Abstract
Although clinical and basic studies show that parental trauma, fear and anxiety may be transmitted to offspring, the neurobiology of this transmission is still not well understood. We recently demonstrated in an animal model that infant rats acquire threat responses to a distinct cue if a mother expresses fear to this cue in their presence. This ability to acquire maternal fear through social learning is present at birth and, as we previously reported, depends on the pup's amygdala. However, the remaining neural mechanisms underlying social fear learning in infancy remain elusive. Here, using 14C 2-deoxyglucose autoradiography, we show that the mother-to-infant transmission of fear in preweaning rats is associated with a significant increase of activity in the subregions of the lateral septum, nucleus accumbens, bed nucleus of stria terminalis, retrosplenial cortex, paraventricular nucleus of the thalamus, mediodorsal and intralaminar thalamic nuclei, medial and lateral preoptic nuclei of the hypothalamus, and the lateral periaqueductal gray. In contrast to studies of adult social fear learning demonstrating the role of the anterior cingulate cortex and possibly the insular cortex, or research of infant classical fear conditioning showing the role of the posterior piriform cortex, no changes of activation in these areas were observed. Our results indicate that the pup's exposure to maternal fear activates a number of areas involved in processing threat, stress or pain. This pattern of activation suggests a unique set of neural mechanisms underlying social fear learning in the developing brain.
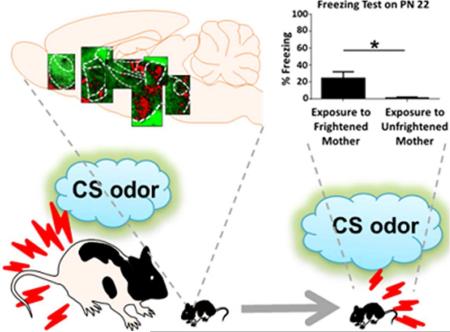
Graphical Abstract
Exposure to mother expressing fear in response to the previously conditioned stimulus (CS) odor activates a number of pain, stress and fear processing areas in the brain of the infant rat and produces fear responses to this odor that last at least until the post-weaning period.
Clinical studies report that emotional trauma and fear may be transmitted across generations (Yehuda et al., 2005; Murray et al., 2008; Roberts et al., 2012; Eley et al., 2015). Parental history of posttraumatic stress disorder (PTSD) increases the child's risk of developing PTSD (Roberts et al., 2012), whereas parental diagnosis of a specific phobia increases the occurrence of phobia in the offspring (Murray et al., 2008; Lara et al., 2012). One of the features shared by PTSD and specific phobias is a presence of distinct, trauma- (as in PTSD) or phobia-related cues that trigger threat responses. Intergenerational transmission of stress, fear and anxiety may be explained by a wide range of mechanisms, including genetic and epigenetic mechanisms, environmental factors, or gene environment interactions (Bowers and Yehuda, 2016). Recent studies suggest that parental modeling, such as a display of anxious behaviors in the child's presence and the children's ability to learn these behaviors from parents are major ways of the intergenerational transmission of fear and anxiety (de Rosnay et al., 2008; Aktar et al., 2013; Eley et al., 2015). Although existing research sheds some light on the biological basis of intergenerational transfer of nonspecific stress and anxiety (Klengel et al., 2015), little is known about the mechanisms controlling intergenerational social transmission of specific threat responses. To study neural mechanisms of the intergenerational social transmission of fear we used a social fear learning (SFL) paradigm.
Social fear learning, also referred to as vicarious fear learning, is a behavioral tool well suited for investigating mechanisms of intergenerational transmission of threat responses triggered by distinct cues, such as in PTSD and phobias (Olsson and Phelps, 2007). In SFL, an animal acquires fear responses to a neutral cue through exposure to a conspecific expressing fear to this cue. SFL is thus a form of associative fear learning in which a classical noxious unconditioned stimulus (Debiec et al., 2010) is replaced with an expression of fear by a conspecific animal. Although SFL has been shown by a number of studies across animal species, including humans (Mineka & Cook, 1993; Knapska et al., 2006; Jones et al., 2014; Molapour et al., 2015), the neural mechanisms of SFL remain to be elucidated. Research suggests that SFL and classical fear conditioning share similar neural mechanisms, e.g. studies in adult humans and rodents show that both SFL and classical fear conditioning engage the amygdala, the hippocampus, the affective pain processing systems, including the midline and intralaminar thalamic nuclei (MITN), the anterior cingulate cortex (ACC), and the midbrain structures, such as the periaqueductal gray subdivisons, which are directly involved in controlling threat responses (Olsson et al., 2007; Jeon et al., 2010; Molapour et al., 2015). Despite the growing number of studies of SFL in adults, little is known about the mechanisms of social transmission of fear in infancy.
We recently reported that infant rats can acquire fear responses from their mother through SFL (Debiec and Sullivan, 2014). This mother-to-infant fear transmission was mediated by alarm chemosignaling and depended on the pup's amygdala (Debiec and Sullivan, 2014). Specifically, we found that the acquisition of SFL in preweaning rats was accompanied by increased activity in the lateral, basal, central, medial and cortical nuclei of the amygdala, and that a pharmacological inactivation of the lateral nucleus of the amygdala prevented the acquisition of SFL (Debiec and Sullivan, 2014). Although in our previous study we observed that SFL occurs at the end of the first week of life (Debiec and Sullivan, 2014), it is still to be determined whether rats are born with the ability to acquire specific threat responses through social learning. We hypothesize that SFL mechanisms in pups are functional at birth. Also, the involvement of neural sites, other than the amygdala, in early SFL needs to be characterized. Previous fear conditioning studies in rodents show the key role of the olfactory processing areas, such as the posterior pririform cortex (PPir) (e.g. Raineki et al., 2009), yet, the role of the PPir in SFL has not been determined. Also, it is unlikely that the hippocampus or the neocortical structures, such as the ACC, which contribute to the formation of fear memories in adults (Toyoda et al., 2011), are involved in the infant SFL, since they are not fully functional until after weaning (Rudy, 1993; Raineki et al., 2010). We hypothesize that SFL in infancy occurs without the involvement of the hippocampus or neocortical structures. In this study we address the two questions raised above: whether SFL is present at birth and what extra-amygdala structures are potentially involved in SFL in infancy. We show that SFL occurs on the day of birth suggesting that pups are born with the ability to acquire SFL. To assess neural sites activated during infant SFL we used the 14C 2-deoxyglucose (2-DG) autoradiograms obtained during the previously described experiment (Debiec and Sullivan, 2014). 2-DG autoradiography is a method allowing the assessment of activity by measuring the activity-dependent uptake of the radiographic marker. 2-DG has been especially useful in the whole-brain analysis of neural activity in rodent pups (Sullivan et al., 2000; Raineki et al., 2009; Marquez et al., 2013). Using 2-DG autoradiography, we identify several neural structures activated in the pup's brain during the mother-to-infant transmission of fear through social learning.
