Abstract
Objective To investigate how individuals with diabetes and diabetes educators reason about data collected through self-monitoring and to draw implications for the design of data-driven self-management technologies.
Materials and Methods Ten individuals with diabetes (six type 1 and four type 2) and 2 experienced diabetes educators were presented with a set of self-monitoring data captured by an individual with type 2 diabetes. The set included digital images of meals and their textual descriptions, and blood glucose (BG) readings captured before and after these meals. The participants were asked to review a set of meals and associated BG readings, explain differences in postprandial BG levels for these meals, and predict postprandial BG levels for the same individual for a different set of meals. Researchers compared conclusions and predictions reached by the participants with those arrived at by quantitative analysis of the collected data.
Results The participants used both macronutrient composition of meals, most notably the inclusion of carbohydrates, and names of dishes and ingredients to reason about changes in postprandial BG levels. Both individuals with diabetes and diabetes educators reported difficulties in generating predictions of postprandial BG; their predictions varied in their correlations with the actual captured readings from r = 0.008 to r = 0.75.
Conclusion Overall, the study showed that identifying trends in the data collected with self-monitoring is a complex process, and that conclusions reached by both individuals with diabetes and diabetes educators are not always reliable. This suggests the need for new ways to facilitate individuals’ reasoning with informatics interventions.
Keywords: chronic disease, self-care
1. BACKGROUND AND SIGNIFICANCE
Diabetes continues to be one of the most devastating chronic diseases, with a significant impact on the affected individuals, their families, and communities. As of 2014, 29.1 million people or 9.3% of the United States population has diabetes,1 with ethnic minorities disproportionally affected.2 Diabetes is associated with a number of complications, including heart disease and stroke, high blood pressure, kidney disease, and nervous system disease, and can lead to blindness and amputations.3 Finally, diabetes has a devastating economic impact; in 2012 alone its costs were estimated at $246 billion.4
Self-monitoring has long been accepted as a critical component of self-management for a variety of diseases and health conditions, and particularly for diabetes.5–7 Previous studies conducted with individuals with diabetes suggested that self-monitoring of blood glucose (BG) levels is associated with better glycemic control and improved clinical outcomes.8–10 Researchers in personal informatics proposed that self-monitoring in diabetes can lead to increased self-knowledge, and to heightened awareness of an individual’s current health status (e.g., current levels of BG) and changes in this status over time.11 Moreover, combining BG data with records of daily activities, such as meals and exercise, can help individuals examine the comparative impact of these activities on BG levels and identify behaviors and activities with either particularly beneficial or particularly detrimental impact on glycemic control.12–14 This knowledge can help individuals make informed choices about future actions and refine treatment choices, e.g., in nutritional therapy.15 Given the growing popularity and availability of self-monitoring technologies,16 these new abilities can have a significant impact on diabetes self-management and help to reduce its significant burden.
However, to realize their full potential to improve health management, these new technologies need to help individuals translate captured data into insight and discovery. Yet the path for this transition remains unclear. Previous studies of personal informatics solutions in chronic disease self-management have demonstrated that these technologies are limited in providing explicit support for discovery of patterns and associations in the captured data.11,12 Moreover, even though pattern management is encouraged, there exist only a few published studies on how individuals with chronic diseases reason about data captured through self-monitoring, find patterns, and make discoveries.17
The long-term goal of this research is to develop novel informatics solutions that can help individuals with diabetes engage in analysis and reflection on data collected through self-monitoring, and use these data to improve their self-management behaviors and, ultimately, their health. As the first step toward this goal, we conducted a mixed-methods study investigating how individuals with diabetes and experienced diabetes educators engage with data collected through self-monitoring. In this mixed methods study, 12 participants (n = 12), including individuals with diabetes (n = 10), and diabetes educators (n = 2) were asked to examine a collection of dietary records, combined with BG records before and after each meal (2 such datasets were used in the study). Our specific questions included: 1) what aspects/properties of captured activities individuals include in their reasoning about the impact of these activities on changes in BG levels; 2) how they use these data to identify recurring trends and patterns; and 3) to what degree they can incorporate lessons learned from past records to project the impact of future actions. These questions are important because they can help identify what properties of individuals’ activities, specifically meals, need to be captured by self-monitoring technologies. In addition, they can guide the development of new methods for automatically detecting recurring patterns and correlations in the data collected through self-monitoring, identifying activities that have a significant impact on glycemic control, and helping individuals make more informed self-management decisions.
