Abstract
Although mobile health (mHealth) devices offer a unique opportunity to capture patient health data remotely, it is unclear whether patients will consistently use multiple devices simultaneously and/or if chronic disease affects adherence. Three healthy and three chronically ill participants were recruited to provide data on 11 health indicators via four devices and a diet app. The healthy participants averaged overall weekly use of 76%, compared to 16% for those with chronic illnesses. Device adherence declined across all participants during the study. Patients with chronic illnesses, with arguably the most to benefit from advanced (or increased) monitoring, may be less likely to adopt and use these devices compared to healthy individuals. Results suggest device fatigue may be a significant problem. Use of mobile technologies may have the potential to transform care delivery across populations and within individuals over time. However, devices may need to be tailored to meet the specific patient needs.
Keywords: mHealth, health apps, data collection, health promotion, self-monitoring, mobile health, informatics
INTRODUCTION
Advances in information technology are enabling the development and delivery of affordable health interventions beyond the traditional office visit and across populations. Over one billion users have mobile broadband and connect with mobile application marketplaces, and an estimated 75% of the world population has access to mobile communications.1,2 Healthcare providers have an opportunity to use these interactive capabilities to connect with patients and enable personalized health interventions in real-time. However, maximizing interactive capabilities will require both patient engagement with mobile health devices and meaningful communication of their health-related information to physicians and medical record systems. The first step in developing this new area of health care, known as mobile health or “mHealth,” is to identify devices that patients can effectively, comfortably, and confidently integrate into their daily routines. The second step is to create an infrastructure and interface for data transfer to occur.
Mobile health devices include smartphones, wearable activity trackers, wireless connected scales, blood pressure cuffs, pulse oximeters, and glucometers. Despite the excitement about the potential for these devices to improve health, their successful adoption by consumers and patients for routine self-monitoring remains uncertain.
Access to mobile platforms has the potential to aid in modifying self-care behaviors related to diet, exercise, weight management, and lifestyle choices such as not smoking, which have been shown to prevent nearly 80% of chronic diseases.12 For patients with chronic illnesses, mobile platforms could allow personalized coaching and goal monitoring by care managers in real-time, as well as active surveillance of those patients at highest risk for hospital admission. Mobile health platforms may also promote a greater individual sense of empowerment over managing disease. For example, patients with congestive heart failure could benefit from in home wireless scales that enable monitoring of daily fluid retention.3 Patients with diabetes, performing up to 95% of their own diabetes management at home and spending only a few hours a year in clinic visits, could be monitored for fluctuations in blood sugar.4–6 Evidence remains mixed for the efficacy of home-health monitoring of most chronic diseases, although general consensus suggests self-monitoring improves outcomes for diabetes, hypertension, and weight loss.7–9
To leverage mobile health as a tool to promote chronic disease management, further study is required of adherence to use of these devices over time and the feasibility to collect, display, and secure data in a unified system. Furthermore, mobile health practices must demonstrate flexibility to respond to regulatory hurdles in an environment where rules and regulations are quickly changing. The dual purpose of this pilot feasibility study was to develop a technological infrastructure to collect and analyze mobile health data from multiple devices available to the public and to examine the consistency over time with which healthy and chronically ill patients used applications (apps) on those devices to monitor their personal health data.
METHODS
Study design and recruitment
Six adult patients in an academic primary care setting were recruited by their primary care providers to participate in testing the feasibility of capturing multiple types of mobile health data from four devices over 4 weeks. Selection criteria included an equal number of healthy and chronically ill (obesity, diabetes, hyperlipidemia, and/or hypertension) participants, availability of both a smartphone with a data and text-messaging plan and Wi-Fi at home, and ability to read and speak English.
To comply with US Food and Drug Administration guidelines on mobile medical applications and both institutional review board and information security requirements, the informed consent process was designed to ensure that potential participants were aware that their health data would be collected by third party companies over which the medical center had no control. For example, data from a wrist-worn accelerometer, such as Fitbit®, would be transferred to that company first, before residing in the research database. All participants provided written informed consent to participate and were provided with four mobile health devices from two companies. Participants were compensated with $50 or the option to keep one device.