MATERIALS AND METHODS
Animals
Animals were male and female Long–Evans rats born and bred in our colony (originally acquired from Harlan Laboratories). Mothers or ‘substitute mothers’ were multiparous females. Animals were housed in polypropylene cages (34 × 29 × 17 cm) with an abundant amount of wood shavings for nest building. Rats were kept in a 20 ± 1°C environment with a 12 h light/dark cycle. Food and water were provided ad libitum. To prevent litter effects on statistical analysis, no more than one female and one male from a litter was used in each experimental condition. All animal care and experimental procedures were in accordance with the National Institutes of Health guidelines for the care and use of experimental animals and were approved by the Institutional Animal Care and Use Committee.
Fear conditioning of mothers
Maternal olfactory fear conditioning took place in a conditioning chamber constructed of aluminum and Plexiglas walls (Coulbourn Instruments, Allentown, Pennsylvania) with a metal stainless steel rod flooring that was attached to a shock generator (Model H13-15; Coulbourn Instruments). The chamber was enclosed within an acoustic-isolation ventilated cubicle (Model H10-24A; Coulbourn Instruments). Stimuli presentations were controlled by Freezeframe and behavior was recorded. Conditioned stimulus (CS) odor (pure peppermint, McCormick, Hunt Valley, Maryland;) was delivered by a flow dilution olfactometer controlled by a ChronTrol (ChonTrol Corporation, San Diego, California), at a 2 L/min flow rate and at a concentration of 1:10 peppermint vapor. The unconditioned stimulus (US) was a 0.5-s (0.6-mA) electric foot shock delivered through a grid floor. In this study, we used a “substitute mother” procedure. “Substitute mothers” were dams matched with pups’ mothers for postpartum period and diet. Previous studies have shown that mothers accept all pups and pups fail to distinguish between their mother and a substitute mother matched for the same postpartum period and the same diet (Moriceau and Sullivan, 2006; Debiec and Sullivan, 2014). “Substitute mothers” were conditioned during the lactation period. Animals were placed in the conditioning chamber, and given a 10-minute acclimation period. Conditioning consisted of 6 conditioning trials using a 30-second CS odor which co-terminated with a US shock (the inter trial interval, ITI, was randomly generated and was 4 minutes on average). “Unfrightened mothers” were dams that did not receive fear conditioning but instead were exposed to the equivalent number of the CS odor presentations (Fig. 1) or the equivalent number of unpaired CSs and USs (Fig. 2), and did not express fear during a subsequent re-exposure to the CS in the pups’ presence (Tab. 1; Debiec and Sullivan, 2014).
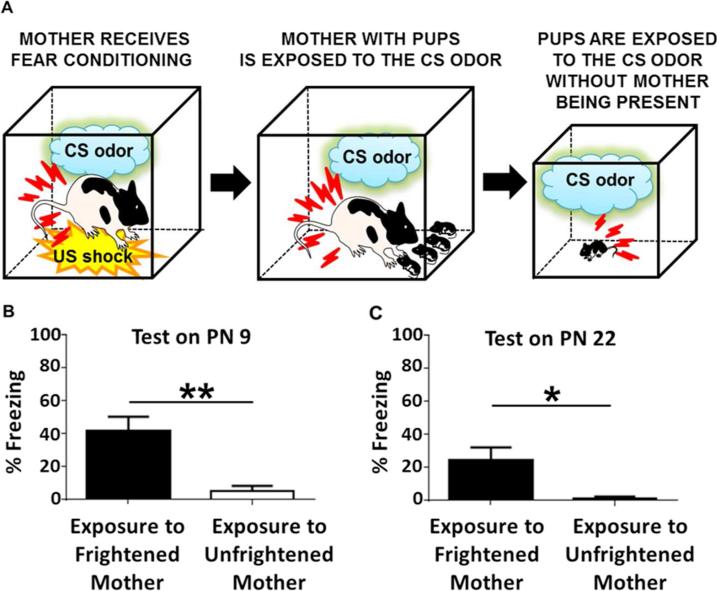
Fig. 1.
Mother-to-infant social transfer of fear on the day of birth. A: Schematic diagram illustrating behavioral procedures used in the mother-to-infant transmission of fear experiment. Postnatal day (PN) 0 pups were exposed to mother previously fear conditioned and re-exposed to the conditioned stimulus (CS) in pups presence (“Exposure to Frightened Mother”). Another group of PN 0 pups were exposed to mother with prior exposure to the CS alone (without the unconditioned stimulus, US) and then re exposed to this CS while with the pups (“Exposure to Unfrightened Mother”). A part of animals from each experimental group was re-exposed to the odor that served as the CS in maternal fear conditioning on PN 9, whereas the other part was re-exposed to the same CS on PN 22. B: PN 0 pups from the “Exposure to Frightened Mother” group show significantly higher levels of freezing behavior upon CS presentation than the “Exposure to Unfrightened Mother” pups on PN 9 (n = 6 for each group). C: PN 0 pups from the “Exposure to Frightened Mother” group show significantly higher levels of freezing behavior upon CS presentation than the “Exposure to Unfrightened Mother” pups on PN 22 (n = 9 for each group). All bars indicate mean ± SEM. *P < 0.05, **P < 0.01.
