Abstract
PURPOSE
To compare the late complications in the Ahmed Baerveldt Comparison Study during 5 years of follow-up.
DESIGN
Multicenter, prospective, randomized clinical trial.
METHODS
SETTINGS
Sixteen international clinical centers.
STUDY POPULATION
Two hundred seventy six subjects aged 18 to 85 years with previous intraocular surgery or refractory glaucoma with intraocular pressure of > 18 mmHg.
INTERVENTIONS
Ahmed Glaucoma Valve FP7 or Baerveldt Glaucoma Implant BG 101-350.
MAIN OUTCOME MEASURES
Late postoperative complications (beyond 3 months), reoperations for complications, and decreased vision from complications.
RESULTS
Late complications developed in 56 subjects (46.8 ± 4.8 5 year cumulative % ± SE) in the Ahmed Glaucoma Valve group and 67 (56.3 ± 4.7 5 year cumulative % ± SE) in the Baerveldt Glaucoma Implant group (P = 0.082). The cumulative rates of serious complications were 15.9% and 24.7% in the Ahmed Glaucoma Valve and Baerveldt Glaucoma Implant groups respectively (P = 0.034) although this was largely driven by subjects who had tube occlusions in the two groups (0.8% in the Ahmed Glaucoma Valve group and 5.7% in the Baerveldt Glaucoma Implant group, P = 0.037). Both groups had a relatively high incidence of persistent diplopia (12%) and corneal edema (20%), although half of the corneal edema cases were likely due to pre-existing causes other than the aqueous shunt. The incidence of tube erosion was 1% and 3% in the Ahmed Glaucoma Valve and Baerveldt Glaucoma Implant groups, respectively (P = 0.04).
CONCLUSIONS
Long term rates of vision threatening complications and complications resulting in reoperation were higher in the Baerveldt Glaucoma Implant than the Ahmed Glaucoma Valve group over 5 years of follow-up.
INTRODUCTION
The use of aqueous shunts for surgical glaucoma management has increased dramatically in the past 20 years. In a recent analysis of the U.S. Medicare database, the number of aqueous shunt implantations performed annually has risen from 2,356 in 1994 to 12,021 in 2012.1 There are numerous aqueous shunts in use but, at least in the U.S. market, two models are used most commonly, the Ahmed Valve model FP7 (New World Medical, Rancho Cucamonga, CA) and the Baerveldt BG 101-350 (Abbott Medical Optics, Abbott Park, IL). A 2008 survey of the American Glaucoma Society membership showed that approximately half of respondents favored the Ahmed Glaucoma Valve and half preferred the Baerveldt Glaucoma Implant when operating on patients with previous incisional eye surgery or refractory glaucoma.2 In that same year, the American Academy of Ophthalmology published a Technology Assessment article reviewing aqueous shunts and concluded that “Too few high-quality direct comparisons of various available shunts have been published to assess the relative efficacy or complication rates of specific devices….”3 While there have been several retrospective studies comparing the Ahmed and Baerveldt implants, these have been of relatively short duration and inconclusive as to the relative success rates and complications of these two implants.4–8 In addition, these earlier studies used older models with different materials and designs, which have been improved upon to address prior concerns. Lastly, these studies suffer from the selection bias inherent to all retrospective analyses of existing medical record data.
The Ahmed Baerveldt Comparison (ABC) Study was a prospective multicenter randomized surgical trial that compared the safety and efficacy of the Ahmed Glaucoma Valve FP7 and Baerveldt Glaucoma Implant BG 101-350 in patients with previous incisional eye surgery or refractory glaucoma.9 These two implants have markedly different design features. The Ahmed Glaucoma Valve FP7 is a valved implant with a 184mm2 endplate and the Baerveldt Glaucoma Implant BG 101-350 is a non-valved implant with a 350mm2 endplate. At five years, the Baerveldt Glaucoma Implant group had an average IOP that was 2 mmHg lower than the Ahmed Glaucoma Valve FP7 group and, at most time points, subjects in the Baerveldt Glaucoma Implant BG 101-350 group were, on average, taking fewer medications than the Ahmed Glaucoma Valve FP7 group.10 Over the five years of follow-up, the two treatment groups failed at the same rate, approximately 8% per year, but failures in the two groups occurred for different reasons. Subjects in the Ahmed Glaucoma Valve FP7 group failed more often than the Baerveldt Glaucoma Implant BG 101-350 group due to high IOP endpoints (persistently elevated IOP and reoperation for IOP elevation), while subjects in the Baerveldt Glaucoma Implant BG 101-350 group failed more often than the Ahmed Glaucoma Valve FP7 group due to safety endpoints such as persistent hypotony or loss of light perception vision.
However, in any surgical comparison study, it is important to weigh the relative efficacy against the relative risks of each procedure. The intraoperative and early postoperative complications (within three months of surgery) in the ABC study were reported along with the one-year outcomes.11 The purpose of the current study was to examine the long-term (five-year) complications of the Ahmed FP7 and Baerveldt BG 101-350 implants in subjects with prior incisional surgery or refractory glaucoma.
METHODS
This prospective randomized prospective clinical trial was approved at the Institutional Review Boards at 16 clinical centers and each patient gave informed consent. The study was registered at www.clinicaltrials.gov (identifier NCT00376363). The design and methods of the ABC Study are described in detail in the baseline methodology paper,9 and are summarized as follows.
Randomization, Eligibility, and Treatment
Subjects age 18–85 years with refractory glaucoma and IOPs greater than or equal to 18 mmHg in whom aqueous shunt surgery was planned were enrolled in the study. Subjects with primary glaucomas with a previous failed trabeculectomy or who had previous intraocular surgery were included. Also, subjects without previous intraocular surgery were eligible if they had secondary glaucomas known to be refractory to trabeculectomy such as neovascular glaucoma (NVG), uveitic glaucoma, or glaucoma associated with iridocorneal endothelialization (ICE) syndrome.
