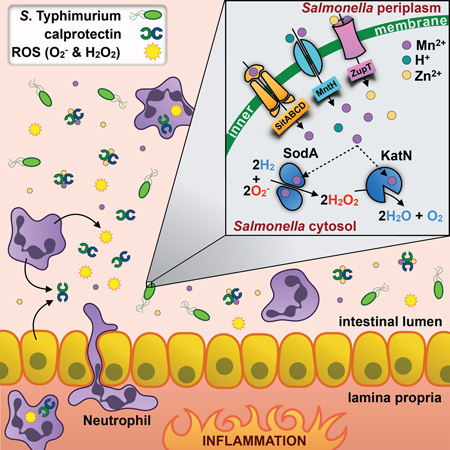
SUMMARY
Neutrophils hinder bacterial growth by a variety of antimicrobial mechanisms, including the production of reactive oxygen species and the secretion of proteins that sequester nutrients essential to microbes. A major player in this process is calprotectin, a host protein that exerts antimicrobial activity by chelating zinc and manganese. Here we show that the intestinal pathogen Salmonella enterica serovar Typhimurium employs specialized metal transporters to evade calprotectin sequestration of manganese, allowing the bacteria to outcompete commensals and thrive in the inflamed gut. The pathogen’s ability to acquire manganese in turn promotes function of SodA and KatN, enzymes that utilize the metal as a cofactor to detoxify reactive oxygen species. This manganese-dependent SodA activity allows the bacteria to evade neutrophil killing mediated by calprotectin and reactive oxygen species. Thus, manganese acquisition enables S. Typhimurium to overcome host antimicrobial defenses and support its competitive growth in the intestine.
Graphical abstract
INTRODUCTION
Humans infected with Salmonella enterica serovar Typhimurium (S. Typhimurium; STM) develop acute gastroenteritis, an illness characterized by inflammatory diarrhea (Scallan et al., 2011). Many arms of the host response to STM infection (e.g., IL-17-mediated recruitment of neutrophils; (Raffatellu et al., 2008)) have beneficial effects for the host, as inferred by clinical observations that STM usually remains localized to the gut in healthy individuals (Scallan et al., 2011). However, gut inflammation also creates an environment in which STM thrives and better competes with the microbiota (Ali et al., 2014; Barman et al., 2008; Lawley et al., 2008; Lupp et al., 2007; Maier et al., 2013; Nuccio and Bäumler, 2014; Raffatellu et al., 2009; Stecher et al., 2007; Thiennimitr et al., 2011; Winter et al., 2010). Even so, in order to prosper in the inflamed gut, STM must resist the antimicrobials released into the intestinal lumen by neutrophils and intestinal epithelial cells.
One of the most abundant host antimicrobial proteins released during STM infection is lipocalin-2 (LCN2) (Raffatellu et al., 2009), which limits bacterial access to iron (Fe) by sequestering siderophores (Flo et al., 2004; Goetz et al., 2002). However, enteric pathogens including STM can evade this host response; in this capacity, evasion of LCN2-mediated Fe starvation promotes the growth of STM in the inflamed gut over competing, susceptible microbes (Behnsen et al., 2014; Raffatellu et al., 2009).
Calprotectin (CP), a heterodimer of the two EF-hand calcium-binding proteins S100A8 and S100A9 (Teigelkamp et al., 1991), is another abundant host antimicrobial found in the intestinal lumen during STM infection. CP’s antimicrobial activity is dependent on its ability to bind both zinc (Zn) and manganese (Mn), thereby limiting the availability of these essential metal nutrients to microbes and inhibiting their growth (Corbin et al., 2008; Kehl-Fie et al., 2011; Lusitani et al., 2003; Sohnle et al., 2000; Steinbakk et al., 1990; Urban et al., 2009). Nevertheless, some microbes can evade CP’s activity (Hood et al., 2012; Liu et al., 2012; Stork et al., 2013); e.g., STM utilizes ZnuABC, a high affinity Zn transporter, to overcome CP-mediated Zn sequestration and colonize the inflamed gut (Liu et al., 2012).
