SUMMARY
Pathogenic H7N9 avian influenza viruses continue to represent a public health concern and several candidate vaccines are currently being developed. It is vital to assess if protective antibodies are induced following vaccination, and to characterize the diversity of epitopes targeted. Here we characterized the binding and functional properties of twelve H7-reactive human antibodies induced by a candidate A/Anhui/1/2013 (H7N9) vaccine. Both neutralizing and non-neutralizing antibodies protected mice in vivo during passive transfer challenge experiments. Mapping the H7 hemagglutinin antigenic sites by generating escape mutant variants against the neutralizing antibodies identified unique epitopes on the head and stalk domains. Further, the broadly cross-reactive non-neutralizing antibodies generated in this study were protective through Fc-mediated effector cell recruitment. These findings reveal important properties of vaccine-induced antibodies and provide a better understanding of the human monoclonal antibody response to influenza in the context of vaccines.
INTRODUCTION
Influenza epidemics result in 250,000–500,000 deaths annually (World Health Organization, 2014). Vaccination offers the most effective protection against infection but vaccines have to be reformulated every year due to antigenic drift. (Krammer and Palese, 2015). In addition to seasonal epidemics, influenza virus strains that are antigenically divergent can arise, leading sporadically to pandemics. The surface glycoprotein hemagglutinin (HA) is the main target of neutralizing antibodies (Kaur et al., 2011). Seasonal vaccination generally induces a narrow, strain-specific response against the highly variable head domain of HA, whereas broadly neutralizing antibodies specific to the more conserved stalk domain are typically rare (Henry Dunand et al., 2015; Krammer and Palese, 2013; Wilson and Andrews, 2012). Although in vitro neutralization traditionally correlates with protection against infection in humans (Couch and Kasel, 1983), recent work has highlighted the importance of non-neutralizing antibodies (Jegaskanda et al., 2013a; Krammer et al., 2014b; Terajima et al., 2015). A more complete understanding of all types of protective antibodies is critical for the improvement of existing influenza vaccines and the development of new ones.
A novel reassortant avian H7N9 virus crossed the species barrier and caused a zoonotic epidemic in China in 2013 (Gao et al., 2013; Watanabe et al., 2013). This virus reemerged in 2014 and 2015 in a seasonal pattern, causing morbidity and mortality in humans (World Health Organization, 2015). Although virus transmission occurs primarily through poultry exposure, its continuous circulation in poultry and the large number of sporadic human infections increase the chance of a new reassortment or the acquisition of mutations that could change the properties of the virus (Hu et al., 2014). To prevent H7N9 influenza infections, a live-attenuated A/Anhui/1/2013 H7N9 virus vaccine candidate was developed (Chen et al., 2014b) and evaluated in healthy individuals (Sobhanie et al., 2015).
Induction of potent humoral immune responses with H7 vaccines has proven to be problematic due to the poor immunogenicity of novel avian HAs (Cox et al., 2009). Several studies using inactivated or live attenuated H7 vaccines (Couch et al., 2012; Cox et al., 2009; Karron et al., 2009; Rudenko et al., 2014; Talaat et al., 2009; Treanor et al., 2006) showed similarly modest results. However, an H7N7 live-attenuated virus vaccine led to long-term cross-reactive immune memory (Babu et al., 2014) and a strong recall response with high-affinity H7 head and stalk domain-specific serum antibodies (Halliley et al., 2015). Given that candidate H7N9 vaccines are currently being developed, it is vital to assess if protective antibodies are induced following vaccination, and additionally, to characterize the diversity of epitopes targeted on the HA protein.
In this study, we mapped the H7 HA antigenic sites using human monoclonal antibodies (mAbs) isolated from individuals who had received an H7N9 vaccine. Twelve mAbs with particularly high potency and/or breadth of reactivity to multiple influenza strains were chosen for in-depth characterization. These mAbs bound to various epitopes on the head and the stalk domains and were found to be both neutralizing and non-neutralizing in vitro. Importantly, passive transfer of both categories of mAbs protected mice in vivo against a stringent lethal challenge. This work identifies potential therapeutic mAbs and provides a better understanding of the antibody response to H7 viruses.
RESULTS
Generation and characterization of human monoclonal antibodies after H7N9 immunization
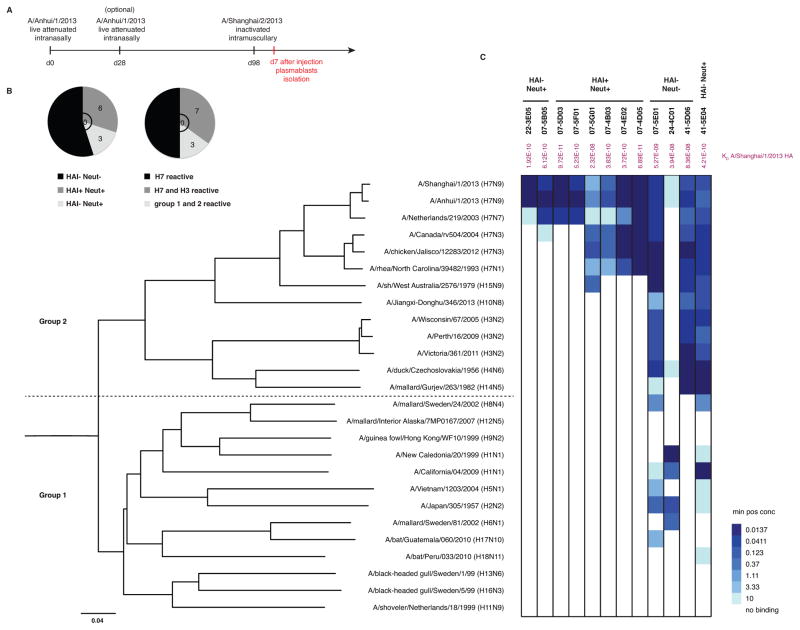
Four healthy individuals were primed with either one or two doses of a live attenuated cold-adapted influenza A/Anhui/1/2013 (H7N9) vaccine (Chen et al., 2014b). The subjects were then boosted 12 weeks later with an inactivated virus vaccine based on the closely related A/Shanghai/2/2013 (H7N9) strain (Sanofi Pasteur) (Figure 1A). Plasmablasts were isolated 7 days after administration of the inactivated vaccine and mAbs were cloned as previously described (Smith et al., 2009; Wrammert et al., 2008). Twenty mAbs bound A/Anhui/1/2013 (H7N9) HA by ELISA and were then screened using three criteria: HAI activity, neutralization activity, and cross-reactivity to diverse influenza A HAs (group 1 and group 2). Nine out of the 20 mAbs displayed neutralization activity, with 6 displaying HAI activity (Figure 1B). Half of the mAbs (10/20) were cross-reactive within group 2 HAs (H3 and H7) and/or between group 1 and 2 HAs (Figure 1B). Twelve influenza virus-positive mAbs with particularly high potency (HAI and microneutralization) and/or breadth were then chosen for full characterization: 22-3E05, 07-5B05, 07-5D03, 07-4D05, 07-5F01, 07-5G01, 07-4B03, 07-4E02, 07-5E01, 41-5D06, 41-5E04 and 24-4C01 (Figure 1C). Four of the mAbs (22-3E05, 07-5B05, 07-5D03, 07-5F01) were restricted in binding to H7 strains from the Eurasian lineage (H7N9 and H7N7). Four antibodies (07-5G01, 07-4B03, 07-4E02 and 07-4D05) bound to strains from both the Eurasian and the North American lineages (H7N3 and H7N1). Three of the 12 mAbs were broadly cross-reactive (07-5E01, 41-5D06 and 41-5E04) and bound to various HAs from group 1 and group 2 HAs (Figure 1C). None of them bound to influenza B HA (data not shown). The binding affinity of each mAb to A/Shanghai/1/2013 (H7N9) HA was determined using biolayer interferometry (Table S1). The majority of the mAbs bound with subnanomolar affinities (KD values ranging from 5.27 × 10−9 M to 9.72 × 10−11 M) except 07-5G01 (KD = 2.32 × 10−8 M), 41-5D06 (KD = 8.36 × 10−8 M) and 24-4C01 (KD = 3.94 × 10−8 M).