Individuals enrolled in the study were randomized in a 1:1 ratio to placement of an Ahmed Glaucoma Valve FP7 or Baerveldt Glaucoma Implant BG 101-350 according to a permuted variable block randomization scheme, stratified by surgeon within one of 16 clinical centers and type of glaucoma. Subjects were allocated to one of 4 strata according to their type of glaucoma, as follows: (1) Primary glaucomas with previous intraocular surgery; (2) Secondary glaucomas (excluding uveitic glaucoma and NVG); (3) NVG; and (4) Uveitic glaucoma. Subjects were excluded if they lacked light perception vision, were unwilling or unable to give informed consent, lived out of the area and were expected to be unavailable for follow-up visits, underwent a previous cyclodestructive procedure or previous aqueous shunt implanted in the same eye, underwent a prior scleral buckling procedure or other external impediment to superotemporal device implantation, had silicone oil in the eye, had vitreous in the anterior chamber sufficient to require a vitrectomy, had uveitis associated with a systemic condition like juvenile rheumatoid arthritis, had nanophthalmos, had Sturge-Weber syndrome or other conditions associated with elevated episcleral venous pressure, or needed aqueous shunt surgery combined with other ocular procedures. For subjects in whom both eyes were eligible for enrollment, only the first eligible eye to be implanted was enrolled. Neither the subject nor investigator was masked to the randomization assignment. Details of the surgical procedures for Ahmed Glaucoma Valve and Baerveldt Glaucoma Implant BG 101-350 implantation used in this study are described in the baseline paper.9
Patient Visits
Follow-up visits were scheduled one day, one week, one month, three months, six months, one year, 18 months, two years, three years, four years, and five years postoperatively. Detailed information about data obtained at baseline and follow-up visits is contained in the baseline paper.9
Postoperative Interventions
At each follow-up visit, investigators were asked about interventions performed since the subject’s last visit. There were specific questions about whether anterior chamber reformation or intravitreal injections had been performed as well as an open ended “Other Interventions” category. Postoperative interventions were counted as such in the analysis if the intervention was deemed related to the original surgery, needed for further IOP lowering but not incisional surgery (such as laser trabeculoplasty), or needed for a complication of the surgery.
Definition of Complications
The current analysis only includes reoperations or loss of vision if they were attributable to the aqueous shunt surgery. Early complications were those that were recorded by the 3-month follow-up visit. These were reported in the 1-year outcomes paper.11 Late complications were those that were experienced after the 3-month followup visit. A serious complication was defined as any complication, early or late, that was associated with a 2-line Snellen acuity decrease or a return to the operating room for a surgical procedure to manage the complication. A revision in the operating room to manage an occluded tube was considered a reoperation for a complication. The Snellen visual acuity (VA) decrease was assessed at the 5-year visit. If the patient did not have a 5-year visit, then the patient’s complication could not be categorized as serious by vision loss, but could by virtue of reoperation.
Statistical Analysis
Univariate comparisons between treatment groups were made using the 2-sided Student t test, X2 test, or Fisher exact test. Subjects’ data were analyzed in the group to which they were assigned during randomization (intent-to-treat analysis). A P value of 0.05 or less was considered statistically significant in our analyses.
RESULTS
A total of 276 subjects were enrolled between October 2006 and April 2008. One hundred forty three subjects (52%) were randomly assigned to placement of an Ahmed Glaucoma Valve FP7 and 133 (48%) to a Baerveldt Glaucoma Implant BG 101-350. The disposition of subjects is summarized in the Consort Flow Diagram (Supplemental material available at AJO.com). Intraoperative complications,9 early postoperative complications,11 and visual acuity results10 have been described in detail previously.
Postoperative Interventions
Table 1 lists postoperative interventions that occurred over 5 years of follow-up, excluding those included in the1 year report.11 The total number of subjects requiring interventions beyond one year was 16 in the Ahmed Glaucoma Valve FP7 group and 25 in the Baerveldt Glaucoma Implant BG 101-350 group, a difference that was not statistically significant (P = 0.21, Fishers Exact Test). Excluding cataract extraction (detailed below), there were only seven surgical interventions needed between three and five years of follow-up.
Table 1.
Postoperative Interventions after 12 months in the Ahmed Baerveldt Comparison Study
| Intervention | Ahmed (n = 143) | Baerveldt (n = 133) |
|---|---|---|
| Anterior chamber reformation | 0 | 1 (1%) |
| Intravitreal Injection | 0 | 2 (2%) |
| Needling at slit lamp | 1 (1%) | 0 |
| Macular laser for CME | 1 (1) | 0 |
| Laser Trabeculoplasty | 0 | 1 (1%) |
| Corneal Scraping for Band Keratopathy | 0 | 1 (1%) |
| Total Number of Patients with Interventions | 2 (1.4%) | 5 (3.8%) |
P = 0.21, Fisher Exact Test
CME – Cystoid Macular Edema
Late Postoperative Complications
Table 2 details the cumulative five year incidence of late (after 3 months) complications by randomized treatment group. Late complications developed in 56 subjects (46.8 ± 4.8 5 year cumulative % ± SE) in the Ahmed Glaucoma Valve group and 67 subjects (56.3 ± 4.7 5 year cumulative % ± SE) in the Baerveldt Glaucoma Implant BG 101-350 group during 5 years of follow-up (P = 0.082). The overall incidence of late postoperative complications was similar between treatment groups.
Table 2.