In addition to binding Zn, CP can bind Mn and limit its availability to pathogens (Brophy et al., 2012; Brophy et al., 2013; Damo et al., 2013; Hayden et al., 2013; Kehl-Fie et al., 2011). Although Mn is a cofactor for a number of bacterial proteins, its best-studied function is its essential role in the detoxification of reactive oxygen species (ROS) (Aguirre and Culotta, 2012; Papp-Wallace and Maguire, 2006). For example, Mn sequestration by neutrophil-derived CP inhibits the growth of Staphylococcus aureus in tissue abscesses and increases the pathogen’s susceptibility to oxidative stress (Corbin et al., 2008; Kehl-Fie et al., 2011).
STM has two high affinity Mn transporters, MntH and SitABCD (Kehres et al., 2002; Kehres et al., 2000): MntH is a proton-dependent Mn2+ transporter homologous to vertebrate NRAMP1, and can be found in pathogenic and non-pathogenic bacterial species (Cellier et al., 2001; Kehres et al., 2000); SitABCD is an ABC-type Mn transporter found mostly in pathogenic species; in Salmonella it is encoded within pathogenicity island 1 (Zhou et al., 1999). STM also expresses ZupT, a broad-specificity divalent cation transporter of the ZIP family (Grass et al., 2005), which mediates transport of Mn (Karlinsey et al., 2010). Individually, each of these transporters is required for STM virulence in the mouse typhoid model of infection (Boyer et al., 2002; Cerasi et al., 2014; Janakiraman and Slauch, 2000; Karlinsey et al., 2010; Zaharik et al., 2004), wherein STM colonizes the spleen and liver but does not trigger intestinal inflammation (Tsolis et al., 2011). Still, the role of Mn acquisition during infection, and whether it contributes to evasion of host antimicrobial defenses such as ROS, is unknown. Moreover, the ROS-detoxifying enzymes that utilize Mn as a cofactor (i.e., the superoxide dismutase SodA and the catalase KatN) have not been shown to contribute to STM virulence in vivo.
Here we set out to investigate the contribution of SitABCD, MntH, and ZupT to STM colonization of the inflamed intestine, to define additional mechanisms by which STM evades CP-mediated metal chelation, and to determine the mechanisms by which Mn acquisition contributes to STM’s evasion of neutrophil killing in the inflamed intestine. We show that STM utilizes specialized metal transporters to evade CP-mediated sequestration of Mn, an essential cofactor for the ROS-detoxifying enzymes SodA and KatN. By using mice either deficient in neutrophil recruitment or in neutrophil function, we also demonstrate that Mn transporters and SodA allow STM to evade neutrophil-dependent killing that is mediated in concert by ROS and CP, thereby promoting STM’s competitive growth in the inflamed intestine.
RESULTS
Mn transporters promote growth of STM under nutrient-limiting conditions
We constructed STM mutants deficient in one or multiple Mn transporters (sitABCD, mntH, zupT; Table S1, S2), then tested their growth in rich media (LB) and in minimal media (M9), the latter of which contains only trace amounts of metal micronutrients (Fig. S1). Whereas all strains grew to similar levels in LB (Fig. S1), in M9 we observed a significant growth defect of STM sitA (Fig. S1A), but not of the STM mntH or STM zupT mutants (Fig. S1D, E). Furthermore, the sitA mntH mutant exhibited a greater growth defect in M9 than STM sitA (Fig. S1B), while growth of STM sitA mntH zupT was not inhibited further (Fig. S1C). Intracellular Mn concentrations were lower in the sitA and mntH mutants, and addition of MnCl2 rescued growth to WT levels (Fig. S1A–C and data not shown), indicating that growth defects in M9 resulted from Mn limitation. In addition, trans complementation with the sitABCD operon on a low-copy plasmid restored growth of each mutant to WT levels (Fig. S1). These data are consistent with previous studies and show that SitABCD and MntH non-redundantly promote STM growth in Mn-limited media.