2. METHODS
Participants
Twelve participants (n = 12), including 2 certified diabetes educators (CDEs) and 10 individuals with diabetes, were recruited for a mixed-methods study. The participants with diabetes (both types 1 and 2) were recruited among members of TuDiabetes community, an online health forum for individuals with diabetes, using an advertisement on the forum’s website. Two of these individuals (both with type 2 diabetes) had previously participated in a self-monitoring study that generated datasets for the reasoning study described here. The datasets included images of meals captured by the participants with their brief textual descriptions, and BG levels before and after meals. The participants were instructed to capture at least 1 but possibly more postprandial BG levels, one at 2 h postmeal, and other at participants’ discretion. The self-monitoring study was conducted in October–November of 2014; the reasoning study described here was conducted in May–July of 2015. The other 8 participants with diabetes (both types 1 and 2) did not participate in the self-monitoring study and were not previously exposed to the data collected during that study.
Study Design
Each of the 12 participants took part in an interview that included 2 essential phases, a retrospective association analysis phase, and a prospective forecasting phase. During the first phase, the retrospective association analysis, the participants reviewed images of 5 days of meals and BG readings before and after these meals (see Figure 1). The 2 participants who collected the data were each presented with their own datasets; the other participants with diabetes were randomly assigned to 1 of the 2 available datasets. Each CDE reviewed both datasets, each during separate interviews. The participants were asked to review each image and its description, examine BG readings before and after the meal, and explain what properties or components of the meal could have been associated with the recorded changes in postprandial BG level. At the end of this phase, each participant was asked to discuss any recurring patterns associated with both desirable and undesirable changes in BG levels. During the next phase, the prospective forecasting, the participants were presented with a different set of images for another 5 days from the same dataset (not included in the first part) and their descriptions and premeal BG level, and were asked to predict postprandial BG levels and explain their prediction. We limited the data used in the study to 10 days (out of 28 days with records), mostly due to pragmatic considerations. Discussing each meal took 1–2 min; as a result, each interview lasted between 1 and 1.5 h. Including more data in the interviews would have extended them beyond what was reasonable.
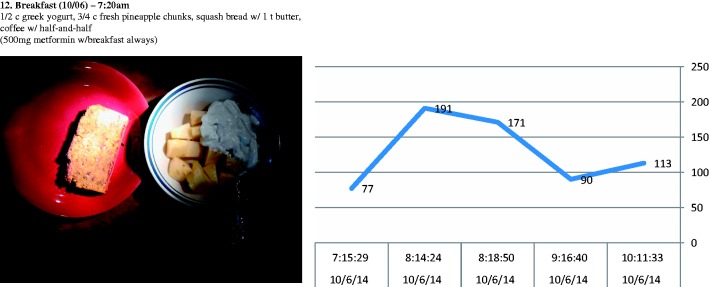
Figure 1:
Screenshot of the data presented to the participants during the retrospective association analysis part of the interviews.
The reasoning interviews were conducted either in person or over the phone or Skype and were audio recorded and transcribed verbatim for analysis. The Institutional Review Board of the Columbia University Medical Center approved the study.
Data Analysis
The analysis of the data included several distinct steps.
First, 2 registered dietitians assessed the meals in the datasets on the inclusion of macronutrients commonly associated with glycemic control (carbohydrate, protein, fat, and fiber).
Next, all captured meals were grouped according to the premeal/postmeal BG differences (which we will refer to as a meal’s glycemic impact) into four distinct categories: 1) stable: meals with a glycemic impact of<20 mg/dl; 2) low impact: meals with a glycemic impact between 20 and 39 mg/dl; 3) medium impact: meals with a glycemic impact between 40 and 59 mg/dl, and 4) high impact: meals with a glycemic impact of 60 mg/dl and above. These categories were developed together with the 2 diabetes educators on our team; they are representative of the way diabetes educators typically classify meals recorded by their patients.