5-year Incidence of Late Complications in the Ahmed Baerveldt Comparison Study
| Year 5 N, Cumulative proportion (SE) | log-rank p-value | Risk Ratio* | 95% CI | ||
|---|---|---|---|---|---|
| Complication | Ahmed | Baerveldt | |||
| Tube occlusion | 1, 0.8% (0.8%) | 6, 5.7% (2.3%) | 0.037 | 6.93 | 0.83, 57.5 |
| Choroidal effusion | 0, 0.0% NA | 2, 1.8% (1.2%) | 0.16 | NA | NA |
| Endophthalmitis | 0, 0.0% NA | 2, 2.2% (1.6%) | 0.16 | NA | NA |
| Cystoid macular edema | 6, 6.2% (2.5%) | 7, 7.2% (2.7%) | 0.81 | 1.14 | 0.38, 3.40 |
| Shallow anterior chamber | 2, 2.2% (1.5%) | 3, 3.7% (2.1%) | 0.64 | 1.53 | 0.26, 9.16 |
| Hypotony maculopathy | 0, 0.0% NA | 1, 0.8% (0.8%) | 0.30 | NA | NA |
| Diplopia | 16, 12.7% (3.0%) | 14, 11.8% (3.0%) | 0.81 | 0.92 | 0.45, 1.88 |
| Corneal edema – All | 18, 20.1% (4.4%) | 18, 20.4% (4.3%) | 0.71 | 1.13 | 0.59, 2.18 |
| Corneal edema – Likely attributable to implant | 9, 11.9% (3.8%) | 9, 11.7% (3.7%) | 0.82 | 1.10 | 0.49, 2.44 |
| Tube-corneal touch | 4, 3.5% (1.7%) | 4, 3.7% (1.8%) | 0.91 | 1.08 | 0.27, 4.34 |
| Corneal graft rejection | 8, 7.1 (2.4%) | 8, 7.0% (2.4%) | 0.96 | 0.98† | 0.37, 2.62 |
| Band keratopathy | 1, 1.2% (1.2%) | 2, 2.0% (1.4%) | 0.57 | 1.99 | 0.18, 21.9 |
| Corneal neovascularization | 0, 0.0% NA | 1, 1.0% (1.0%) | 0.33 | NA | NA |
| Tube erosion | 3, 2.9% (1.7%) | 1, 1.0% (1.0%) | 0.33 | 0.34 | 0.04, 3.31 |
| Encysted bleb | 1, 0.9% (0.9%) | 0, 0.0% NA | 0.32 | NA | NA |
| Recurrent or persistent iritis | 7, 6.2% (2.3%) | 6 5.5% (2.2%) | 0.83 | 0.89 | 0.30, 2.63 |
| Phthisis bulbi | 1, 0.8% NA | 6, 5.7% (2.3%) | 0.037 | 6.93 | 0.83, 57.5 |
| Hyphema | 2, 1.5% (1.1%) | 2, 1.6% (1.1%) | 0.97 | 1.04 | 0.15, 7.40 |
| Vitreous hemorrhage | 3, 2.7% (1.5%) | 3, 2.5% (1.4%) | 0.96 | 1.04 | 0.21, 5.17 |
| Pupillary membrane | 1, 0.8% (0.8%) | 0, 0.0% NA | 0.33 | NA | NA |
| Epiretinal membrane | 0, 0.0% NA | 1, 0.8% (0.8) | 0.31 | NA | NA |
| Retinal detachment | 2, 1.6% (1.1%) | 2, 1.7% (1.2%) | 0.98 | 1.02 | 0.14, 7.25 |
| Corneal blood staining | 0, 0.0% NA | 2, 1.6% (1.1%) | 0.15 | NA | NA |
Ahmed is the reference group.
Adjusted for previous PKP prior to enrollment and implantation of study GDI, which was itself highly significantly related to corneal graft failure (p<0.001).
N – Number
CI – Confidence Interval
SE – Standard Error
NA – Not applicable
Tube occlusion (p = 0.037, Fisher’s exact test) and phthisis bulbi (p = 0.037, Fisher’s exact test) were late postoperative complications that occurred with significantly greater frequency in the Baerveldt Glaucoma Implant BG 101-350 group than the Ahmed Glaucoma Valve group. The issue of tube occlusion was discussed in the 1-year outcomes paper11 and there were no additional tube occlusions beyond the 1 year time point. Phthisis bulbi was found in 6 subjects in the Baerveldt Glaucoma Implant BG 101-350 group compared to 1 subject in the Ahmed Glaucoma Valve FP7 group. These same subjects were counted as failures due to persistent hypotony due to severe vision loss in all of these subjects.
Persistent corneal edema was found in 20.1% and 20.4% of subjects in the Ahmed Glaucoma Valve FP7and Baerveldt Glaucoma Implant BG 101-350 groups, respectively, although this was attributed to non-implant causes in 50% of these so the percentage of subjects with persistent corneal edema attributable to the aqueous shunt was closer to 12%. Diplopia was found in 12.7% and 11.8% of subjects in the Ahmed Glaucoma Valve FP7 and Baerveldt Glaucoma Implant BG 101-350 groups, respectively, and there was no difference between the two groups in the incidence of diplopia. Cystoid macular edema was the third most frequent complication, occurring in 6.2% of subjects in the Ahmed Glaucoma Valve group and 7.2% of subjects in the Baerveldt Glaucoma Implant BG 101-350 group. It was difficult to determine whether this was surgically related or caused by underlying conditions such as diabetic retinopathy, uveitis, or neovascular glaucoma, all of which were common in this cohort and are also associated with cystoid macular edema.
Serious Complications
Table 3 shows serious complications resulting in reoperation and/or vision loss. Complications were classified as serious if they were associated with either reoperation for complication or if they were associated with a Snellen acuity loss of 2 or more lines at their last study visit that could be attributed to a complication of tube implantation. The incidence of serious complications was higher in the Baerveldt Glaucoma Implant BG 101-350 group. Serious complications were observed in 17 (15.9%) subjects in the Ahmed Glaucoma Valve group and 29 (24.7%) subjects in the Baerveldt Glaucoma Implant BG 101-350 group (p = 0.034, log rank test adjusted for stratum). Figure 1 shows the cumulative rates of serious complications for each group. Persistent corneal edema was the most common cause for both reoperation for a complication and loss of 2 or more lines of Snellen VA in both groups (Table 3). As reported previously,10 approximately 40% of subjects in both groups lost 2 or more lines of Snellen VA after five years, but the vast majority of these were attributable to their underlying ocular disease rather than the aqueous shunt surgery. Sixteen subjects in the Ahmed Glaucoma Valve group and 24 subjects in the Baerveldt Glaucoma Implant BG 101-350 group underwent reoperations for complications. A total of 22 eyes experienced complications during follow up resulting in the loss of 2 or more lines of Snellen VA. The study PI (DLB) reviewed all of these, masked to randomized treatment assignment and the first three months of follow up (during which eyes implanted with Baerveldt tubes could be expected to have high IOP), and attributed the acuity loss to glaucoma progression (N = 4; Ahmed Glaucoma Valve FP7:3, Baerveldt Glaucoma Implant BG 101-350:1), progressive retinal disease (N = 4; Ahmed Glaucoma Valve FP7:2, Baerveldt Glaucoma Implant BG 101-350:2), other causes (N = 7; Ahmed Glaucoma Valve FP7:2, Baerveldt Glaucoma Implant BG 101-350:5), and in one case the cause could not be determined as follow up information was obtained from a non-study ophthalmologist. The other causes of acuity loss not attributed to the GDI implantation included corneal decomposition secondary to ICE (N = 2), corneal epitheliopathy secondary to dry eye (N = 1), pre-existing corneal disease (N = 2), posterior corneal opacification (N = 1), and aphakia (N = 1).
