described a 55% loss in the IgG-binding affinity of Protein A after it was cross-linked to lanthanide-labelled BSA. However in the case of LPG, the detection reagent (PG) is genetically-fused to the carrier molecule (linker) which circumvents protein cross-linking. To our knowledge, this property is unique to the LPG fusion protein and has not been reported for other detection reagents used for TGL microscopy. Traditional carrier molecules also tend to have large masses and varying stabilities that make it difficult to maintain their solubility after conjugation. The linker overcomes these complications due to its relatively small molecular mass (~10 kDa) and high hydrophilicity. Accordingly, LPG is theoretically 41% more soluble than PG (based on their GRAVY index36) which may allow more flexible conjugation procedures and higher labelling ratios with less soluble lanthanide binding ligands (e.g., BHHCT).
LA-LPG represents a novel indirect universal detection reagent that rapidly labels virtually any antibody capable of binding to Protein G with luminescence within seconds. This is property is due to the two different regions of LA-LPG. The Protein G region has the ability to bind to a broad range of mammalian IgGs with high affinity and binding specificity, allowing for an easy interchange of IgGs for different targets. The linker region of LA-LPG functions as a genetically-fused carrier molecule that can be loaded with lanthanide chelates to maximise luminescence for TGL bioimaging. LA-LPG can be used to directly label antibodies for several applications, e.g., bioimaging, cell labelling and detection, and flow cytometry.
Methods
Source of materials
BHHTEGST was synthesised as described previously20. Recombinant LPG was produced in E. coli and purified by ion exchange chromatography as described previously15. Recombinant PG was purchased from Sigma-Aldrich (Australia).
Cells and antibodies
Cryptosporidium oocysts and Giardia cysts, Cryptosporidium monoclonal antibodies CRY104 and CRY104-conjugated to fluorescein isothiocyanate (CRY104-FITC) specific to the walls of Cryptosporidium oocysts, and the Giardia monoclonal antibody G203 specific to the walls of Giardia cysts were purchased from BTF Pty Ltd (Sydney, Australia). Cells from the cyanobacterium Synechococcus were kindly provided by Dr Deepa Varkey (Macquarie University).
Conjugation of proteins with BHHTEGST
BHHTEGST contains an N-hydroxysuccinimide ester that enables its attachment to proteins via the amino group of lysine residues. Assuming that all of the lysine residues were accessible for reaction, each protein (PG without linker, 19 residues and LPG, 27 residues) was conjugated with three different molar ratios of the BHHTEGST ligand per lysine residue. This is referred to here as the BHHTEGST:Lysine ratio. All conjugation reactions were performed in triplicate. In the conjugation reaction, 100 μg protein was exchanged into 100 mM NaHCO3, pH 8.5 and then mixed with different molar excesses of BHHTEGST. After incubation for 1 h at 37 °C the reaction mixtures were passed through a Sephadex G-25 column in water to remove excess unconjugated BHHTEGST. The fractions corresponding to labelled conjugates were detected using a spectrophotometer (280 and 320 nm), combined and concentrated to 100 μL using an Amicon Ultra centrifugal filter (10 kDa cut-off, Millipore).
Relative quantification of BHHTEGST ligands attached to proteins
The UV spectrum of BHHTEGST exhibits a maximum absorption at 335 nm and partial absorption at 280 nm, which overlaps with that from both proteins (PG and LPG). Thus, to account for the partial absorption of BHHTEGST in conjugated proteins, molar extinction coefficients for BHHTEGST were calculated from standard curves at both 320 nm and 280 nm as described previously20. The concentration of BHHTEGST was calculated from the absorbance of conjugates at 320 nm and the extinction coefficient of BHHTEGST at 320 nm. Then partial absorption of BHHTEGST at 280 nm was identified from its standard curve (at 280 nm). The final protein concentration was obtained after subtracting the absorbance of BHHTEGST from the absorbance of conjugated protein at 280 nm. The average number of BHHTEGST molecules per protein was then determined by dividing the BHHTEGST concentration by the protein concentration.
Standard cell-labelling procedure
To couple the conjugates to cell-specific antibodies, each conjugate (~2 μg) was mixed with antibody (2 μg) in 100 mM phosphate-buffered saline, pH 7.4 to a final volume of 10 μL and incubated for 30 s at room temperature. To label cells, 5 μL of the conjugate-coupled antibodies were loaded onto a microscope slide containing fixed target cells and incubated for 1 min at room temperature. Cell fixation was carried out by gently drying a 10 μL cell sample on a slide with a hot plate at 40 °C for 20 s. The slide was gently rinsed with MilliQ water. Then 3 μL of 22 mM europium(III) chloride (EuCl3) was added to the slide and allowed to react with the ligands for 30 s to form the luminescent chelates. The labelled cells were examined using bright-field, fluorescence and TGL microscopy.
