Abstract
Purpose of review
We review our current understanding of abnormal γ band oscillations in schizophrenia, their association with symptoms and the underlying cortical circuit abnormality, with a particular focus on the role of fast-spiking parvalbumin gamma-aminobutyric acid (GABA) neurons in the disease state.
Recent findings
Clinical electrophysiological studies of schizophrenia patients and pharmacological models of the disorder show an increase in spontaneous γ band activity (not stimulus-evoked) measures. These findings provide a crucial link between preclinical and clinical work examining the role of γ band activity in schizophrenia. MRI-based experiments measuring cortical GABA provides evidence supporting impaired GABAergic neurotransmission in schizophrenia patients, which is correlated with γ band activity level. Several studies suggest that stimulation of the cortical circuitry, directly or via subcortical structures, has the potential to modulate cortical γ activity, and improve cognitive function.
Summary
Abnormal γ band activity is observed in patients with schizophrenia and disease models in animals, and is suggested to underlie the psychosis and cognitive/perceptual deficits. Convergent evidence from both clinical and preclinical studies suggest the central factor in γ band abnormalities is impaired GABAergic neurotransmission, particularly in a subclass of neurons which express parvalbumin. Rescue of γ band abnormalities presents an intriguing option for therapeutic intervention.
Keywords: γ band oscillations, interneurons, N-methyl-D-aspartate receptor, parvalbumin, schizophrenia
INTRODUCTION
The purpose of this article is to review recent clinical and basic science findings concerning the relationship between schizophrenia and γ band oscillations (GBOs). Schizophrenia is a complex and devastating neuropsychiatric disorder, which is believed to stem from a combination of genetic, developmental, and environmental factors. Although often clinically identified by the appearance of positive symptoms (psychosis, hallucinations, and paranoia), schizophrenia patients also suffer from negative symptoms (flat affect, impaired attention, and motivation), and deficits in fundamental cognitive processes (working memory, executive function). Abnormal GBO have been postulated to be important in all these symptoms. There is a particularly strong clinical correlation between GBO and perceptual impairment/auditory hallucinations in schizophrenia patients [1]. More recent studies suggest a link between GBO activity and cognitive performance [2], supporting the long-standing hypothesis that GBO represent a key neural substrate for cognition [3,4]. Convergent evidence from preclinical and clinical experiments suggests that impaired inhibition mediated by fast-spiking parvalbumin positive neurons is central to these abnormalities. Current schizophrenia therapeutics, including both first and second-generation antipsychotics, do not adequately address many of the disease symptoms [5]. Thus, improved therapies which better address all schizophrenia symptoms are urgently required. Complex neuronal information processing is critically dependent on the precise spatial and temporal coordination of neural activity [6–8]. Thus, GBO activity offers an intriguing target for potential therapeutic intervention.
KEY POINTS.
MRI-based studies increasingly support impaired GABAergic neurotransmission in cortical regions of schizophrenia patients, and correlation of the degree of impairment with GBO activity level.
Broadband spontaneous (induced) GBO is elevated in the left STG of schizophrenia patients during presentation of 40 Hz auditory steady-state stimuli, which correlated with positive symptom severity, especially auditory hallucinations.
Ketamine treatment, modeling schizophrenia-like NMDAR hypofunction, increased broadband spontaneous (not stimulus-evoked, resting-state) GBO activity in healthy individuals, and increased functional connectivity between cortical and subcortical structures.
Recent clinical and preclinical findings suggest that stimulation of cortical circuitry, either directly or via subcortical projections, has the potential to generate/modulate GBO activity.
IMPORTANCE OF γ BAND OSCILLATION ACTIVITY IN BRAIN CIRCUIT COMMUNICATION AND ITS DYSFUNCTION IN SCHIZOPHRENIA
GBO activity occurs in the frequency range 30–100 Hz as recorded in scalp electroencephalography, centered at around 40 Hz. The clinically active psychiatrist may wonder why GBO are important for his/her knowledge, what function they serve, and how they enter into psychopathology. Functionally, GBO serve to unify and to make coherent, synchronous neuronal communication from one brain region to another. Coherence is important since, for example, when different sensory inputs are united to form a complete rendering of perception, neurons transmitting information about the visual aspects of the scene must discharge synchronously to register faithfully the visual aspects of the environment to be combined with the auditory and somatosensory inputs in the parietal cortex. This process is called feature binding and is an important example of how GBO activity functions to produce synchronous neuronal discharge [9].