Table 3.
Serious Complications Associated with Reoperation and/or Vision Loss in the Ahmed Baerveldt Comparison Study
| Ahmed Group (n = 143) | Baerveldt Group (n = 133) | |
|---|---|---|
|
| ||
| Reoperation for complications | 16 (14.3%) | 24 (19.5%) |
|
| ||
| Vision loss of ≥ 2 Snellen lines | ||
| Persistent corneal edema | 1 | 1 |
| Persistent corneal edema + hypotony maculopathy | 0 | 1 |
| Persistent corneal edema + tube-corneal touch | 0 | 2 |
| Cystoid macular edema | 0 | 1 |
|
| ||
| Total number of subjects with serious complications† | 17 (15.9%) | 29 (24.7%) |
Data censored after a reoperation for glaucoma.
P = 0.034 for the difference in 5-year cumulative serious complication rates between treatment groups from Kaplan-Meier analysis (log rank test adjusted for stratum).
Figure 1.
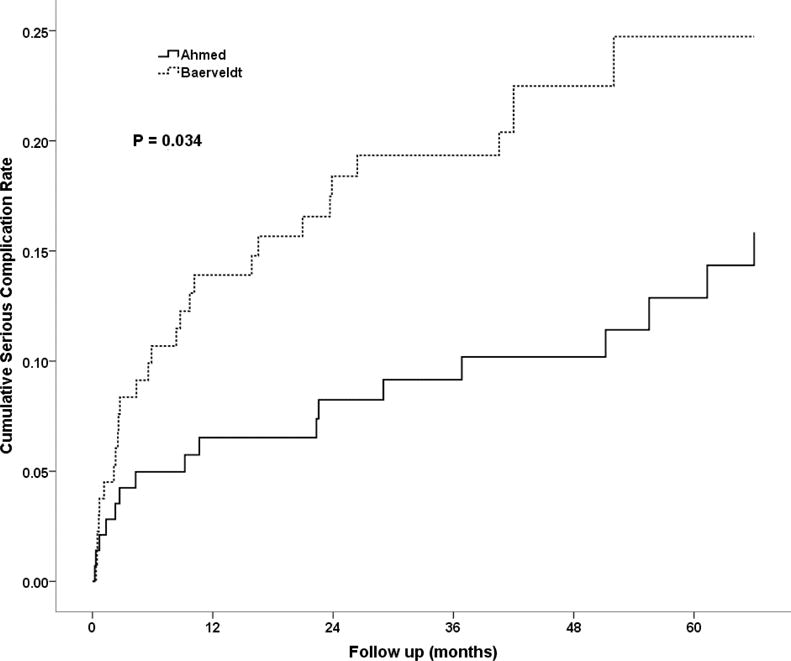
Cumulative probability of experiencing a serious complication within five years of surgery in the Ahmed Baerveldt Comparison Study.
Reoperation for Complications
At year five, the cumulative proportion (SE) of subjects with complication-related reoperations, including explantations, in the Ahmed Glaucoma Valve group was 14.3% (3.5%), N = 16, compared to 19.5% (3.6%), N = 24, in the Baerveldt Glaucoma Implant BG 101-350 group (p = 0.109, log-rank test). Figure 2 shows the cumulative proportion of subjects in each group who required reoperation for a complication throughout five years of follow-up. The risk ratio was 0.60 with 95% confidence interval 0.32 to 1.13 (Cox proportional hazard regression), so a clinically significant difference in favor of Ahmed Glaucoma Valve implantation having a risk that was 1/3rd that of Baerveldt Glaucoma Implant BG 101-350 implantation with respect to complication reoperations cannot be excluded. There was no significant difference by stratum (p = 0.98, cox regression accounting for randomized treatment group), nor was there a significant interaction between randomized treatment group and stratum (p = 0.38, cox regression). This implies that diagnostic stratum, particularly neovascular glaucoma, did not affect the likelihood of having a reoperation for complications. After 5 years of follow up there were no significant differences (all p>0.1) in rates of late onset complications between clinical centers or between surgeons more experienced with the randomized aqueous shunt (≥20 prior implantations) compared to those with less experience.
Figure 2.
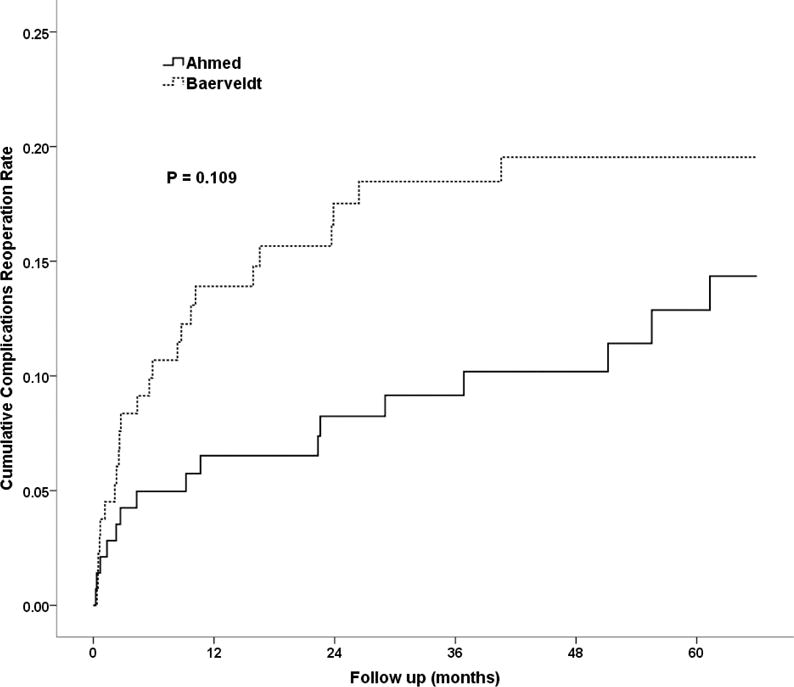
Cumulative probability of requiring an operation for complications within five years of surgery in the Ahmed Baerveldt Comparison Study.