Figure 1. Characterization of human H7-reactive antibodies.
(A) Human monoclonal antibodies (mAbs) were cloned from plasmablasts isolated at day 7 after the inactivated H7N9 vaccine. (B) Twenty H7-reactive mAbs were screened for HAI, neutralization and cross-reactivity to diverse influenza A HAs (group 1 and 2). (C) Binding to recombinant HA proteins was tested by ELISA. Representative minimum positive concentrations (μg/ml) from 3 independent experiments are plotted as a heatmap. The different HAs were clustered by amino acid sequences phylogeny and multiple alignments were performed using the CLUSTALW algorithm. Phylogenetic rooted tree was constructed using the neighbor-joining method and was visualized using FigTree v1.4.0 software. mAbs are grouped by individuals (22, 07, 24 and 41) and HAI+ Neut+, HAI− Neut+ and HAI− Neut− categories are mentioned. Affinity measurements (KD) determined by biolayer interferometry for A/Shanghai/1/2013 (H7N9) HA are displayed in purple. See also Table S1 and Figure S1.
In vitro HAI and neutralization activities were then determined. 07-5D03, 07-5F01, 07-5G01, 07-4B03, 07-4E02 and 07-4D05 displayed HAI and neutralization activities with A/Shanghai/1/2013 (H7N9) viruses, with 07-4B03, 07-4E02 and 07-4D05 being the more potent (minimum positive concentration <0.5 μg/ml for HAI and IC50 < 0.1μg/ml for neutralization) (Figure 2A). 22-3E05, 07-5B05 and 41-5E04 were neutralizing (IC50 < 10μg/ml) but had no HAI activity. Finally, 41-5D06, 07-5E01 and 24-4C01 had neither neutralizing nor HAI activity. Thus, we identified three classes of H7-induced mAbs: HAI+ Neut+, HAI− Neut+ and HAI− Neut−. Interestingly, there was no significant correlation between KD and IC50 of the neutralizing mAbs (Figure S1). The mAbs were then tested in vitro against various representative strains from group 1 and 2, based on the cross-reactivity previously observed by ELISA (Figure 2B). As expected, 07-5G01 neutralized H15 and 41-5E04 neutralized H1 and H3 viruses. In line with our results using the A/Shanghai/1/2013 (H7N9) strain, 41-5D06 and 07-5E01 had no detectable neutralizing activity. 24-4C01 did not neutralize nor inhibit hemagglutination of A/California/04/09 (H1N1) virus (Figure 2B, and HAI data not shown). The HAI+ Neut+ mAbs were tested for HAI with multiple H7 strains (Figure 2C). 07-5D03 and 07-5F01 had HAI activity only for the A/mallard/Netherlands/12/2000 (H7N3) strain (Eurasian lineage) but not for any strains from the North American lineage (H7N3 and H7N1). 07-5G01, 07-4B03 and 07-4E02 had HAI activity for all the strains from both lineages except for A/rhea/NC/39482/1993 (H7N1), despite the binding observed by ELISA. This loss of HAI activity might have been caused by steric hindrance from an N-linked glycan near the antibody epitope (Goff et al., 2013) that was not present in the other tested H7 strains. The HAI+ mAbs were also tested against an A/Anhui/1/2013 antigenic site A (R149G) escape mutant virus. 07-4D05 inhibited hemagglutination with the wild-type A/Anhui/1/2013 (H7N9) virus but not with the A/Anhui/1/2013 escape mutant virus, suggesting that this mAb bound to the antigenic site A of the globular head (Figure 2D). The five remaining HAI+ Neut+ mAbs retained HAI activity with the mutant. For all mAbs, similar HAI activity was observed for the wild-type A/Anhui/1/2013 (H7N9) strain and the A/Shanghai/1/2013 (H7N9) strain (Figure 2A and 2D).
Figure 2. Human H7-reactive antibodies exhibit various in vitro functional characteristics.
(A–D) Minimum positive concentrations in μg/ml (mean± SEM) are reported for HAI; IC50 in μg/ml (mean± SEM) are reported for neutralization. Experiments were done in duplicate 3 times. (A) HAI and neutralization assays were performed with A/Shanghai/1/2013 (H7N9) virus. (B) Neutralization with various group1 and 2 viruses based on previous ELISA results; n.d. for not determined. In color are reported previous neutralization results from (A) with H7N9. (C) HAI was performed with various H7 viruses from the Eurasian (H7N7) and North American (H7N3 and H7N1) lineages. Only the HAI+ Neut+ mAbs were tested. (D) HAI was performed with wild-type A/Anhui/1/2013 (H7N9) virus and with an escape mutant virus that displays mutations in the H7 HA antigenic site A (R149G). Only the HAI+ Neut+ mAbs were tested. No HAI activity was observed for 07-4D05 with the escape mutant (R149G). See also figure S1.
In vivo protective activity of H7-reactive antibodies is not exclusively dependent on neutralization
The in vivo protective efficacy of the mAbs against H7N9 infection was tested prophylactically in mice. Doses as low as 0.3 mg/kg (07-5F01, 07-5G01, 07-4B03, 22-3E05 and 07-5B05) or 1 mg/kg (07-5D03 and 41-5E04) fully protected mice from mortality upon H7N9 challenge, with little or modest weight loss (Figure 3A to 3G). Treatment with the non-neutralizing mAbs (41-5D06, 07-5E01 and 24-4C01) led to more pronounced weight loss than treatment with the neutralizing ones, but still resulted in increased survival and reduced weight loss compared to the control treatment (Figure 3H, 3I and 3J). Control mice succumbed to infection by day 7. 07-4D05 was not tested as it has been previously shown that an antibody targeting the same residue in the antigenic site A provided protection against H7N9 viruses (Schmeisser et al., 2015). Moreover we tested only one of the two clonally related mAbs 07-4B03 and 07-4E02, which was defined by identical VH and JH usage (heavy chain), identical VL and JL usage (light chain) and CDR3 length, as well as CDR3 sequences that differ by no more than the general frequency of somatic hypermutation (Table 1). Because all the H7-reactive mAbs, independently of in vitro neutralization activity were offering protection in vivo, we wanted to determine if the breadth of reactivity could explain protection. Mice treated with an HAI− Neut− H7 specific-reactive mAb (non-broadly cross-reactive) in the same challenge model succumbed to infection by day 7, whereas all mice treated with 07-5E01 (non-neutralizing but cross-reactive) survived (Figure S2A).
