Abstract
The diabetes healthcare provider plays a key role in interpreting blood glucose trends, but few institutions have successfully integrated patient home glucose data in the electronic health record (EHR). Published implementations to date have required custom interfaces, which limit wide-scale replication. We piloted automated integration of continuous glucose monitor data in the EHR using widely available consumer technology for 10 pediatric patients with insulin-dependent diabetes. Establishment of a passive data communication bridge via a patient’s/parent’s smartphone enabled automated integration and analytics of patient device data within the EHR between scheduled clinic visits. It is feasible to utilize available consumer technology to assess and triage home diabetes device data within the EHR, and to engage patients/parents and improve healthcare provider workflow.
Keywords: electronic health records, patient generated health data, mobile applications, blood glucose, clinical informatics
INTRODUCTION
Type 1 diabetes is one of the most common chronic diseases of childhood, and its incidence and prevalence continue to rise.1–3 Tight control of hyperglycemia (high blood glucose) with intensive insulin therapy, including in early childhood, decreases the risk of serious long-term diabetes complications.4–6 However, aggressive insulin dosing may result in hypoglycemia (low blood glucose) with risk of adverse changes in the central nervous system.7,8 As a result, self-monitoring of blood glucose is critical for affected children and their parents to guide mealtime insulin dosing and to facilitate interventions to avoid hypoglycemia.4,8 This chronic and intense requirement for interpretation of fluctuating data is stressful for many families, particularly caregivers of young children.9,10 The diabetes healthcare provider plays a key role in interpreting blood glucose trends, but most do not have easy access to patient data between visits. Although cloud-based diabetes data applications are available, consistent use by healthcare providers has been limited, as the electronic health record (EHR) has become the center of clinician workflow.11–14
Few institutions have successfully integrated patients’ home glucose data in the EHR. Previously published implementations have required custom interfaces, which limit wide-scale replication.15–17 Given the increasing number of children with Type 1 diabetes and the increasing adoption of EHRs by physicians, we predict a rising need for home glucose data interpretation that may be addressed through EHR integration and analytics.1–3,11,18 In this brief report, we detail how widely available consumer technology can enable automated integration of patient device data into a health system EHR in order to triage care between scheduled clinic visits and simultaneously improve healthcare provider workflow.
MATERIALS AND METHODS
In 2014, a major mobile device company (Apple, Cupertino, CA, USA) announced an operating system update that enables health data interoperability (“HealthKit”). When our EHR vendor (Epic, Verona, WI, USA) announced that its patient portal app (“MyChart”) would be HealthKit compatible, our team recognized the opportunity to use this platform for integration of patient device data into the EHR.19 Subsequently, a major continuous glucose monitor (CGM) device company (Dexcom, San Diego, CA, USA) announced compliance of its patient-facing app with the described platform, and we launched a pilot initiative to assess the feasibility of EHR integration of home-based continuous glucose monitoring. Our Institutional Review Board exempted this quality improvement initiative from oversight.
Architecture
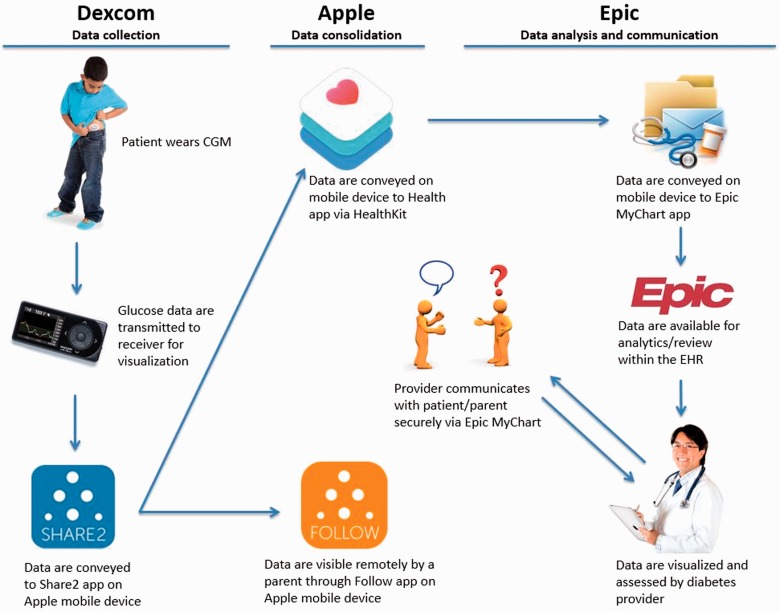
See Figure 1 for an overview of the architecture.