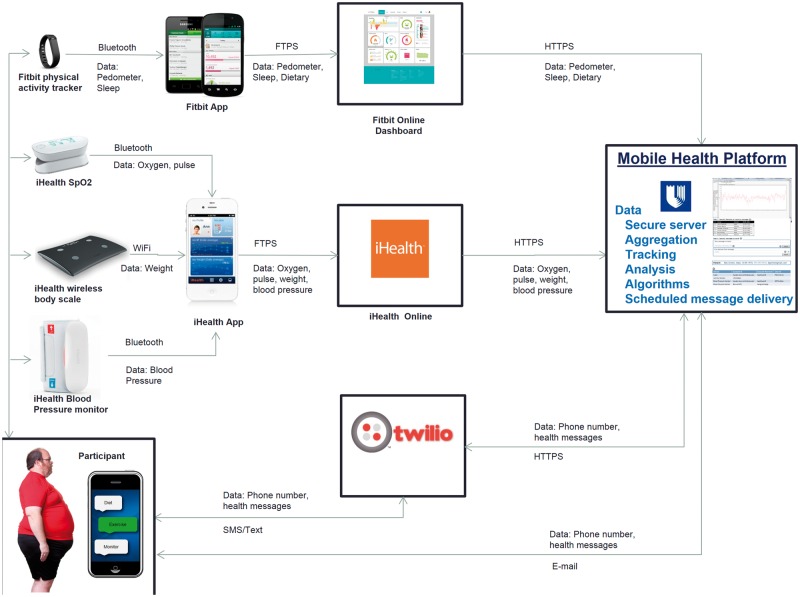
Device data were collected using a researcher-developed web-based software platform that assembled data from all participants and their devices into a single aggregate secure database. The platform connected to the third party devices’ respective application program interfaces (Figure 1).
Figure 1:
Assembled data from all participants and their devices into a single aggregate secure database.
Measures
The study used four devices, one diet app, and two data transmission apps from Fitbit® and iHealth® that are widely available for consumers and permit data extraction from the participants’ devices. Participants were requested to track 11 daily health indicators over 4 weeks: distance (miles and step count), physical activity intensity (minutes both sedentary and active) and quantity of sleep (minutes) via a wrist-worn accelerometer (Fitbit®); blood pressure via a Bluetooth-enabled monitor (iHealth®); pulse and peripheral capillary oxygen saturation (SpO2) via a wireless oximeter (iHealth®); weight and body mass index (with self-reported height) via a wireless analysis scale (iHealth®); and fluid intake via a dietary app (Fitbit®). A research coordinator trained participants to use the devices and install required apps (see Appendix). Regular charging varied from every 5–7 days for the Fitbit® to once every few weeks for the pulse oximeter and blood pressure cuff.
Tracking varied by device. The Fitbit®, which participants wore and synced via Bluetooth to their phone, permitted passive tracking of distance and intensity but required manual activation of sleep monitoring on the associated Fitbit® app. The Fitbit® app, which was also a diet app, required manual entry of fluid intake into participants’ phones. Among iHealth® devices, the pulse oximeter, blood pressure cuff and wireless scale required use once daily, and synced via Bluetooth or Wi-Fi to two apps on their phone (see Appendix).
Following data collection, participants returned the devices and completed an interview to discuss how effective they felt the devices had been for managing health, experiences with using multiple devices, and the positives and negatives of study participation. Interviews were recorded.
Data analysis
Descriptive statistics summarized baseline demographic (age, gender) and clinical (height, chronic illnesses) characteristics. Weekly averages of 11 health indicators were calculated over the 4 weeks and by chronic disease status (Table 1). Adherence was operationalized as the actual number of days out of seven that data were collected on or transmitted via each device or app. Data received on at least 4 of 7 days implied adherence (Table 2). Overall adherence was calculated as the summed adherence to each device across all weeks divided by six participants. Adherence among healthy compared to chronically ill participants was calculated the same way but divided by three participants each. Due to differences in behavior tracking that may occur by time of the week,10 we also explored calculated adherence during 5 days of a typical work week vs 2-day weekends. Interview data were analyzed using conventional content analysis, a data reduction technique to identify recurring themes.