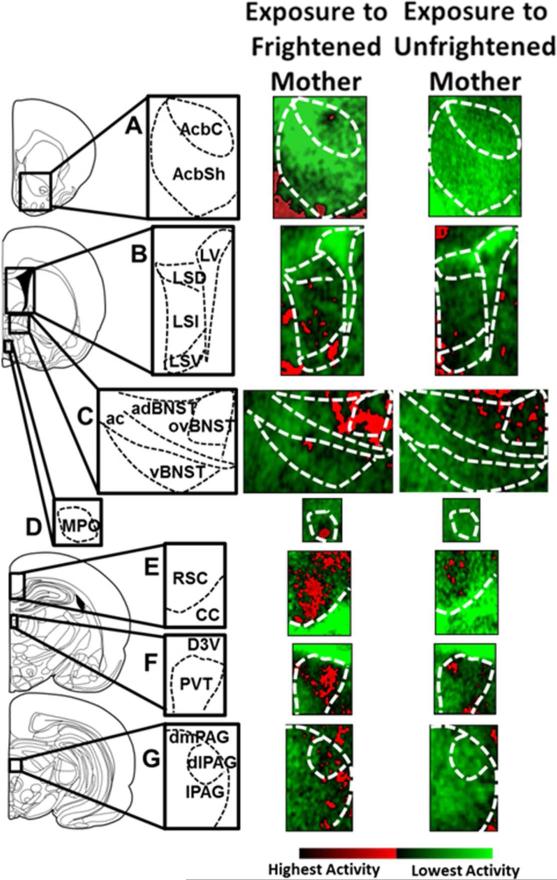
Fig. 2.
Autoradiography of mother-to-infant transfer of fear. 14C 2-deoxyglucose (2-DG) autoradiography images were taken from postnatal day (PN) 13-14 pups either exposed to mother previously fear conditioned and re exposed to the conditioned stimulus (CS) in pups presence (“Exposure to Frightened Mother”) or staying with mother not expressing fear while with the pups (“Exposure to Unfrightened Mother”). Left column shows the locations of the selected examined brain areas. Middle and right columns show representative 14C 2-deoxyglucose (2-DG) autoradiography images of the selected brain areas. A: Nucleus accumbens core (AcbC) and shell (AcbSh). B: Lateral septal nucleus: dorsal, lateral and ventral parts (LSD, LSI and LSV, respectively; LV – lateral ventricle). C: Bed nucleus of stria terminalis: anterodorsal and ventral portions, and the oval nucleus (adBNST, vBNST and ovBNST, respectively; ac – anterior commissure). D: Medial preoptic nucleus of the hypothalamus, MPO. E: Retrosplenial cortex, RSC; CC – corpus callosum. F: Paraventrical nucleus of the thalamus; PVT; D3V – dorsal 3rd ventricle. G: Periaqueductal gray: dorsomedial, dorsolateral and lateral (dmPAG, dlPAG and lPAG, respectively). Color gradation showing neural activity (pseudocolor images displayed using ImageJ Red/Green Lookup Table: from light green (no activity) to dark red (highest activity).
Table 1.
Maternal Behavior during Fear-Inducing CS Odor Exposure
| Percent of Observation Periods in Which Behaviors Occurred | ||
|---|---|---|
| Maternal Behaviors | Frightened Mother | Unfrightened Mother |
| Fearful / Defensive | 53 | 0 |
| Rough / Abusive | 0 | 0 |
| Nurturing | 0 | 52 |
| Neutral | 47 | 48 |
| Mother and pup in the nest | 28 | 36 |
Maternal behavior (%) in the presence of the pups during re-exposure to the conditioned (Frightened Mother) or neutral (Unfrightened Mother) cue. Fearful / Defensive behaviors include: freezing, startle, escaping, covering the source of odor, covering pups with bedding; Rough / Abusive behaviors include: stepping / jumping on pup, throwing / dropping / dragging / pushing away / rough handling pup; Nurturing behaviors include: nursing, grooming / licking / retrieving pup; Neutral behaviors: sleeping, resting, self-grooming, eating and drinking (see: Materials and Methods).
Mother-to-infant social transmission of fear
Exposure of pups and mothers to the CS took place in the home cage on postnatal day (PN) 0 (Fig. 1) or PN 13-14 (Fig. 2). For the duration of the experiment, a biological mother was replaced in the home cage with a “substitute mother”. The biological mother then remained in the colony in the new home cage, whereas pups in the cage with the “substitute mother” were moved to the experimental room. Animals were given a 1 hour adaptation period after which all mothers were settled and were nursing the litter. The 4, 30s CS odor presentations were delivered by an olfactometer (concentration of 1:10 peppermint vapor; 2 L/min flow rate) to mother and pups with a 10 min ITI. Immediately after, pups were removed from the cage with a ‘substitute mother’ and returned to the colony room and were placed in the home cage with their mother.
Fear memory test
On PN 9 or PN 22, pups were individually placed in a 600-mL clear plastic beaker and given a 2 min adaptation period. Subsequently, pups received three presentations of a peppermint CS odor (30 s) delivered by an olfactometer (concentration of 1:10 peppermint vapor; 2 L/min flow rate) with a 4-min ITI. After the completion of the experiment, pups were returned to the dam. The freezing behavior was videotaped and scored by a blind observer. An average of the three scores for each CS for each pup was used for the statistical analysis. Behavior was displayed as percent of freezing during the CS exposure.