Table 4 outlines the specific operations performed for complications in each of the two groups. Figure 2 presents the cumulative rates of reoperation for complications in the two groups. The higher rate of complications in the Baervledt Glaucoma Implant BG 101-350 group, which approached statistical significance at three years (p = 0.053, log-rank test12), decreased at five years (p = 0.109, log-rank test) although the 95% confidence interval around the relative risk (1.67, Cox survival regression) of increased complications resulting in reoperation in the Baerveldt Glaucoma Implant BG 101-350 group still includes a possibly substantially higher risk (95% CI:0.9, 3.1).
Table 4.
Reoperations for Complications in the Ahmed Baerveldt Comparison Study
| Ahmed Group (n = 143) | Baerveldt Group (n = 133) | |
|---|---|---|
| Corneal transplant procedure* | 3 | 4 |
| Pars plana vitrectomy | 1 | 3 |
| Surgery for tube occlusion | 1 | 7 |
| YAG laser to clear vitreous from tube | 1 | 1 |
| Surgical iridectomy | 1 | 0 |
| Tube tied off to repair hypotony | 1 | 1 |
| Repair of wound leak | 1 | 1 |
| Tube repositioning/extension | 3 | 1 |
| Revision for tube erosion | 2 | 3 |
| Drainage of suprachoroidal hemorrhage | 0 | 1 |
| Implant explantation | 3 | 3 |
| Total number of patients (cumulative percentage) with reoperations for complications† | 16 (14.3%) | 24 (19.5%) |
Data censored after a reoperation for glaucoma.
Includes penetrating keratoplasty and Descemet’s stripping automated endothelial keratoplasty.
One subject in each group had a penetrating keratoplasty and pars plana vitrectomy performed at the same surgery, explaining why there are more complications listed for each group than subjects with complications.
P = 0.109 for the difference in 5-year cumulative reoperations for complication rates between treatment groups from Kaplan-Meier analysis (log rank test adjusted for stratum).
Cataract Surgery during Follow-Up
There were 91 phakic eyes enrolled. Of those, 34 had cataract extraction, censored at glaucoma reoperation. Seventeen were in the Ahmed Glaucoma Valve FP7 group and 17 were in the Baerveldt Glaucoma Implant BG 101-350 group. The cumulative proportions receiving cataract extraction at 5 years were 49.5% (SE = 9.0%) and 43.6% (SE = 8.1%) in the Ahmed Glaucoma Valve FP7 and Baerveldt Glaucoma Implant BG 101-350 groups, respectively (P = 0.88, log rank test, Figure 3).
Figure 3.
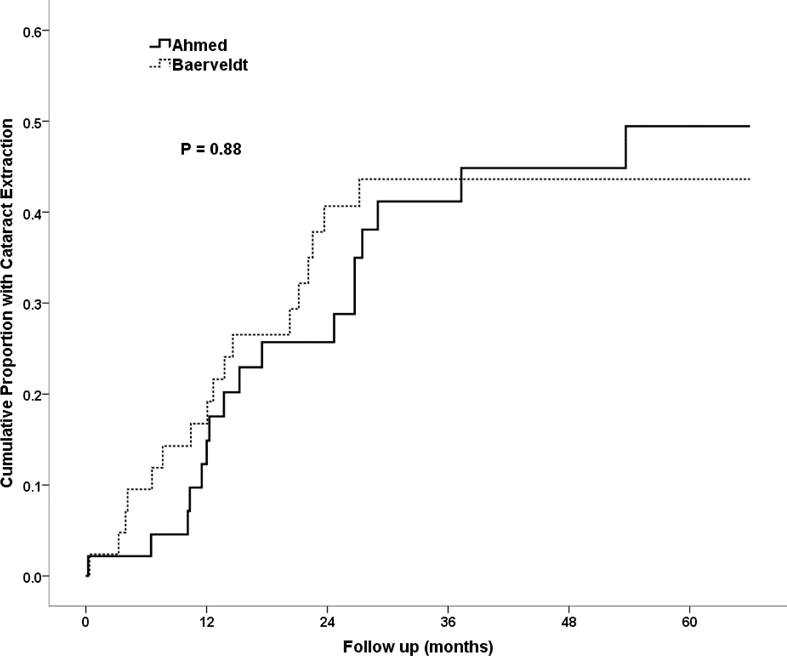
Cumulative probability of phakic subjects requiring cataract extraction within five years of surgery in the Ahmed Baerveldt Comparison Study.
DISCUSSION
The ABC Study is the largest and longest prospective clinical trial comparing two aqueous shunts, with an enrollment of 276 subjects followed over 5 years. The study was designed to compare the safety and efficacy of the Ahmed Glaucoma Valve model FP7 and the Baerveldt Glaucoma Implant BG 101-350. The 5 year treatment outcomes, reported earlier, found that the Baerveldt Glaucoma Implant BG 101-350 had fewer failures over time with a slightly lower average IOP (2 mmHg) on fewer medications at most time points.10 However, previous reports on the safety of these two implants at 1 and 3 years have shown a higher incidence of interventions and serious vision-threatening complications with the Baerveldt Glaucoma Implant BG 101-350 compared with the Ahmed Glaucoma Valve FP7.10–14 In the analysis of complications out to 5 years, this trend continued in the ABC study, with more subjects in the Baerveldt Glaucoma Implant BG 101-350 group undergoing reoperation for implant-related complications as well as loss of 2 or more lines of visual acuity related to implant complications.
There appeared to be more tube occlusions in the Baerveldt Glaucoma Implant BG 101-350 group (6) compared to the Ahmed Glaucoma Valve FP7 group (1) for reasons that are unclear. In addition, there were more cases of phthisis bulbi in the Baerveldt Glaucoma Implant BG 101-350 group (6) than the Ahmed Glaucoma Valve FP7 group (1), perhaps due to the fact that the Baerveldt Glaucoma Implant BG 101-350 group had a similar proportion of subjects experiencing failures due to persistent hypotony. It may be that the larger end plate of the Baerveldt Glaucoma Implant BG 101-350 implant, which is generally considered to provide lower long-term IOPs,15 appears to put patients at increased risk of persistent hypotony and phthisis bulbi as well.