Table 1:
Weekly averages and changes by 11 health indicators by patient
| Averages (total per 7 days divided by 7) (Standard deviation per 7 days)a | Weekly change | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy 1 | Distance (miles; SD) | Steps (SD) | Minutes sedentary (SD) | Minutes very active (SD) | BP (SD) | Pulse (SD) | SpO2 (SD) | Minutes sleeping (SD) | Diet: water (Oz; SD) | Weight (lbs) | BMI (%) |
| Week 1 | 5.1 (1.5) | 10 571 (3006) | 1057 (190) | 19 (15) | 122/84 (7/3) | 76 (7.4) | – | – | 36.6 (10.2) | −1 | 0 |
| 2 | 5.4 (1.5) | 11 333 (3014) | 1004 (237) | 19.4 (21.9) | 122/81 (7.6/4) | 77 (13.5) | – | 498 | 38 (12.1) | −1 | 0 |
| 3 | 4.1 (2.0) | 8564 (4236) | 1140 (134) | 7.6 (10.7) | 123/83 (8/4) | 74 (6.5) | – | – | 23.4 (24.9) | +2 | 0 |
| 4 | 5.8 (0.9) | 12 077 (1514) | 1018 (194) | 25.9 (24.1) | 117/81 (4/5) | 80 (8.9) | – | – | 32 (20.4) | −2 | 0 |
| Healthy 2 | |||||||||||
| 1 | 4.9 (1.1) | 11 407 (2446) | 692 (227) | 47 (30.6) | 111/76 (7/6) | 66 (5.1) | 99 (0.53) | 339 (62.0) | 62 (16.2) | 0 | 0 |
| 2 | 4.9 (0.94) | 11 470 (2179) | 681 (174) | 52 (41.8) | 112/72 (5/4) | 63 (3.9) | 99 (0.52) | 347 (48.4) | 41 (18.1) | −1 | 0 |
| 3 | 4.7 (0.82) | 10 842 (1922) | 718 (156) | 52 (39.5) | 108/71 (6/6.6) | 61 (3.1) | 98 (0.49) | 330 (31.6) | 26 (16.1) | +1 | 0 |
| 4 | 5.3 (0.97) | 12 219 (2252) | 700 (214) | 41 (48.5) | 111/74 (2.6/4) | 62 (6.8) | 99 (0.7) | 339 (79.9) | 25 (17.3) | −1 | 0 |
| Healthy 3 | |||||||||||
| 1 | 3.2 (1.2) | 6974 (2535) | 658 (115) | 5 (3.3) | 127/81 (7/5) | 63 (8.9) | 95 (6.3) | 415 (88.0) | 63 (21.5) | −4 | −1 |
| 2 | 3 (0.92) | 6531 (2003) | 805 (239) | 4 (4.6) | 124/78 (4/3) | 62 (9.9) | 94 (8.96) | 376 (161.5) | 57 (30.9) | 0 | 0 |
| 3 | 3.2 (0.91) | 6897 (1992) | 647 (98) | 2 (0.76) | 127/78 (3.4/3 | 67 (6.2) | 96 (0.9) | 426 (103.0) | 48 (32.8) | +1 | 0 |
| 4 | 3.6 (1.3) | 7898 (2867) | 598 (208) | 10 (21) | 129/80 (6.6/2.5) | 68 (5.7) | 96 (0.71) | 431 (98.8) | 41 (35) | 0 | 0 |
| Co-morbid 1 | |||||||||||
| 1 | 3.6 (1.14) | 8886 (3071) | 1039 (177) | 15 (13.5) | 118/75 (11/5.5) | 88 (5.8) | 94 | 373 (21.2) | 10 (21) | 0 | 0 |
| 2 | 1.8 (1.84) | 4499 (4725) | 1068 (293) | 4 (10.6) | 125/81 (8/3.5) | 92 (1.9) | 95 (1.4) | 488 (141.4) | – | +1 | 0 |
| 3 | 0.6 (1.46) | 1613 (3738) | 1255 (309) | 4 (9.7) | 130/83 | 81 (1.4) | 94 | 720 | – | −2 | 0 |
| 4 | 1.2 (1.56) | 3131 (3992) | 1347 (116) | 6 (10.2) | 116/72 | 93 (6.4) | 95 | – | – | −1 | 0 |
| Co-morbid 2 | |||||||||||
| 1 | 1.8 (1.8) | 4499 (4725) | 1068 (293) | 4 (10.6) | – | – | – | 498 (127.3) | – | – | – |