Assessment of neural correlates using autoradiography
For the assessment of neural activity we used autoradiograms obtained in the previously reported experiment (Debiec and Sullivan, 2014). PN 13-14 pups were injected with 14C 2-deoxyglucose (2-DG) (20 μCi/100 g, s.c.) 5 min before the mother-to-infant social transmission of fear procedure (see above). Immediately after the social transmission of fear procedure, pups were euthanized, and their brains were quickly removed, frozen in 2-methylbutane (−45°C), and stored in a −70°C freezer. Subsequently, brains were sectioned (20 μm) in a −20°C cryostat, and every other section was placed on a coverslip and exposed for 5 d along with standards (14C standards 10 × 0.02 mCi; American Radiolabeled Chemicals) to x-ray film. 2-DG uptake was assessed using ImageJ software (NIH). The uptake of 2-DG was expressed relative to uptake in the corpus callosum (CC). 2-DG uptake in the CC did not vary across conditions. 2-DG uptake was measured by an observer blind to the experimental condition. For image analysis a default grey scale was used. For a better display, the images shown in Figure 2 were converted to pseudocolor using the ImageJ Green/Red Lookup Table (see: Fig. 2 legend).
Data analysis
Data were analyzed with GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA) using the t-test. Differences were considered significant when p < 0.05.
RESULTS
Mother-to-Infant Social Transfer of Fear Occurs on the Day of Birth
PN 0 pups exposed to a mother expressing fear to the previously trained CS odor (“Exposure to Frightened Mother”; n = 6), as well as pups exposed to a mother that was not frightened (“Exposure to Unfrightened Mother”; n = 6), showed negligible levels of freezing behavior prior to the presentation of the same CS odor on PN 9 (percent of freezing behavior during a 1 min. time interval prior to the presentation of the first CS for the “Exposure to Frightened Mother” group was 3.89 ± 2.36 and for the “Exposure to Unfrightened Mother” group was: 2.59 ± 0.88, respectively;, P > 0.05). However, during subsequent presentations of the CS odor “Exposure to Frightened Mother” pups displayed significantly higher levels of freezing behavior as compared to the “Exposure to Unfrightened Mother” controls (Student t-test, P < 0.01) (Fig. 1B; for maternal behavior during the CS odor re-exposure see: Tab. 1). Testing on PN 22 of another group of pups that had been exposed on PN 0 to a mother frightened by a presentation of the previously conditioned CS odor (“Exposure to Frightened Mother”; n = 9) as compared to pups exposed to a mother which had not been frightened (“Exposure to Unfrightened Mother”; n = 9) revealed that pups with a history of exposure to a frightened mother on PN 0 expressed significantly higher levels of freezing behavior while presented with the same CS odor (Student t-test, P < 0.05; Fig. 1C).
Brain Autoradiography of Mother-to-Infant Social Transfer of Fear
The analysis of the 2-DG autoradiograms results using t-test shows that PN 13-14 pups exposed to a frightened mother (“Exposure to Frightened Mother”; n = 7) as compared to pups exposed to an unfrightened mother (“Exposure to Unfrightened Mother”; n = 6) display significant increase in neural activity in several sites, including the nucleus accumbens core (AcbC) (P < 0.05), nucleus accumbens shell (AcbSh) (P < 0.05), lateral septum portions: dorsal (LSD) (P < 0.001), lateral (LSL) (P < 0.001) and ventral (LSV) (P < 0.05), bed nucleus of stria terminalis parts: anterodorsal (adBNST) (P < 0.05) ventral (vBNST) (P < 0.05) and the oval nucleus (ovBNST) (P < 0.05), retrosplenial cortex (RSC) (P < 0.01), hypothalamic nuclei: medial preoptic (MPO) (P < 0.01), lateral preoptic (LPO) (P < 0.01) and paraventricular (PVN) (P < 0.05), thalamic nuclei: paraventricular (PVT) (P < 0.05) and mediodorsal / intralaminar (MITN) (P < 0.05), and the lateral portion of the periaqueductal gray (lPAG) (P < 0.05). No significant differences between the experimental groups (P > 0.05) were observed in the prelimbic cortex (PL), anterior cingulate cortex (ACC), insular cortex (IC), anterior piriform cortex (APir), posterior piriform cortex (PPir), dorsal hippocampus, ventral hippocampus, primary auditory cortex (Au1), ventral posterolateral (VPN) and ventral posteromedial (VPM) nuclei of the thalamus, medial geniculate nucleus of the thalamus (MG), ventrolateral or dorsomedial periaqueductal gray (vlPAG and dmPAG, respectively). The complete list of examined structures with corresponding results is presented in Tab. 2.; Fig. 2 includes representative autoradiography images from selected areas showing significant increase of neural activity during the exposure to a frightened mother.
Table 2.
| Region | Paired-CS | CS only |
|---|---|---|
| Forebrain and Cortex | ||
| Prelimbic cortex (PL) | 1.959 ± 0.270 | 1.764 ± 0.191 |
| Anterior cingulate cortex (ACC) | 2.524 ± 0.224 | 1.987 ± 0.081 |
| Insular cortex | 1.829 ± 0.058 | 1.701 ± 0.033 |
| Anterior piriform (APir) | 2.791 ± 0.193 | 2.886 ± 0.159 |
| Posterior piriform (PPir) | 2.311 ± 0.207 | 2.123 ± 0.151 |
| Nuc. Accumbens core (AcbC) | 2.268 ± 0.175* | 1.791 ± 0.029 |
| Nuc. Accumbens shell (AcbSh) | 2.276 ± 0.166* | 1.793 ± 0.046 |
| Lateral septum dorsal (LSD) | 1.708 ± 0.032*** | 1.312 ± 0.061 |
| Lateral septum lateral (LSL) | 2.311 ± 0.119*** | 1.610 ± 0.093 |
| Lateral septum ventral (LSV) | 1.723 ± 0.087* | 1.380 ± 0.068 |
| Anterodorsal BNST (adBNST) | 3.024 ± 0.405* | 1.892 ± 0.149 |
| Oval nucleus BNST (ovBNST) | 3.929 ± 0.565* | 1.879 ± 0.128 |
| Ventral BNST (vBNST) | 1.690 ± 0.072* | 1.479 ± 0.060 |
| Retrosplenial cortex (RSC) | 2.498 ± 0.132** | 2.009 ± 0.060 |
| Dorsal hippocampus | 1.825 ± 0.094 | 1.712 ± 0.038 |
| Ventral hippocampus | 3.237 ± 0.384 | 2.277 ± 0.131 |
| Primary aud. Cortex (Aul) | 2.800 ± 0.184 | 2.279 ± 0.151 |
| Hypothalmus | ||
| Medial preoptic nucleus (mPO) | 2.995 ± 0.262** | 1.650 ± 0.150 |
| Lateral preoptic nucleus (IPO) | 2.305 ± 0.121** | 1.790 ± 0.098 |
| Paraventricular nucleus (PVN) | 2.469 ± 0.217* | 1.690 ± 0.061 |
| Thalamus | ||
| Paraventricular nucleus thalamus (PVT) | 3.773 ± 0.330* | 2.691 ± 0.223 |
| Mediodorsal and intralaminar thalamic nuclei (MITN) | 2.770 ± 0.255* | 1.984 ± 0.087 |
| Ventral posterolateral nucleus (VPN), Ventral posteromedial nucleus (VPM) | 3.213 ± 0.177 | 3.068 ± 0.230 |
| Medial geniculate nucleus (MG) | 4.215 ± 0.469 | 4.280 ± 0.445 |
| Periaqueductal gray | ||
| Dorsomedial PAG (dmPAG) | 2.044 ± 0.187 | 1.807 ± 0.088 |
| Lateral PAG (IPAG) | 2.610 ± 0.185* | 2.017 ± 0.126 |
| Ventrolateral PAG(vlPAG) | 2.142 ± 0.108 | 1.949 ± 0.214 |
Group means were tested using the t test with Welch's correction for unequal variance. Data are presented as means ± SEM.