Figure 1.
Overview of the CGM data communication bridge architecture.
Dexcom G4 Platinum CGM data collection
The continuous glucose monitor is a popular sensor technology that enables frequent measurement (5-minute intervals) of interstitial glucose concentration.20 CGM accuracy is recently approaching that of standard finger-stick glucometers, and its use provides real-time glucose data to patients/parents and retrospective data to healthcare providers.20–22 The child/adolescent wears an interstitial glucose sensor that is connected to a transmitter. Sensors are inserted subcutaneously by a patient or family member at home. Each sensor is approved for 1 week of use. The transmitter sends glucose information by radio frequency to a patient’s wireless receiver for display of CGM values. The receiver sends the glucose information (including concentration and trend) by Bluetooth connection to the Dexcom Share2 app on a paired Apple mobile device.
Apple mobile device data consolidation to the Epic EHR
Following a one-time enable step, the Dexcom Share2 app passively shares glucose values, including date and time, on the device with the Apple Health app via the HealthKit interface (https://developer.apple.com/healthkit/). The glucose data is stored locally on the iOS device, but is not shared with Apple. There is a 3-hour time lag in data transfer from Share2 to HealthKit as mandated by Dexcom receiver Food and Drug Administration device classification. Once the glucose values reach HealthKit, they are passively shared with the Epic MyChart app (https://www.epic.com/software-phr.php). The MyChart patient portal is a component of the Epic EHR and uses the same database, and the CGM values populate a standard glucose flowsheet in the patient’s chart. This connection is initially established when a provider places an order in a patient’s electronic chart, resulting in a request to the patient within the MyChart app. Once the patient or patient proxy (parent) accepts this connection request on the mobile device, a communication bridge is established between HealthKit and MyChart enabling population of CGM data as frequently as every 5 minutes. All provider workflow is in the EHR.
Patient enrollment
To assess this communication bridge and optimize analytic tools in our EHR, we conducted a quality improvement pilot limited to 10 patients (Table 1) from a single provider (R.B.K.). The lead author selected the first 10 interested patients during standard pediatric diabetes clinic visits who were already using a Dexcom CGM and used an iOS device. Those patients whose CGM receivers did not have Bluetooth-enabled functionality (2 patients) were loaned an equipped receiver for the duration of the 3-month pilot.
Table 1:
Pilot group demographics and disease characteristics
| Age | Age at diabetes diagnosisa | Gender | Insulin method |
|---|---|---|---|
| 21 months | 4 months | M | MDI |
| 5 years | 21 months | M | MDI |
| 9 years | 7 years | F | MDI |
| 9 years | 7 years | M | Pump |
| 10 years | 5 years | F | Pump |
| 11 years | 8 years | M | Pump |
| 12 years | 10 years | F | MDI |
| 15 years | 12 years | M | MDI |
| 16 years | 13 years | F | Pump |
| 17 years | 14 years | F | MDI |
aOne participant has partial pancreatic agenesis and the rest have type 1 diabetes; MDI = multi-daily insulin injections; Pump = insulin pump.