STM utilizes Mn transporters to evade CP-mediated growth inhibition
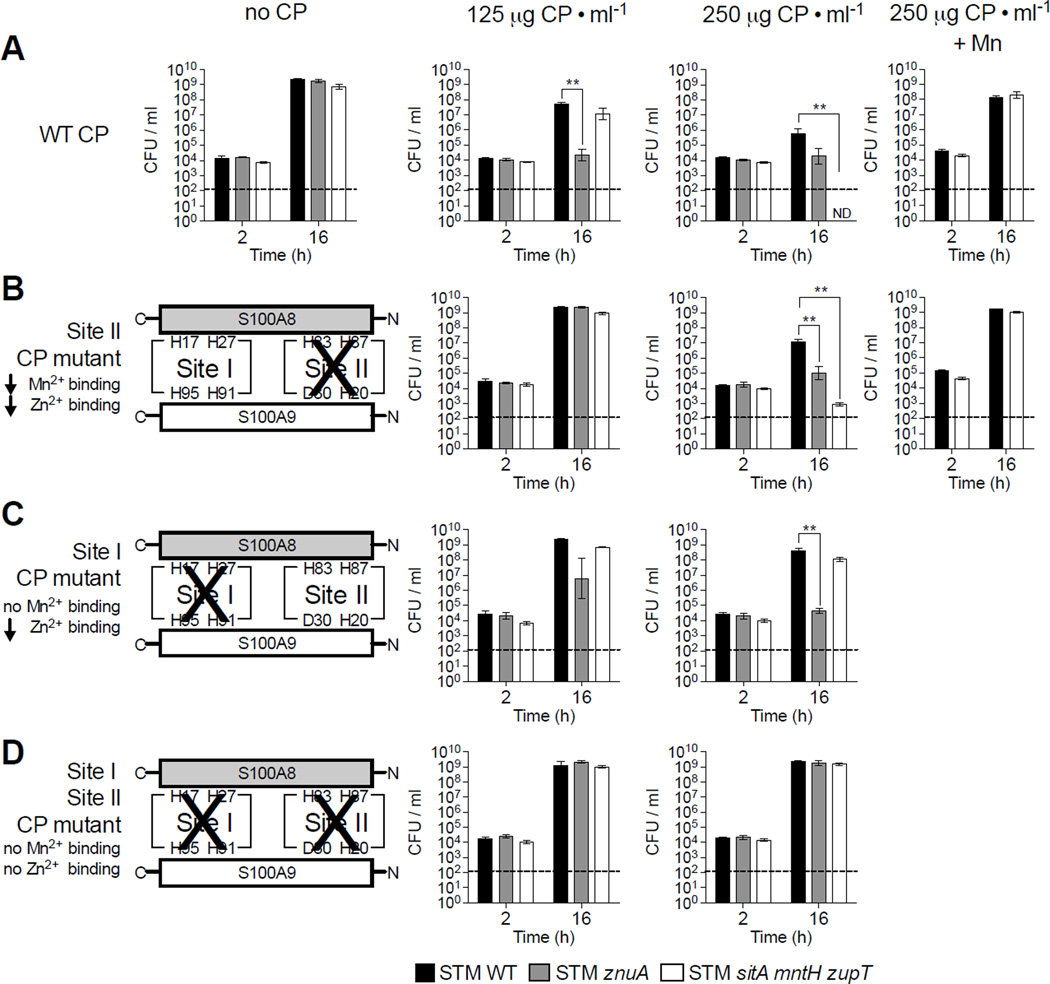
CP-mediated Mn chelation is a general host strategy to limit Mn availability during infection (Corbin et al., 2008). Thus, we investigated whether Mn transporters are required for optimal growth of STM in rich media + CP (Fig. 1).