Figure 3. Human H7-reactive antibodies confer protection in vivo.
6–8 week old female BALB/c mice were injected with 5, 1 or 0.3 mg/kg of each mAb and then infected with 7.5 LD50 of A/Shanghai/1/2013 (H7N9) virus. Values represent mean ± SEM (n=5 mice per group). (A–J) Percent of initial weight and percent survival are plotted for each mAb: (A) 07-5D03, (B) 07-5F01, (C) 07-5G01, (D) 07-4B03, (E) 22-3E05, (F) 07-5B05, (G) 41-5E04, (H), 41-5D06, (I) 07-5E01 and (J) 24-4C01. Mice that received an H3-reactive but not H7-reactive mAb (011-2C01) died at day 6 after the challenge. The same control group of mice was used for all panels. See also Figure S2.
Table 1.
Genetic characteristics of H7-reactive antibodies
| Antibody | VH | JH | VH mutations | CDR3 | VL | JL |
|---|---|---|---|---|---|---|
| 07-5D03 | VH3-30-3 | JH4 | 10 | REESSQYYFDN | VL3-1 | JL2 |
| 07-5F01 | VH4-31 | JH4 | 9 | RGNSSSWYPYYFDY | VL1-51 | JL3 |
| 07-5G01 | VH3-48 | JH6 | 12 | RALTNNKDYFYFMDV | VK3-11 | JK4 |
| 07-4B03 | VH1-46 | JH5 | 11 | RDQRYPQGPFDYGEFYGDWFDP | VL1-40 | JL3 |
| 07-4E02 | VH1-46 | JH5 | 11 | RDQRYPQGPFDYGEFYGDWFDP | VL1-40 | JL3 |
| 07-4D05 | VH3-33 | JH5 | 12 | RGAMGYNWFDP | VK1-5 | JK4 |
| 22-3E05 | VH3-13 | JH6 | 6 | RGCAASLVPRRYYYGLDV | VL3-1 | JL2 |
| 07-5B05 | VH3-21 | JH4 | 9 | RDVEDYGGNSGGPFDY | VL4-69 | JL1 |
| 41-5E04 | VH3-53 | JH6 | 29 | RDFLRGPIHDYFFYMDV | VK3-11 | JK3 |
| 41-5D06 | VH1-8 | JH5 | 13 | RGSGGDYVPRYWFDP | VK3-11 | JK2 |
| 07-5E01 | VH4-39 | JH6 | 16 | RSDGDYDYHYYMDV | VK3-15 | JK4 |
| 24-4C01 | VH3-33 | JH4 | 20 | KEVLYGGYYFDY | VK3-15 | JK1 |
Immunoglobulin sequences were analyzed with VGenes, software developed by P.C.W, and with International Immunogenetics information system (IMGT).
Additionally to assess the mAbs ability to clear infection, we analyzed the lung viral titers of mice prophylactically treated with each mAb (Figure S2B). We detected decreased viral titers at day 3 in the lungs of all mice treated with H7-reactive mAbs, compared to the control mice. Analysis of the day 6 viral lung titers of mice treated with neutralizing mAbs (HAI+ or HAI−) revealed less replicating virus in the lungs, which demonstrated that neutralizing mAbs cleared the viruses faster than the non-neutralizing protective ones in vivo (p<0.05).
In addition to the A/Shanghai/1/2013 (H7N9) challenge, 41-5E04 was tested in a prophylactic setting against infection with a reverse genetics derived A/Jiangxi-Donghu/346/2013 (H10N8) virus (Chen et al., 2014a; Wohlbold et al., 2015a; Wohlbold et al., 2015b). The mice were fully protected at 5 mg/kg, while mice treated with the same dose of 22-3E05 (not reactive to H10N8) died before day 7 (Figure S2C). Finally the protective efficacy of the different mAbs was tested in a therapeutic setting. For all five HAI+ Neut+ mAbs, the mice lost minimal weight (~ 5%) and all such treated mice survived the challenge (Figure S2D). Similar results were observed when the mice were treated with the HAI− Neut+ mAbs (Figure S2D). For the HAI− Neut− mAbs 41-5D06 and 24-4C01, the mice had more pronounced weight loss (10–15%); only 07-5E01 inhibited weight loss similar to that observed with the neutralizing mAbs. These results correlate with the outcome of the prophylactic experiments at the highest dose (5 mg/kg). In conclusion our results demonstrate that both the neutralizing and non-neutralizing but broadly cross-reactive mAbs elicited after H7 vaccination protect mice in vivo.
Neutralizing antibodies induced by H7N9 vaccine bind to various epitopes on the head and stalk domain of hemagglutinin
To determine how the functionally distinct H7N9-reactive mAbs bound and protected against influenza virus infection, we generated escape mutant variants of the A/Shanghai/1/2013 (H7N9) virus and determined the critical residues for binding (Figure 4A and 4B). The putative H7 antigenic sites (sites A, B, C, D and E) on the HA globular head have been predicted using the highly similar structure of H3 subtype HA (Goff et al., 2013). 07-4D05 bound to a previously described epitope involving the 149 residue in the antigenic site A of the head domain (Figure 2D) (Schmeisser et al., 2015). The G132R substitution was identified in the escape mutants generated with 07-5D03. The Gly132 residue was not in a “classical” antigenic site (A to E) but was located in the receptor-binding domain and was conserved between H7 strains from both lineages. Similarly, the G137E substitution observed for viruses that escaped from 07-5G01 was outside of the classical antigenic sites and overlapped with the receptor-binding domain. The Gly137 residue was conserved in H7 strains from both lineages, explaining the broader binding pattern of 07-5G01 (Figure 1). This mutation has been previously described in the literature (He et al., 2013). The R65K substitution was identified in the escape mutants generated with 07-5F01. This epitope is located outside the receptor-binding domain. S152P and S153P/A440T substitutions were observed for the escape mutants generated with the clonally related mAbs 07-4B03 and 07-4E02, respectively. Both residues (Ser152 and Ser153) were located in the antigenic site A but the epitope targeted might differ slightly from the one bound by 07-4D05. This would explain the difference in HAI activity observed against the R149G escape mutant between 07-4D03/07-4E02 and 07-4D05 (Figure 2D). These mAbs could be approaching the hemagglutinin from different angles.
Figure 4. Human H7-neutralizing antibodies bind to various epitopes on the HA stalk and head domains.
Escape mutant variants with A/Shanghai/1/2013 (H7N9) virus were generated for the neutralizing mAbs. Sequences for each mutant were compared to the wild-type virus and unique mutations were reported. (A) Critical residues for binding of Neut+ mAbs are reported. Nomenclature for substitutions follows the rule of original residue, position of the amino acid, new residue (H3 numbering). (B–C) Modeling of A/Shanghai/1/2013 H7 HA was done using PyMOL (PDB ID 4LN3). HAI+ Neut+ critical residues are shown in red and HAI− Neut+ residues are shown in yellow. The Arg65 residue, (HAI+ and HAI−) is shown in orange. See also Figure S3.