Further, interview transcripts were analyzed to identify properties and components of meals used by the participants to construct their explanations; in the rest of this paper, we refer to these simply as “key terms.” For example, for the following explanation: “And I expect there’s probably a little bit of a contribution from the carrots and red peppers and maybe from the tomato but generally, I think it’s mostly from the rice in the gumbo,” we would extract the following words and phrases: “carrots,” “red peppers,” “tomato,” “rice,” and “gumbo” as key terms that were used to explain the impact of this meal.
To identify key terms that were mentioned as recurring reasons for changes (or lack thereof) in postprandial BG levels, all meals for each dataset were categorized by glycemic impact category (stable, low, medium, and high as discussed above), and key terms for each of the categories were aggregated across meals.
Finally, we extracted all statements that expressed participants’ perceptions regarding recurring patterns in the datasets, as well as their general beliefs about the impact of nutrition on postprandial BG levels, for example: “Protein slows down absorption [of glucose in food] and delays rise of BG levels.”
Evaluation with Data Science Methods
Two different approaches were used to evaluate participants’ intuitions recorded during the reasoning study with computational data analysis. First, we examined correlations between macronutrient content of the recorded meals and changes in individuals’ postprandial BG levels using Spearman’s correlation test.
Second, we examined correlations between predictions generated by the participants with the actual postmeal BG levels using Spearman correlation tests for nonparametric data.
To account for multiple tests, we used a Bonferroni correction: for the total of 21 tests of correlation, the P value was reduced to 0.002381.
4. RESULTS
4.1. Participant Demographics
Two CDEs and 10 individuals with diabetes took part in this study. Each CDE had over 20 years of practice and research experience in diabetes education. One educator was trained as a nurse practitioner, and another as a dietitian; both were certified by the National Certification Board for Diabetes Educators. Among the participants with diabetes, six had type 1 diabetes (P3, P4, P5, P7, P8, P10), and four had type 2 diabetes (P1, P2, P6, P9). The average age of the participants with diabetes was 55 years (ranging from 45 to 70), and their average time from diagnosis was 30 years (ranging from 10 to 50). All of the participants with diabetes self-identified as committed to proactive diabetes self-management (as is also indicated by their membership in the online diabetes forum), and all had some experience with self-monitoring (kept records of BG readings at least on paper).
4.2. Retrospective Association Analysis
When asked to explain differences between the premeal and postmeal (postprandial) BG levels associated with different meals, most participants began by identifying sources of carbohydrates in the meals and estimating their potential impact. Several participants considered carbohydrates the only source of change in postprandial BG levels. Others included other macronutrients in their analysis as factors that could either amplify the impact of carbohydrates or reduce it, for example: “Mayonnaise will slow the absorption because of the fat” (P11).
Both CDEs and 3 participants with diabetes spontaneously engaged in carbohydrate counting—estimating the actual weight of carbohydrate in each meal—and used that estimate to explain the impact of the meal on postprandial BG levels. Others approached it more holistically, without generating precise numeric estimates: “Scrambled eggs, pretty neutral in terms of blood glucose and carbo wise, … they have a combination of strawberries and blueberries, is going to have a significant impact on blood glucose” (P5).
In addition, some participants paid attention to the glycemic index of the meal, which they defined as the rate of absorption of carbohydrates. However, these participants reported general lack of knowledge and of reliable information on the glycemic index of different foods.
The analysis of the 10 most common key terms used to explain changes in postprandial BG levels are presented in Table 1. Notably, carbohydrates are at the top of this list for both CDEs and participants with diabetes for both datasets. However, other items beyond carbohydrates vary considerably between the 2 datasets and between CDEs and participants with diabetes. For example, while the names of macronutrients are consistent between the datasets, the names of the actual foods are specific to the dietary choices of the individuals who collected the data (e.g., “squash bread” and “half-and-half” in Dataset 1 and “apple” and “almond butter” in Dataset 2). In addition, whereas CDEs tended to refer to macronutrients, the participants with diabetes more often referred to the actual products and dishes, such as “squash bread” and “strawberries.”