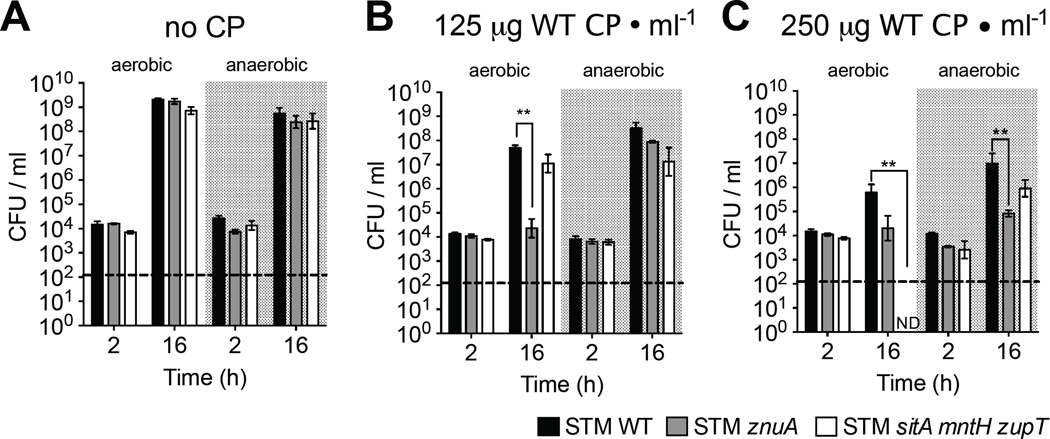
Figure 1. STM utilizes Mn transporters to evade CP-mediated growth inhibition in vitro.
STM strains grown in LB + (A) WT CP, (B) Site II CP mutant, (C) Site I CP mutant, or (D) Site I/Site II CP double mutant; also, when indicated (+Mn) + 50µM MnCl2. CFUs were determined by plating dilutions on selective media. Bars = mean CFU/ml culture of ≥3 biological replicates ± SEM. * (P ≤ 0.05), ** (P ≤ 0.01). ND = not detected. Dashed line = limit of detection. (See also Fig. S1, S2, S3 and Tables S1, S2)
The means by which CP binds Zn and Mn were recently demonstrated (Brophy et al., 2012; Brophy et al., 2013; Damo et al., 2013; Hayden et al., 2013; Kehl-Fie et al., 2011). The two subunits of CP, S100A8 and S100A9, interact to form two metal-binding sites: Site I and Site II (see schemes in Fig. 1B–D). When Site I is mutated, CP binding to Mn is abolished while binding to Zn is reduced (Damo et al., 2013). In contrast, when Site II is inactivated, CP’s high affinity binding of both Mn and Zn is retained, albeit with reduced capacity. When Site I and II are both mutated, CP loses all capacity to bind Zn and Mn with high affinity (Damo et al., 2013; Kehl-Fie et al., 2011).
To determine whether STM evades CP-mediated Mn sequestration, we monitored growth of WT and Mn transporter-deficient STM strains in rich media (i.e., not limited in Mn or other metals) supplemented with WT or mutant CP (Fig. 1, S2, S3). As a control, we measured growth of an STM mutant lacking the high-affinity Zn transporter ZnuABC (STM znuA), which is inhibited by WT CP at concentrations similar to those in the inflamed gut (125–250µg/ml; (Liu et al., 2012)). As we previously observed (Liu et al., 2012), STM WT was only minimally inhibited by 125µg/ml CP, whereas STM znuA was significantly impaired (Fig. 1A, S2). Similar to STM WT, growth of STM sitA mntH was only minimally attenuated by CP (Fig. S3A). This latter finding suggested that other metal transporters, such as ZupT, contributed to evasion of CP-mediated Mn chelation in a manner similar to that shown in metal-chelated medium (Karlinsey et al., 2010). Consistent with our prediction, although growth of STM zupT was not inhibited by CP (Fig. S3B), the sitA mntH zupT mutant was significantly inhibited at higher CP concentrations (≥250µg/ml), with CFUs falling below the limit of detection 16h after inoculation (Fig. 1A, S2). Of note, CP has two binding sites for Zn and one for Mn (Damo et al., 2013), which correlates with our finding that more CP is needed to hinder growth of STM sitA mntH zupT than of STM znuA.