| 2 | 0.6 (1.5) | 1613 (3738) | 1255 (309) | 4 (9.7) | – | – | – | 720 | – | – | – |
| 3 | 1.2 (1.6) | 3131 (3992) | 1347 (116) | 6 (10.2) | – | – | – | – | – | – | – |
| 4 | 0.5 (0.95) | 1372 (2422) | 1398 (81) | 1 (2.99) | – | – | – | – | – | – | – |
| Co-morbid 3 | |||||||||||
| 1 | – | – | – | – | – | – | – | – | 34 (35) | – | – |
| 2 | – | – | – | – | – | – | – | – | 57 (29.8) | – | – |
| 3 | – | – | – | – | – | – | – | – | 38 (35) | – | – |
| 4 | – | – | – | – | – | – | – | – | 72 (52.9) | – | – |
aNo standard deviation indicates only one measurement during the week period
Note: SD = standard deviation (calculated per 7 days of data); oz = ounces; lbs = pounds; BMI = body mass index.
Table 2.
Weekly averages of adherence by week to wearing or using the mobile health devices and applications by patient
| Adherence to using the device and applications per week (%) |
|||||||
|---|---|---|---|---|---|---|---|
| Healthy 1 | Accelerometer | Sleep | Weight | BP | SpO2 | Diet app | Number of devices or apps used at least 4 of 7 days |
| Week 1 | 100 | 0 | 100 | 100 | 0 | 100 | 4 |
| 2 | 100 | 14 | 57 | 100 | 0 | 100 | 4 |
| 3 | 100 | 0 | 71 | 86 | 0 | 57 | 4 |
| 4 | 100 | 0 | 71 | 100 | 0 | 71 | 4 |
| Healthy 2 | |||||||
| 1 | 100 | 71 | 100 | 86 | 86 | 100 | 6 |
| 2 | 100 | 86 | 100 | 86 | 71 | 100 | 6 |
| 3 | 100 | 86 | 100 | 86 | 86 | 100 | 6 |
| 4 | 100 | 71 | 71 | 43 | 29 | 71 | 4 |
| Healthy 3 | |||||||
| 1 | 100 | 100 | 86 | 100 | 100 | 57 | 6 |
| 2 | 100 | 86 | 71 | 100 | 100 | 57 | 6 |
| 3 | 100 | 100 | 100 | 100 | 100 | 29 | 5 |
| 4 | 100 | 100 | 71 | 71 | 71 | 0 | 5 |
| Co-morbid 1 | |||||||
| 1 | 100 | 29 | 14 | 43 | 14 | 0 | 1 |
| 2 | 100 | 29 | 43 | 43 | 29 | 0 | 1 |
| 3 | 29 | 14 | 29 | 14 | 14 | 0 | 0 |
| 4 | 57 | 0 | 0 | 14 | 14 | 0 | 1 |
| Co-morbid 2 | |||||||
| 1 | 100 | 29 | 0 | 0 | 0 | 0 | 1 |
| 2 | 29 | 14 | 0 | 0 | 0 | 0 | 0 |
| 3 | 57 | 0 | 0 | 0 | 0 | 0 | 1 |
| 4 | 29 | 0 | 0 | 0 | 0 | 0 | 0 |
| Co-morbid 3 | |||||||
| 1 | 0 | 0 | 0 | 0 | 0 | 43 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 86 | 1 |
| 3 | 0 | 0 | 0 | 0 | 0 | 43 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 71 | 1 |
Note: Adherence was measured as the actual number of days out of seven that data were collected on each device or application. Participants were instructed to use the scale, blood pressure cuff, and pulse oximeter at least one time per day and to record at least 50% (4 of 7 days) of their weekly physical activity, sleep, or diet.