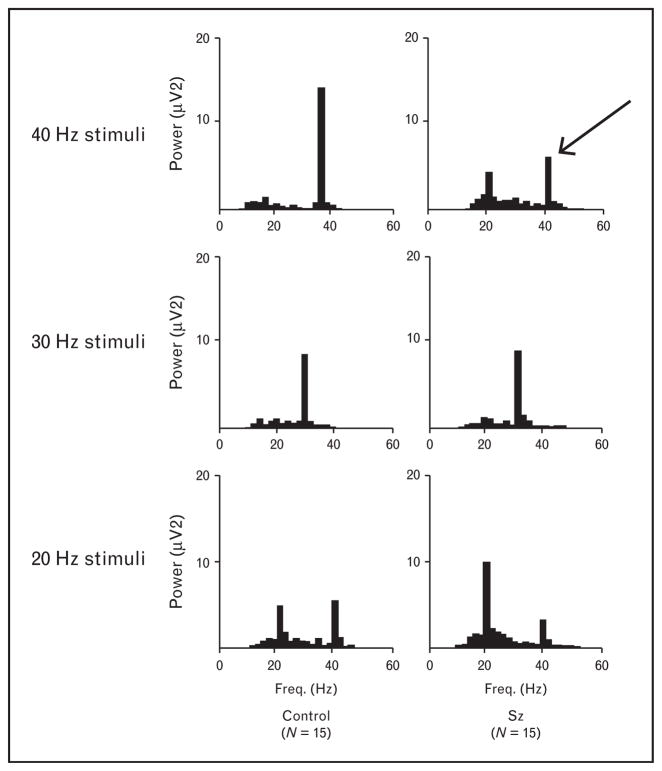
Imagine what would happen if information were not faithfully transmitted from one brain region to another – this would likely result in an ‘integration disorder’, which is aptly used as the Japanese term for what most Americans psychiatrists term ‘schizophrenia’. In our opinion, schizophrenia is best translated from the Greek as ‘shattered mind’. Indeed this viewpoint led us to rigorously measure the capacity of patients with schizophrenia to respond to GBO frequency stimuli using an auditory steady-state response (ASSR) protocol [10]. Here, we observed that there was a deficit in the in response 40 Hz audible clicks in the GBO range in schizophrenia patients in comparison to the response of healthy controls, whereas those at lower frequencies (30 and 20 Hz) were unaffected. We illustrate this early study in Fig. 1, since we believe it conveys simply and directly the results. This finding has been repeatedly confirmed [11,12], and has been cited in numerous publications.
FIGURE 1.
Auditory steady-state response is impaired in the γ band oscillation frequency range in schizophrenia patients. Figures show mean power spectra for EEGs recorded during trains of clicks at the following three stimulus rates: 40 Hz (upper), 30 Hz (middle), and 20 Hz (lower). Schizophrenia patients show decreased power at 40-Hz stimulation (arrow) compared with control study participants, while there was no difference between groups at 30 Hz stimulation. Note: controls show a prominent 40 Hz harmonic to 20 Hz stimulation, whereas schizophrenia patients do not, further evidence of a 40 Hz impairment. (Adapted from Kwon et al. [10].)
The clinical implications and basic science-related studies of GBO have become a topic of intense interest; a PubMed search in December 2015 on ‘γ frequency and schizophrenia’ showed 301 cited papers, with only six published prior to the study by Kwon et al. [10] and its presentation at national conferences. Magnetoencephalographic (MEG) studies confirm the GBO abnormality in schizophrenia, showing reduced amplitude at 80 Hz, the higher frequency GBO, as well as 40 Hz [13]. Generally, the ASSR phase locking factor (PLF) has been found to be superior in sensitivity to a Fourier power analysis in analyzing the ASSR – the PLF measures the consistency of time-locked responses to each individual stimulus in the ASSR and is reduced in schizophrenia. Importantly, the ASSR abnormality in schizophrenia appears not to be an artifact of medication [14] and is reduced in unaffected and psychotropic medication naïve 1st degree relatives of schizophrenia patients [15]. Psychotic bipolar patients also show GBO deficits but do not show the schizophrenia-specific lateralized pattern of greater deficits in the left hemisphere, where numerous MRI studies show a similarly left-lateralized reduction of cortical gray matter [14].
The research psychiatrist will find GBO to be a superb topic for the research domain criteria format, as described in the 2011 National Institute of Mental Health cognitive systems workshop proceedings [16]. This is because it is an example of a multilevel neural circuit construct having defined relationship with molecules, neurons, physiology and symptoms; moreover, it crosses diagnostic boundaries with GBO abnormality being present in autism, Alzheimer’s, and other neuropsychiatric disorders. Furthermore, in schizophrenia patients the GBO response is abnormal not only with auditory stimuli but also during processing of visual gestalt stimuli, such as illusory squares [17]. Here, the GBO occur at a lower frequency and with a lower degree of phase locking, a feature which is associated with slower reaction time and symptoms of visual hallucinations, thought disorder, and disorganization. Taylor et al. [18] analyzed the early auditory GBO occurring during presentation of standard (noncounted) stimuli during the auditory oddball P300 paradigm in which the counted oddball stimuli differ in frequency from the standard stimuli; they found both reduced power and lower PLF in first-episode schizophrenia, confirming previous studies in patients with chronic schizophrenia. No abnormalities were found in a similar study of individuals at clinical high risk for psychosis [19]. Of interest is the temporal overlap of this simple stimulus with the timing of the P1 and P50 evoked potentials suggesting that GBO play a role even in simple sensory stimuli whose dysfunction may be responsible for the P50 failure to gate in schizophrenia. Indeed MEG studies found both left hemisphere GBO abnormalities in response to speech sounds [20] and also left hemisphere failure to gate P50 to speech, a feature that was associated with auditory hallucinations [21].
A new and important line of clinical research asks what is the level of ‘induced’ GBO in patients with schizophrenia [22▪]. Induced (nonphase locked to stimuli) GBO activity represents that which is not directly evoked by stimuli. Here, they confirmed a reduction in the evoked response to 40 Hz stimulation but, importantly, also observed an increase in 30–100 Hz spontaneous (non-evoked) broadband power in patients during performance of the ASSR task, but not during rest, with a negative correlation between the spontaneous broadband power and the PLF of the 40 Hz ASSR computed as originating in the auditory cortex of the left superior temporal gyrus (STG) by a dipole model. This suggested the possibility of an increased broadband noise in the γ generating neuronal circuit, a possibility that is addressed in the N-methyl-D-aspartate (NMDA) and ketamine discussion below. In this Hirano et al. [22▪] study the degree of auditory hallucinations was positively correlated with the degree of spontaneous (induced) left hemisphere sources of the 40 Hz ASSR. Moreover, the failure to find a significant increase in spontaneous broadband power during rest raised the possibility of a heightened wakefulness-induced increase in the responsible neural circuit, whose origin we will discuss below.