Table 1:
Ten most common key terms used to explain the impact of the meals on postprandial BG levels; the numbers indicate percent of this particular term to all key terms recorded by this user group for each of the datasets
| Dataset 1 | |||
|---|---|---|---|
| CDEs | % | Participants | % |
| Carbohydrates | 7.3 | Carbohydrates | 7.3 |
| Fat | 4.7 | Protein | 4.1 |
| Protein | 4.4 | Squash bread | 3.4 |
| Fiber | 3.6 | Strawberries | 3.4 |
| Squash bread | 2.5 | Blueberries | 2.9 |
| Vegetables | 2.5 | Yogurt | 2.7 |
| Half and half | 2.2 | Fat | 2.4 |
| Strawberries | 2.2 | Chicken | 2.2 |
| Sugar | 2.2 | Salad | 2.0 |
| Yogurt | 2.2 | Sugar | 2.0 |
| Dataset 2 | |||
|---|---|---|---|
| Carbohydrates | 10.4 | Carbohydrates | 13.0 |
| Fat | 5.5 | Apple | 5.1 |
| Fiber | 4.3 | Pears | 4.3 |
| Apple | 3.7 | Protein | 3.6 |
| Protein | 3.7 | Salsa | 3.6 |
| Vegetables | 4.3 | Medication | 3.2 |
| Cheese | 3.1 | Almond butter | 2.9 |
| Almond butter | 2.5 | Bread | 2.9 |
| Avocado | 2.5 | Ham | 2.9 |
| Bread | 2.5 | Vegetables | 2.5 |
The results are presented separately for the two datasets, and for CDEs and participants with diabetes. The numbers indicate the percent of the given factor to all the factors extracted from the transcripts.
The 10 most popular key terms identified separately for meals that led to a significant rise in BG levels (over 60 mg/dl) and meals that did not lead to a considerable rise in BG levels (< 20 mg/dl) across participants produced the results shown in Table 2.
Table 2.
Common terms used in explanations of meals with high impact on BG (over 60 mg/dl difference in BG between premeal and postmeal) and meals with stable impact on BG (under 20 mg/dl difference in BG between premeal and postmeal)
| High—Dataset 1 | High—Dataset 2 | ||
|---|---|---|---|
| Term | Frequency | Term | Frequency |
| Carbohydrates | 17 | Apple | 13 |
| Squash bread | 10 | Carbohydrates | 9 |
| Yogurt | 9 | Almond butter | 8 |
| Protein | 9 | Avocado | 6 |
| Pancake | 8 | Medication | 5 |
| Blueberries | 7 | Fat | 4 |
| Half and half | 7 | Low carbohydrate | 4 |
| Sugar | 7 | Protein | 4 |
| Fat | 6 | Wine | 4 |
| Peanut butter | 6 | Celery | 3 |
| Stable—Dataset 1 | Stable—Dataset 2 | ||
|---|---|---|---|
| Carbohydrates | 15 | Carbohydrates | 17 |
| Fat | 12 | Pears | 16 |
| Protein | 11 | Salsa | 8 |
| Almonds | 5 | Protein | 7 |
| Butter | 5 | Bread | 6 |
| Chicken | 5 | Fat | 6 |
| Raisins | 5 | Medication | 6 |
| Avocado | 4 | Fiber | 5 |
| Gelato | 4 | Ham | 5 |
| Salmon | 4 | Wine | 5 |
Here, beside the typically frequent use of the term “carbohydrates,” the terms used to explain high-impact meals and low-impact meals differed considerably between Datasets 1 and 2. In Dataset 1, the 4 most frequently used key terms to explain high-impact meals (besides “carbohydrates”) included “squash bread” (n = 10), “protein” (n = 9), “yogurt” (n = 9), and “pancake” (n = 8). In Dataset 2, the 4 other most frequent terms included “apple” (n = 13), “almond butter” (n = 8), “avocado” (n = 6), and “medication” (n = 5). Similarly, for meals in the “Stable” category, the 4 most frequent terms for Dataset 1 were “fat” (n = 12); “protein” (n = 11); “almonds” (n = 5); and “butter,” “chicken,” and “raising” (each with n = 5). These terms for Dataset 2 included “pears” (n = 16); “salsa” (n = 8); “protein” (n = 7); and “bread,” “fat,” and “medication” (all with n = 6).