Adding MnCl2 to CP-containing media rescued the sitA mntH zupT mutant (Fig. 1A), indicating growth inhibition was due to CP chelating Mn. To confirm this, we measured growth of STM sitA mntH zupT in media + Site I mutant CP, which does not bind Mn (Damo et al., 2013). As predicted, Site I mutant CP did not inhibit growth of STM sitA mntH zupT but still inhibited STM znuA, although higher mutant CP concentrations were needed due to reduced Zn-binding capacity (Fig. 1C, S2C, S3C). In contrast, Site II mutant CP impaired growth of the znuA and sitA mntH zupT mutants, albeit more mutant CP was needed due to diminished capacity for binding Zn or Mn (Fig. 1B, S2B, S3D). As with WT CP, adding MnCl2 rescued STM sitA mntH zupT growth in media + CP Site II mutant (Fig. 1B). No growth inhibition was observed in media + CP Site I/Site II double mutant (Fig. 1D, S2D), further demonstrating the importance of metal-binding to CP’s antimicrobial activity. Overall, these results show that the SitABCD, MntH and ZupT transporters help STM overcome growth inhibition by CP-mediated Mn sequestration.
Mn transporters give STM a competitive advantage in the inflamed gut
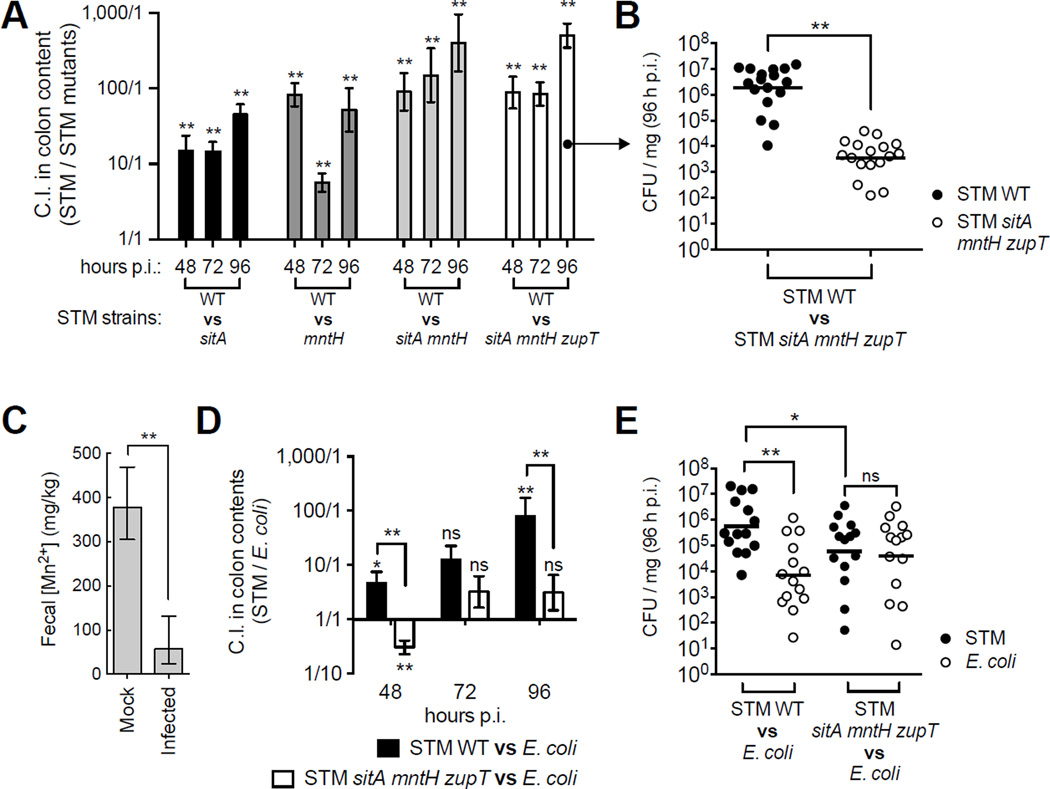
Having shown that SitABCD, MntH and ZupT enable evasion of CP-mediated Mn sequestration in vitro, we investigated if Mn levels were reduced in the inflamed gut upon STM infection. To this end, we employed the streptomycin-treated mouse model of STM colitis (Barthel et al., 2003), wherein mice develop intestinal inflammation characterized by neutrophil influx and high levels of fecal CP (125–250µg/ml; (Liu et al., 2012)) (Fig. 2). As we previously observed for Zn (Liu et al., 2012) and for Fe (Deriu et al., 2013), fecal Mn levels were significantly reduced upon STM infection, dropping to ~57mg/kg from 378mg/kg in uninfected mice (Fig. 2C).
Figure 2. Mn transporters enhance STM’s competitive advantage in the inflamed gut.
(A) C57BL/6 mice coinfected 1:1 with STM WT and indicated mutant (n >7/group). Competitive index (C.I.) = output ratio (CFU STM WT / mutant) divided by input ratio. Bars = mean C.I. ± SEM. (B) CFUs in colon contents of individual mice from A. Bars = geometric mean. (C) ICP-MS measurement of fecal Mn levels in mock (n = 4) or STM-infected (n = 4) mice 96h p.i. Bars = geometric mean ± S.D. (D) Mice coinfected 1:1 with commensal E. coli JB2 and STM strains. Bars = mean C.I. (CFU STM / E. coli) ± SEM. (E) CFUs in colon contents of individual mice from D. * (P ≤ 0.05), ** (P ≤ 0.01). NS = not significant. (See also Fig. S4)
