Abstract
Objective Personal health applications have the potential to help patients with chronic disease by improving medication adherence, self-efficacy, and quality of life. The goal of this study was to assess the impact of MyMediHealth (MMH) – a website and a short messaging service (SMS)-based reminder system – on medication adherence and perceived self-efficacy in adolescents with asthma.
Methods We conducted a block-randomized controlled study in academic pediatric outpatient settings. There were 98 adolescents enrolled. Subjects who were randomized to use MMH were asked to create a medication schedule and receive SMS reminders at designated medication administration times for 3 weeks. Control subjects received action lists as a part of their usual care. Primary outcome measures included MMH usage patterns and self-reports of system usability, medication adherence, asthma control, self-efficacy, and quality of life.
Results Eighty-nine subjects completed the study, of whom 46 were randomized to the intervention arm. Compared to controls, we found improvements in self-reported medication adherence (P = .011), quality of life (P = .037), and self-efficacy (P = .016). Subjects reported high satisfaction with MMH; however, the level of system usage varied widely, with lower use among African American patients.
Conclusions MMH was associated with improved medication adherence, perceived quality of life, and self-efficacy.
Trial Registration This project was registered under http://clinicaltrials.gov/ identifier NCT01730235.
Keywords: adherence, biomedical informatics, adolescent, clinical trial, asthma, mHealth, mobile technology, personal health records
INTRODUCTION
Treatment of chronic illnesses is a major healthcare expense in the United States, accounting for 75% of the $2.7 trillion spent on health care.1 Poor adherence, defined as the extent to which behavior opposes medical advice,2 is a significant cause of this cost.3 It especially affects children and adolescents, who are challenged by routine completion of self-care tasks,4 and is known to affect health outcomes.5–7
Various projects have used technology to address adherence in pediatric chronic illness. For example, home Internet education and asynchronous video evaluation of inhaler use have demonstrated improved outcomes in young families,8,9 including those with asthma.10 Researchers also have examined applications that monitor asthma symptoms at home or school,8,11 enhance self-management skills, teach about asthma and its management,12–15 promote collaborative decision-making between providers and families,16 and support health behaviors, including medication adherence, all with encouraging results.17–20
Text messaging or short-message service (SMS) has been leveraged to address previous limitations in behavior change technologies and reduce barriers to medication adherence.17,20 Mobile phones provide near-ubiquitous access to patients; text messaging is a core feature of these devices. Because text messaging does not require a smart phone, it has good potential for use in studies serving low-income and underserved populations.21–23 Text messaging now plays an important role in translating behavior change theory and empirical findings into feasible patient support systems. Efficacious short communications have been scheduled to address forgetting in adult populations, a common barrier to medication adherence.24 Individually tailored communications also may address beliefs related to taking a medication, knowledge associated with outcomes, or problem-solving related to reducing side-effects of medications.17,24–27
The primary goal of this study was to examine the impact of a text-message-based reminder system on adherence rates of adolescents with asthma, a chronic disease affecting 12% of high school students28 with an overall prevalence of 9.5% in children.29 Patients with asthma are reported to have adherence rates as low as 40% for medications that prevent symptoms.30 Low adherence is strongly associated with unnecessary visits to emergency departments30 and contributes to preventable admissions annually.31
We hypothesized that compared with patients who did not receive text-message-based medication reminders, patients who received these reminders would have higher rates of reported medication administration adherence, a higher reported self-efficacy, and a higher rate of reported asthma control and disease-related quality of life.
METHODS
We compared medication adherence when using text-message reminders vs usual treatment, between April and November 2012. Our study utilized a personal health application called MyMediHealth (MMH).32 MMH is a system to help manage medications and dosing reminders.
Appendix 1 provides some screen snapshots of MMH. MMH is a web application designed to run on a tablet or desktop computer. It contains tools for patients to create and print a structured medication list, to attach a dosing schedule to each medication, to request a text-message reminder for each dose, and to visualize medication adherence performance for each medication in the system. It also provides features such as a “vacation” feature that uses prescription information (dose count) entered by the patient to determine if a refill will be needed before a particular date. To use MMH, a patient creates a secure, password-protected profile including a cell phone number. The system automatically sends a text message to the number requesting a verification response. Upon verification, the patient can log in and create a medication list and schedule. All medication names are coded using the RxNorm standard.33 Next, MMH generates dosing reminders based on the requested time of administration. Multiple medication reminders can be combined into one message. Users may reply to a reminder by typing a letter: (T)aking, (S)kipping, or (H)olding a dose one time. MMH also records natural language responses and user assertions about medication use.34 This approach allows a user to record the use of a listed medication that is not prescheduled (e.g., an as-needed bronchodilator.) When a dose is taken or skipped, MMH creates a note in a medication administration log. When a dose is held, MMH asks the user when s/he expects to take the drug (in hours from time of response) and automatically generates a reminder for that time. The hold duration is designed not to overlap with the time/day of the next scheduled dose.