As far as recurring trends and patterns in the datasets, for Dataset 1, most participants mentioned significant amounts of carbohydrates in breakfasts as the main reason for frequent high after-breakfast readings. In addition, a long time interval between lunch and dinner was viewed as a potential reason for high after-dinner readings. For Dataset 2, most participants noticed a recurring spike in BG levels after lunch meals that included a very limited amount of carbohydrates and generated several theories explaining these trends, from over-restriction in carbohydrates (P2, CDE1, CDE2) to suboptimal dose of morning medication (P4, P6) to lack of exercise in the afternoons (P2, P4, P6, P10). The full set of recurring trends reported by the participants is included in Appendix A.
4.3. Prospective Forecasting
All participants reported considerable difficulties generating predictions of postprandial BG levels for specific meals; this was the case even for the 2 participants whose datasets were used in the study. First, they found it difficult to identify the exact composition of the meals: “… being able to look at a plate to say what’s in it is something that comes with both experience knowledge and it’s trial and error on top of that, but that one is a very key part of the whole big picture on that when it comes to meals.” Second, the participants found it difficult to determine the unique reaction of the person who collected the data to different ingredients in the meals. Finally, all participants with diabetes commented on the difficulties in disambiguating the impact of meals from other factors that influence BG: “I could never quite figure out why the same meal wouldn’t necessarily have the same effect, and trying to figure out what made the difference is tricky” (P1).
These challenges were not specific to the study, but common in participants’ everyday lives: “ … every meal is a stress because you have to sit down and calculate the stuff out. The more comfortable you are with feeling that, the better off you’re going to be, but you also have to accept that you’re going to make a lot of mistakes” (P5).
Notably, when the readings in the dataset were inconsistent with participants’ expectations, they used trends in the data to inform their predictions. For example, most of the participants who examined dataset 2 noticed frequent spikes in after-lunch BG levels following low-carbohydrate lunch meals. While many participants found this trend puzzling, they nonetheless used it to predict similarly high postprandial BG levels after low-carbohydrate meals: “I’m going to give that 160 as well because the person tends to—the blood sugar tends to go up a little after lunch, even though there’s nothing in there to really kick it up” (P6).
4.4. Validation of participants’ perceptions
4.4.1. Impact of macronutrients
To examine the associations between the inclusion of different macronutrients and changes in individuals’ premeal/postmeal BG levels, we used Spearman correlation analysis for each of the macronutrients (carbohydrates, protein, fat, and fiber) and differences between postmeal and premeal BG levels. The results of this analysis are presented in Table 3.
Table 3.
Correlations between the amount of different macronutrients and the magnitude of change in premeal/postmeal BG levels
| Dataset1 1 h | Dataset1 2 h | Dataset2 2 h | ||||
|---|---|---|---|---|---|---|
| R | P-value | r | P-value | r | P-value | |
| Carbohydrates | 0.31 | .003* | 0.14 | .23 | 0.56 | < .001* |
| Protein | 0.12 | .23 | 0.16 | .17 | −0.5 | .69 |
| Fat | 0.17 | .17 | 0.27 | .02* | 0.1 | .4 |
| Fiber | 0.24 | .023* | 0.1 | .37 | 0.38 | .001* |
*The asterisks (or minus signs) indicate negative correlation with a negative correlation coefficient r.
Consistently with the participants’ expectations, the amount of carbohydrates was positively correlated with high glycemic impact for both datasets. In Dataset 1, this impact was present only at 1 h postmeal and was diminished by the 2-h mark; for Dataset 2, it was still significant at the 2-h mark. However, other theories generated by the participants were only partially confirmed, or not confirmed at all. For example, fat was indeed correlated with high postprandial BG levels at 2 h postmeal, but only in Dataset 1. Protein was not correlated with changes in BG levels in either dataset, and fiber was positively correlated with high glycemic impact, despite the common perception that fiber minimizes the impact of carbohydrates.
4.4.1. Accuracy of predictions
The results of correlation analysis between predictions generated using different methods and the actual captured postmeal BG levels (for 1 and 2 h postmeal for Dataset 1 and for 2 h postmeal for Dataset 2) are presented in Table 4.
Table 4.