RESULTS
We recruited three male and three female participants whose ages were 62, 43, 38, 33, 28, and 25. Three participants were healthy, and three had one or more chronic illness(es). Average weekly health indicator scores and change in scores are presented by participant in Table 1. Those with and without chronic disease appeared to use their devices differently, with the healthy group producing more data over 4 weeks. All healthy participants used the accelerometer peripheral device (Fitbit®) for the duration of the study period, whereas chronically ill participants demonstrated diminishing or no accelerometer use across weeks. All participants demonstrated decreased use of the SpO2 pulse oximeter and diet app from weeks 1 to 4. One participant with chronic illness used only one mobile health device (the diet app) during the entirety of the study period. Weekly weight averages fluctuated across the weeks, but the overall average change in weight among participants was 0 pounds with no observed change in BMI. Sleep monitoring varied across participants, but was more likely to be used by the healthier participants.
Average weekly adherence to use of each device or app that measured a health indicator is presented by participant and week in Table 2. Among all participants, average percentage of daily adherence to use of each device or app to measure specific indicators over 4 weeks was: accelerometer 71%, BP cuff 49%, weight scale 45%, fluid intake app 41%, sleep 35%, oximeter 30% (not shown in table). Healthy participants showed an overall average weekly adherence to use of devices of 76%, compared with 16% for those with chronic illnesses. No notable differences were found by weekdays versus weekend adherence.
Post-study interviews indicated that participants generally liked the devices. In particular, the Fitbit® was found to be the most useful in terms of monitoring health. Participants reported that they initially felt overwhelmed by having multiple devices and began to use only the devices with data of interest to them, such as the Fitbit®. All six participants (100%, N = 6) reported some level of technical difficulty with using the devices, particularly connecting the wireless scale. They noted that the devices needed improvements to ease setup, especially for connecting to Wi-Fi and synchronizing via Bluetooth to their phones, which consumed battery life. Following the intervention, all six participants (100%, N = 6), even those with lower rates of adherence, stated they would recommend the devices for monitoring health.
DISCUSSION
The present study was the first to test the feasibility of patient usage of multiple wireless apps and devices to feed health data over time to a secure database via software that complied with US Food and Drug Administration guidelines and institutional review board and information security requirements. With the exception of the Fitbit®, use of mobile health devices was <50% over 1 month of study. Over time, all participants showed decreased use of all devices except the Fitbit®, with the exception of one participant’s utilization of tracked fluid intake. Among healthy participants, step counts generally increased, although neither weight nor BMI changed across the month.
Concerns remain about the general effectiveness and adoption of these devices in all patients, particularly those with chronic disease states. Given the potential benefit of frequent monitoring and lifestyle health interventions in this group, additional efforts are likely required to support their engagement with mobile health technology. Device fatigue could be a significant challenge, which was apparent after only 4 weeks. Interview results highlighted the burden participants felt by the number of devices and their neglect of those whose data they thought less meaningful. Ultimately, fewer devices may be more effective in terms of device adherence. Prior studies have shown that merely tracking a behavior can effect positive change,11,12 which may explain increased step counts in the absence of instruction to lose weight or to change behaviors.
Future research should focus on longer-term use of devices and applications in larger samples to compare healthy and chronically ill patients’ behavior and change as well as to examine device and data overload. Devices whose measures are passive- versus action-oriented should be compared on adherence rates. While we did not find any differences within a week; that is, weekdays versus weekend days, this potential effect requires more focused attention. Finally, future research should also examine the impact of numeracy skills and health and digital literacy.
We note three limitations to our results. First, this feasibility study was not powered to detect statistically significant differences between healthy and chronically ill groups or across time. Second, the Mobile Health Platform is an experimental software program capable only of collecting, storing, and transmitting limited data at this time. Third, patients received no instructions on interpretation of data from the devices.
CONCLUSION
The use of mobile and wireless technologies to assess biological states and assist in behavior modification in patients’ everyday environments in real time has the potential to transform medical research and care delivery across populations and within individuals over time.13 This may contribute to intra-individual tailoring of health interventions, known as personalized medicine. However, patient commitment to the use of mobile devices remains a challenge. mHealth technology may be less effective when implemented ubiquitously throughout populations, particularly with regard to adherence to use in patients with chronic diseases. This study supports the feasibility of research on integrating mobile health technology into patient’s lives to support their health.