MMH is a product of significant user-centered design as described elsewhere,32 consisting initially of focus groups that informed the feature set, followed by user testing of the scheduling application and web site. Before conducting this study, the final MMH web site and application was reviewed by eight families of adolescents who had asthma, who suggested refinements in the text messaging components (specifically, a method to combine notifications into one message, as well as the feature of “holding” a dose and repeating the notification at a specified interval). All features were tested by the project team before enrolling patients.
Study Design
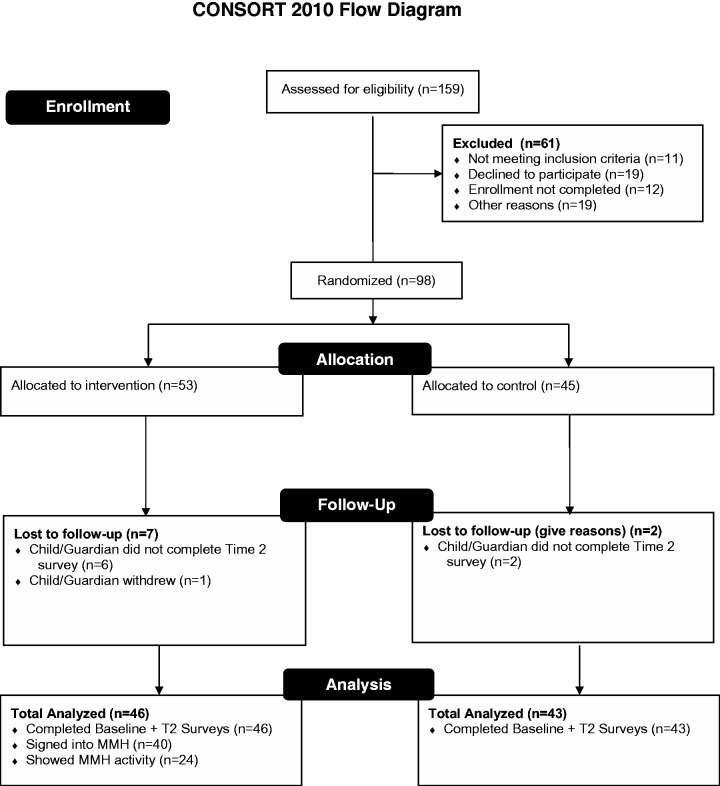
This randomized, controlled study took place in pediatric outpatient settings at an academic medical center. Figure 1 summarizes the study design. Participants were recruited with flyers, interest card boxes, an advertisement, and letters of invitation. Participants from a previous related study35 who had indicated interest in future studies also were contacted. A research assistant assessed all respondents for eligibility over the phone. Eligible participants were English speaking, aged 12–17 years, prescribed an asthma medication, able to access the Internet, and in possession of a cell phone with an SMS plan. Verbal consent was obtained from parents or guardians and assent was obtained from adolescents. The parent and adolescent then completed baseline online surveys. Completing the survey over the phone was also possible. Participation in the trial lasted for 3 weeks; a follow-up survey containing some of the same measures collected at baseline was completed at study’s end. Three weeks was the duration of participation estimated to allow an adolescent to interact with the scheduling system, determine if the timing or messaging was appropriate, modify the scheduling of reminders if needed, and then ascertain if there was benefit. Participants were block-randomized automatically36 to the intervention or control group after completion of the baseline survey, to ensure equivalent randomization during the entire recruitment period.
Figure 1:
MyMediHealth study eligibility and enrollment overview.
We followed a protocol for supporting participants that would be feasible to implement for population-scale availability of MMH. Participants in the intervention group were instructed to create a MMH account. Instructions were sent via email, which included a phone number for 24-h support, a demonstration video, and detailed directions for testing the text message reminder system. A research coordinator staffed a “help desk” and monitored participant activity for the first week, contacting inactive participants to troubleshoot and document problems. Participants who actively chose not to continue were dropped from the study. In addition, if a participant was not accessible after 1 week, the coordinator made approximately three attempts at contact, after which the participant was dropped. Intervention participants were responsible for any text message costs. Participants in the control group did not receive additional medication management support beyond usual care, but did receive online educational materials about asthma medication management. Parents were compensated at the rate of $20 at enrollment and at completion of the trial. Adolescents were compensated at the rate of $20 at enrollment and $40 at completion of the trial. Parents were instructed not to use the application on behalf of their child, although they were allowed to answer questions posed by their child about the use of the application.