Our finding that Mn is limited in the inflamed gut prompted us to investigate whether Mn transporters help STM colonize this environment. First, we compared STM CFUs in the cecum of mice infected with STM WT or mutants lacking one or multiple Mn transporters. Each mutant induced gut inflammation similar to WT levels (Fig. S4B), indicating they all retained virulence. Furthermore, all mutants colonized the inflamed gut to WT levels (Fig. S4A), suggesting possible compensatory effects by other metal transporters. However, when mice were infected 1:1 with STM WT and a Mn transporter mutant, WT significantly outcompeted each mutant in the cecal content at every time point analyzed (Fig. 2A). Moreover, WT’s colonization advantage was greater in competition with mutants where multiple Mn transporters were inactivated (Fig. 2A, B). This outcome was not due to changes in inflammatory status as pathology was comparable to mice infected with a single strain of STM (Fig. S4C). Furthermore, without streptomycin treatment (and thus no intestinal inflammation), WT’s competitive advantage was reduced and did not reach statistical significance at 48h and 96h post-infection (p.i.) (Fig. S4E).
Having established that Mn acquisition provides a competitive advantage to STM in the inflamed gut, we next determined whether Mn transporters help STM outgrow Escherichia coli, a related commensal that can also bloom in the inflamed gut (Winter et al., 2013). We inoculated mice 1:1 with a commensal E. coli strain previously isolated from our mouse colony (Behnsen et al., 2014) and either STM WT or the sitA mntH zupT mutant. Consistent with our previous results (Behnsen et al., 2014), STM WT outcompeted E. coli in the inflamed gut (Fig. 2D, E). In contrast, STM sitA mntH zupT did not outcompete E. coli (Fig. 2D) as both strains colonized to similar levels (Fig. 2E). Collectively, our results indicate that Mn acquisition via SitABCD, MntH and ZupT enhances STM growth in the inflamed gut. Moreover, these transporters provide a competitive advantage over other bacteria that thrive in the same environment.