We analyzed the binding of the mAbs to their respective escape mutants by immunofluorescence and found that the mutations resulted in loss of binding for all the HAI+ Neut+ mAbs (Figure S3). The virus variants generated with the HAI− Neut+ mAbs each displayed multiple substitutions (Figure 4A). To determine which residue was critical for binding, we cloned and expressed these HA variant proteins on the surface of cells. R65W and F535L substitutions were observed for the virus variant generated with 07-5B05 and a reduction in binding was witnessed when cells expressed hemagglutinin proteins with an R65W substitution (Figure S3). The same Arg65 residue was critical for binding of 07-5F01 (HAI+). R149S and K182N substitutions were observed for the virus variant generated with 22-3E05 and loss of binding was visualized for cells expressing K182N (Figure 4A and Figure S3). The Lys182 residue was located in the antigenic site E on globular head of H7 HA. Finally, two substitutions, A143T and D358N, were identified for the virus variant generated with 41-5E04. The Asp358 residue was critical for binding to H7 HA (Figure S3), suggesting that this broadly neutralizing (group 1 and 2) mAb targeted the HA stalk domain. To confirm this epitope, a competition assay was performed (Figure 5A and Figure S4A) and 41-5E04 competed with CR9114, a well-known broadly neutralizing stalk-reactive mAb (Dreyfus et al., 2012). These results confirmed that 41-5E04 and CR9114 bound to overlapping epitopes on the stalk domain of HA. We conclude from these mapping studies that the H7 viruses can be neutralized by mAbs binding to the predicted antigenic sites surrounding the receptor binding site, to the HA stalk, and to epitopes outside the receptor binding site.
Figure 5. Neutralizing and non-neutralizing H7-reactive antibodies bind to different epitopes on hemagglutinin.
(A) Biotinylated mAbs 41-5E04 (HAI− Neut+) and 41-5D06 (HAI− Neut−) were tested for binding to A/Shanghai/1/2013 HA by ELISA with or without the presence of a competitor mAb. The experiment was done in duplicate 3 times. The percentage of competition between the 41-5E04 and 41-5D06 (in red) and the non-neutralizing mAbs or CR9114 is shown. Absorbance value of each mAb against itself is scored at 100% inhibition and comparison of different mAbs was done as a percentage of this 100% inhibition. (B) Purified virus preparations were treated with various pH-buffered solutions (from pH 7.0 to 4.4) and binding (at pH 7.4) to HA was measured by ELISA. Antibody binding was tested in duplicate and shown here is one representative of two independent experiments. See also Figure S4.
Neutralizing and non-neutralizing antibodies induced by H7N9 vaccine bind to different epitopes on hemagglutinin
Due to the lack of selective pressure, escape mutants could not be generated towards non-neutralizing mAbs. To gain insight into the binding sites, a competition assay was performed using the stalk-reactive 41-5E04 (HAI− Neut+) and 41-5D06 (HAI− Neut−) mAbs (Figure 5A and Figure S4A). 41-5E04 (HAI− Neut+) did not compete with the non-neutralizing mAbs 41-5D06 or 24-4C01 but did compete partially with 07-5E01 (42%). 41-5D06 (HAI− Neut−) did not compete with 24-4C01 (HAI− Neut−), showed almost no competition with the neutralizing stalk-reactive mAbs (41-5E04, 27% and CR9114, 22%) and did partially compete with the 07-5E01 (HAI− Neut−). In addition, the non-neutralizing mAbs were tested by immunofluorescence against all of the escape mutant plasmids expressed in cells and none of them showed any reduction in binding (data not shown), suggesting that they bound different epitopes.
At low pH, the HA stalk domain undergoes conformational changes. Neutralizing mAbs binding to the stalk region can prevent membrane fusion by inhibiting these conformational changes (Dreyfus et al., 2012; Ekiert et al., 2011; Tan et al., 2012). It is unknown if non-neutralizing protective mAbs inhibit membrane fusion in the same manner. We treated purified virus preparations with various pH-buffered solutions (from pH 7.0 to 4.4), returned the pH to neutral, and then measured binding of the mAb (at pH 7.4) to the HA. Exposure to low pH converts the HA to the post-fusion state and prevents mAbs that recognize conformational epitopes on the pre-fusion HA from binding (Figure 5B). 41-5E04 (stalk-reactive) bound to the virus treated with pH 7.0, 5.8 and 5.4 but did not bind virus treated with pH 5.0 or below, suggesting that the conformational epitope recognized was not maintained at low pH when the HA is in a fusion-active conformation. A similar binding pattern was observed with the control stalk-reactive mAb CR8020. In contrast, the binding of the neutralizing mAbs to the head region of HA was independent of the pH (Figure 5B). Interestingly, the non-neutralizing mAbs bound to the HA in a post-fusion state (pH 5.0 and 4.4), suggesting that the epitope targeted was not affected by the conformational change and might even become more accessible in the post-fusion state. Despite the difference in the binding affinity observed for 41-5D06 between the native and post-fusion states as measured by biolayer interferometry, the binding affinity of the non-neutralizing mAbs was not significantly different between the two states (p>0.05) (Figure S4B). We conclude from these experiments that the non-neutralizing mAbs bind to epitopes outside of the traditional binding sites.
Mechanism of protection of the non-neutralizing antibodies
Recent work has demonstrated the importance of Fc-FcγR interactions in the clearance of influenza virus infections in vivo (DiLillo et al., 2014). To investigate whether the Fc-FcγR interactions were critical for protection by the non-neutralizing mAbs, we cloned 41-5D06, 07-5E01 and 24-4C01, as well as one head-reactive (07-5G01), into a murine IgG2a expression vector (Fc domain engaging activating FcγR) and a murine IgG2a vector with the D265A mutation that abrogates FcγR binding (Baudino et al., 2008; DiLillo et al., 2014). To ensure that the Fab function was unaffected by altering the Fc region, we verified that the different forms of the mAbs expressed retained binding to A/Shanghai/1/2013 (H7N9) virus (Figure 6A) and, for the control HAI+ Neut+ antibody 07-5G01, neutralization of H7N9 virus (Figure 6B). Mice that received 07-5E01 IgG2a or 41-5D06 IgG2a mAbs had minimal weight loss following infection with A/Shanghai/1/2013 virus compared to mice treated with the irrelevant control mAb (Figure 6C). In contrast, mice treated with 07-5E01 and 41-5D06 with the D265A Fc mutation showed similar weight loss as compared to the mice treated with an irrelevant mAb. These results demonstrate that Fc-FcγR interactions are important for in vivo protection by these broadly cross-reactive non-neutralizing mAbs. No difference was observed between mice treated with 24-4C01 as wild-type IgG2a versus Fc-D265A mutation. Interestingly this mAb had limited cross-reactivity compared to the other two non-neutralizing antibodies. Recent work showed that Fc-FcγR interactions were not essential for in vivo protection by strain-specific head-reactive or strain specific anti-NA mAbs (DiLillo et al., 2016; DiLillo et al., 2014). As a control in our experiment, mice that received 07-5G01 (HAI+ Neut+), either wild-type IgG2a or with the Fc-D265A mutation, showed no weight loss and were protected against H7N9 infection (Figure 6C).
Figure 6. Fc-FcγR interactions are important for in vivo protection by non- neutralizing antibodies.