IMPAIRED GABAergic INTERNEURONAL ACTIVITY LEADS TO ABNORMAL γ BAND OSCILLATIONS
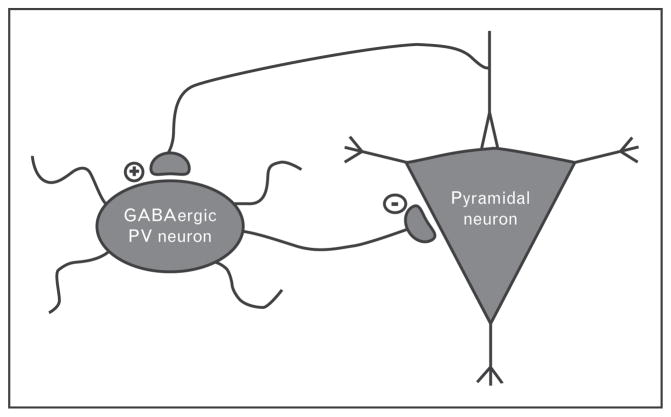
Numerous studies suggest that GBO abnormalities underlie both the psychosis and impairments in higher cognitive function associated with this disease [23–26], and have additionally been linked to negative schizophrenia symptoms [27]. Therefore, GBO provides an intriguing target for novel therapeutic strategies. The abnormalities in GBO observed in schizophrenia are believed to be caused by impairments to the cortical circuitry responsible for their generation [26]. Such oscillations are primarily governed by the complex interplay between excitatory pyramidal cells, and inhibitory GABA-ergic interneurons (see Fig. 2). Although these two neuronal subtypes make up the core of the cortical circuit, a number of factors serve to modulate the ability of the local neuronal population to entrain to synchronous activity.
FIGURE 2.
Oversimplified model of the cortical circuitry responsible for generating of γ oscillations. The cortical circuitry consists principally of excitatory pyramidal neurons and inhibitory GABAergic interneurons. The behavior of these circuits is defined by the interaction of these two neuronal subtypes. Of particular interest are interneurons containing the calcium-binding protein parvalbumin (PV), which appear to be essential for the generation of rhythmic activity because of their fast-action firing as well as the perisomatic location of their synapses. Postmortem studies have found altered parvalbumin, GAD67, and NMDA receptor subunit levels in these neurons in individuals with schizophrenia.
Of particular importance are a subclass of cortical GABAergic interneurons which express the calcium-binding protein parvalbumin (parvalbumin neurons). Convergent lines of evidence from a number of studies suggests parvalbumin neurons play a central role in synchronizing neuronal network activity, and appear to be functionally impaired in schizophrenia [26,28]. The fast action potential firing capabilities of parvalbumin neurons allow them to fire at a rate capable of entraining GBO [29]. As such, in in-vitro studies parvalbumin neurons have been observed to fire with almost every cycle of GBO [30–33]. Cortical parvalbumin neurons possess mutual inhibitory innervation, and are further connected via gap junctions [34,35]. Further, cortical parvalbumin neurons show extensive axonal arborization, forming perisomatic GABAergic terminals on a large subset of pyramidal neurons (>200) [36]. Together, these properties allow the recruitment of parvalbumin neurons via phasic inputs from pyramidal neurons, which provides strong and fast feedback inhibition, generating near synchronous inhibition of a large subset of excitatory and inhibitory neurons in the local network [37].
Numerous studies have reported schizophrenia-like abnormalities in transgenic mice with disruption targeting cortical parvalbumin neuron function [38–42]. More directly, in-vivo optogenetic techniques have been employed to either inhibit or drive parvalbumin neuron activity [43,44]. These studies clearly indicate that the activity of cortical parvalbumin neurons is central to the generation of GBO activity in mice.
POSTMORTEM STUDIES OF GABA NEURONS
Postmortem data from a number of studies consistently point toward the involvement of the GABA system in neural circuit dysfunction in schizophrenia [45]. A consistently replicated postmortem finding in the cortex of individuals with schizophrenia is the reduced expression of mRNA encoding the 67-kD isoform of glutamic acid decarboxylase (GAD67), the enzyme principally responsible for the synthesis of GABA. This deficit is prominent in the parvalbumin subset of GABA neurons and conserved with similar magnitude across multiple cortical regions [46▪,47,48]. GAD67 expression has been found to be undetectable in ~50% of parvalbumin interneurons in cortical tissue from individuals with schizophrenia [49]. Further, decreased parvalbumin mRNA expression and altered GABA receptor expression has also been observed to be lower in cortical parvalbumin neuron in schizophrenia patients [50]. As the expression of GAD67 and parvalbumin expression appear to be activity dependent, their decreased expression in schizophrenia may represent a compensatory change resultant from impaired activity of parvalbumin neurons caused by other upstream factors [51,52]. Taken together, the above postmortem findings suggest a weakening of parvalbumin neuron mediated GABAergic inputs to pyramidal neurons in schizophrenia.
Given these findings, it is not surprising that a prominent hypothesis in the field is the presence of a reduction in GABAergic neurotransmission in patients with schizophrenia, which may be linked to cognitive and perceptual impairments. Indeed, clinical studies have shown that decreasing GABA-ergic neurotransmission exacerbated symptoms in schizophrenia patients [53]. Conversely, increasing GABAergic neurotransmission increased evoked GBO activity in a cognitive control experimental paradigm [54].