Mn transporters help STM resist IL-22-dependent antimicrobial mechanisms
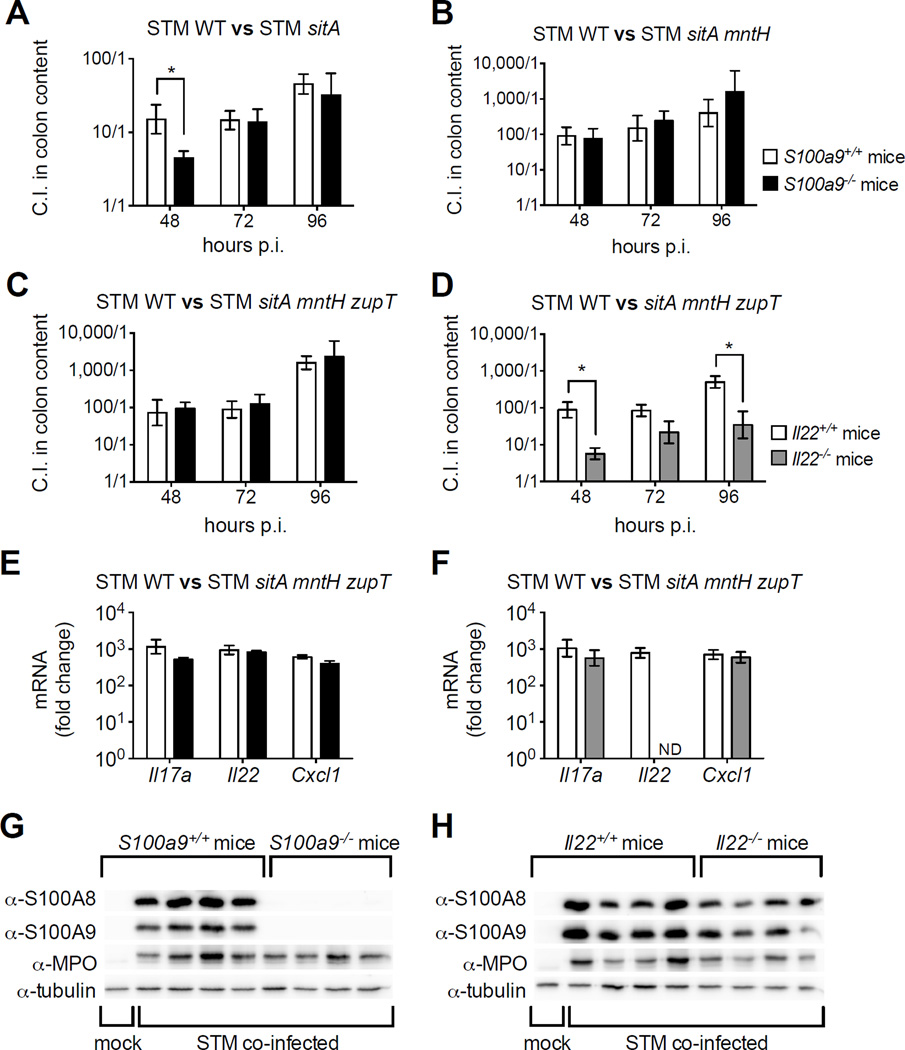
As Mn acquisition promotes growth of STM in the inflamed gut, we next determined whether the colonization defect of Mn transporter mutants could be rescued in mice that do not express CP (Fig. 3). For this, we employed S100a9−/− mice, which lack expression of S100A9 and S100A8 (Liu et al., 2012; Manitz et al., 2003). To our surprise, STM WT outcompeted Mn transporter mutants to a similar extent in the colon content of both S100a9−/− and S100a9+/+ mice, excepting a partial rescue of STM sitA at 48h p.i (Fig. 3A–C). Consistent with our prior findings, S100a9+/+ and S100a9−/− mice exhibited similar induction of pro-inflammatory cytokine transcripts such as Il17a and Il22, as well as of the neutrophil chemoattractant Cxcl1 (Fig. 3E). As expected, both CP subunits were highly induced in S100a9+/+ but not S100a9−/− mice upon STM infection, with comparable neutrophil recruitment as indicated by myeloperoxidase detection (Fig. 3G, S5E) and by histopathology (Fig. S5A–C). Taken together, these results suggest that additional host factors can limit Mn availability or enhance STM’s requirement for Mn.