Participation required confirmation of Bluetooth pairing of the CGM receiver to a mobile device, updating the mobile device with the most recent version of the operating system, Dexcom Share2 app, Epic MyChart app, and confirming or establishing a username and password for all accounts, including a parent’s/adolescent’s Epic MyChart account. Setup time averaged 45–60 minutes in addition to the scheduled clinic visit. During this time, there was specific verbal and written notification to the patients/parents that the diabetes healthcare team would not be actively monitoring or have real-time access to CGM data, which was out of scope for this pilot. The patients/parents were advised that they should continue to contact the diabetes care team by established means for any urgent questions/concerns. Additionally, patients/parents were advised to maintain updates for their linked mobile devices, including the latest operating system and app updates, to maintain communication of CGM data.
EHR visualization and analytics
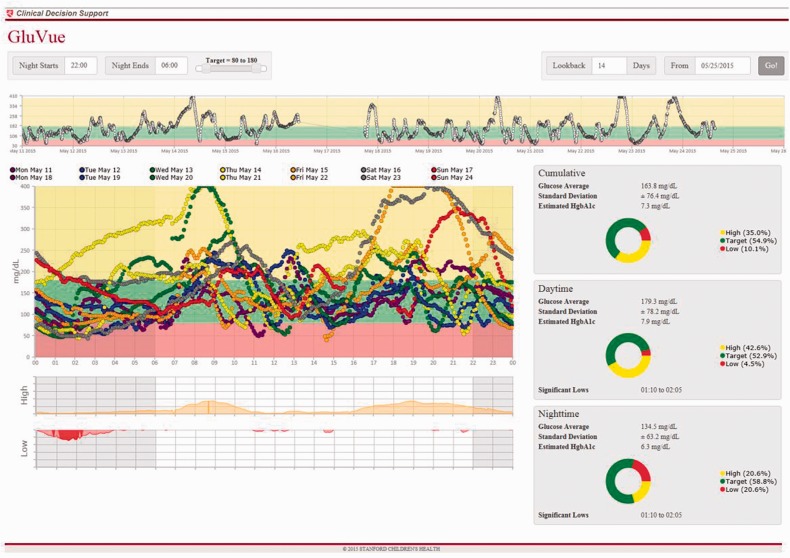
Given the data volume of up to 288 glucose readings per day, the standard flowsheet did not support visualizing a patient’s trends over weeks to months. Therefore, we implemented modal day visualization with a custom web-service embedded in the EHR (Figure 2). Design of this clinical decision support tool could be a barrier for other healthcare delivery systems that might want to replicate our workflow. We therefore have made it publically available at https://gluvue.stanfordchildrens.org/. This tool enables a provider to designate target glucose range and define nighttime hours when visualizing data over a given date range.
Figure 2.
Modal day visualization of 2 weeks of CGM data for a single participant with a custom web-service embedded in the EHR (available at https://gluvue.stanfordchildrens.org) A pattern of nocturnal hypoglycemia is readily identified.
An analytic report was also designed to triage patients based on glycemic control at home. The intention of this functionality was not to substitute regular monitoring by patients/parents, but rather to identify actionable trends by retrospective review between scheduled quarterly clinic visits. As such, no alerts or provider in-basket messages were utilized to identify specific glucose values out of a patient’s target range. At setup, we used verbal and written notification to establish appropriate patient/parent expectations regarding only intermittent provider monitoring. The report pulled data from the standard glucose flowsheets in the EHR and displayed days of sensor use/available glucose readings, estimated hemoglobin A1c values (derived from mean CGM glucose value), percent hypoglycemia, and episodic nocturnal hypoglycemia for each patient. This report was generated every 2 weeks, though specific glucose data was reviewed earlier if prompted by a patient/parent.
Provider workflow
When prompted by patient/parent request, or by summary information in the analytic report, the pediatric diabetes provider assessed glucose patterns using modal day visualization within the EHR. The provider sent messages summarizing the assessment from the EHR to the parent/adolescent via MyChart. Messages also included questions regarding specific trends, insulin dosing parameters, and other recommendations. The provider was alerted within the EHR in-basket if a parent/adolescent did not read the message within 48 hours. MyChart messages can be pasted into more formal documentation formats for billing purposes.