Data Sources/Collection and Measures
Adolescents and parents completed questions about family demographics, medication regimen, asthma control test (ACT37 plus one question about compliance with their asthma controller in the last week). They were also asked about Perceptions of Asthma Medication using a 5-point scale ranging from 1 to 5, with 5 being the most negative (DePaola et al.38; Cronbach’s α 0.70 for both measures). These surveys items were administered to both child (C-PAM) and parent (A-PAM). In addition, at baseline, adolescents completed questions about asthma management self-efficacy using the Child Asthma Self-Efficacy Scale (5-point scale ranging from 1 = “Not at all sure” to 5 = “Completely sure”)39 with Cronbach’s α reliability at 0.87; quality of life (mini PAQLQ, 13 items, 7-point scale scored from least to highest quality,40 with Cronbach’s α 0.80). The Illness Management Survey was used to measure barriers to adherence and has a Cronbach’s α of 0.87 (Logan et al.41 five items on a scale of 1–5, with 5 being the most impairment) and completed items related to their use of mobile phones and the Internet.
At the end of the trial, we collected usage information, including activity on the MMH website (e.g., medications entered, number of reminders established) and text message reminders/ responses for the last 3 weeks of the trial. We excluded the first week’s data to allow for variation in participants’ time to set up the system. In addition, adolescents completed a usability survey.
All study methods were approved by the Vanderbilt University Institutional Review Board.
Data Analysis
The study analysis followed an intention-to-treat approach. Intervention and control characteristics collected at baseline were summarized with descriptive statistics (mean ± SD or frequency). We used a Wilcoxon test for continuous variables, Pearson’s chi-squared test for categorical variables, and the proportional odds model for ordinal variables between groups. We used the Wilcoxon test for significance to assess the change from the baseline survey to the follow-up survey results, and to assess MMH impact on asthma control, medication adherence asthma self-efficacy, and quality-of-life. MMH usage patterns were examined and categorized qualitatively by K.B.J. and Y.X.H. Differences in usage and responses to the usability scale items were examined and compared between subgroups in the intervention group using Wilcoxon and Pearson tests. Comments were reviewed and described qualitatively.
RESULTS
Figure 1 summarizes enrollment. A total of 98 adolescents and parents were randomized. Table 1 summarizes demographic and other information for control and intervention groups. There were no statistically significant differences between groups. Notably, both groups had similar scores of self-reported asthma control and medication adherence.
Table 1:
Summary of control and intervention group baseline characteristics
| N | Control (N = 43) | Intervention (N = 46) | P-value | |
|---|---|---|---|---|
| Age | 89 | 13.93 ± 1.54 | 14.17 ± 1.83 | 0.644a |
| Gender | 89 | |||
| Male | 53% (23) | 48% (22) | ||
| Female | 47% (20) | 52% (24) | ||
| Race | 89 | 0.162b | ||
| White | 47% (20) | 46% (21) | ||
| African American | 47% (20) | 50% (23) | ||
| Hispanic | 0% (0) | 4%(2) | ||
| Otherc | 7% (3) | 0% (0) | ||
| Family Income | 74 | 0.389b | ||
| <$20 000 | 33% (11) | 37% (15) | ||
| $20 001–$40 000 | 21% (7) | 32% (13) | ||
| $40 001–$70 000 | 24% (8) | 17% (7) | ||
| >$70 000 | 21% (7) | 15% (6) | ||
| Parent/Guardian Education | 89 | 0.422b | ||
| Some high school | 33% (14) | 48% (22) | ||
| High school degree | 9% (4) | 11% (5) | ||
| Some college, no degree | 28% (12) | 24% (11) | ||
| College degree | 21% (9) | 15% (7) | ||
| Graduate degree | 9% (4) | 2% (1) | ||
| Adolescent needs to earn cell phone | 89 | 0.083b | ||
| Yes | 19% (8) | 7% (3) | ||
| No | 81% (35) | 93% (43) | ||
| Type of asthma inhaler | 89 | 0.250b | ||
| Rescue | 33% (14) | 22% (10) | ||
| Rescue + Control | 67% (29) | 78% (36) | ||
| Asthma control Test | 89 | 19.37 ± 3.75 | 19.13 ± 3.96 | 0.951a |
| Adherence last 7 days | 65 | 5.17 ± 2.22 | 4.25 ± 2.06 | 0.058a |
| Self-efficacy | 89 | 4.31 ± 0.45 | 4.04 ± 0.66 | 0.089a |
| Perceptions about Medication | 89 | 2.13 ± 0.51 | 2.30 ± 0.59 | 0.161a |
| Quality of Life | 89 | 5.90 ± 090 | 5.36 ± 1.36 | 0.107a |
| Illness management | 89 | 2.37 ± 1.01 | 2.66 ± 0.91 | 0.200a |
x ± s: Mean ± 1 SD
N: number of patients answering each question. Medication adherence was assessed only for patients who self-identified as taking a controller (daily) medication.
aWilcoxon test; bPearson test
cOther include: Native American, Asian, and Pacific Islander
Overall Use of MyMediHealth
Fifty-two patients were randomized to the MMH user group (53 allocated to intervention with one withdrawal). We included 46 MMH users and parents who completed follow-up in our analysis. Of this group, 6 (13%) never signed into MMH. One adolescent’s mobile carrier (T-Mobile) did not support our text messaging service. Fifteen (38%) MMH users did not enter any medications, 11 could not be contacted, 3 (parents) stated that they were too busy to use the system, and 1 (adolescent) was unable to set up a reminder successfully. Six (15%) patients who entered medications did not create reminders. Two of these did not believe they needed the reminders to help them take medications, while the other four did not provide an explanation.