MRI AND BRAIN GABA MEASUREMENTS
Recently, a number of groups have utilized magnetic resonance spectroscopy (MRS) to measure GABA in specific brain regions of interest (ROI). Table 1 compares subjects with schizophrenia vs. healthy controls on MRS GABA measurements within specific ROI (dlPFC and mPFC = dorsolateral and medial prefrontal cortex, respectively) [55–60]. The table and Kegeles et al.’s [60] study indicate antipsychotic medication is a potential serious confound. A more detailed review of MRS in schizophrenia is in [61]. It should be noted that, while postmortem measurements can specify which neurotransmitter-specific neuronal cell types are deficient in neurotransmission relevant enzymes, MRS measures all GABA within the ROI; some GABA concentrations are directly relevant to neurotransmission, such as vesicular GABA, but others do not have clear information as to their relevance, such as those in the cytoplasmic pool. Despite these limitations, several such studies have suggested lower cortical GABA levels in individuals with schizophrenia [56,59], and that frontal lobe GABA levels are correlated with working memory performance [55].
Table 1.
Magnetic resonance spectroscopy GABA in schizophrenia
| Study | Medication | Regions of interest | Schizophrenia vs. healthy controls |
|---|---|---|---|
| Goto et al. (2009) [55] | + | Striatum | Decreased |
| Yoon et al. (2010) [56] | + | Occipital | Decreased |
| Tayoshi et al. (2010) [57] | + | Striatum | Same |
| Ongur et al. (2010) [58] | + | mPFC | Increased |
| Rowland et al. (2013) [59] | + | mPFC | Decreased |
| Kegeles et al. (2012) [60] | − | mPFC | Increased |
| Kegeles et al. (2012) [60] | + | mPFC | Same |
| Kegeles et al. (2012) [60] | − | dlPFC | Same |
| Kegeles et al. (2012) [60] | + | dlPFC | Same |
dlPFC, dorsolateral prefrontal cortex; mPFC, medial prefrontal cortex.
To attempt to provide information on in-vivo neurotransmission of GABA, Frankle and coworkers [46▪] gave patients and controls tiagabine, a GABA membrane transport blocker to increase extracellular GABA, and measured the binding of [11C] flumazenil a specific positron emission tomography ligand for GABAA receptors before and after tiagabine. There was blunting of the effect of tiagabine in antipsychotic naïve patients. The authors interpreted this finding as suggesting that low levels of GABA led to upregulation of receptors as a potential compensatory mechanism. Of note, GBO activity was correlated with the degree of baseline binding in the antipsychotic naïve group. However, as noted in an editorial accompanying this study, there are some methodological issues and also alternative explanations for the findings that need to be considered [62].
N-METHYL-D-ASPARTATE RECEPTOR HYPOFUNCTION MAY LEAD TO γ BAND OSCILLATION ABNORMALITIES
In this section, we focus on glutamatergic input to cortical parvalbumin neurons. Although the above findings suggest a GABAergic basis for GBO abnormalities in schizophrenia, increasing evidence indicates that abnormal glutamatergic neurotransmission may play an upstream role; particularly that mediated by the N-methyl-D-aspartate receptor (NMDAR) on parvalbumin neurons (see Fig. 2). The NMDAR hypofunction theory of schizophrenia stems from early findings that NMDAR antagonists (e.g., ketamine and phencyclidine) produce symptoms similar to a psychotic episode in healthy humans and exacerbate symptoms of schizophrenia patients [63–66]. More recently large-scale genetic studies have found that genes related to glutamatergic, and not GABAergic, neuronal signaling are related to schizophrenia susceptibility [67]. Further, postmortem studies of schizophrenia patients have found evidence for altered NMDAR expression specific to cortical parvalbumin neurons [68]. Specifically, lower expression of the NMDA receptor subunit 2A was found in dorsolateral prefrontal cortex parvalbumin neurons in schizophrenia individuals [69]. Additionally, several lines of evidence suggest that NMDAR has a more robust effect on cortical parvalbumin neurons than pyramidal cells [70,71]. Thus, NMDAR hypofunction may underlie a number of the core features of schizophrenia.
Abnormal GBO activity has been observed in both pharmacological as well as genetic NMDAR hypofunction models of schizophrenia [5,26,72]. This work supports the hypothesis that NMDAR on parvalbumin neurons can function as sensors of local pyramidal neuron activity [73]. In brief, parvalbumin neuron NMDAR hypofunction, as suggested to occur in schizophrenia, would be sensed by parvalbumin neurons as a lack of pyramidal neuron activity, resulting in a compensatory decreased inhibitory tone. Initially, this would lead to disinhibition of pyramidal neurons, likely increasing GBO. Recent MRS findings provide clinical support for this hypothesis, as elevated glutamate levels have been observed in early-stage schizophrenia patients [74]. Over time increased pyramidal cell excitation may lead to changes in the synaptic connectivity of local cortical circuits or even excitotoxic neural degeneration [26,75]. Additionally, schizophrenia-related NMDAR hypofunction may impair the normal developmental maturation and/or maintenance of excitatory synaptic inputs received by cortical parvalbumin neurons [50].