Corneal edema has been a concern after aqueous shunt surgery, primarily through loss of endothelial cell density, which has been demonstrated to occur.16–18 We found a 20% rate of persistent corneal edema after tube implantation in the current study at 5 years. This was similar to the 5-year rate in the tube group in the TVT study, which was 16%.19 We did not find a difference between the two treatment arms in the ABC study, however. When we examined the reason for corneal edema, half of the cases had a reason other than the presence of a tube in the anterior chamber that could have explained the corneal edema such as pre-existing corneal transplants which could have failed, pre-existing corneal diagnoses such as ICE syndrome, or the presence of an anterior chamber intraocular lens, all of which are equally as likely to cause persistent corneal edema. In the 5-year TVT results,19 the tube and trabeculectomy groups had the same rate of persistent corneal edema, suggesting that corneal edema may not be related simply to the presence of a tube in the anterior chamber but possibly due to hypotony or pre-existing conditions. Results from the GDI arm of the ongoing Primary Tube Versus Trabeculectomy study, which enrolled subjects without prior intraocular surgery and without high risk for treatment failures, may help elucidate this issue.
Late-onset endophthalmitis, a significant concern following trabeculectomy, particularly in the antifibrotic era,20 was seen in only 1 of the 276 subjects in the current study. This may be because of the early and aggressive intervention for exposed implant tubes and explants practiced as part of current practice patterns.21 It seems that the concerns regarding long-term endophthalmitis with relation to trabeculectomy do not follow for aqueous shunts.
Diplopia is a more common complication of glaucoma surgery performed with GDIs compared to trabeculectomy22 and, indeed, the 5-year results of the TVT study found a three times greater incidence in the tube (6%), compared to the trabeculectomy (2%), group.19 The current study found an equal, approximate 12% cumulative risk of persistent diplopia in the two groups, contrary to older, non-randomized studies that found a higher incidence with the Baerveldt Glaucoma Implant than the Ahmed Glaucoma Valve.23 However, these older reports were all based on the old design of the Baerveldt Glaucoma Implant, which did not have fenestrations in the end plate, specifically designed to reduce the height of the bleb and minimize restrictive strabismus. The cause of diplopia in patients undergoing GDIs is likely a restrictive strabismus, either from the bleb itself or the plate impinging on the muscle insertion.22 Unlike the TVT Study, the current study did not do formal motility examination but relied on a forced choice question regarding double vision conducted at each visit. Assessing motility disturbances in this way might actually underestimate the true incidence of motility disturbances, particularly since patients with refractory glaucomas like those in the current study may have advanced visual field loss from glaucoma or central visual acuity loss from other underlying conditions and may even be monocular. The risk of diplopia is significantly high and relatively unique to aqueous shunts to warrant discussion of this possibility during the risk/benefit discussion with patients preoperatively, at least in those with good binocular vision.
Tube and plate erosion are a concern when using an extraocular implant. Prior to the use of tissue patch graft material to cover the tube, the initial experience with GDIs showed a tube erosion rate of 30%.24 The rate in the current series at 5 years was 3% in the Ahmed Glaucoma Valve FP7 group and 1% in the Baerveldt Glaucoma Implant BG 101-350 group. In all cases, scleral reinforcement with a tissue patch was used. At 5 years, the rate in the Baerveldt Glaucoma Implant BG 101-350 group of the TVT study was 5%.19 Previously identified risk factors for erosion include prior ocular surgery, neovascular glaucoma, Hispanic ancestry, and combining GDI with another surgery.25,26 The mechanism for tube erosion has been postulated to be either mechanical or immunological.27,28 Unproven strategies for reducing the risk of tube erosion include routing the tube directly superiorly to reduce rubbing by the eyelid margin, placing the tube in the sulcus when possible, or tunneling the tube and also covering it with a tissue patch graft.
The ABC Study has several limitations. First, there was no ability to mask subjects or investigators to the treatment assignment. Second, although the results have generalizability across geographic regions and across many surgeons, they cannot be generalized to other models of GDIs. Third, since patients undergoing combined GDI implantation with concomitant other ocular surgery, this large group of patients cannot be generalized to. And lastly, the results cannot be generalized to patients who have not had prior eye surgery in low risk groups. The GDI arm of the ongoing Primary TVT study will provide more information as to the complications in this population.
In summary, when the eye surgeon needs to decide which glaucoma implant an individual patient should receive, it is important to consider the success and failure rates, the final IOP and number of medications needed, and the risk of complications of various devices. The ABC Study has demonstrated comparable success rates between the Ahmed Glaucoma Valve FP7 and Baerveldt Glaucoma Implant BG 101-350 implants with different reasons for failures in the two groups at five years.10 The Ahmed Glaucoma Valve FP7 tended to fail due to inadequate control of IOP resulting in reoperation whereas the Baerveldt Glaucoma Implant BG 101-350 tended to fail due to safety endpoints such as hypotony, need for explantation of the device, and loss of light perception vision.10 While the Baerveldt Glaucoma Implant BG 101-350 provided an additional 2 mmHg of IOP lowering compared to the Ahmed Glaucoma Valve FP7,10 the current study demonstrates an increased risk of serious complications needing operative correction or resulting in loss of some visual acuity associated with the Baerveldt Glaucoma Implant BG 101-350. All of these factors should be considered when choosing between these two commonly used aqueous shunts.
Supplementary Material
Acknowledgments
Disclosure:
Funding Support: NIH P30 EY014801 (University of Miami), unrestricted grants from Research to Prevent Blindness, NY, NY, USA (University of North Carolina and University of Miami), and an unrestricted grant from New World Medical, Inc., Rancho Cucamonga, CA, USA (University of Miami). Some clinical centers received free implants for the study from New World Medical, Rancho Cucamonga, CA, USA and Abbott Medical Optics, Abbott Park, IL, USA.