The HAI− Neut− mAbs and one HAI+ Neut+ antibody were cloned in an IgG2a mouse construct with or without the D265A mutation (DiLillo et al., 2014). (A) Binding of the mouse mAbs to A/Shanghai/1/2013 (H7N9) viruses by ELISA. Values represent mean ± SEM from triplicate wells. (B) In vitro MN with A/Shanghai/1/2013 (H7N9) viruses. Values represent mean ± SEM from triplicate wells. (C) 6–8 week old female BALB/c mice were injected with one dose of each mouse mAb and then infected with a sublethal dose of A/Shanghai/1/2013 (H7N9) virus. Values represent mean ± SEM (n=4 mice per group). Percent of initial weight are plotted for each mAb: 3 mg/kg for 07-5E01, 4 mg/kg for 41-5D06, 3 mg/kg for 24-4C01 and 4 mg/kg 07-5G01. Mice that received an irrelevant H6-reactive IgG2a mAb were used as a control. The same control group of mice was used for all panels. See also Figure S5 and Figure S6.
As Fc-FcγR interactions contribute to in vivo protection, we hypothesized that the mAbs recruit effector cells that induce killing by antibody-dependent cellular cytotoxicity (ADCC) and/or induce activation of complement leading to complement-dependent lysis (CDL). ADCC activity was tested in vitro using a human FcγRIIIa (activating receptor) mediated ADCC assay (Figure S5A). Interestingly, neither of the non-neutralizing mAbs mediated ADCC activity through this receptor (fold induction <5). In accordance with previous work (DiLillo et al., 2014), none of the HAI+ Neut+ antibodies had high ADCC activity either (fold induction <10). In comparison, stalk-reactive 41-5E04 and control stalk-reactive mAbs CR8020 and CR9114 did mediate ADCC activity through FcγRIIIa activation (fold induction >20). As our human mAbs showed protection in vivo in mice and some crosstalk has been demonstrated between human Fc regions and mouse FcγR (Overdijk et al., 2012), we performed a mouse FcγRIV (activating receptor) ADCC assay. The results obtained were similar to the human ADCC assay in which ADCC activity was detectable only with the stalk-reactive neutralizing mAbs (Figure S5A). In addition we tested the ability of the mAbs to induce CDL in vitro, after infection of the human lung epithelial cell line A549 with A/Shanghai/1/2013 (H7N9) viruses. We observed that the HAI− Neut− mAbs had low CDL activity (percent of specific lysis between 20 and 40) but only at high concentration (20μg/ml) (Figure S5B). Two control mAbs reactive to H1N1 viruses did not induce CDL activity with H7N9 viruses in vitro. CR9114 induced CDL activity, at a concentration as low as 0.1μg/ml. The fact that we did not observe CDL activity with the HAI− Neut− mAbs at lower concentrations suggests that CDL might not contribute to protection observed in vivo. We conclude from these results that ADCC and CDL are not the main mechanisms by which the non-neutralizing mAbs protect.
Phagocytosis can be induced via engagement of FcγR and most FcγR isoforms are able to activate professional phagocytes, monocytes/macrophages and neutrophils (Garcia-Garcia and Rosales, 2002). To investigate whether this Fc-mediated mechanism might contribute to protection by non-neutralizing mAbs, we used a robust flow-based assay to measure antigen-specific antibody-mediated phagocytosis in vitro (Ackerman et al., 2011; Gach et al., 2014). We observed that all three non-neutralizing mAbs 41-5D06, 07-5E01 and 24-4C01 were able to induce phagocytic activity (phagocytic score >2) in a dose-dependent fashion (Figure S6). 24-4C01 had the lowest score (3.7) in this assay (starting at 3 μg/ml of antibody). This mAb also had the lowest affinity for H7 HA and was the least efficient at protecting mice in vivo. Both 41-5D06 and 07-5E01 had higher scores (>5) and they had similar scores compared to the neutralizing mAbs tested: 22-3E05, 07-5B05, 41-5E04, CR9114 and CR8020. We also observed that the HAI+ Neut+ mAbs were able to induce phagocytosis as efficiently as the HAI− ones (phagocytic scores between 4 and 7). Furthermore, in order to address the mechanism of protection for the non-neutralizing mAbs in vivo, we conducted a passive transfer experiment and tested mouse serum for reactivity to purified H7 HA at day 7 and 10 post-vaccination. We observed that the serum reactivity on day 7 was higher for the mice that received each of the non-neutralizing mAbs, compared to the mice treated with an irrelevant mAb (Figure S6D). At day 10, this difference was lost (data not shown). These results indicate that the non-neutralizing mAbs drive a faster endogenous response, likely through efficient immune complex formation and improved activation of helper T cells upon peptide presentation after phagocytosis. As these mAbs are not able to induce ADCC or CDL in vitro, uptake of immune complexes by phagocytes is most likely contributing to protection.
DISCUSSION
In the present study, we characterize the protective response to an H7N9 vaccine through a high-resolution monoclonal antibody-based approach. Our results suggest that non-neutralizing antibodies, a class of antibodies typically not examined in assessments of vaccine efficacy, may contribute to protection in vivo. This is a noteworthy finding as clinical trials with vaccines based on pre-pandemic avian strains have been shown to be poorly immunogenic when efficacy is measured using the traditional HAI assay (Couch et al., 2012; Cox et al., 2009; Mulligan et al., 2014). Inducing high titers of HAI active antibodies with classical inactivated avian influenza vaccines has been especially challenging even when administered with strong adjuvants (Mulligan et al., 2014). We suggest that a proportion of protective immunity against H7 might be achieved by antibodies that are missed in a classical HAI assay. However, assessing which non-neutralizing antibodies contribute to protection and how to measure their significant contribution in vaccinees remains a difficult challenge.
The characterization of the neutralizing mAbs allowed us to generate a detailed map of the antigenic sites on the H7 HA and reveal four previously uncharacterized epitopes. The Arg65 residue plays a role in the binding of two mAbs with diverse functionality (HAI+ versus HAI-). It has been previously shown that different angles of approach can in part explain why antibodies have distinct sensitivities to epitope mutations affecting their neutralization activity (Friesen et al., 2014; Tan et al., 2014). Importantly, escape mutations sometimes arise at positions that are not in the center of the antibody footprint but might change the footprint by network interactions. This has been shown for conformational epitopes of stalk-reactive mAbs (Henry Dunand et al., 2015; Tan et al., 2012).
Our results strongly suggest that cross-reactive non-neutralizing mAbs target previously unrecognized epitopes on the HA protein. Analysis of somatic mutations in the heavy chain variable region reveal that the average number of mutations of the non-neutralizing mAbs (16.3 mutations ± 2) was significantly higher than the average of the head-reactive neutralizing ones (9.9 mutations ± 0.8) (p<0.01) but not statistically different compared to the average number of mutations (25.8 mutations ± 4.6) of a group of stalk-reactive neutralizing mAbs (including 41-5E04 and three other H7N9 neutralizing stalk-reactive mAbs (Henry Dunand et al., 2015)). Collectively, the high cross-reactivity and the number of somatic mutations support a model in which the plasmablasts producing the non-neutralizing antibodies arose from pre-existing cross-reacting memory B cells, as shown with pandemic H1N1 infection (Wrammert et al., 2011). Recent studies analyzing stalk-reactive neutralizing mAbs have shown that only a few “founder mutations” maximize binding to the eliciting antigen, followed by the accumulation of additional favorable mutations conferring breadth to different HA subtypes (Avnir et al., 2014; Pappas et al., 2014). Thus, extensive accumulation of mutations may be a common feature of all broadly protective antibodies.