MMH users logged into the site an average of 2.5 times (range: 1–6) over the study period. Twenty MMH users added controller medications to their medication lists, 18 added rescue inhalers, and 8 added allergy medications. MMH users added a median of 2 (SD = 1.4) medications to their medication lists (range: 0–5).
A total of 24/46 MMH users participated in at least one text message exchange. Participating users were similar to users who did not log into MMH with regard to age, gender, family income, education, cell phone plan, child’s need to earn cell phone, or inhaler type. However, 77% (17) of MMH users who did not log in were African American, compared to 25% (6) of African Americans who actively used the system (P = .001). Over the trial period, three usage patterns emerged. One group of eight users had no problems receiving or responding to alerts. A second group of three had challenges that resolved over the first week. A third group of eight users had persistent problems responding to alerts, but no challenges receiving them. Five users managed as-needed medications only and had no problems doing so.
Of 21 (46%) MMH users who set up medication reminders, 17 successfully adopted this feature. Participants received an average of 12 initial reminders (with subsequent SMS dialog as shown in the Appendix) during the 2-week trial period. Based on responses to medication reminders accepted by the system, users took their daily medications an average of 10 times over 2 weeks.
MMH was set up by 18 (39%) patients to support rescue medication use. Five users attempted to log their use of a rescue inhaler during the study period, and all succeeded.
Impact on Asthma Management
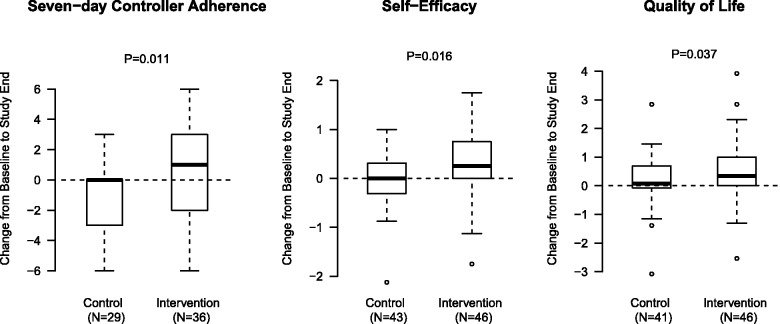
Table 2 provides a summary of the trial results. Compared with control patients, intervention patients had a significant improvement in self-reported 7-day adherence (Figure 2), with an average gain of 1 day of adherence, and a median change from 4 to 6 days, compared with no median change in the control group (P = .011; median data not shown). These numbers were consistent with observed behaviors documented through MMH for the intervention group. Parent and adolescent responses showed good agreement with measures of adherence (Cohen’s κ = 0.52) and a positive correlation for ACT change (ρ = 0.69.)
Table 2:
Summary of MyMediHealth use (Intervention) impact
| Measure | Control | Intervention | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | T1 | T2 | Change | N | T1 | T2 | Change | P | |
| Asthma Control Test | 43 | 19.37 | 21.12 | 1.74 | 46 | 19.13 | 20.78 | 1.65 | 0.728 |
| Adherence in last 7 Days – Adolescent on Controller | 29 | 5.17 | 3.83 | −1.345 | 36 | 4.25 | 4.86 | 0.611 | 0.011 |
| Self Efficacy | 43 | 4.305 | 4.276 | 0.0291 | 46 | 4.038 | 4.321 | 0.2826 | 0.016 |
| Quality of Life | 43 | 5.902 | 6.053 | 0.0957 | 44 | 5.355 | 5.885 | 0.5301 | 0.037 |
Figure 2:
Control versus Intervention group perceptions of medication adherence, self-efficacy, and quality of life.
Adolescent perceptions of self-efficacy rose from a median of 4.1–4.4 (between “quite sure” and “completely sure” of self-efficacy in asthma management; P = .016). Quality of life increased from a median of 5.7–6.3 (on a 7 point scale) in the intervention group, compared with the control group (P = .037). Patients who never received reminders from MMH had unchanged measures of change in 7-day self-reported adherence and perception of self-efficacy.