P <0.05
P< 0.01
P<0.00 as compared to ‘Exposure to Unfrightened Mother’.
DISCUSSION
In the present study, we showed that PN 0 pups may acquire maternal CS-specific threat responses through social fear learning (Fig. 1). Maternal expression of fear to the CS in the newborns’ presence (Tab. 1) was sufficient to produce CS-controlled freezing responses that persisted at least until after weaning on PN 22 (Fig. 1). These results are consistent with our previous findings showing that pups during the first postnatal week may acquire from their mother long lasting CS-specific threat responses (Debiec and Sullivan, 2014). The current study extends our earlier findings and suggests that pups are born with an ability to learn about threats through social learning. This is in stark contrast with studies on classical fear conditioning which demonstrate that until PN 10 electric shock-reinforced aversive learning is physiologically attenuated (Sullivan et al., 2000). The present study, together with our previous work (Debiec and Sullivan, 2014), points at unique role of maternally transmitted emotional learning which enables infants to develop defense responses against possible environmental threats when they are still with the mother in the nest, and before they are able to learn through their own harmful experiences, such as these occurring in fear conditioning. However, given the small sample size, the interpretation of these data is limited and future research with a larger sample size should substantiate our current findings.
Most of what we know about the neural mechanisms of social fear learning comes from studies of adult organisms. We previously showed that transfer of maternal fear responses in infancy through SFL is mediated by alarm chemosignaling and depends on the pup's amygdala (Debiec and Sullivan, 2014). A surgical disruption of alarm pheromone processing pathways or pharmacological inactivation of the amygdala in preweaning pups prevented social transmission of fear using alarm pheromone (an odor of the frightened mother). However, both alarm chemosignaling and amygdala-dependent fear learning, including social fear learning, engage a number of neural sites and systems (Kiyokawa et al., 2005; Herry and Johansen, 2014). Our experiment using 2-DG autoradiography allowed identification of several extra-amygdala sites in the pup's brain that were activated by the maternal expression of fear (Tab. 2; Fig. 2).
Neural Activity in the Cortex and the Hippocampus
Previous research has identified several neural sites in the forebrain subserving fear learning. A recent study reported that pharmacological inactivation of the ACC in adult mice prevented the acquisition of social fear learning suggesting that the ACC plays a critical role in SFL (Jeon et al., 2010). In accordance with this report, a functional imaging study in adult human subjects showed activation in the ACC during a social fear learning task (Olsson et al., 2007). The same study reported increased activity in the IC during SFL. Indeed, evidence from human and rodent research suggests that the ACC and IC play a role in assessing potential threats (Fiddick, 2010). Another neocortical structure known to be involved in the acquisition and expression of classical fear conditioning in the adults is the PL (Sotres-Bayon et al., 2012; Sharpe and Killcross, 2015). However, in our study we did not observe any changes of activity in the pup's ACC, IC or PL during the mother-to-infant social transmission of fear (Tab. 2). It is likely that SFL in preweaning pups occurs independently of the neocortical structures, ACC, IC or PL, since mature cerebral metabolism emerges in rats around PN 20 and maturation of the neocortex is completed around PN 90 (Watson et al., 2006). Similarly, an acquisition of maternal fear responses was not accompanied by an increased 2-DG uptake in the pup's primary auditory cortex or hippocampus (Tab. 2). Although, the hippocampus and the auditory cortex play important roles in adult fear conditioning (context fear conditioning and auditory fear conditioning, respectively), the auditory modality is not functional until the postnatal week 3 and the hippocampus-dependent aversive learning emerges around / after weaning at PN 21 (Rudy, 1993; Raineki et al., 2010). However, we did observe increased 2-DG uptake in the RSC (Tab. 2; Fig. 2) which was shown to be involved in context fear conditioning in adults (Kwapis et al., 2015), yet its role in other forms of fear learning is not so well understood (Tab. 2; Fig. 2E).