Antibodies targeting epitopes on the HA stalk region are preferentially encoded by VH1-69 or the highly similar VH1-18 gene segments (Dreyfus et al., 2012; Ekiert et al., 2009; Li et al., 2012; Wrammert et al., 2011). These mAbs were induced by and/or were highly reactive to group 1 influenza strains. Previous studies including ours have described group 2-neutralizing mAbs that were not encoded by such gene segments (Ekiert et al., 2011; Friesen et al., 2014; Henry Dunand et al., 2015). Moreover none of the mAbs described here, and induced by H7N9 virus, were encoded by VH1-69 or VH1-18 gene segments. Differences in the HA stalk epitope of group 2 viruses as compared to group 1 viruses likely account for the altered antibody sequence requirements. It will be of significant interest in future studies to determine the structural basis for the difference between the group 1 and group 2 stalk epitopes.
Two of the non-neutralizing mAbs reported here had a clear dependence on FcγR engagement for in vivo protection. It has been shown that stalk-reactive but not head-reactive antibodies require Fc-FcγR interactions and can efficiently induce ADCC (DiLillo et al., 2014). In addition, the presence of cross-reactive serum antibodies with ADCC activity was associated with protection in humans (Jegaskanda et al., 2013b) and macaques (Jegaskanda et al., 2013c). Surprisingly we did not observe induction of ADCC or CDL in vitro but we did observe phagocytosis in vitro. We observed a trend in the correlation between affinity, phagocytosis and protection for non-neutralizing mAbs. Future studies with a large number of mAbs are needed to address the relative contribution of phagocytosis and epitope specificity to protection. Because the non-neutralizing mAbs drove a faster endogenous immune response in mice, we suggest that immune complexes formed by non-neutralizing mAbs enable recruitment of effector cells via Fc-FcγR interactions. Interestingly, neutralizing HA stalk-specific and head-specific mAbs also induced phagocytosis, despite head-specific mAbs not requiring Fc-FcγR interactions for protection in vivo ((DiLillo et al., 2014) and Figure S5 herein). The greater breadth of FcγR isoforms able to mediate phagocytosis, including FcγRI, IIa and IIIa may account for differences in the induction of ADCC versus phagocytosis activities.
In conclusion, this study demonstrate that non-neutralizing and non-HAI active antibodies may be important when considering immune responses to influenza virus vaccines. Our results suggest that the standard surrogate marker of protection - hemagglutination inhibiting antibodies – is an incomplete measure for pre-pandemic influenza virus vaccines of avian origin and possibly also for seasonal influenza virus vaccines. Therapeutics or vaccines must provide durable protection against mutating virus; thus a cocktail of antibodies binding non-overlapping but protective epitopes both neutralizing or not would best avoid immune escape due to antigenic variation. Together, these findings provide important information for the development of therapeutics and the design of broadly protective influenza vaccines, both against seasonal as well as those with pandemic potential.
EXPERIMENTAL PROCEDURES
Additional experimental procedures are provided in the supplemental materials.
Clinical study
The candidate A/Anhui/1/2013 (H7N9) pandemic live attenuated influenza vaccine study was conducted at the University of Rochester Medical Center in accordance with a protocol approved by the University of Rochester Research Subjects Review Board and registered on www.clinicaltrials.gov (NCT01995695). Samples were collected from healthy adults volunteers and one tube of blood was sent to the University of Chicago. Plasmablasts were isolated at day 7 after the inactivated vaccine (Figure 1A) and mAbs were generated as previously described (Smith et al., 2009) in accordance with the University of Chicago Institutional Review Board.
Cells, viruses and recombinant HA proteins
293T and Madin Darbey Canine Kidney (MDCK) cells were obtained from the American Type Culture Collection (ATCC). H7N9 virus expressing the HA and NA of A/Shanghai/1/13 and the internal genes from A/Puerto Rico/8/34 and H10N8 virus expressing the HA and NA of A/Jiangxi-Donghu/346/2013 and the internal genes from A/Puerto Rico/8/34, were rescued as previously described (Krammer et al., 2014a). All influenza virus stocks used for the assays were freshly grown in specific pathogen-free eggs, purified and titered. Recombinant HA proteins derived from influenza A virus strains A/Wisconsin/57/05 (H3N2), A/Perth/16/2009 (H3N2), A/Victoria/361/2011 (H3N2), A/Canada/rv444/2004 (H7N3), A/Netherlands/219/2003 (H7N7) and A/Vietnam/1203/2004 (H5N1) were obtained from BEI resources. The other recombinant HA proteins were expressed in house in the baculovirus expression system as previously described (Margine et al., 2013).
Generation of escape mutant variants
MDCK cells in 24 well plates were infected with A/Shanghai/1/2013 virus for 45 minutes. After the incubation, Tosyl Phenylalanyl Chloromethyl Ketone (TPCK)-treated trypsin containing MEM media with or without antibody at a concentration equivalent to one IC50 was added. Twenty-four hours post-infection, supernatants were harvested and used to infect fresh cells that were again overlayed with TPCK-treated trypsin containing Minimum Essential Media (MEM), with or without one ~IC50 of the respective antibody. After six passages, the antibody concentration was increased to ~ two IC50. Approximately three IC50 concentrations were used for passage 7 and 8. Viruses from passage 8 were plaque purified and grown in ten-day old embryonated chicken eggs for 48 hours. Alternatively, one IC50 of antibodies with hemagglutination inhibition activity were passaged with 106 PFU of virus in specific-pathogen free embryonated chicken eggs and plaque purified. The presence of virus was confirmed using an HA assay. RNA was extracted from allantoic fluid using TriZol reagent (Invitrogen), cDNA was generated using Superscript III reverse transcriptase (Invitrogen) and HA segments were subjected to Sanger sequencing. Wild-type virus passaged in parallel in the absence of antibody was used to distinguish between mutations resulting from the adaptation to cell culture and mutations contributing to abrogating antibody binding.
Mus IgG2a and Mus IgG2a D265A antibodies
The human heavy (VH) and kappa (VK) variable regions were amplified by PCR and cloned into IgG2a mammalian expression vectors (pFUSEss-CHIg-mG2a and pFUSE2ss-CLIg-mK respectively, Invivogen). The D265A mutation was introduced by site-directed mutagenesis. Antibodies were expressed and purified as previously described (Smith et al., 2009).
In vivo studies
All animal experiments were performed in accordance with the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee (IACUC). Groups of 5 female BALB/c mice (Jackson Laboratories) aged 6–8 weeks received a dose of 0.3, 1 or 5mg/kg of purified antibody intraperitoneally. Control mice received human non-H7 binding H3N2 neutralizing mAb (011-10069 2C01, IgG1) at a 5mg/kg dose. Two hours post treatment, mice were deeply anesthetized using a ketamine/xylazine mixture and infected with 7.5 LD50 of A/Shanghai/1/2013 (H7N9) viruses diluted in PBS (pH 7.4). The same protocol was followed with the mouse IgG2a and IgG2a D265A mAbs with a sublethal dose of virus and only one dose per antibody. In a therapeutic setting, mice received a 15mg/kg dose of each mAb individually 48 hours post-infection (h.p.i.). The mice were monitored daily for survival and weight loss until day 14 post-infection. Animals that lost more than 20% of their initial body weight were euthanized.