Microscopy and image analysis
All microscopy and imaging was performed on an Olympus BX51 fluorescence microscope with a UPLSAPO 100 X oil immersion objective lens. Colour images were captured by an Olympus 12.8 megapixel DP72 camera with a sensor resolution of 4140 × 3096 and stored in the TIFF format. To view TGL, a previously described Gated Auto-synchronous Luminescence Detector (GALD) was inserted into the DIC slot of the microscope nosepiece3. Cells labelled with luminescence were detected using the GALD without any fluorescence filter and exposure times of 3 s. Fluorescence imaging was carried out using a 100 W mercury arc lamp and a FITC filter set with 200 ms exposure times. The reduction in exposure time from 3 s to 200 ms was required to attain comparable image/pixel brightness. All images in this work are as originally sampled and without post processing.
The luminescent labelling of cells was quantified by analysing the raw digital images with the freeware ImageJ downloaded from the NIH website (http://rsb.info.nih.gov/ij). In each image, a line-plot was placed across the cells. The 8-bit value of the pixels in the red channel was used to attain the peak intensity and was defined as the ‘signal’. The mean intensity from a selected section in the darkest region (cell free) was defined as the ‘noise’. These values were then used to calculate signal-to-noise ratios (SNR). SNRs were normalised to 1 by dividing the ‘signal’ value by the ‘noise’ value (an example is provided in Supplementary Information).
Additional Information
How to cite this article: Sayyadi, N. et al. A Novel Universal Detection Agent for Time-Gated Luminescence Bioimaging. Sci. Rep. 6, 27564; doi: 10.1038/srep27564 (2016).
Supplementary Material
Acknowledgments
The authors would like to thank Dr N. Cordina for her expertise and assistance with the PyMOL software.
Footnotes
Author Contributions All authors were involved in designing the experiments. N.S., R.E.C. and A.C.T. carried out the ligand synthesis. A.C., P.L.B. and A.S. designed the LPG biomolecule. N.S. and A.C. collected all the data. N.S., A.C. and A.S. performed the data analysis. A.C., P.L.B. and A.S. wrote the manuscript. All authors reviewed the manuscript.
References
- Jin D. & Piper J. A. Time-gated luminescence microscopy allowing direct visual inspection of lanthanide-stained microorganisms in background-free condition. Anal. Chem. 83, 2294–2300 (2011). [DOI] [PubMed] [Google Scholar]
- Eliseeva S. V. & Bünzli J. C. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 39, 189–227 (2010). [DOI] [PubMed] [Google Scholar]
- Connally R. A device for gated autosynchronous luminescence detection. Anal. Chem. 83, 4782–4787 (2011). [DOI] [PubMed] [Google Scholar]
- Hagan A. K. & Zuchner T. Lanthanide-based time-resolved luminescence immunoassays. Anal. Bioanal. Chem. 400, 2847–2864 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connally R., Veal D. & Piper J. Flash lamp-excited time-resolved fluorescence microscope suppresses autofluorescence in water concentrates to deliver an 11-fold increase in signal-to-noise ratio. J. Biomed. Opt. 9, 725–734 (2004). [DOI] [PubMed] [Google Scholar]
- Connally R., Veal D. & Piper J. Time-resolved fluorescence microscopy using an improved europium chelate BHHST for the in situ detection of Cryptosporidium and Giardia. Microsc. Res. Tech. 64, 312–322 (2004). [DOI] [PubMed] [Google Scholar]
- Wu F. B. & Zhang C. A new europium β-diketone chelate for ultrasensitive time-resolved fluorescence immunoassays. Anal. Biochem. 311, 57–67 (2002). [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang Y., Ye Z., Jin D. & Yuan J. New class of tetradentate β-diketonate-europium complexes that can be covalently bound to proteins for time-gated fluorometric application. Bioconjugate Chem. 23, 1244–1251 (2012). [DOI] [PubMed] [Google Scholar]
- Hollinshead J., Sanderson M. & Vaux D. J. Anti-biotin antibodies offer superior organelle-specific labeling of mitochondria over avidin or streptavidin. J. Histochem. Cytochem. 45, 1053–1057 (1997). [DOI] [PubMed] [Google Scholar]
- Matsumoto V. Simultaneous inhibition of endogenous avidin-binding activity and peroxidase applicable for the avidin-biotin system using monoclonal antibodies. Histochemistry 83, 325–330 (1985). [DOI] [PubMed] [Google Scholar]
- Wood, S. G. & Warnke R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. JJ. Histochem. Cytochem. 29, 1196–1204 (1981). [DOI] [PubMed] [Google Scholar]
- Jiang L. et al. Development of a visible-light-sensitized europium complex for time-resolved fluorometric application. Anal. Chem. 82, 2529–2535 (2010). [DOI] [PubMed] [Google Scholar]