Research on olfactory learning in preweaning pups indicates the involvement of the piriform cortex (part of the olfactory cortex), with the APir being implicated in the odor preference learning (Roth and Sullivan, 2005; Raineki et al., 2009; Morrison et al., 2013), and the PPir in the odor aversive learning (Raineki et al., 2009). Our published study indicates that a Grueneberg ganglion (GG) olfactory subsystem that processes alarm chemosignaling is involved in pups in the mother-to-infant social transmission of fear (Debiec and Sullivan, 2014). However, in the current study, an exposure to a frightened mother did not produce any changes in the activity of the APir or PPir (Tab. 2). Although, earlier research suggests that classical fear conditioning and SFL share similar neural mechanisms (Olsson and Phelps, 2007), the observed lack of activation in the PPir suggests that olfaction-mediated infant social fear learning is controlled by neural systems, at least in part, distinct from the classical olfactory aversive learning mechanisms. The lack of activation in the hippocampus, the PL, IC or the ACC, which are all involved in aversive learning in adults, also points at the distinct neural mechanisms supporting SFL in infancy.
Neural Activity in the Lateral Septum
We found that exposure to a frightened mother is accompanied by increased 2-DG uptake in the LSD, LSL and LSV (Tab. 2; Fig 2B). Lateral septum (LS) nuclei are reciprocally connected with the main olfactory bulb, cingulate cortex, amygdala, hippocampus, hypothalamus, thalamus and midbrain areas, and have been shown by previous research to be involved in the affect regulation (Sheehan et al., 2004). Specifically, studies in rodents show that the lateral septum plays an important role in the social modulation of fear (Guzman et al., 2014; Zoicas et al., 2014). Our findings show the activation of the LS during SFL, however, the possible role of the LS in the mother infant transmission of fear has to be determined by further research.
Neural Activity in the Nucleus Accumbens and the Bed Nucleus of Stria Terminalis
We previously demonstrated that early SFL is associated with activation of several amygdala nuclei (Debiec and Sullivan, 2014). Here we assessed neural activation in the extended amygdala areas, including the nucleus accumbens shell and stria terminalis subregions. It is well known that the bed nucleus of stria terminalis (BNST) plays an important role in stress, fear and anxiety (Daniel and Rainnie, 2015). We found that mother-to-infant social transmission of fear was associated with increased 2-DG uptake in the BNST subregions: adBNST, ovBNST and vBNST (Tab. 2; Fig 2C). This is consistent with a recent study showing that an exposure to alarm pheromone induces expression of the early expression gene c-Fos in the BNST in adult rats (Kiyokawa et al, 2005). Our data show that early SFL is accompanied by increased activity in the AcbC and AcbSh (Tab. 2; Fig 2A). Although, the role of the nucleus accumbens (Acb) in fear learning is not so well understood, recent studies in adult rats show that inactivation of the Acb disrupts the acquisition of fear-potentiated startle (Schwienbacher et al., 2004) and that active avoidance of threat is associated with increased neural activity in the Acb, especially in the AcbSh (Ramirez et al., 2015). We found that infant SFL is associated with increased activity in the BNST and the Acb subregions. Recent studies highlight the role of the BNST and the Acb systems in controlling fear and anxiety states (Daniel and Rainnie, 2015; Ramirez et al., 2015). Our findings suggest that the BNST and the Acb systems may be involved in social learning in infancy.
Neural Activity in the Hypothalamus
It is well established that hypothalamic structures are involved in processing fear, stress and pain (Gross and Canteras, 2012). We found that early SFL increased 2-DG uptake in the hypothalamic nuclei: MPO, LPO and PVN (Tab. 2; Fig 2). Although, the role of the hypothalamus in SFL is unknown, several hypothalamic structures are critical parts of a defensive circuitry and control social or predator fear (Gross and Canteras, 2012). A recent study showed that social defeat stress task in adult rats increased c-Fos immunoreactivity in MPO, LPO and PVN (Lkhagvasuren et al., 2014). In addition, increased c-Fos expression was observed in the LPO and PVN during predator exposure in adult mice (Martinez et al., 2008). Increased activity in the MPO, LPO and PVN during an exposure to a frightened mother suggests that these hypothalamic nuclei may be a part of neural networks supporting SFL in infancy.
Neural Activity in the Thalamus
Previous research has shown that the MITN, which are a thalamic part of the affective pain processing system, are involved in SFL in adult mice (Jeon et al., 2009). Consistent with this finding, we observed that mother-to-infant social transmission of fear was accompanied by increased 2-DG uptake in the MITN (Tab. 2). However, we did find any augmentation of neural activity during SFL in other hypothalamic pain systems, the VPN and VPM (Gauriau and Bernard, 2002). Our 2-DG analysis showed that infant SFL was accompanied by the PVT activation (Tab. 2; Fig 2F). The PVT is vastly connected with the Acc, BNST and the amygdala, and there is increasing evidence coming from adult studies showing the role of the PVT in regulating fear and anxiety (Kirouac, 2015). The role of the PVT in infant fear is yet to be determined. Although, the auditory thalamus plays a key role in adult auditory fear conditioning (Apergis-Schoute et al., 2005), consistent with previous studies showing that auditory modality is not functional until the postnatal week 3 (Rudy, 1993), we did not observe any activation in the auditory thalamus (MG) during infant SFL (Tab. 2). In accordance with adult SFL findings, our data suggest that the thalamus is a part of the social transmission of fear circuitry in infancy.
Neural Activity in the Periaqueductal Gray
It is well known that the periaqueductal gray (PAG) is a part of pain and fear processing systems, and through its connections with the amygdala is an important part of classical fear conditioning circuitry (Herry and Johansen, 2014). However, the role of the PAG in SFL has not yet been studied. We found that mother to infant social transmission of fear was associated with increased neural activity in the lPAG but not dmPAG or vlPAG (Tab. 2; Fig 2G), although all these parts of the PAG are involved in classical fear conditioning in adults. Our findings suggest that the PAG nociceptive and fear processing circuits may play a role in the infant SFL, as they do in classical fear conditioning in adults. The fact that SFL, as compared to fear conditioning, is associated with the more selective activation of the PAG (activation of the lPAG but no activation of the dmPAG or vlPAG) suggests that the plausible involvement of the periaqeductal gray in controlling socially transmitted fear is distinct from its role in fear conditioning.