Correlations between predictions generated by CDEs (E1,E2) and participants with diabetes (P1-P10) and the actual captured postmeal BG readings
| Dataset 1 | Dataset 2 | |||||
|---|---|---|---|---|---|---|
| 1 h postmeal | 2 h postmeal | 2 h postmeal | ||||
| R | P-value | r | P-value | r | P-value | |
| E 1 | 0.17 | .52 | 0.008 | .98 | 0.72 | < .001* |
| E 2 | 0.69 | .002* | −0.06 | .82 | 0.75 | < .001* |
| P 1** | 0.56 | .019 | 0.12 | .42 | N/A | N/A |
| P 2** | N/A | N/A | N/A | N/A | 0.73 | .002* |
| P 3 | 0.57 | .017 | 0.068 | .8 | N/A | N/A |
| P 4 | N/A | N/A | N/A | N/A | 0.55 | .034 |
| P 5 | 0.55 | .024 | 0.45 | .084 | N/A | N/A |
| P 6 | N/A | N/A | N/A | N/A | 0.58 | .023 |
| P 7 | 0.67 | .003* | 0.018 | .95 | N/A | N/A |
| P 8 | N/A | N/A | N/A | N/A | 0.71 | .003 |
| P 9 | 0.49 | .073 | 0.094 | .74 | N/A | N/A |
| P 10 | N/A | N/A | N/A | N/A | 0.57 | .029 |
*Results with statistical significance from adjusted significance level of P = .0024.
**Participants who took part in the self-monitoring study.
Participants’ predictions varied in their accuracy between low (r = 0.018) to high (r = 0.75). Despite the participants’ confusion about the mysterious spikes after low-carbohydrate lunches in Dataset 2, most predictions for this dataset were highly correlated with the actual recorded readings. In contrast, for Dataset 1, while most participants were able to relatively accurately predict postprandial BG levels at 1 h postmeal, their predictions for BG levels at 2 h postmeal were considerably off-mark with correlations for that time point, ranging from r = 0.008 to r = 0.45.
5. DISCUSSION
The increasing volumes of person-generated data open new horizons for promoting self-knowledge and self-awareness.12 This has a particular significance for individuals with chronic diseases who could potentially use such data to identify beneficial self-management strategies. However, to take advantage of these data, individuals need to be able to examine and analyze it, identify and test recurring patterns and associations between their activities and changes in relevant biomarkers, and incorporate their discoveries into their future choices. In this study, we took initial steps toward understanding these processes with the goal of informing the design of future self-monitoring and self-management technologies.
The study included 3 types of participants who in our view represent the 3 most typical scenarios in which self-monitoring technologies can be used. The 2 individuals who participated in the self-monitoring study are exemplars of experienced individuals with diabetes who reflect on their own records, arguably the most typical case of self-monitoring. The other participants with diabetes were representative of newly diagnosed individuals, who have no developed intuitions about their body’s reactions to dietary changes. Finally, the experience of 2 diabetes educators was similar to a typical consultation between a new patient with diabetes and their diabetes educator. As a result, the findings discussed here can inform new solutions in all 3 of these spaces.
In regards to the retrospective association analysis, the study suggested that the participants used both macronutrient composition of meals and specific ingredients to reason about the meals’ impact on BG levels. However, whereas participants with diabetes often relied on ingredients and food groups, experts’ explanations more frequently included macronutrients. This distinction is important, as reasoning on the macronutrient level can help individuals generalize between different products similar in their macronutrient composition.
Across datasets and participants, the inclusion of carbohydrates was the single most important and consistent information used to explain the change in postprandial BG levels. This intuition was confirmed by quantitative analysis of the datasets; the amount of carbohydrates was strongly and positively correlated with postprandial BG. However, beyond carbohydrates, the participants’ perceptions regarding the impact of macronutrients were varied, often contradictory, and not always supported by the data. For example, in both datasets, fat and protein were noted as common key terms associated with meals with low glycemic impact, yet data analysis did not detect any correlation between protein and changes in postprandial BG levels, and fat was positively correlated with high postprandial BG levels in Dataset 1 at 2 h postmeal. A similar lack of consistency was typical for participants’ general beliefs about the impact of nutrition on BG levels; e.g., their beliefs about alcohol ranged from “raises BG levels” to “lowers BG levels” to “has no impact on BG.” This suggests the need for further research and education on the impact of nutrition on BG levels, especially as it relates to macronutrients and meal composition.