Although the literature examining GBO abnormalities in schizophrenia is quite complex, the majority of clinical studies have found impaired GBO in response to stimuli or during the performance of cognitive tasks [1]. This directly contradicts the findings from the majority of pharmacologic and genetic animal studies modeling schizophrenia-like NMDAR hypofunction which observe an increase in GBO activity. This disparity likely stems from the fact that most clinical studies follow GBO evoked by stimuli, as opposed to modeling work, which focuses mainly on spontaneous, or nonstimulus originating GBO. Spontaneous GBO activity can be further subdivided into activity that is occurring at rest (resting-state GBO), and induced GBO activity, which is activity that occurs during, but not phase-locked to a stimulus (see [22▪]). This level of complexity illustrates the importance of clearly defining the type of GBO one is examining. The idea that schizophrenia affects these distinct aspects of GBO differently is supported by recent basic science and clinical work. Several studies of in-vivo models of NMDA hypofunction have observed both increased spontaneous GBO and decreased task-evoked GBO power [76,77]. Additionally, as described above, clinical ASSR findings reveal increased spontaneous (induced) GBO activity together with reduced stimulus evoked during ASSR stimulation [22▪,78].
As a further attempt to establish the link between preclinical findings and clinical results from schizophrenia patients, a recent study has investigated the impact of acute ketamine on spontaneous (resting-state) γ activity in MEG recordings from healthy individuals [79▪]. Here, the authors observed a pronounced increase in broadband power across the GBO frequency range (30–90 Hz), and a strong reduction in β-band activity. These findings are in agreement with recently published work examining the effects of ketamine in vivo in rodents [80], as well as preliminary findings from our group [81]. Interestingly, Rivolta et al. [79▪] also report an increase in functional connectivity following ketamine treatment, which suggests that the increased spontaneous GBO they observe is generated/modulated by both cortical and subcortical areas. They speculate that a reduction in the GABA release from parvalbumin neurons in the thalamic reticular nucleus would lead to increased firing of relay neurons in other thalamic nuclei. This could result in a pathological activation of thalamocortical circuits, leading to widespread shift in excitability levels and increased broadband GBO. The role of subcortical regions in GBO has been proposed by a number of groups [82–84], and the potential of these regions to modulate cortical GBO activity will be discussed below.
POTENTIAL FOR RESCUE OF NMDA HYPOFUNCTION-INDUCED CORTICAL CIRCUIT DYSFUNCTION
As suggested by the above studies, NMDA hypofunction can alter the delicate balance in activity between the inhibitory and excitatory components of the cortical circuitry (E/I balance), principally through reducing the activity of parvalbumin neurons. As discussed, the increase in background GBO during auditory steady state stimulation in schizophrenia was interpreted by Hirano et al. [22▪] as a disruption in E/I balance. Such a disruption could increase background GBO activity leading to a decrease in the signal to noise ratio in cortical circuit activity, which may interfere with incoming sensory information and impact the ability to properly distinguish salient from nonsalient information [26]. Clinical MEG studies have shown excessive spreading of neural activity as indexed by event-related fields during sensory processing in first-episode schizophrenia patients supporting this idea of inappropriate cortical circuit activation [85]. Thus, abnormal E/I balance may constitute a pathophysiological mechanism which underlies impaired cognition and other clinical symptoms of schizophrenia.
Excitingly, several studies suggest the potential to restore/reset the E/I balance in the cortex, and improve schizophrenia-related cognitive impairment. Cho and colleagues [86▪] recently utilized a transgenic mouse line with impaired expression of the transcription factors Dlx5 and Dlx6. These transcription factors are involved in development of GABAergic neurons derived from the medial ganglionic eminence, which includes cortical parvalbumin neurons. Here, they found that in transgenic mice where the activity of the transcription factors Dlx5/6 was impaired, parvalbumin interneuronal activity became abnormal following adolescence, similar to the age of onset of schizophrenia symptoms in patients. This effect was coupled with a reduction in evoked GBO activity, and the onset of cognitive inflexibility, shown using an intradimensional set-shifting behavioral task (animal analogue of the clinical Wisconsin card sorting task). Interestingly, they found that optogenetic stimulation of cortical parvalbumin neurons at GBO frequencies (40 Hz) was capable of restoring cognitive flexibility both immediately and days following such treatment. Although questions remain concerning the relevance of the animal model used in this study to the schizophrenia disease state, their findings directly implicate GBO activity to schizophrenia-like cognitive deficits, and suggest a therapeutic role for direct PFC interneuron-driven GBO that has the potential to provide long-lasting procognitive effect.
Although the Cho et al. study examined the effects of directly stimulating cortex to rescue abnormal GBO, subcortical structures can also play a role in generation and modulation of such activity [79▪,83]. The bulk of this research focuses on the thalamus and associated structures. However, recent evidence suggests that the basal forebrain plays a role in promoting cortical GBO. The basal forebrain region is involved in cortical activation and attention [87]. Cortically projecting basal forebrain GABAergic neurons containing parvalbumin are one component of this system. Recent work from our group has revealed that these wake-active neurons target cortical parvalbumin neurons [88▪], giving them the power to directly influence cortical circuit activity. In fact, optogenetic stimulation specific to basal forebrain parvalbumin neurons in mice preferentially increased cortical GBO power by entraining a cortical oscillator with a resonant frequency of ~40 Hz, in both rhythmic and nonrhythmic stimulation paradigms. In addition, our preliminary findings revealed that tonic low-level optogenetic excitation of basal forebrain parvalbumin neurons was capable of increasing spontaneous broadband GBO activity, whereas inhibition of these neurons partially rescued the increase in broadband, spontaneous GBO elicited by systemic ketamine [81]. These finding suggest that basal forebrain parvalbumin neurons may represent a novel therapeutic target to rescue impaired E/I balance, and abnormal GBO in schizophrenia without direct manipulation of the cortical circuitry. Schizophrenia impairments span numerous cortical regions. Thus, targeting stimulation of subcortical structures, which project to multiple cortical regions, may provide a more effective means for restoration of E/I balance than direct stimulation of individual cortical structures.