RESULTS
Over a 45-day period we enrolled 10 patients ranging in age from 21 months to 17 years. Six patients utilized their own Apple iPhone with a cellular data plan, 2 conveyed glucose readings intermittently using a parent’s iPhone with a cellular data plan, and 2 conveyed glucose readings intermittently using an iPhone without a cellular data plan (WiFi only). Upon establishing the communication bridge for the initial 2 patients, the system imported 2623 and 3857 glucose readings previously recorded in their respective CGM receivers. This initial high-volume data communication resulted in Epic MyChart app error messages and functionality freezing. For the subsequent patients, we limited retrospective glucose reading import to the preceding 24 hours (ranging from 4 to 257 initial data points), and there were no further reports of error messages. For those patients whose CGM receiver had only intermittent proximity to an iPhone, prospective data integration was also truncated to the preceding 24 hours. Accordingly, we asked the parents of these 4 patients to assure proximity of their CGM receiver to the paired iPhone, and internet connection for the phone, at least once daily to facilitate communication of all available glucose readings. Of note, there is now the option of using a smartphone as the receiver (http://www.dexcom.com/g5-mobile-cgm). For all patients, glucose data were not available for durations when a CGM transmitter was out of range of the CGM receiver, e.g., when a patient left the receiver at home or it was charging in a different room. Some patients chose not to wear a sensor daily, but most utilized the CGM near daily for the duration of the pilot. No participant expressed challenges in maintaining updates for the apps or mobile device operating system.
The pediatric endocrinologist providing recommendations for these patients (R.B.K.) found that after successful enrollment, the system enabled secure communication, timely access to information, and enhanced interpretation of large volumes of patient device data. Despite tens of thousands of data points per patient recorded in the EHR glucose flowsheet by the end of the pilot, chart access was not slowed, although viewing this specific flowsheet (rather than using the modal day viewer, which has rapid display) did take 1 to 2 minutes to display.
For one toddler, intermittent nocturnal hypoglycemia was identified, prompting communication with his mother. Discussion revealed that on occasions when the patient had hyperglycemia at bedtime, his family administered additional rapid-acting insulin using his dinnertime dose regimen. Less aggressive late-evening dosing was recommended, and a new insulin dose calculation sheet was securely forwarded to the family via MyChart. Subsequent monitoring demonstrated resolution of nocturnal hypoglycemia.
Unlike typical experiences with conventional communication, the 4 adolescents in the pilot were more actively engaged in their care between visits using MyChart messaging. A conversation was initiated with 1 teen and her mother to explore notable post-mealtime hyperglycemia. Despite the recommendation of more aggressive insulin dosing for mealtime carbohydrate (carb) intake, remote monitoring demonstrated only minimal improvement. Further discussion helped to identify that she was not including number of servings when calculating mealtime carb content. This prompted a nutritionist visit to review carb-counting technique, resulting in an improvement in post-meal hyperglycemia. Another teen was noted to have recurring nocturnal hypoglycemia on 2 specific weeknights. When he and his mother were made aware of this pattern, he offered that the highlighted nights followed sports practice and likely a related decrease in insulin requirement. He suggested a decreased dose of basal insulin on those specific weeknights from that point forward, resulting in resolution of the pattern.
DISCUSSION
We believe this is the first published report detailing how widely available consumer technology can enable automated integration of patient data into a health system EHR. Here we demonstrate two things: first, continuous information delivery is feasible through the use of commonly owned mobile devices. Second, passive EHR-based data delivery, coupled with automated triage and intuitive visualization, facilitates more efficient provider workflow for reviewing data and improved communication with patients. In our pilot, this was associated with better care between scheduled clinic visits.