In regards to prospective forecasting, all participants reported experiencing significant challenges in generating predictions for glycemic impact of different meals. Perhaps as a result, these predictions varied in their correlation with the actual captured BG levels from correlation coefficient r close to 0 to r as high as 0.75. Many of the predictions were informed by the nutritional composition of meals. Others were based on consistent trends observed within the datasets, even when these trends were contradictory to the participants’ prior expectations or knowledge of diabetes pathophysiology, as was the case with the consistently high impact of low-carbohydrate meals in Dataset 2. Yet not all acknowledged trends led to high-accuracy predictions: while most participants observed that BG in Dataset 1 often returned to premeal levels by the 2-h mark, they did not incorporate this discovery into their predictions.
Overall, these findings showed that reasoning about data collected through self-monitoring is challenging and conclusions reached with such data can be less than reliable. This underscores the need for more systematic methods for using data to generate conclusions and discoveries and has several implications for the design of future informatics interventions for data-driven health self-management. First, it suggests the importance of both rich textual descriptions and macronutrient composition of meals to individuals’ ability to analyze captured records for trends and associations. Yet the vast majority of diet-monitoring technologies, including computational methods for automated analysis of captured meals, continue to focus on caloric assessment of meals18,19; this information is important for weight loss, but less valuable for diabetes self-management. Refocusing these technologies from general calorie counts to macronutrient composition could significantly reduce the burden associated with diet monitoring and provide individuals with the necessary information. Second, it suggests that names of dishes and ingredients, often captured as part of diet tracking, can provide additional valuable information to facilitate reasoning and decision-making. These descriptions could be analyzed by natural language processing and data mining methods on their associations with high or low glycemic impact in a way similar to how they were used by the participants in the study. These computational methods, however, would need to be combined with interaction mechanisms to examine and validate the discoveries. Finally, it suggests the need for new ways to help individuals anticipate the glycemic impact of their future meals. Previously, researchers proposed computational endocrine models that can forecast glycemic response to nutrition in meals, particularly to the amount of carbohydrates.20,21 While these models are still in their infancy, they can provide another venue for reducing the burden associated with diabetes self-management.
Together, these new methods can build a foundation for creating personalized informatics interventions for diabetes self-management. In recent years, precision medicine—an approach to the delivery of medical treatment that is tailored to an individual’s genetic and molecular makeup—has been suggested as the way that medicine will be practiced in the future.22 In a similar manner, we propose that data collected with self-monitoring can enable precision health informatics, in which behavioral strategies for self-management of chronic diseases are tailored based on individuals’ personal data. However, further research is needed to make this vision a reality.
This study has a number of limitations. First, it included a limited number of participants who all had substantial experience (typically over 20 years) of managing diabetes, and as a result may have been more knowledgeable about diabetes self-management than an average individual with diabetes. The reasoning and judgment may be different for individuals who are newly diagnosed. In addition, it was based on only 2 datasets, which may have had atypical patterns. Reasoning on different datasets may be different as well.
6. CONCLUSIONS
This study examined how individuals with diabetes and diabetes educators reason and form conclusions about data collected with self-monitoring, including dietary records and BG readings captured before and after meals. Overall, the study showed that identifying trends in the data collected with self-monitoring is a complex process, and that conclusions reached by both individuals with diabetes and diabetes educators are not always reliable. This suggests the need for new ways to facilitate individuals’ reasoning with informatics interventions. We propose that these methods could focus on helping individuals determine the nutritional composition of their meals, using Natural Language Processing (NLP) methods to identify associations between key words describing meals and undesired changes in BG levels, and using predictive endocrine models to forecast an individual’s reaction to future meals based on previous records.
FUNDING
This work was funded by the National Library of Medicine grant “Training in Biomedical Informatics at Columbia University,” T15 LM007079; National Institute of Diabetes and Digestive and Kidney Disease grant, 1R01DK090372-01A1; and National Library of Medicine grant “Discovering and applying knowledge in clinical databases”, R01 LM06910.
CONTRIBUTORS
All authors designed the study. L.M. served as main research investigator in conducting all the qualitative research activities. L.M. wrote the first draft of the manuscript. A.M.S. and P.D. served as domain experts and were responsible for providing domain expertise during interpretation sessions. All authors participated in interpretation of study findings, formulating study conclusions, and preparation of the manuscript. There are no collaborators beyond the co-authors of the paper.