The concept of rescuing abnormal GBO activity and impaired E/I balance is supported by several recent clinical studies. First, cognitive remediation therapy findings in schizophrenia patients showed that training which targets auditory and verbal learning enhanced sensory response in both the auditory and dorsolateral prefrontal cortex, accompanied by an increase in evoked GBO [89]. This neural circuit enhancement was additionally associated with an increase in executive function. Second, brain stimulation via repetitive transcranial magnetic stimulation (rTMS) has been employed to rescue cognitive impairment [90]. Such studies have shown that rTMS can be employed to modulate GBO activity in the dorsolateral prefrontal cortex [91]. More recently, bilateral cortex rTMS was observed to improve performance in a visual n-back task (pre vs. post) in schizophrenia patients [92]. These studies provide evidence that rTMS and potentially other stimulation paradigms could be used to manipulate GBO activity in the cortex, and restore cognitive impairment in schizophrenia. However, it remains essential to further investigate the treatment paradigms and targets of such intervention, to determine if they provide long-lasting and clinically relevant improvement in cognition [24].
CONCLUSION
There is now considerable evidence supporting the role of abnormal GBO in schizophrenia and GBO as a potential target for symptom remediation. However, numerous preclinical and clinical research questions remain. Perhaps the most obvious is a demonstration of the association of reduced left STG and Heschl gyrus MRI gray matter volume with abnormalities in spontaneous and induced GBO. Moreover, a direct functional association of MRS with electrophysiological measures of evoked GBO would be important. Additionally, further research opportunities exist in better describing the mechanisms underlying the development of the impaired parvalbumin neuron activity and other cortical circuit abnormalities which lead to abnormal GBO in schizophrenia.
Acknowledgments
None.
Financial support and sponsorship
The review was supported by the Department of Veterans Affairs (VA Merit Award to R.W.M., and VA Career Development Award to J.M.M.) and National Institute of Health R01 MH039683 to R.W.M.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 2.Herman AB, Houde JF, Vinogradov S, Nagarajan SS. Parsing the phonological loop: activation timing in the dorsal speech stream determines accuracy in speech reproduction. J Neurosci. 2013;33:5439–5453. doi: 10.1523/JNEUROSCI.1472-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llinas RR, Pare D. Of dreaming and wakefulness. Neuroscience. 1991;44:521–535. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- 4.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 5.Pratt J, Winchester C, Dawson N, Morris B. Advancing schizophrenia drug discovery: optimizing rodent models to bridge the translational gap. Nat Rev Drug Discov. 2012;11:560–579. doi: 10.1038/nrd3649. [DOI] [PubMed] [Google Scholar]
- 6.Fries P, Nikolic D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Traub RD, Whittington MA, Stanford IM, Jefferys JG. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–624. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- 8.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 9.Fries P, Roelfsema PR, Engel AK, et al. Synchronization of oscillatory responses in visual cortex correlates with perception in interocular rivalry. Proc Natl Acad Sci U S A. 1997;94:12699–12704. doi: 10.1073/pnas.94.23.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon JS, O’Donnell BF, Wallenstein GV, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner CA, Kieffaber PD, Clementz BA, et al. Event-related potential abnormalities in schizophrenia: a failure to ‘gate in’ salient information? Schizophr Res. 2009;113:332–338. doi: 10.1016/j.schres.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamm JP, Gilmore CS, Picchetti NA, et al. Abnormalities of neuronal oscillations and temporal integration to low- and high-frequency auditory stimulation in schizophrenia. Biol Psychiatry. 2011;69:989–996. doi: 10.1016/j.biopsych.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuchimoto R, Kanba S, Hirano S, et al. Reduced high and low frequency gamma synchronization in patients with chronic schizophrenia. Schizophr Res. 2011;133:99–105. doi: 10.1016/j.schres.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rass O, Forsyth JK, Krishnan GP, et al. Auditory steady state response in the schizophrenia, first-degree relatives, and schizotypal personality disorder. Schizophr Res. 2012;136:143–149. doi: 10.1016/j.schres.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NIMH cognitive systems workshop proceedings; 2011 October 23–25; 2011. [Google Scholar]
- 17.Spencer KM, Nestor PG, Perlmutter R, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor GW, McCarley RW, Salisbury DF. Early auditory gamma band response abnormalities in first hospitalized schizophrenia. Suppl Clin Neurophysiol. 2013;62:131–145. doi: 10.1016/b978-0-7020-5307-8.00009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez VB, Roach BJ, Woods SW, et al. Early auditory gamma-band responses in patients at clinical high risk for schizophrenia. Suppl Clin Neurophysiol. 2013;62:147–162. doi: 10.1016/b978-0-7020-5307-8.00010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirano S, Hirano Y, Maekawa T, et al. Abnormal neural oscillatory activity to speech sounds in schizophrenia: a magnetoencephalography study. J Neurosci. 2008;28:4897–4903. doi: 10.1523/JNEUROSCI.5031-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirano Y, Hirano S, Maekawa T, et al. Auditory gating deficit to human voices in schizophrenia: a MEG study. Schizophr Res. 2010;117:61–67. doi: 10.1016/j.schres.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 22▪.Hirano Y, Oribe N, Kanba S, et al. Spontaneous gamma activity in schizophrenia. JAMA Psychiatry. 2015;72:813–821. doi: 10.1001/jamapsychiatry.2014.2642. The study examined both spontaneous GBO and evoked GBO activity in the auditory cortex of schizophrenia patients. Compared to healthy controls, spontaneous (induced) GBO activity was increased during 40 Hz auditory state stimulation, potentially contributing to the observed deficit in evoked GBO response. The authors suggest that this effect reflects an imbalance of excitation and inhibition in cortical circuitry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senkowski D, Gallinat J. Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biol Psychiatry. 2015;77:1010–1019. doi: 10.1016/j.biopsych.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 25.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNally JM, McCarley RW, Brown RE. Impaired GABAergic neurotransmission in schizophrenia underlies impairments in cortical gamma band oscillations. Curr Psychiatry Rep. 2013;15:346. doi: 10.1007/s11920-012-0346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77:1031–1040. doi: 10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penttonen M, Kamondi A, Acsady L, Buzsaki G. Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur J Neurosci. 1998;10:718–728. doi: 10.1046/j.1460-9568.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- 30.Gloveli T, Dugladze T, Saha S, et al. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol. 2005;562(Pt 1):131–147. doi: 10.1113/jphysiol.2004.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulyas AI, Szabo GG, Ulbert I, et al. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30:15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tukker JJ, Lasztoczi B, Katona L, et al. Distinct dendritic arborization and in vivo firing patterns of parvalbumin-expressing basket cells in the hippocampal area CA3. J Neurosci. 2013;33:6809–6825. doi: 10.1523/JNEUROSCI.5052-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hajos N, Palhalmi J, Mann EO, et al. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci. 2004;24:9127–9137. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hormuzdi SG, Pais I, LeBeau FE, et al. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron. 2001;31:487–495. doi: 10.1016/s0896-6273(01)00387-7. [DOI] [PubMed] [Google Scholar]
- 35.Traub RD, Whittington MA, Buhl EH, et al. A possible role for gap junctions in generation of very fast EEG oscillations preceding the onset of, and perhaps initiating, seizures. Epilepsia. 2001;42:153–170. doi: 10.1046/j.1528-1157.2001.26900.x. [DOI] [PubMed] [Google Scholar]
- 36.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 37.Hajos N, Paulsen O. Network mechanisms of gamma oscillations in the CA3 region of the hippocampus. Neural Netw. 2009;22:1113–1119. doi: 10.1016/j.neunet.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Belforte JE, Zsiros V, Sklar ER, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlen M, Meletis K, Siegle JH, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Pino I, Garcia-Frigola C, Dehorter N, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79:1152–1168. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Korotkova T, Fuchs EC, Ponomarenko A, et al. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Billingslea EN, Tatard-Leitman VM, Anguiano J, et al. Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacology. 2014;39:1603–1613. doi: 10.1038/npp.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardin JA, Carlen M, Meletis K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benes FM. The GABA system in schizophrenia: cells, molecules and microcircuitry. Schizophr Res. 2015;167:1–3. doi: 10.1016/j.schres.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 46▪.Frankle WG, Cho RY, Prasad KM, et al. In vivo measurement of GABA transmission in healthy subjects and schizophrenia patients. Am J Psychiatry. 2015;172:1148–1159. doi: 10.1176/appi.ajp.2015.14081031. The study utilized a novel positron emission tomography paradigm to investigate the physiological properties of GABAergic neurotransmission in schizophrenia patients. The authors demonstrated in-vivo GABAergic impairment in schizophrenia individuals (particularly antipsychotic naïve), which appear to be linked to abnormal GBO and cognition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curley AA, Arion D, Volk DW, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto T, Volk DW, Eggan SM, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woo TU, Spencer K, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010;18:173–189. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benson DL, Huntsman MM, Jones EG. Activity-dependent changes in GAD and preprotachykinin mRNAs in visual cortex of adult monkeys. Cereb Cortex. 1994;4:40–51. doi: 10.1093/cercor/4.1.40. [DOI] [PubMed] [Google Scholar]
- 52.Volk DW, Lewis DA. Prenatal ontogeny as a susceptibility period for cortical GABA neuron disturbances in schizophrenia. Neuroscience. 2013;248:154–164. doi: 10.1016/j.neuroscience.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahn K, Gil R, Seibyl J, et al. Probing GABA receptor function in schizophrenia with iomazenil. Neuropsychopharmacology. 2011;36:677–683. doi: 10.1038/npp.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewis DA, Cho RY, Carter CS, et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goto N, Yoshimura R, Moriya J, et al. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophr Res. 2009;112:192–193. doi: 10.1016/j.schres.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 56.Yoon JH, Maddock RJ, Rokem A, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tayoshi S, Nakataki M, Sumitani S, et al. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr Res. 2010;117:83–91. doi: 10.1016/j.schres.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Ongur D, Prescot AP, McCarthy J, et al. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry. 2010;68:667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rowland LM, Edden RA, Kontson K, et al. GABA predicts inhibition of frequency-specific oscillations in schizophrenia. J Neuropsychiatry Clin Neurosci. 2013;25:83–87. doi: 10.1176/appi.neuropsych.11120368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kegeles LS, Mao X, Stanford AD, et al. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- 61.Wijtenburg SA, Yang S, Fischer BA, Rowland LM. In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: application to schizophrenia. Neurosci Biobehav Rev. 2015;51:276–295. doi: 10.1016/j.neubiorev.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abi-Dargham A. Imaging the ‘GABA Shift’ in Schizophrenia. Am J Psychiatry. 2015;172:1062–1063. doi: 10.1176/appi.ajp.2015.15081088. [DOI] [PubMed] [Google Scholar]