Workflow integration has repeatedly been shown to be a critical success factor for clinical decision support systems, yet the workflow used for diabetes care today is far from optimized.23–25 The current standard for reporting interval CGM data requires patients to perform multiple manual steps. As a result, this data is generally available to providers only at quarterly clinic visits. Given the voluminous amount of data trends over the preceding 3 months, diabetes providers can only realistically focus on the most recent 2 to 4 weeks of glucose values, which often are biased by recent illness, changes in diet and/or activity during school breaks, and other factors. In contrast, our model eliminates the need for intermittent CGM device download. As an additional clinic time-saver, descriptions of glycemic trends and any medical advice provided since the preceding visit are documented in MyChart messages, negating the need for lengthy additional documentation and further increasing available face-to-face time with patients.
Our patient portal not only served as the infrastructure for sharing data, but simultaneously facilitated secure discussion among adolescents, parents, and providers. Adolescents are adept with electronic media, and the majority have their own mobile phone – including youth from low-income families, who are more likely to access the internet from their phone than a computer.26,27 Given the leading role that adolescents play in their own diabetes care, we recognize this tool as an additional means of building their self-management skills before adulthood.28 However, establishing patient portal access for this unique age group is challenging, given the importance of privacy and confidentiality issues specific to their health and proxy access. Our health institution systematically addressed these concerns to facilitate secure access for all of our pediatric patients.29
In reviewing previous efforts to integrate home glucose data in the EHR, we learned the importance of managing patient/parent expectations to enrich their experience and decrease medicolegal concerns.30 We stressed both verbally and via written patient notification (After Visit Summary) that real-time glucose monitoring was out of scope of this pilot (although certainly something we will explore in future deployments and studies) and advised that patients/parents continue to contact their diabetes care team by established means for any urgent questions/concerns. While hyperglycemia and hypoglycemia values were transmitted for every patient during the pilot, no patient or parent expressed concern or frustration that they were not contacted regarding each instance. Rather, participants frequently expressed gratitude that actionable trends were brought to their attention. Some also forgot they were transmitting CGM data and were pleased when they received our intermittent retrospective data review.
Importantly, the workflow described here requires no institutional-level customization and is agnostic to EHR vendor. While HealthKit is only available with Apple devices, we anticipate that Android mobile devices will offer a similar secure exchange platform for diabetes data in the future (https://developers.google.com/fit/?hl=en). The only customization we developed was the modal day glucose viewer (Figure 2), which we have now released as a cloud-based clinical decision support tool available to all healthcare delivery systems. Thus, we expect that others can easily leverage our methods for similar delivery of clinically relevant data to improve workflow.
Beyond the enormous potential to assess actionable trends on a continuous basis, the data-driven model deployed and piloted here supports proposed criteria for meaningful use of EHRs.31,32 With this approach patients/parents actively participate in their health management and have direct engagement with healthcare providers from a smartphone or computer via the secure patient portal, and their data is stored and easily retrieved. This methodology also holds the potential to facilitate telehealth diabetes care in regions with insufficient access to pediatric diabetes providers, and for those patients who do not benefit from quarterly visits.33
CGM integration with the EHR may raise concern for strained provider resources, as most diabetes care between visits (including review of insulin pump data) is a nonbillable service. However, analysis and interpretation of CGM data is a billable service (relative value units = 1.22) up to monthly by a physician or midlevel provider using Current Procedural Terminology code 95251.34,35 This analysis does not need to be performed in person, and reimbursement for review of CGM data between visits, in addition to accomplished meaningful use criteria, may protect or increase provider resources for enhanced diabetes care.35 With evolving technology and healthcare practices, we are optimistic that national reimbursement models for pediatric chronic disease will be updated to facilitate effective precision medicine.31
This brief report demonstrates that it is feasible to utilize available consumer technology to integrate home glucose data in the EHR and enhance healthcare team workflow. Implementation on a larger scale of providers and patients is necessary to demonstrate impact on diabetes outcome measures.