COMPETING INTERESTS
There are no competing interests.
REFERENCES
- 1.US Centers for Disease Control. National Diabetes Statistics Report, 2014. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 5, 2015.
- 2.Statistics About Diabetes. American Diabetes Association website. http://www.diabetes.org/diabetes-basics/statistics/. Accessed January 5, 2015.
- 3.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14 (Suppl 5):S1–S85. [PubMed] [Google Scholar]
- 4.The Cost of Diabetes. American Diabetes Association website. http://www.diabetes.org/advocate/resources/cost-of-diabetes.html. Accessed January 5, 2015.
- 5.Glasziou P, Irwig L, Mant D. Monitoring in chronic disease: a rational approach. BMJ. 2005;330:644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drummond N, Abdalla M, Beattie JAG. Effectiveness of routine self monitoring of peak flow in patients with asthma. Grampian Asthma Study of Integrated Care (GRASSIC). BMJ. 1994;308:564–567. [PMC free article] [PubMed] [Google Scholar]
- 7.Karter AJ, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry. Am J Med. 2001;111:1–9. [DOI] [PubMed] [Google Scholar]
- 8.Martin S, et al. Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: an epidemiological cohort study. Diabetologia. 2006;49:271–278. [DOI] [PubMed] [Google Scholar]
- 9.O’Kane MJ, Bunting B, Copeland M, Coates VE. Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008;336:1174–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welschen LMC, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin A systematic review. Dia Care. 2005;28:1510–1517. [DOI] [PubMed] [Google Scholar]
- 11.MacLeod H, Tang A, Carpendale S. Personal informatics in chronic illness management. In Proceedings of Graphics Interface. Toronto, Canada: Canadian Information Processing Society, 2013. 2013:149–156. [Google Scholar]
- 12.Li I, Dey A, Forlizzi J. A stage-based model of personal informatics systems. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems.(CHI'06), ACM, New York, NY, USA, 2010:557–566. [Google Scholar]
- 13.Mamykina L, Mynatt ED, Kaufman DR. Investigating health management practices of individuals with diabetes. in Proceedings of the SIGCHI Conference on Human Factors in Computing Systems (CHI'06), ACM, New York, NY, USA, 2006:927–936. [Google Scholar]
- 14.Polonsky WH, Skinner TC. Perceived treatment efficacy: an overlooked opportunity in diabetes care. Clin Diabetes. 2010;28:89–92. [Google Scholar]
- 15.Sevick MA, Stone RA, Novak M, Piraino B. A PDA-based dietary self-monitoring intervention to reduce sodium intake in an in-center hemodialysis patient. PPA. 2008;2:177–184. [PMC free article] [PubMed] [Google Scholar]
- 16.Ledger D. Inside Wearables - Part 2. Endeavour Partners LLC website. http://endeavourpartners.net/assets/Endeavour-Partners-Inside-Wearables-Part-2-July-2014.pdf. Accessed January 5, 2015. [Google Scholar]
- 17.Paterson B, Thorne S. Expert decision making in relation to unanticipated blood glucose levels. Res Nurs Health. 2000;23:147–157. Accessed January 1, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Noronha J, Hysen E, Zhang H, Gajos KZ. Platemate: crowdsourcing nutritional analysis from food photographs. In Proceedings of the 24th Annual ACM Symposium on User Interface Software and Technology. ACM; 2011: 1–12. [Google Scholar]
- 19.Wang D-H, Kogashiwa M, Kira S. Development of a new instrument for evaluating individuals’ dietary intakes. J Am Diet Assoc. 2006;106:1588–1593. [DOI] [PubMed] [Google Scholar]
- 20.Dalla Man C, Raimondo DM, Rizza RA, Cobelli C. GIM, simulation software of meal glucose-insulin model. J Diabetes Sci Technol. 2007;1:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sturis J, Polonsky KS, Mosekilde E, Van Cauter E. Computer model for mechanisms underlying ultradian oscillations of insulin and glucose. Am J Physiol. 1991;260:E801–E809. [DOI] [PubMed] [Google Scholar]
- 22.Hamburg MA, Collins FS. The path to personalized medicine. New Engl J Med. 2010;363:301–304. [DOI] [PubMed] [Google Scholar]