- Financial Disclosures:
- D. Budenz: Consulting fees – Alcon Labs, Ft. Worth, TX, USA; Travel grant – New World Medical, Rancho Cucamonga, CA, USA; Data Safety Monitoring Board fees – Ivantis, Irvine, CA, USA. Research grants – New World Medical Inc., Rancho Cucamonga, CA, National Eye Institute (NIH)
- W. Feuer: Research grants – New World Medical, Rancho Cucamonga, CA, USA; National Eye Institute (NIH), Bethesda, MD, USA; Research to Prevent Blindness, NY, NY, Department of Defense, Washington, DC, USA.
- K. Barton: Consulting fees – Alcon Labs, Ft. Worth, TX, USA; Ivantis, Irvine, CA, USA; Aquesys, Aliso Viejo, CA, USA. Advisory Board fees – Glaukos, Laguna Hills, CA, USA; Kowa, Aichi, Japan; Amakem, Diepenbeek, Belgium; Laboratoires Thea, Clermont-Ferrand, France; Travel grant – New World Medical, Rancho Cucamonga, CA, USA; Supplied Implants for research – New World Medical, Rancho Cucamonga, CA, USA; Abbott Medical Optics, Abbott Park, IL, USA; Research grant – Merck & Co., Kenilworth, NJ, USA. Stock and options – Aquesys Inc, Ophthalmic Implants Pte, Vision Futures Ltd, Vision Medical Events Ltd.
- J. Schiffman, V. Costa, D. Godfrey, Y. Buys – Nothing to disclose.
Biography

Donald Budenz is Kittner Family Distinguished Professor and Chairman, Department of Ophthalmology, UNC Chapel Hill School of Medicine. His areas of research include clinical trials in glaucoma, imaging in glaucoma, and glaucoma epidemiology. He has received awards for teaching from the Department of Ophthalmology, University of Pennsylvania and the Department of Epidemiology and Public Health, University of Miami School of Medicine and humanitarian awards from the American Glaucoma Society and the American Academy of Ophthalmology.
APPENDIX: The Ahmed versus Baerveldt Comparison Study Group
Clinical Centers:
Bascom Palmer Eye Institute, University of Miami, Donald Budenz, MD, MPH,i Steven J. Gedde, MD, Fouad El Sayyad, MDii
Duke University Eye Center, Leon Herndon, MD
Glaucoma Associates of Texas, David Godfrey, MD, Ronald Fellman, MD
Medical College of Wisconsin, The Eye Institute, James Robinson, MD, David Dueker, MDiii
Minnesota Eye Consultants, Patrick Riedel, MD, Thomas Samuelson, MD
NIHR Biomedical Research Centre for Ophthalmology, Moorfields Eye Hospital, Keith Barton, MD, Renata Puertas, MD
National University Hospital, Department of Ophthalmology, Paul Chew, MD, Cecilia Aquino, MD
Southern California Glaucoma Consultants, Alfred M Solish, MD
Toronto Western Hospital Eye Clinic, Yvonne Buys, MD, Graham Trope, MD
University of California Davis Medical Center, Department of Ophthalmology, James D Brandt, MD, Michele Lim, MD
University of California Los Angeles, Jules Stein Eye Institute, Simon Law, MD
University of Campinas, Ophthalmology, Vital Costa, MD
University of Oklahoma, Dean A. McGee Eye Institute, Steve Sarkisian, MD
University of Southern California, Doheny Eye Institute, Vikas Chopra, MD,iv Brian Francis, MD,iv Mario Meallet, MD,v Rohit Varma, MD, MPHvi
University of Tennessee at Memphis, Department of Ophthalmology, Peter Netland, MD, PhD,vii Sarwat Salim, MDviii
University of Texas Houston, Cizik Eye Clinic, Robert Feldman, MD, Nicholas Bell, MD
Safety and Data Monitoring Committee, Voting Members: Philip Chen, MD, University of Washington, Department of Ophthalmology; Dale Heuer, MD, The Eye Institute, Medical College of Wisconsin; Kuldev Singh, MD, MPH, Department of Ophthalmology Stanford University; Martha Wright, MD, University of Minnesota, Department of Ophthalmology. Non-voting members: Donald L. Budenz, MD, MPH, William J. Feuer, MS, Joyce C. Schiffman, MS
Steering Committee: Keith Barton, MD, Donald L Budenz, MD, MPH, William J. Feuer, MS
Statistical Coordinating Center: William J. Feuer, MS, Joyce C. Schiffman, MS, Wei Shi, MS; Coordinator, Luz Ajuria; Database Manager, Yolanda Silva, BS, Bascom Palmer Eye Institute, University of Miami
iCurrently affiliated with University of North Carolina at Chapel Hill
iiCurrently affiliated with Department of Ophthalmology, University of Florida
iiiCurrently affiliated with King Khaled Eye Specialty Hospital
ivCurrently affiliated with Doheny Eye Institute, University California Los Angeles
vCurrently affiliated with A Center for Vision, North Hollywood, CA
viCurrently affiliated with Department of Ophthalmology, Keck School of Medicine, University of Southern California
viiCurrently affiliated with Department of Ophthalmology, University of Virginia
viiiCurrently affiliated with The Eye Institute, Medical College of Wisconsin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material available at AJO.com
References
- 1.Arora KS, Robin AL, Corcoran KJ, Corcoran SL, Ramulu PY. Use of various glaucoma surgeries and procedures in Medicare Beneficiaries from 1994 – 2012. Ophthalmology. 2015;122(8):1615–1624. doi: 10.1016/j.ophtha.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Desai MA, Gedde SJ, Feuer WJ, Shi W, Chen PP, Parrish RK., 2nd Practice preferences for glaucoma surgery: a survey of the American Glaucoma Society in 2008. Ophthalmic Surg Lasers Imaging. 2011;42(3):202–208. doi: 10.3928/15428877-20110224-04. [DOI] [PubMed] [Google Scholar]
- 3.Minckler DS, MS, Francis BA, Hodapp EA, et al. Aqueous shunts in glaucoma: A report by the American Academy of Ophthalmology. Ophthalmology. 2008;115(6):1089–1098. doi: 10.1016/j.ophtha.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 4.Tsai JC, Johnson CC, Dietrich MS. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma. Ophthalmology. 2003;110(9):1814–1821. doi: 10.1016/S0161-6420(03)00574-8. [DOI] [PubMed] [Google Scholar]