Acknowledgments
We would like to thank the patients and their parents for their participation and time. In the Stanford Children’s Health Information Services Department, we thank Ed Kopetsky, Joshua Faulkenberry, Tom McPherson, Simrat Ghuman, Arash Anoshiravani, MD, Jon Palma, MD, Mark Amey, Josh Glandorf, Rosalia Sandoval, Amit Borcar, and Rajesh Sharma. In the Stanford Children’s Health Pediatric Diabetes Program we thank Kathryn Cefalu, RN, CDE, and Suruchi Bhatia, MD. We also thank Dr Darrell Wilson for support and guidance throughout this process. The authors gratefully acknowledge technical support from Lucas Bannister and Raghavendran Mani at Epic, Nathaniel Heintzman and Jorge Valdes at Dexcom, and Afshad Mistri and Michael O’Reilly, MD, at Apple.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
COMPETING INTERESTS
R.B.K. is an elected, non-paid member of the Epic Systems advisory board for pediatric endocrinology. The authors have no other competing interests to declare.
CONTRIBUTORS
C.A.L. and R.B.K. conceived of and conducted the quality improvement project and authored the initial manuscript drafts. N.D.G., D.E.S., and D.P.W. helped to formulate the pilot design and provided editorial input on the manuscript with their unique background perspectives. All authors read and approved the final manuscript.
REFERENCES
- 1.Centers for Disease Control and Prevention. Diabetes Report Card 2014. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services, 2015:5. http://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf. Accessed for verification February 2016.
- 2.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imperatore G, Boyle JP, Thompson TJ, et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35(12):2515–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White NH, Cleary PA, Dahms W, et al. Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Pediatr. 2001;139(6):804–812. [DOI] [PubMed] [Google Scholar]
- 5.Silverstein J, Klingensmith G, Copeland K, et al. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28(1):186–212. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of medical care in diabetes—2015: summary of revisions. Diabetes Care. 2015;38(Suppl 1):S4. [DOI] [PubMed] [Google Scholar]
- 7.Bjørgaas MR. Cerebral effects of severe hypoglycemia in young people with type 1 diabetes. Pediatr Diabetes. 2012;13(1):100–107. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Glycemic targets. Diabetes Care. 2015;38(Suppl 1):S33–S40. [DOI] [PubMed] [Google Scholar]
- 9.Patton SR, Dolan LM, Henry R, et al. Fear of hypoglycemia in parents of young children with type 1 diabetes mellitus. J Clin Psychol Med Settings. 2008;15(3):252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hullmann SE, Wolfe-Christensen C, Ryan JL, et al. Parental overprotection, perceived child vulnerability, and parenting stress: a cross-illness comparison. J Clin Psychol Med Settings. 2010;17(4):357–365. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura MM, Harper MB, Castro AV, et al. Impact of the meaningful use incentive program on electronic health record adoption by US children’s hospitals. JAMIA. 2015;22(2):390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diasend. https://www.diasend.com/us/. Accessed for verification February 2016.
- 13.Glooko. https://www.glooko.com/. Accessed for verification February 2016.
- 14.Tidepool. http://tidepool.org/. Accessed for verification February 2016.
- 15.Gómez EJ, Hernando ME, García A, et al. Telemedicine as a tool for intensive management of diabetes: the DIABTel experience. Comput Methods Programs Biomed. 2002;69(2):163–177. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg HI, Ralston JD, Hirsch IB, et al. Using an Internet comanagement module to improve the quality of chronic disease care. Jt Comm J Qual Saf. 2003;29(9):443–451. [DOI] [PubMed] [Google Scholar]
- 17.Bellazzi R, Arcelloni M, Ferrari P, et al. Management of patients with diabetes through information technology: tools for monitoring and control of the patients’ metabolic behavior. Diabetes Technol Ther. 2004;6(5):567–578. [DOI] [PubMed] [Google Scholar]
- 18.Adler-Milstein J, DesRoches CM, Furukawa MF, et al. More than half of US hospitals have at least a basic EHR, but stage 2 criteria remain challenging for most. Health Aff. 2014;33(9):1664–1671. [DOI] [PubMed] [Google Scholar]
- 19.Apple. Apple announces iOS 8 available September 17: introduces new Messages & Photo features, QuickType keyboard, extensibility, iCloud Drive & new Health app. Cupertino, CA: Apple, 2014. http://www.apple.com/pr/library/2014/09/09Apple-Announces-iOS-8-Available-September-17.html. Accessed for verification February 2016.