Summary and Conclusions
Children are very vulnerable to parental stress, fear and trauma. The impact of maternal stress on the child's behavior and emotion regulation can been observed as early as in infancy (Bosquet Enlow et al., 2011). The effects of parental stress and fear on the offspring are mediated by hereditary, genetic or non genetic, as well as non-hereditary mechanisms, such as social learning. Although the ability to acquire social fear learning is present across the lifespan, the young child's dependence on the caregiver and the distinctive sensitivity to the caregiver's emotions determine a special role of social fear learning in infancy and early childhood. This unique character of SFL in childhood allows the offspring to learn early from parents about possible threats in the surrounding world (Debiec and Sullivan, 2014). Our data indicate the unique nature of social fear learning which is present at birth, in contrast to classical fear conditioning that emerges in pups during the second week of life. Infant rats can thus learn from the mother about environmental threats before their sensory and motor development allows them to acquire aversive learning through their own interactions with the environment outside their nest. However, the same ability to develop adaptive threat responses in conjunction with exposures to parental maladaptive fear may mediate the parent-child transmission of disordered fear and anxiety. Indeed, a recently published study of children-of-twins shows that children may acquire maladaptive anxiety from their parents through social fear learning, independently of hereditary mechanisms (Eley et al., 2015). Characterization of the mechanisms of SFL in infancy and early childhood is thus critical for the understanding of the social transmission of adaptive and maladaptive fears.
We previously demonstrated that the amygdala plays a critical role in social transmission of fear in infancy (Debiec and Sullivan, 2014). Here, we show that several neural sites which are involved in processing fear, stress and pain, and which, in major part, are either directly or indirectly connected with the amygdala, are activated in the pup's brain during the acquisition of maternal threat responses. The current study provides candidate regions for further research of neural mechanisms of social fear learning in humans. Identification of the behavioral, neural and molecular mechanisms of social fear learning in infancy and early childhood may help develop early preventive and treatment interventions aimed at disrupting the transfer of parental maladaptive fears to children.
SIGNIFICANCE STATEMENT.
Children learn adaptive and maladaptive anxious behaviors from their parents. However, neural mechanisms of this parent-child fear transmission remain elusive. Here, using a rodent model of mother-to-infant social transmission of fear and autoradiography imaging, we identify a number of threat and stress processing areas in the infant brain activated by an exposure to a frightened mother. Understanding the neurobiology of the parent-child fear transmission will contribute to the development of novel methods aimed at prevention and treatment of the intergenerational transmission of maladaptive fear, stress and trauma.
OTHER ACKNOWLEDGEMENTS
We thank Dr. Regina M. Sullivan for her valuable comments and discussions, and Lisa Salstein for her technical assistance in preparation of the 2-DG autoradiograms.
Supported by: K08 MH014743 01A1, NARSAD Young Investigator Award from the Brain & Behavior Research Foundation and Todd Ouida Clinical Scholar Award in Childhood Anxiety and Depression to J.D.
ROLE OF AUTHORS
D.C. and J.D. conceived and designed the study. D.C. acquired the data and conducted the statistical analysis. D.C. and J.D. analyzed and interpreted the data. D.C. prepared the figures and J.D. wrote the manuscript. J.D. obtained funding and supervised the study.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflict of interest
REFERENCES
- Aktar E, Majdandzic M, de Vente W, Bogels SM. The interplay between expressed parental anxiety and infant behavioural inhibition predicts infant avoidance in a social referencing paradigm. J Child Psychol Psychiatry. 2013;54(2):144–156. doi: 10.1111/j.1469-7610.2012.02601.x. [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute AM, Debiec J, Doyere V, LeDoux JE, Schafe GE. Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: a role for presynaptic plasticity in the fear system. J Neurosci. 2005;25(24):5730–5739. doi: 10.1523/JNEUROSCI.0096-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet Enlow M, Kitts RL, Blood E, Bizarro A, Hofmeister M, Wright RJ. Maternal posttraumatic stress symptoms and infant emotional reactivity and emotion regulation. Infant Behav Dev. 2011;34(4):487–503. doi: 10.1016/j.infbeh.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers ME, Yehuda R. Intergenerational Transmission of Stress in Humans. Neuropsychopharmacology. 2016;41(1):232–244. doi: 10.1038/npp.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel SE, Rainnie DG. Stress Modulation of Opposing Circuits in the Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.178. doi: 10.1038/npp.2015.178. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Diaz-Mataix L, Bush DE, Doyere V, Ledoux JE. The amygdala encodes specific sensory features of an aversive reinforcer. Nat Neurosci. 2010;13(5):536–537. doi: 10.1038/nn.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Sullivan RM. Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(33):12222–12227. doi: 10.1073/pnas.1316740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rosnay M, Cooper PJ, Tsigaras N, Murray L. Transmission of social anxiety from mother to infant: an experimental study using a social referencing paradigm. Behav Res Ther. 2006;44(8):1165–1175. doi: 10.1016/j.brat.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Eley TC, McAdams TA, Rijsdijk FV, Lichtenstein P, Narusyte J, Reiss D, Spotts EL, Ganiban JM, Neiderhiser JM. The Intergenerational Transmission of Anxiety: A Children-of-Twins Study. The American journal of psychiatry. 2015;172(7):630–637. doi: 10.1176/appi.ajp.2015.14070818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiddick L. There is more than the amygdala: potential threat assessment in the cingulate cortex. Neurosci Biobehav Rev. 2011;35(4):1007–1018. doi: 10.1016/j.neubiorev.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87(2):251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci. 2012;13(9):651–658. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- Guzman YF, Tronson NC, Sato K, Mesic I, Guedea AL, Nishimori K, Radulovic J. Role of oxytocin receptors in modulation of fear by social memory. Psychopharmacology. 2014;231(10):2097–2105. doi: 10.1007/s00213-013-3356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci. 2014;17(12):1644–1654. doi: 10.1038/nn.3869. [DOI] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, Shin HS. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nature neuroscience. 2010;13(4):482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Riha PD, Gore AC, Monfils MH. Social transmission of Pavlovian fear: fear-conditioning by-proxy in related female rats. Anim Cogn. 2014;17(3):827–834. doi: 10.1007/s10071-013-0711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac GJ. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci Biobehav Rev. 2015;56:315–329. doi: 10.1016/j.neubiorev.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Mapping the neural circuit activated by alarm pheromone perception by c-Fos immunohistochemistry. Brain Res. 2005;1043(1-2):145–154. doi: 10.1016/j.brainres.2005.02.061. [DOI] [PubMed] [Google Scholar]