Usability of the MyMediHealth website
Attitudes towards the system ranged from neutral to positive, depending on the aspect of the system. Table 3 shows that usability scores were the same among active and less active users, with the exception of frequent users being less able to tell when they used the website incorrectly (P = .048). One nonuser said that MMH “didn’t work on my phone.” Two subjects found the medication selection process confusing. A third user had problems responding to reminders for medications scheduled at the same time. After several attempts, she gave up on simultaneously scheduling the medications. However, the subject was still able to receive and respond to reminder messages for medications that were scheduled individually. A total of 78% (18) of users and 86% (18) of nonusers expressed interest in continuing to use MMH.
Table 3:
MyMediHealth web site usability assessment
| Active Users (N = 23) | Non-Users (N = 21) | P-value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| I felt comfortable interacting with the MyMediHealth website | 81.2 (24.7) | 79.2 (22.9) | 0.801 |
| MyMediHealth is easy to learn. | 83.7 (20.9) | 81.5 (23.2) | 0.900 |
| I was able to create my medication schedule easily. | 72.2 (32.0) | 71.9 (29.5) | 0.795 |
| I found the medication summary report to be useful. | 73.7 (26.8) | 78.0 (19.9) | 0.704 |
| When using the MyMediHealth website, I was able to tell when I made an error or mistake. | 56.7 (30.1) | 72.7 (25.3) | 0.048 |
| The website effectively alerts me to any potential errors or problems. | 67.5 (27.6) | 68.1 (29.2) | 0.768 |
| If I noticed an error or was alerted by the website that there was an error or problem, I was able to make the changes needed to fix the problem. | 56.0 (36.4) | 69.1 (30.8) | 0.194 |
| MyMediHealth allows me to do what I need to do with this website. | 75.4 (29.0) | 73.7 (24.6) | 0.515 |
| MyMediHealth provides me with all of the information I need in order to use the website effectively. | 73.0 (29.2) | 79.6 (24.1) | 0.391 |
| MyMediHealth is easy to start up (e.g., setting up my medication schedule) and begin using. | 76.3 (31.3) | 75.0 (28.2) | 0.711 |
| MyMediHealth allows me to work quickly when I am busy or do not have much time. | 72.3 (27.6) | 74.8 (26.0) | 0.867 |
| The website has a pleasing and appropriate appearance. | 85.5 (20.2) | 82.9 (24.2) | 0.803 |
All values reported on a 0–100 scale from strongly disagree to strongly agree.
P-values are based on Wilcoxon test.
DISCUSSION
Different studies have addressed the potential benefits of mobile phone applications for patients with chronic illness.17,42 Though this study is small, it is one of the first to demonstrate even a short-term impact on pediatric medication adherence and perceptions of self-efficacy in a randomized, controlled fashion. In particular, this study is the first to involve adolescents, and the first to report changes related to perceived quality of life and self-efficacy – both of which are more predictive of larger downstream impact and behavior change.
The use of SMS-based reminders for medication management holds promise, given the pervasive nature of mobile phones and computer literacy among young adults and children.43 Recent studies44 provide theoretical support for this technology as a behavior-changing mechanism, and are good starting points for identifying patient beliefs and barriers to self-care Mulvaney, 2012 #4563}.25,45 Still, few randomized trials have focused on pediatric-aged patients with asthma.46,47 Pediatric intervention research in this area is in early stages but promising larger randomized studies have been initiated.
As with any effectiveness study, there were a number of results that should inform other attempts to manage medication adherence using SMS reminders. First, despite performing task analysis with adolescents early in the project32 and pilot testing this intervention before conducting this study, and iteratively improving the design based on these results, a few patients struggled to identify the right medication from the graphical depiction of inhalers or the search tool. We believe that searching will always be a challenge, and that multiple methods will need to be considered and possibly implemented based on literacy, numeracy, and language/cultural differences. Second, a few adolescents had difficulty setting up and addressing reminders. We struggled with the optimal way to present a reminder for multiple medications scheduled for administration at the same time. Additional work is clearly needed to accomplish the goal of engaging a person in dialog related to sets of medications (e.g., “Hold this but not that” or “I took this but not that.”)
Our findings need to be considered in light of the study’s limitations. First, as mentioned above, usability problems were identified during the usability assessment. It is likely that other users had initial challenges using MMH that might be easily remedied with more testing and feedback.
Second, although we used an intention-to-treat approach, a subset of participants in the intervention arm never used MMH. This subset was disproportionately African American, with no other distinguishing characteristic (other than a slight increase in the need for adolescents to earn the right to use their cell phones.) Although text messaging is considered a useful technology to address healthcare disparities, the integration of the web-based component may have limited access for some families. Other studies48 have reported similar issues; this is an area in which further research is indicated.