- Deng W., Jin D., Drozdowicz-Tomsia K., Yuan J. & Goldys E. M. Europium chelate (BHHCT-Eu3+) and its metal nanostructure enhanced luminescence applied to bioassays and time-gated bioimaging. Langmuir 26, 10036–10043 (2010). [DOI] [PubMed] [Google Scholar]
- Lu Y. et al. Automated detection of rare-event pathogens through time-gated luminescence scanning microscopy. Cytometry Part A 79, 349–355 (2011). [DOI] [PubMed] [Google Scholar]
- Sunna A., Chi F. & Bergquist P. L. A linker peptide with high affinity towards silica-containing materials. N. Biotechnol. 30, 485–492 (2013). [DOI] [PubMed] [Google Scholar]
- Sunna A., Chi F. & Bergquist P. L. Efficient capture of pathogens with a zeolite matrix. Parasitol. Res. 112, 2441–2452 (2013). [DOI] [PubMed] [Google Scholar]
- Lu Y. et al. Tunable lifetime multiplexing using luminescent nanocrystals. Nat. Photonics 8, 32–36 (2014). [Google Scholar]
- Care A., Chi F., Bergquist P. L. & Sunna A. Biofunctionalization of silica-coated magnetic particles mediated by a peptide. J. Nanopart. Res. 16, 2543 (2014). [Google Scholar]
- Care A., Nevalainen H., Bergquist P. L. & Sunna A. Effect of Trichoderma reesei proteinases on the affinity of an inorganic-binding peptide. Appl. Biochem. Biotechnol. 173, 2225–2240 (2014). [DOI] [PubMed] [Google Scholar]
- Sayyadi N., Connally R. E. & Try A. A novel biocompatible europium ligand for sensitive time-gated immunodetection. Chem. Commun. 52, 1154–1157 (2016). [DOI] [PubMed] [Google Scholar]
- Yuan J., Matsumoto K. & Kimura H. A new tetradentate β-diketonate-europium chelate that can be covalently bound to proteins for time-resolved fluoroimmunoassay. Anal. Chem. 70, 596–601 (1998). [DOI] [PubMed] [Google Scholar]
- Goward C. R., Irons L. I., Murphy J. P. & Atkinson T. The secondary structure of protein G′, a robust molecule. Biochem. J. 274, 503–507 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer-Eriksson A. E., Kleywegt G. J., Uhlén M. & Jones T. A. Crystal structure of the C2 fragment of streptococcal protein G in complex with the Fc domain of human IgG. Structure 3, 265–278 (1995). [DOI] [PubMed] [Google Scholar]
- Markela E., Ståhlberg T. H. & Hemmilä I. Europium-labelled recombinant protein G. A fast and sensitive universal immunoreagent for time-resolved immunofluorometry. J. Immunol. Methods 161, 1–6 (1993). [DOI] [PubMed] [Google Scholar]
- Ius A., Ferrara L., Meroni G. & Bacigalupo M. A. Immunoaffinity for IgG antibodies of protein A labelled with 4,7-bis(chlorosulfophenyl)-1,10-phenanthroline-2,9-dicarboxylic acid. Fresenius J. Anal. Chem. 336, 235–237 (1990). [Google Scholar]
- Ius A., Ferrara L., Meroni G. & Bacigalupo M. A. Eu-conjugated protein-A as a label of IgG antibodies. Fresenius Z. Analyt. Chem. 322, 509–510 (1985). [Google Scholar]
- Bacigalupo M. A., Iacobello C., Meroni G. & Ius A. Affinity of europium-labelled proteins A and G for immunoglobulin G from seven mammalian species. Eur. J. Clin. Chem. Clin. Biochem. 30, 529–530 (1992). [PubMed] [Google Scholar]
- Goward C. R., Murphy J. P., Atkinson T. & Barstow D. A. Expression and purification of a truncated recombinant streptococcal protein G. J. Biol. Chem. 267, 171–177 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerström B. & Björck L. A physicochemical study of protein G, a molecule with unique immunoglobulin G-binding properties. J. Biol. Chem. 261, 10240–10247 (1986). [PubMed] [Google Scholar]
- Sloan D. J. & Hellinga H. W. Dissection of the protein G B1 domain binding site for human IgG Fc fragment. Protein Sci. 8, 1643–1648 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ius A., Ferrara L., Meroni G. & Bacigalupo M. A. Evaluation of time-resolved fluoroimmunoassay with Eu-labelled protein-A for serum progesterone. J. Steroid Biochem. 33, 101–103 (1989). [DOI] [PubMed] [Google Scholar]
- Bacigalupo M. A., Ferrara L., Meroni G. & Ius A. Time-resolved fluoroimmunoassay of progesterone by Eu-labelled protein-A. Fresenius Z. Analyt. Chem. 328, 263–264 (1987). [Google Scholar]
- Connally R., Veal D. & Piper J. High resolution detection of fluorescently labeled microorganisms in environmental samples using time-resolved fluorescence microscopy. FEMS Microbiol. Ecol. 41, 239–245 (2002). [DOI] [PubMed] [Google Scholar]
- Hemminki A. et al. Introduction of lysine residues on the light chain constant domain improves the labelling properties of a recombinant Fab fragment. Protein Eng. 8, 185–191 (1995). [DOI] [PubMed] [Google Scholar]
- Scorilas A., Bjartell A., Lilja H., Moller C. & Diamandis E. P. Streptavidin-polyvinylamine conjugates labeled with a europium chelate: Applications in immunoassay, immunohistochemistry, and microarrays. Clin. Chem. 46, 1450–1455 (2000). [PubMed] [Google Scholar]
- Kyte J. & Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132 (1982). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.