Statistical analysis
Results from multiple experiments are presented as mean ± SEM with the exception of those noted as single representative experiments. Student’s t-test was used to test for statistical differences in the mean values. Data were analyzed with Graphpad Prism software and p values of < 0.05 were considered to be statistically significant.
Supplementary Material
Acknowledgments
This project has been funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under CEIRS contracts No HHSN272201400006C (P.W., J.T. and D.T.) and HHSN272201400008C (P.P. and F.K.); NIH U19AI109946-01 (P.W., P.P. and F.K.), P01AI097092-03 (P.W. and P.P.), P01AI097092-04S1 (P.E.L.) and U19AI057266-11 (P.W.). Clinical trial NCT01995695 was supported by the Division of Intramural Research, NIAID, NIH and by the Biomedical Advanced Research and Development Authority, HHS under contract #HHSN272200900026C. We thank Donna Neu, Mary Dawn T. Co, the Center for Therapeutic Antibody Development, the Flow Cytometry Center of Research Excellence and the Microscopy Core at the Icahn School of Medicine at Mount Sinai and the University of Chicago Flow Cytometry Core.
Footnotes
ACCESSION NUMBERS
Antibody sequences were deposited in GenBank with accession numbers KU987551-KU987574.
AUTHOR CONTRIBUTIONS
C.J.H.D and P.E.L. designed and performed experiments, analyzed data, and wrote the manuscript, M.H., A.C., V.C., I.Y.H., G.S.T., J.C., A.H., N.Y.Z and C.M. performed experiments and/or made reagents, F.A.E. and M.T. designed and analyzed the CDL experiment, J.J.T., D.J.T and K.S. designed and performed the vaccine study and P.P., F.K. and P.C.W. designed and directed the study.
Supplemental Information includes six figures and one table, Supplemental Experimental Procedures and Supplemental References.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods. 2011;366:8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avnir Y, Tallarico AS, Zhu Q, Bennett AS, Connelly G, Sheehan J, Sui J, Fahmy A, Huang CY, Cadwell G, et al. Molecular signatures of hemagglutinin stem-directed heterosubtypic human neutralizing antibodies against influenza A viruses. PLoS pathogens. 2014;10:e1004103. doi: 10.1371/journal.ppat.1004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu TM, Levine M, Fitzgerald T, Luke C, Sangster MY, Jin H, Topham D, Katz J, Treanor J, Subbarao K. Live attenuated H7N7 influenza vaccine primes for a vigorous antibody response to inactivated H7N7 influenza vaccine. Vaccine. 2014;32:6798–6804. doi: 10.1016/j.vaccine.2014.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudino L, Shinohara Y, Nimmerjahn F, Furukawa J, Nakata M, Martinez-Soria E, Petry F, Ravetch JV, Nishimura S, Izui S. Crucial role of aspartic acid at position 265 in the CH2 domain for murine IgG2a and IgG2b Fc-associated effector functions. J Immunol. 2008;181:6664–6669. doi: 10.4049/jimmunol.181.9.6664. [DOI] [PubMed] [Google Scholar]
- Chen H, Yuan H, Gao R, Zhang J, Wang D, Xiong Y, Fan G, Yang F, Li X, Zhou J, et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet. 2014a;383:714–721. doi: 10.1016/S0140-6736(14)60111-2. [DOI] [PubMed] [Google Scholar]
- Chen Z, Baz M, Lu J, Paskel M, Santos C, Subbarao K, Jin H, Matsuoka Y. Development of a high-yield live attenuated H7N9 influenza virus vaccine that provides protection against homologous and heterologous H7 wild-type viruses in ferrets. Journal of virology. 2014b;88:7016–7023. doi: 10.1128/JVI.00100-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch RB, Kasel JA. Immunity to influenza in man. Annual review of microbiology. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- Couch RB, Patel SM, Wade-Bowers CL, Nino D. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PloS one. 2012;7:e49704. doi: 10.1371/journal.pone.0049704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RJ, Madhun AS, Hauge S, Sjursen H, Major D, Kuhne M, Hoschler K, Saville M, Vogel FR, Barclay W, et al. A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine. 2009;27:1889–1897. doi: 10.1016/j.vaccine.2009.01.116. [DOI] [PubMed] [Google Scholar]
- DiLillo DJ, Palese P, Wilson PC, Ravetch JV. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. The Journal of clinical investigation. 2016;126:605–610. doi: 10.1172/JCI84428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nature medicine. 2014;20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, et al. Highly conserved protective epitopes on influenza B viruses. Science. 2012;337:1343–1348. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011;333:843–850. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen RH, Lee PS, Stoop EJ, Hoffman RM, Ekiert DC, Bhabha G, Yu W, Juraszek J, Koudstaal W, Jongeneelen M, et al. A common solution to group 2 influenza virus neutralization. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:445–450. doi: 10.1073/pnas.1319058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gach JS, Achenbach CJ, Chromikova V, Berzins B, Lambert N, Landucci G, Forthal DN, Katlama C, Jung BH, Murphy RL. HIV-1 specific antibody titers and neutralization among chronically infected patients on long-term suppressive antiretroviral therapy (ART): a cross-sectional study. PloS one. 2014;9:e85371. doi: 10.1371/journal.pone.0085371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. The New England journal of medicine. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia E, Rosales C. Signal transduction during Fc receptor-mediated phagocytosis. J Leukoc Biol. 2002;72:1092–1108. [PubMed] [Google Scholar]
- Goff PH, Krammer F, Hai R, Seibert CW, Margine I, Garcia-Sastre A, Palese P. Induction of cross-reactive antibodies to novel H7N9 influenza virus by recombinant Newcastle disease virus expressing a North American lineage H7 subtype hemagglutinin. Journal of virology. 2013;87:8235–8240. doi: 10.1128/JVI.01085-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliley JL, Khurana S, Krammer F, Fitzgerald T, Coyle EM, Chung KY, Baker SF, Yang H, Martinez-Sobrido L, Treanor JJ, et al. High-Affinity H7 Head and Stalk Domain-Specific Antibody Responses to an Inactivated Influenza H7N7 Vaccine After Priming With Live Attenuated Influenza Vaccine. The Journal of infectious diseases. 2015 doi: 10.1093/infdis/jiv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Prabakaran M, Tan Y, Indira K, Kumar SR, Kwang J. Development of dual-function ELISA for effective antigen and antibody detection against H7 avian influenza virus. BMC Microbiol. 2013;13:219. doi: 10.1186/1471-2180-13-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry Dunand CJ, Leon PE, Kaur K, Tan GS, Zheng NY, Andrews S, Huang M, Qu X, Huang Y, Salgado-Ferrer M, et al. Preexisting human antibodies neutralize recently emerged H7N9 influenza strains. The Journal of clinical investigation. 2015;125:1255–1268. doi: 10.1172/JCI74374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhu Y, Zhao B, Li J, Liu L, Gu K, Zhang W, Su H, Teng Z, Tang S, et al. Limited human-to-human transmission of avian influenza A(H7N9) virus, Shanghai, China, March to April 2013. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2014;19 doi: 10.2807/1560-7917.es2014.19.25.20838. [DOI] [PubMed] [Google Scholar]