- 63.Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 64.Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the non-competitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 65.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 66.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woo TU, Shrestha K, Amstrong C, et al. Differential alterations of kainate receptor subunits in inhibitory interneurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Schizophr Res. 2007;96:46–61. doi: 10.1016/j.schres.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bitanihirwe BK, Lim MP, Kelley JF, et al. Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC Psychiatry. 2009;9:71. doi: 10.1186/1471-244X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grunze HC, Rainnie DG, Hasselmo ME, et al. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bubenikova-Valesova V, Horacek J, Vrajova M, Hoschl C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci Biobehav Rev. 2008;32:1014–1023. doi: 10.1016/j.neubiorev.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 73.Lisman JE, Coyle JT, Green RW, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marsman A, van den Heuvel MP, Klomp DW, et al. Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophr Bull. 2013;39:120–129. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olney JW, Labruyere J, Wang G, et al. NMDA antagonist neurotoxicity: mechanism and prevention. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- 76.Saunders JA, Gandal MJ, Siegel SJ. NMDA antagonists recreate signal-to-noise ratio and timing perturbations present in schizophrenia. Neurobiol Dis. 2012;46:93–100. doi: 10.1016/j.nbd.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gandal MJ, Sisti J, Klook K, et al. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl Psychiatry. 2012;2:e142. doi: 10.1038/tp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spencer KM. Baseline gamma power during auditory steady-state stimulation in schizophrenia. Front Hum Neurosci. 2011;5:190. doi: 10.3389/fnhum.2011.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79▪.Rivolta D, Heidegger T, Scheller B, et al. Ketamine dysregulates the amplitude and connectivity of high-frequency oscillations in cortical-subcortical networks in humans: evidence from resting-state magnetoencephalography-recordings. Schizophr Bull. 2015;41:1105–1114. doi: 10.1093/schbul/sbv051. Study examined the effects of the NMDAR antagonist, ketamine, on resting-state activity neural activity in MEG recordings from healthy individuals. Ketamine was found to significantly increase resting-state power across the GBO frequency range (30–90 Hz). This increase was coupled with an elevation of information transfer in the thalamocortical network. This work provides a strong link between clinical schizophrenia studies and preclinical work utilizing NMDAR hypofunction models of schizophrenia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lazarewicz MT, Ehrlichman RS, Maxwell CR, et al. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2010;22:1452–1464. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- 81.McNally JM, Thankachan S, McCarley RW, Brown RE. Neuroscience Meeting Planner 2015. Chicago, Illinois: Society for Neuroscience; 2015. Optogenetic and pharmacological manipulation of cortical excitatory/inhibitory balance: rescuing the effects of acute ketamine on cortical gamma band oscillations. Program No. 479.24. [Google Scholar]
- 82.Hunt MJ, Falinska M, Leski S, et al. Differential effects produced by ketamine on oscillatory activity recorded in the rat hippocampus, dorsal striatum and nucleus accumbens. J Psychopharmacol. 2011;25:808–821. doi: 10.1177/0269881110362126. [DOI] [PubMed] [Google Scholar]
- 83.Pinault D. N-methyl D-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 84.Phillips KG, Cotel MC, McCarthy AP, et al. Differential effects of NMDA antagonists on high frequency and gamma EEG oscillations in a neurodevelopmental model of schizophrenia. Neuropharmacology. 2012;62:1359–1370. doi: 10.1016/j.neuropharm.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 85.Rivolta D, Castellanos NP, Stawowsky C, et al. Source-reconstruction of event-related fields reveals hyperfunction and hypofunction of cortical circuits in antipsychotic-naive, first-episode schizophrenia patients during Mooney face processing. J Neurosci. 2014;34:5909–5917. doi: 10.1523/JNEUROSCI.3752-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86▪.Cho KK, Hoch R, Lee AT, et al. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dl × 5/6(+/−) mice. Neuron. 2015;85:1332–1343. doi: 10.1016/j.neuron.2015.02.019. The work provides the most direct evidence to date suggesting that impaired development of cortical parvalbumin neurons contribute to schizophrenia-like abnormal GBO activity and cognitive impairment. Further, the authors demonstrate that direct stimulation of the prefrontal cortical circuitry, particularly 40 Hz GBO, has therapeutic potential value. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown RE, Basheer R, McKenna JT, et al. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88▪.Kim T, Thankachan S, McKenna JT, et al. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci U S A. 2015;112:3535–3540. doi: 10.1073/pnas.1413625112. In mice, basal forebrain parvalbumin neurons were found to project cortically, and synapse principally with cortical parvalbumin neurons. Steady-state optogenetic stimulation of basal forebrain parvalbumin neurons was capable of entraining cortical oscillations at the stimulation frequency, with a preference for the resonant frequency of ~40 Hz. These findings suggest that basal forebrain parvalbumin neurons may represent a novel therapeutic target to treat disorders involving abnormal GBO, such as schizophrenia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dale CL, Brown EG, Fisher M, et al. Auditory cortical plasticity drives training-induced cognitive changes in schizophrenia. Schizophr Bull. 2016;42:220–228. doi: 10.1093/schbul/sbv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guse B, Falkai P, Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J Neural Transm (Vienna) 2010;117:105–122. doi: 10.1007/s00702-009-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Farzan F, Barr MS, Sun Y, et al. Transcranial magnetic stimulation on the modulation of gamma oscillations in schizophrenia. Ann N Y Acad Sci. 2012;1265:25–35. doi: 10.1111/j.1749-6632.2012.06543.x. [DOI] [PubMed] [Google Scholar]
- 92.Barr MS, Farzan F, Rajji TK, et al. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry. 2013;73:510–517. doi: 10.1016/j.biopsych.2012.08.020. [DOI] [PubMed] [Google Scholar]