- Klengel T, Dias BG, Ressler KJ. Models of Inter- and Transgenerational Transmission of Risk for Psychopathology in Mice. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Nikolaev E, Boguszewski P, Walasek G, Blaszczyk J, Kaczmarek L, Werka T. Between-subject transfer of emotional information evokes specific pattern of amygdala activation. Proc Natl Acad Sci U S A. 2006;103(10):3858–3862. doi: 10.1073/pnas.0511302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Helmstetter FJ. The retrosplenial cortex is involved in the formation of memory for context and trace fear conditioning. Neurobiol Learn Mem. 2015;123:110–116. doi: 10.1016/j.nlm.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara A, Crego A, Romero-Maroto M. Emotional contagion of dental fear to children: the fathers' mediating role in parental transfer of fear. Int J Paediatr Dent. 2012;22(5):324–330. doi: 10.1111/j.1365-263X.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- Lkhagvasuren B, Oka T, Nakamura Y, Hayashi H, Sudo N, Nakamura K. Distribution of Fos-immunoreactive cells in rat forebrain and midbrain following social defeat stress and diazepam treatment. Neuroscience. 2014;272:34–57. doi: 10.1016/j.neuroscience.2014.04.047. [DOI] [PubMed] [Google Scholar]
- Marquez C, Poirier GL, Cordero MI, Larsen MH, Groner A, Marquis J, Magistretti PJ, Trono D, Sandi C. Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl Psychiatry. 2013;3:e216. doi: 10.1038/tp.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez RC, Carvalho-Netto EF, Amaral VC, Nunes-de-Souza RL, Canteras NS. Investigation of the hypothalamic defensive system in the mouse. Behav Brain Res. 2008;192(2):185–190. doi: 10.1016/j.bbr.2008.03.042. [DOI] [PubMed] [Google Scholar]
- Mineka S, Cook M. Mechanisms involved in the observational conditioning of fear. J Exp Psychol Gen. 1993;122(1):23–38. doi: 10.1037//0096-3445.122.1.23. [DOI] [PubMed] [Google Scholar]
- Molapour T, Golkar A, Navarrete CD, Haaker J, Olsson A. Neural correlates of biased social fear learning and interaction in an intergroup context. Neuroimage. 2015;121:171–183. doi: 10.1016/j.neuroimage.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison GL, Fontaine CJ, Harley CW, Yuan Q. A role for the anterior piriform cortex in early odor preference learning: evidence for multiple olfactory learning structures in the rat pup. J Neurophysiol. 2013;110(1):141–152. doi: 10.1152/jn.00072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, de Rosnay M, Pearson J, Bergeron C, Schofield E, Royal-Lawson M, Cooper PJ. Intergenerational transmission of social anxiety: the role of social referencing processes in infancy. Child Dev. 2008;79(4):1049–1064. doi: 10.1111/j.1467-8624.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2(1):3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Social learning of fear. Nat Neurosci. 2007;10(9):1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Raineki C, Holman PJ, Debiec J, Bugg M, Beasley A, Sullivan RM. Functional emergence of the hippocampus in context fear learning in infant rats. Hippocampus. 2010;20(9):1037–1046. doi: 10.1002/hipo.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Shionoya K, Sander K, Sullivan RM. Ontogeny of odor LiCl vs. odor-shock learning: similar behaviors but divergent ages of functional amygdala emergence. Learning & memory. 2009;16(2):114–121. doi: 10.1101/lm.977909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F, Moscarello JM, LeDoux JE, Sears RM. Active avoidance requires a serial basal amygdala to nucleus accumbens shell circuit. J Neurosci. 2015;35(8):3470–3477. doi: 10.1523/JNEUROSCI.1331-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Galea S, Austin SB, Cerda M, Wright RJ, Rich-Edwards JW, Koenen KC. Posttraumatic stress disorder across two generations: concordance and mechanisms in a population based sample. Biol Psychiatry. 2012;72(6):505–511. doi: 10.1016/j.biopsych.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Poorman CE, Marder TJ, Bucci DJ. Identification of functional circuitry between retrosplenial and postrhinal cortices during fear conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(35):12076–12086. doi: 10.1523/JNEUROSCI.2814-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57(8):823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci. 1993;107(5):887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Schwienbacher I, Fendt M, Richardson R, Schnitzler HU. Temporary inactivation of the nucleus accumbens disrupts acquisition and expression of fear-potentiated startle in rats. Brain Res. 2004;1027(1-2):87–93. doi: 10.1016/j.brainres.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Sharpe MJ, Killcross S. The prelimbic cortex uses higher-order cues to modulate both the acquisition and expression of conditioned fear. Front. Syst. Neurosci. 2015;8:235. doi: 10.3389/fnsys.2014.00235. doi: 10.3389/fnsys.2014.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev. 2004;46(1):71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76(4):804–812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407(6800):38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H, Li XY, Wu LJ, Zhao MG, Descalzi G, Chen T, Koga K, Zhuo M. Interplay of amygdala and cingulate plasticity in emotional fear. Neural Plast. 2011;2011:813749. doi: 10.1155/2011/813749. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J Clin Endocrinol Metab. 2005;90(7):4115–4118. doi: 10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]
- Watson RE, Desesso JM, Hurtt ME, Cappon GD. Postnatal growth and morphological development of the brain: a species comparison. Birth Defects Res B Dev Reprod Toxicol. 2006;77(5):471–484. doi: 10.1002/bdrb.20090. [DOI] [PubMed] [Google Scholar]
- Zoicas I, Slattery DA, Neumann ID. Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39(13):3027–3035. doi: 10.1038/npp.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]