ACKNOWLEDGEMENTS
The authors thank Judith Hays, PhD, RN for her editorial support.
CONTRIBUTORS
R.J.S. and D.M.S. contributed to the conception, design and acquisition and interpretation of data, and drafted and revised the manuscript. J.B., F.M., A.G., M.S., S.C.G., G.G.B., and H.B.B. contributed to design, interpretation of data, and critical revisions to the manuscript. T.C. and M.M. contributed to acquisition and interpretation of data, and drafted and revised the manuscript.
FUNDING
This work was supported in part by the National Institute of Nursing Research (NIH P30NR014139), S.L. Docherty and D.E. Bailey Jr., principal investigators, Duke University School of Nursing in addition to funding by the Duke Center for Personalized and Precision Medicine.
COMPETING INTERESTS
S.C.G. receives consulting fees from Gilead Sciences for serving on multiple Data and Safety Monitoring Boards. Although the relationship is not perceived to represent a conflict with the present work, it has been included in the spirit of full disclosure. The other authors declare no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is available online at http://jamia.oxfordjournals.org/.
REFERENCES
- 1.World Bank. Information, Communication Technologies, and infoDev (Program). Information and Communications for Development 2012: Maximizing Mobile. World Bank Publications; 2012. Available at: http://web.worldbank.org/WBSITE/EXTERNAL/TOPICS/EXTINFORMATIONANDCOMMUNICATIONANDTECHNOLOGIES/0,,contentMDK:23190786∼pagePK:210058∼piPK:210062∼theSitePK:282823,00.html.
- 2.PricewaterhouseCoopers, E.I.U. Emerging mHealth: Paths for Growth. 2012. Available at: https://www.pwc.com/gx/en/healthcare/mhealth/assets/pwc-emerging-mhealth-full.pdf.
- 3.Suh M-K, Chen CA, Woodbridge J, et al. A remote patient monitoring system for congestive heart failure. J Med Syst. 2011;35(5):1165–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funnell MM, Anderson RM. MSJAMA: the problem with compliance in diabetes. JAMA. 2000;284(13):1709. [PubMed] [Google Scholar]
- 5.Safford MM, Russell L, Suh DC, et al. How much time do patients with diabetes spend on self-care? J Am Board Fam Pract. 2005;18(4):262–270. [DOI] [PubMed] [Google Scholar]
- 6.Russell LB, Suh DC, Safford MA. Time requirements for diabetes self-management: too much for many? J Fam Pract. 2005;54(1):52–56. [PubMed] [Google Scholar]
- 7.Martin S, Schneider B, Heinemann L, et al. Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: an epidemiological cohort study. Diabetologia. 2006;49(2):271–278. [DOI] [PubMed] [Google Scholar]
- 8.Stergiou GS, Salgami EV, Tzamouranis DG, et al. Masked hypertension assessed by ambulatory blood pressure versus home blood pressure monitoring: is it the same phenomenon? Am J Hypertens. 2005;18(6):772–778. [DOI] [PubMed] [Google Scholar]
- 9.Butryn Ml, Phelan S, Hill JO, et al. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity. 2007;15(12):3091–3096. [DOI] [PubMed] [Google Scholar]
- 10.Orsama AL, Mattila E, Ermes M, et al. Weight rhythms: weight increases during weekends and decreases during weekdays. Obes Facts. 2014;7(1):36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobbs N, Godfrey A, Lara J, et al. Are behavioral interventions effective in increasing physical activity at 12 to 36 months in adults aged 55 to 70 years? A systematic review and meta-analysis. BMC Med. 2013;11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pal S, Cheng C, Egger G, et al. Using pedometers to increase physical activity in overweight and obese women: a pilot study. BMC Public Health. 2009;9:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley WT, Rivera DE, Atienza AA, et al. Health behavior models in the age of mobile interventions: are our theories up to the task? Transl Behav Med, 2011;1(1):53–71. [DOI] [PMC free article] [PubMed] [Google Scholar]