Pilot testing of earlier MMH versions disclosed that like many new technologies, there is a period when text messaging was eagerly accepted, followed by a decrease in the willingness of some users to interact with it, even when there is benefit. This drop-off has been well-described by Thaler and Sunstein49 and others, and, in fact, it is a core motivation for the field of behavioral economics. Studies suggest that the choice of sustaining a beneficial intervention requires additional motivation beyond the scope of this study.49,50 We elected to stop data collection at 3 weeks to avoid the drop-off of use unrelated to its value. This short duration study demonstrated that a subpopulation exists who derives value from text messaging. A larger, longitudinal study should be conducted to better understand how to sustain use over long periods of time in subjects who are seen to derive value from an intervention. Coaching, the provision of rewards for sustaining, or penalties for quitting are common motivators that should be included in longitudinal studies addressing longer-term outcomes.51
Adherence measurement is notoriously difficult to accomplish. Mechanisms such as direct observation, medication possession ratio, and even technology to measure doses augments self-report and improves its reliability. In our own work we have shown that interactive response systems provide a similar level of reliability. In our pilot study,35 we attempted a number of methods for data collection, but none proved as reliable as Asthma Control Test (ACT) or using mobile phones. For that reason, this study relied on only one measure of adherence. However, the control group could have reported an improvement in adherence with standard therapy and education, but it did not. This, in addition to the improved assessment of quality of life and self-efficacy, added some strength to the association of MMH use with the self-reported measure of adherence.
In addition to these methodological or design limitations, it is important to recall that participants were required to have a cell phone with SMS, as well as Internet access in some form. While access to this sort of technology is common, it is not universal. Additionally, some patients have access to the technology but are not facile with its use. Although we provided video tutorials, we did not determine if they were sufficient.
Our earliest prototypes for MMH included features not studied here. For example, we did not include tools for medication reconciliation, links to MEDLINE Plus, or intelligent scheduling interfaces (ensuring that medication timing would not affect bioavailability or increase the risk of adverse events). Given the results here, we believe that a more comprehensive mobile system will be viewed favorably by some patients. However, our results also suggest that a more complex interface will require support from the healthcare system.
Behavior change is a critical subject in health care. We designed the initial version of MMH to address a specific population of patients whose problem with adherence is forgetting to take a routine medication. However, adherence behavior is associated with other barriers, as specified by the Information-motivation and behavioral skills model,52 among others. MMH users might benefit from information about their medications and the importance of dosing regimens, how to schedule medications, and about side effects. They also may benefit from tools or system designs that increase motivation, self-efficacy, and social support, and skill training. We clearly have only begun to explore many of these determinants of consistent health behavior performance with this version of MMH.
CONCLUSION
The MMH intervention was associated with improvement in self-reported controller medication adherence, quality of life, and self-efficacy. Interestingly, we also found a significant racial disparity in the rate of MMH adoption. Our results suggest that a text message medication reminder system such as MMH can potentially promote better asthma management in the adolescent population, though further research is needed to identify and address barriers to adoption.
FUNDING
This project was supported by the Agency for Healthcare Research and Quality: R18 HS018168-03, and by CTSA award No. UL1TR000445 from the NIH National Center for Advancing Translational Sciences.
FINANCIAL DISCLOSURE
The authors have no financial relationships relevant to this article to disclose.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Clinical Trial registry name and number: clinicaltrials.gov: NCT01730235
WHAT IS KNOWN ON THIS SUBJECT?
Text messaging reminders have been proposed as a method to improve medication adherence in a number of patient groups. However, there have been no published randomized trials of this technology in children/adolescents.
CONTRIBUTORS
K.B.J., MD, MS conceived of the project and participated in the design of this trial. He wrote the initial draft of this manuscript.
B.L.P., MD, MD provided clinical insight related to treating asthma, and represented the team at clinic meetings. He reviewed and helped to revise this version of the manuscript
Y.X.H., PhD was the lead analyst of the data collected. She wrote portions of the manuscript related to study results and statistics.
Q.C., PhD was the lead statistician. She designed and provided input to the statistical analysis section of the manuscript.
H.N., MS completed statistical analysis. She provided input to the statistical analysis section of the manuscript, and developed graphics for the manuscript.
C.L.D. was the project coordinator and worked with the development team. He drafted portions of the manuscript related to study design and methods.
J.S., PhD lead the teams effort on usability. He provided input for the manuscript related to usability and user interface.
S.A.M., PhD lead the teams effort related to behavior tracking, survey development, and analysis. She provided input in all sections of the manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material is available online at http://jamia.oxfordjournals.org/.
REFERENCES
- 1.Centers for Disease Control and Prevention. The Power of Prevention. Chronic Disease…the publich health challenge of the 21st century. 2009. http://www.cdc.gov/chronicdisease/pdf/2009-Power-of-Prevention.pdf, Accessed October 14, 2015.