- 20.DeSalvo D, Buckingham B. Continuous glucose monitoring: current use and future directions. Curr Diab Rep. 2013;13(5):657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polonsky WH, Hessler D. What are the quality of life-related benefits and losses associated with real-time continuous glucose monitoring? A survey of current users. Diabetes Technol Ther. 2013;15(4):295–301. [DOI] [PubMed] [Google Scholar]
- 22.Benhamou PY, Catargi B, Delenne B, et al. Real-time continuous glucose monitoring (CGM) integrated into the treatment of type 1 diabetes: consensus of experts from SFD, EVADIAC and SFE. Diabetes Metab. 2012;38(Suppl 4):S67–S83. [DOI] [PubMed] [Google Scholar]
- 23.Jeffery R, Iserman E, Haynes RB, et al. Can computerized clinical decision support systems improve diabetes management? A systematic review and meta-analysis. Diabet Med. 2013;30(6):739–745. [DOI] [PubMed] [Google Scholar]
- 24.Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293(10):1223–1238. [DOI] [PubMed] [Google Scholar]
- 25.Wright A, Ash JS, Erickson JL, et al. A qualitative study of the activities performed by people involved in clinical decision support: recommended practices for success. JAMIA. 2014;21(3):464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan A, Fleegler EW. Using technology to improve adolescent healthcare. Curr Opin Pediatr. 2010;22(4):412–417. [DOI] [PubMed] [Google Scholar]
- 27.Johnson SL, Tandon SD, Trent M, et al. Use of technology with health care providers: perspectives from urban youth. J Pediatr. 2012;160(6):997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Diabetes Association. Children and Adolescents. Diabetes Care. 2015;38(Suppl 1):S70–S76. [DOI] [PubMed] [Google Scholar]
- 29.Anoshiravani A, Gaskin GL, Groshek MR, et al. Special requirements for electronic medical records in adolescent medicine. J Adolesc Health. 2012;51(5):409–414. [DOI] [PubMed] [Google Scholar]
- 30.Ralston JD, Revere D, Robins LS. Patients’ experience with a diabetes support programme based on an interactive electronic medical record: qualitative study. BMJ. 2004;328(7449):1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ralston JD, Coleman K, Reid RJ, et al. Patient experience should be part of meaningful-use criteria. Health Aff. 2010;29(4):607–613. [DOI] [PubMed] [Google Scholar]
- 32.Gray SH, Pasternak RH, Gooding HC, et al. Recommendations for electronic health record use for delivery of adolescent health care. J Adolesc Health. 2014;54(4):487–490. [DOI] [PubMed] [Google Scholar]
- 33.Esmaties E, Jansa M, Roca D, et al. The efficiency of telemedicine to optimize metabolic control in patients with type 1 diabetes mellitus: Telemed study. Diabetes Technol Ther. 2014;16(7):435–441. [DOI] [PubMed] [Google Scholar]
- 34.CMS CY2015 Final Physician Rule – Federal Register Vol. 79; National Physician Fee Schedule Relative Value File January 2015 release; Conversion Factor 35.7547.
- 35.Telgener PC, Lowe S. A look at the current reimbursement environment for continuous glucose monitoring (CGM): understanding the fundamentals. J Diabetes Sci Technol. 2008;2(4):681–684. [DOI] [PMC free article] [PubMed] [Google Scholar]