- 5.Syed HM, Law SK, Nam SH, Li G, Caprioli J, Coleman A. Baerveldt-350 implant versus Ahmed valve for refractory glaucoma: A case-controlled comparison. J Glaucoma. 2004;13(1):38–45. doi: 10.1097/00061198-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Wang J-C, See JL, Chew PTK. Experience with the use of Baerveldt and Ahmed glaucoma drainage implants in an Asian population. Ophthalmology. 2004;111(7):1383–1388. doi: 10.1016/j.ophtha.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Tsai JC, Johnson CC, Kammer JA, Dietrich MS. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma II. Ophthalmology. 2006;113(6):913–917. doi: 10.1016/j.ophtha.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Goulet RJ, 3rd, Phan AD, Cantor LB, WuDunn D. Efficacy of the Ahmed S2 glaucoma valve compared with the Baerveldt 250-mm2 glaucoma implant. Ophthalmology. 2008;115(7):1141–1147. doi: 10.1016/j.ophtha.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Barton K, Gedde SJ, Budenz DL, Feuer WJ, Schiffman J, The Ahmed Baerveldt Comparison Study Group The Ahmed Baerveldt Comparison (ABC) Study: Methodology, baseline patient characteristics, and intraoperative complications. Ophthalmology. 2011;118(3):435–442. doi: 10.1016/j.ophtha.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budenz DL, Barton K, Gedde SJ, et al. Five-year treatment outcomes in the Ahmed Baerveldt Comparison Study. Ophthalmology. 2015;122(2):308–316. doi: 10.1016/j.ophtha.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budenz DL, Barton K, Feuer WJ, et al. Treatment outcomes in the Ahmed Baerveldt Comparison Study after one year of follow-up. Ophthalmology. 2011;118(3):443–452. doi: 10.1016/j.ophtha.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton K, Feuer WJ, Budenz DL, et al. Three-year treatment outcomes in the Ahmed Baerveldt Comparison Study. Ophthalmology. 2014;121(8):1547–1557. doi: 10.1016/j.ophtha.2014.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christakis PG, Tsai JC, Kalenak JW, et al. The Ahmed Versus Baerveldt Study: three-year treatment outcomes. Ophthalmology. 2013;120(11):2232–2240. doi: 10.1016/j.ophtha.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Christakis PG, Kalenak JW, Zurakowski D, et al. The Ahmed Versus Baerveldt Study: One-Year Outcomes. Ophthalmology. 2011;118(11):2180–2189. doi: 10.1016/j.ophtha.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Heuer DK, Lloyd MA, Abrams DA, et al. Which is better? One or two? A randomized clinical trial of single-plate versus double-plate Molteno implantation for glaucomas in aphakia and pseudophakia. Ophthalmology. 1992;99(10):1512–19. doi: 10.1016/s0161-6420(92)31772-5. [DOI] [PubMed] [Google Scholar]
- 16.Kim CS, Yim JH, Lee EK, Lee NH. Changes in corneal endothelial cell density and morphology after Ahmed glaucoma valve implantation during the first year of follow up. Clin Experiment Ophthalmol. 2008;36(2):142–147. doi: 10.1111/j.1442-9071.2008.01683.x. [DOI] [PubMed] [Google Scholar]
- 17.Mendrinos E, Dosso A, Sommerhalder J, Shaarawy T. Coupling of HRT II and AS-OCT to evaluate corneal endothelial cell loss and in vivo visualization of the Ahmed glaucoma valve implant. Eye. 2009;23(9):1836–1844. doi: 10.1038/eye.2008.321. [DOI] [PubMed] [Google Scholar]
- 18.Lee EK, Yun YJ, Lee JE, Yim JH, Kim CS. Changes in corneal endothelial cells after Ahmed glaucoma valve implantation: 2-year follow-up. Am J Ophthalmol. 2009;148(3):361–367. doi: 10.1016/j.ajo.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) Study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–814. doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parrish R, Minckler D. Late endophthalmitis–filtering surgery time bomb? Ophthalmology. 1996;103(8):1167–1168. doi: 10.1016/s0161-6420(96)30527-7. [DOI] [PubMed] [Google Scholar]
- 21.Gedde SJ, Scott IU, Tabandeh H, Luu KK, Budenz DL, Greenfield DS, Flynn HJ. Late endophthalmitis associated with glaucoma drainage implants. Ophthalmology. 2001;108(7):1323–1327. doi: 10.1016/s0161-6420(01)00598-x. [DOI] [PubMed] [Google Scholar]
- 22.Rauscher FM, Gedde SJ, Schiffman JC, Feuer WJ, Barton K, Lee RK, Tube Versus Trabeculectomy Study Group Motility disturbances in the tube versus trabeculectomy study during the first year of follow-up. Am J Ophthalmol. 2009;147(3):458–466. doi: 10.1016/j.ajo.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong CH, Arosemena A, Zurakowski D, Ayyala RS. Glaucoma drainage devices: a systematic literature review and current controversies. Surv Ophthalmol. 2005;50(1):48–60. doi: 10.1016/j.survophthal.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Heuer DK, Budenz D, Coleman A. Aqueous shunt tube erosion. J Glaucoma. 2001;10(6):493–496. doi: 10.1097/00061198-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Byun YS, Lee NY, Park CK. Risk factors of implant exposure outside the conjunctiva after Ahmed glaucoma valve implantation. Jpn J Ophthalmol. 2009;53(2):114–119. doi: 10.1007/s10384-008-0630-y. [DOI] [PubMed] [Google Scholar]
- 26.Koval MS, El Sayyad FF, Bell NP, et al. Risk factors for tube shunt exposure: a matched case-control study. J Ophthalmol [serial online] 2013;2013:196215. doi: 10.1155/2013/196215. Available at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3736453. Accessed September 43 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lankaranian D, Reis R, Henderer JD, Choe S, Moster MR. Comparison of single thickness and double thickness processed pericardium patch graft in glaucoma drainage device surgery: a single surgeon comparison of outcome. J Glaucoma. 2008;17(1):48–51. doi: 10.1097/IJG.0b013e318133fc49. [DOI] [PubMed] [Google Scholar]
- 28.Smith MF, Doyle JW, Ticrney JW. A comparison of glaucoma drainage implant tube coverage. J Glaucoma. 2002;11(2):143–147. doi: 10.1097/00061198-200204000-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