- 2.Modi AC, Pai AL, Hommel KA, et al. Pediatric self-management: a framework for research, practice, and policy. Pediatrics. 2012;129(2):e473–e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization, Sabaté E. Adherence to Long-term Therapies: Evidence for Action. Geneva: World Health Organization; 2003. [Google Scholar]
- 4.Walters BH, Adams SA, Nieboer AP, Bal R. Disease management projects and the Chronic Care Model in action: baseline qualitative research. BMC Health Serv Res. 2012;12:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hood KK, Peterson CM, Rohan JM, Drotar D. Association between adherence and glycemic control in pediatric type 1 diabetes: a meta-analysis. Pediatrics. 2009;124(6):e1171–e1179. [DOI] [PubMed] [Google Scholar]
- 6.Drotar D, Bonner MS. Influences on adherence to pediatric asthma treatment: a review of correlates and predictors. J Dev Behav Pediatr. 2009;30(6):574–582. [DOI] [PubMed] [Google Scholar]
- 7.Aspden P, Institute of Medicine (U.S.). Committee on Identifying and Preventing Medication Errors. Preventing Medication Errors. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 8.Chan DS, Callahan CW, Sheets SJ, Moreno CN, Malone FJ. An internet-based store and forward video home telehealth system for improving asthma outcomes in children. Am J Health Sys Pharm. 2003;60:1976–1981. [DOI] [PubMed] [Google Scholar]
- 9.Wise M, Gustafson DH, Sorkness CA, et al. Internet telehealth for pediatric asthma case management: integrating computerized and case manager features for tailoring a web-based asthma education program. Health Promot Pract. 2007;8(3):282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mangione-Smith R, Schonlau M, Chan KS, et al. Measuring the effectiveness of a collaborative for quality improvement in pediatric asthma care: does implementing the chronic care model improve processes and outcomes of care? Ambul Pediatr. 5(2):75–82. [DOI] [PubMed] [Google Scholar]
- 11.McClure L, Harrington K, Graham H, Gerald L. Internet-based monitoring of asthma syjptoms, peak flow meter readings, and absence data in a school-based clinical trial. Clin Trials. 2008;5(1):31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shegog R, Bartholomew LK, Parcel GS, Sockrider MM, Mâsse L, Abramson SL. Impact of a computer-assisted education program on factors related to asthma self-management behavior. JAMIA. 2001;8(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartholomew LK, Shegog R, Parcel GS, et al. Watch, Discover, Think, and Act: a model for patient education program development. Patient Educ Couns. 2000;39(2-3):253–268. [DOI] [PubMed] [Google Scholar]
- 14.Homer C, Susskind O, Alpert HR, et al. An Evaluation of an innovative multimedia educational software program for asthma management: report of a randomized, controlled trial. Pediatrics. 2000;106(1):210–215. [PubMed] [Google Scholar]
- 15.Krishna S, Francisco BD, Balas EA, Konig P, Graff GR, Madsen RW. Internet-enabled interactive multimedia asthma education program: a randomized trial. Pediatrics. 2003;111(3):503–510. [DOI] [PubMed] [Google Scholar]
- 16.Porter SC, Cai Z, Gribbons W, Goldmann DA, Kohane IS. The Asthma Kiosk: A Patient-centered Technology for Collaborative Decision Support in the Emergency Department. JAMIA. 2004;11(6):458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulvaney SA, Anders S, Smith AK, Pittel EJ, Johnson KB. A pilot test of a tailored mobile and web-based diabetes messaging system for adolescents. J Telemed Telecare. 2012;18(2):115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linn AJ, Vervloet M, van Dijk L, Smit EG, Van Weert JC. Effects of eHealth interventions on medication adherence: a systematic review of the literature. J Med Internet Res. 2011;13(4):e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingerski LM, Hente EA, Modi AC, Hommel KA. Electronic measurement of medication adherence in pediatric chronic illness: a review of measures. J Pediatr. 2011;159(4):528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Britto MT, Munafo JK, Schoettker PJ, Vockell AL, Wimberg JA, Yi MS. Pilot and feasibility test of adolescent-controlled text messaging reminders. Clin Pediatr. 2012;51(2):114–121. [DOI] [PubMed] [Google Scholar]
- 21.Deglise C, Suggs LS, Odermatt P. SMS for disease control in developing countries: a systematic review of mobile health applications. J Telemed Telecare. 2012;18(5):273–281. [DOI] [PubMed] [Google Scholar]
- 22.Deglise C, Suggs LS, Odermatt P. Short message service (SMS) applications for disease prevention in developing countries. J Med Internet Res. 2012;14(1):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA. 2012;307(16):1702–1708. [DOI] [PubMed] [Google Scholar]