- Jegaskanda S, Job ER, Kramski M, Laurie K, Isitman G, de Rose R, Winnall WR, Stratov I, Brooks AG, Reading PC, et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol. 2013a;190:1837–1848. doi: 10.4049/jimmunol.1201574. [DOI] [PubMed] [Google Scholar]
- Jegaskanda S, Laurie KL, Amarasena TH, Winnall WR, Kramski M, De Rose R, Barr IG, Brooks AG, Reading PC, Kent SJ. Age-associated cross-reactive antibody-dependent cellular cytotoxicity toward 2009 pandemic influenza A virus subtype H1N1. The Journal of infectious diseases. 2013b;208:1051–1061. doi: 10.1093/infdis/jit294. [DOI] [PubMed] [Google Scholar]
- Jegaskanda S, Weinfurter JT, Friedrich TC, Kent SJ. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. Journal of virology. 2013c;87:5512–5522. doi: 10.1128/JVI.03030-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron RA, Talaat K, Luke C, Callahan K, Thumar B, Dilorenzo S, McAuliffe J, Schappell E, Suguitan A, Mills K, et al. Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults. Vaccine. 2009;27:4953–4960. doi: 10.1016/j.vaccine.2009.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K, Sullivan M, Wilson PC. Targeting B cell responses in universal influenza vaccine design. Trends in immunology. 2011;32:524–531. doi: 10.1016/j.it.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Albrecht RA, Tan GS, Margine I, Hai R, Schmolke M, Runstadler J, Andrews SF, Wilson PC, Cox RJ, et al. Divergent H7 immunogens offer protection from H7N9 virus challenge. Journal of virology. 2014a;88:3976–3985. doi: 10.1128/JVI.03095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Jul-Larsen A, Margine I, Hirsh A, Sjursen H, Zambon M, Cox RJ. An H7N1 influenza virus vaccine induces broadly reactive antibody responses against H7N9 in humans. Clinical and vaccine immunology : CVI. 2014b;21:1153–1163. doi: 10.1128/CVI.00272-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Current opinion in virology. 2013;3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nature reviews Drug discovery. 2015;14:167–182. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, Lee JH, Huang M, Qu X, Edupuganti S, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margine I, Palese P, Krammer F. Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the baculovirus expression system. Journal of visualized experiments : JoVE. 2013:e51112. doi: 10.3791/51112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MJ, Bernstein DI, Winokur P, Rupp R, Anderson E, Rouphael N, Dickey M, Stapleton JT, Edupuganti S, Spearman P, et al. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA. 2014;312:1409–1419. doi: 10.1001/jama.2014.12854. [DOI] [PubMed] [Google Scholar]
- Overdijk MB, Verploegen S, Ortiz Buijsse A, Vink T, Leusen JH, Bleeker WK, Parren PW. Crosstalk between human IgG isotypes and murine effector cells. J Immunol. 2012;189:3430–3438. doi: 10.4049/jimmunol.1200356. [DOI] [PubMed] [Google Scholar]
- Pappas L, Foglierini M, Piccoli L, Kallewaard NL, Turrini F, Silacci C, Fernandez-Rodriguez B, Agatic G, Giacchetto-Sasselli I, Pellicciotta G, et al. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature. 2014;516:418–422. doi: 10.1038/nature13764. [DOI] [PubMed] [Google Scholar]
- Rudenko L, Kiseleva I, Naykhin AN, Erofeeva M, Stukova M, Donina S, Petukhova G, Pisareva M, Krivitskaya V, Grudinin M, et al. Assessment of human immune responses to H7 avian influenza virus of pandemic potential: results from a placebo-controlled, randomized double-blind phase I study of live attenuated H7N3 influenza vaccine. PloS one. 2014;9:e87962. doi: 10.1371/journal.pone.0087962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser F, Vasudevan A, Verma S, Wang W, Alvarado E, Weiss C, Atukorale V, Meseda C, Weir JP. Antibodies to antigenic site A of influenza H7 hemagglutinin provide protection against H7N9 challenge. PloS one. 2015;10:e0117108. doi: 10.1371/journal.pone.0117108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, Wilson PC. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nature protocols. 2009;4:372–384. doi: 10.1038/nprot.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhanie M, Matsuoka Y, Jegaskanda S, Fitzgerald T, Mallory R, Chen Z, Luke C, Treanor J, Subbarao K. Evaluation of the safety and immunogenicity of a candidate pandemic live attenuated influenza vaccine (pLAIV) against H7N9 influenza. Journal of Infectious Diseases. 2015 doi: 10.1093/infdis/jiv526. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat KR, Karron RA, Callahan KA, Luke CJ, DiLorenzo SC, Chen GL, Lamirande EW, Jin H, Coelingh KL, Murphy BR, et al. A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a Phase I trial in healthy adults. Vaccine. 2009;27:3744–3753. doi: 10.1016/j.vaccine.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GS, Krammer F, Eggink D, Kongchanagul A, Moran TM, Palese P. A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. Journal of virology. 2012;86:6179–6188. doi: 10.1128/JVI.00469-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GS, Lee PS, Hoffman RM, Mazel-Sanchez B, Krammer F, Leon PE, Ward AB, Wilson IA, Palese P. Characterization of a broadly neutralizing monoclonal antibody that targets the fusion domain of group 2 influenza A virus hemagglutinin. Journal of virology. 2014;88:13580–13592. doi: 10.1128/JVI.02289-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terajima M, Co MD, Cruz J, Ennis FA. High Antibody-Dependent Cellular Cytotoxicity Antibody Titers to H5N1 and H7N9 Avian Influenza A Viruses in Healthy US Adults and Older Children. The Journal of infectious diseases. 2015;212:1052–1060. doi: 10.1093/infdis/jiv181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. The New England journal of medicine. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kiso M, Fukuyama S, Nakajima N, Imai M, Yamada S, Murakami S, Yamayoshi S, Iwatsuki-Horimoto K, Sakoda Y, et al. Characterization of H7N9 influenza A viruses isolated from humans. Nature. 2013;501:551–555. doi: 10.1038/nature12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PC, Andrews SF. Tools to therapeutically harness the human antibody response. Nature reviews Immunology. 2012;12:709–719. doi: 10.1038/nri3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlbold TJ, Chromikova V, Tan GS, Meade P, Amanat F, Comella P, Hirsh A, Krammer F. Hemagglutinin stalk- and neuraminidase-specific monoclonal antibodies protect against lethal H10N8 influenza virus infection in mice. Journal of virology. 2015a doi: 10.1128/JVI.02275-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlbold TJ, Hirsh A, Krammer F. An H10N8 influenza virus vaccine strain and mouse challenge model based on the human isolate A/Jiangxi-Donghu/346/13. Vaccine. 2015b;33:1102–1106. doi: 10.1016/j.vaccine.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Influenza (seasonal) fact sheet. 2014. [Google Scholar]
- World Health Organization. WHO risk assessment of human infection with avian influenza A(H7N9) virus. 2015. [Google Scholar]
- Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.