- 24.Vervloet M, Linn AJ, van Weert JC, de Bakker DH, Bouvy ML, van Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. JAMIA. 2012;19(5):696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osborn CY, Mulvaney SA. Development and feasibility of a text messaging and interactive voice response intervention for low-income, diverse adults with type 2 diabetes mellitus. J Diabetes Sci Technol. 2013;7(3):612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrie KJ, Perry K, Broadbent E, Weinman J. A text message programme designed to modify patients' illness and treatment beliefs improves self-reported adherence to asthma preventer medication. Brit J Health Psychol. 2012;17(1):74–84. [DOI] [PubMed] [Google Scholar]
- 27.Johnson KB, Ravert RD, Everton A. Hopkins teen central: assessment of an internet-based support system for children with cystic fibrosis. Pediatrics. 2001;107(2):E24. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC). 1991-2013 High School Youth Risk Behavior Survey Data. Available at http://nccd.cdc.gov/youthonline/. Accessed on October 13, 2015
- 29.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012(94):1–8. [PubMed] [Google Scholar]
- 30.Bender B, Zhang L. Negative affect, medication adherence, and asthma control in children. J Allergy ClinI Immunol. 2008;122(3):490–495. [DOI] [PubMed] [Google Scholar]
- 31.Flores G, Abreu M, Tomany-Korman S, Meurer J. Keeping children with asthma out of hospitals: parents' and physicians' perspectives on how pediatric asthma hospitalizations can be prevented. Pediatrics. 2005;116(4):957–965. [DOI] [PubMed] [Google Scholar]
- 32.Slagle JM, Gordon JS, Harris CE, et al. MyMediHealth - designing a next generation system for child-centered medication management. J Biomed Informatics. 2010;43(5 Suppl):S27–S31. [DOI] [PubMed] [Google Scholar]
- 33.Parrish F, Do N, Bouhaddou O, Warnekar P. Implementation of RxNorm as a terminology mediation standard for exchanging pharmacy medication between federal agencies. AMIA Annu Symp Proc. 2006:1057. [PMC free article] [PubMed] [Google Scholar]
- 34.Stenner SP, Johnson KB, Denny JC. PASTE: patient-centered SMS text tagging in a medication management system. JAMIA. 2012;19(3):368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulvaney SA, Ho YX, Cala CM, et al. Assessing adolescent asthma symptoms and adherence using mobile phones. J Med Internet Res. 2013;15(7):e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Informatics. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clinical Immunol. 2004;113(1):59–65. [DOI] [PubMed] [Google Scholar]
- 38.DePaola LM, Roberts MC, Blaiss MS, Frick PJ, FMcNeal RE. Mothers' and Children's Perceptions of Asthma Medicine. Children's Health Care. 1997;26(4):265–283. [Google Scholar]
- 39.Bursch B, Schwankovsky L, Gilbert J, Zeiger R. Construction and validation of four childhood asthma self-management scales: parent barriers, child and parent self-efficacy, and parent belief in treatment efficacy. J Asthma. 1999;36(1):115–128. [DOI] [PubMed] [Google Scholar]
- 40.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14(1):32–38. [DOI] [PubMed] [Google Scholar]
- 41.Logan D, Zelikovsky N, Labay L, Spergel J. The Illness Management Survey: identifying adolescents' perceptions of barriers to adherence. J Pediatric Psychol. 2003;28(6):383–392. [DOI] [PubMed] [Google Scholar]
- 42.Faridi Z, Liberti L, Shuval K, Northrup V, Ali A, Katz DL. Evaluating the impact of mobile telephone technology on type 2 diabetic patients' self-management: the NICHE pilot study. J Eval Clin Pract. 2008;14(3):465–469. [DOI] [PubMed] [Google Scholar]
- 43.Madden M. Teens and Technology. Washington, DC: Pew Research Center's; 2013. [Google Scholar]
- 44.Sharifi M, Dryden EM, Horan CM, et al. Leveraging text messaging and mobile technology to support pediatric obesity-related behavior change: a qualitative study using parent focus groups and interviews. J Med Internet Res. 2013;15(12):e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foster JM, Smith L, Bosnic-Anticevich SZ, et al. Identifying patient-specific beliefs and behaviours for conversations about adherence in asthma. Int Med J. 2012;42(6):e136–e144. [DOI] [PubMed] [Google Scholar]
- 46.de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, Car J, Atun R. Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev. 2012;12:CD007459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lv Y, Zhao H, Liang Z, et al. A mobile phone short message service improves perceived control of asthma: a randomized controlled trial. Telemed J E Health. 2012;18(6):420–426. [DOI] [PubMed] [Google Scholar]
- 48.Du X, Wang W, Helena van Velthoven M, et al. mHealth Series: Text messaging data collection of infant and young child feeding practice in rural China - a feasibility study. J Global Health. 2013;3(2):20403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Rev. and expanded ed. New York: Penguin Books; 2009. [Google Scholar]
- 50.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiauzzi E, Rodarte C, DasMahapatra P. Patient-centered activity monitoring in the self-management of chronic health conditions. BMC Med. 2015;13:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25(4):462–473. [DOI] [PubMed] [Google Scholar]