Figure 3. Role of Mn uptake in mice lacking CP or IL-22 during STM infection.
(A–C, E, G) S100a9+/+ (white bars) or S100a9−/− (black bars) mice coinfected 1:1 with STM WT and indicated mutant (n ≥5/group). (D, F, H) Il22+/+ (white bars) or Il22−/− (grey bars) mice coinfected 1:1 with indicated STM strains (n ≥7/group). (A–D) Bars = mean C.I. (CFU STM WT / mutant) ± SEM. * (P ≤ 0.05). (E, F) Transcript levels of Il17a, Il22, and Cxcl1 relative to uninfected WT littermates. Bars = mean of ≥4 replicates ± SEM. ND = not detected. (G, H) Immunoblots of cecal tissue extract from STM coinfected mice; MPO = myeloperoxidase. (See also Fig. S5, Table S3)
IL-22 induces the expression of metal-binding proteins such as LCN2 and CP, and thus appears to be a regulator of nutritional immunity (Behnsen et al., 2014). We therefore posited IL-22 induces other factors that limit Mn availability. In agreement with our hypothesis, the sitA mntH zupT mutant was markedly rescued in Il22−/− mice (Fig. 3D). Pro-inflammatory gene upregulation and the degree of inflammation were similar in Il22+/+ and Il22−/− mice upon infection (Fig. 3F, S5D), as in our previous study (Behnsen et al., 2014). We also detected slightly lower expression of CP in the cecum of Il22−/−mice at 96h p.i. (Fig. 3H, S5F). Of note, IL-22 induces expression of CP in intestinal crypts during infection at earlier time points (Behnsen et al., 2014). At later stages of infection, IL-22’s effect on epithelial CP expression is likely masked by neutrophil influx to the intestinal tissue. Nevertheless, our results imply IL-22 modulates unknown host factors that, in addition to CP, limit Mn availability. Furthermore, these findings support the concept that STM utilizes metal transporters to evade host nutritional immune responses and to compete for a niche in the inflamed gut.
CP-mediated Mn sequestration is Less Effective in anaerobiosis
Oxidative radicals are a byproduct of aerobic respiration and require neutralization for bacteria to survive (Imlay, 2013). Similarly, the reactive oxygen and nitrogen species generated by the host can be toxic to microbes. As Mn is involved in free radical detoxification by STM, we next tested whether Mn acquisition is needed to overcome growth inhibition by CP with or without O2 (Fig. 4). To this end, we compared aerobic and anaerobic growth of STM WT, znuA and sitA mntH zupT in rich media + CP.
Figure 4. Mn sequestration by CP is less effective in anaerobiosis.
STM strains grown anaerobically in (A) LB, (B) LB + 125µg CP/ml, or (C) LB + 250µg CP/ml. Aerobic growth (as in Fig. 1A) shown for comparison. Bars = mean CFU/ml culture of ≥3 biological replicates ± SEM. ** (P ≤ 0.01). ND = not detected. Dashed line = limit of detection.
Without CP, all strains grew equally well aerobically and anaerobically (Fig. 4A). The znuA mutant was impaired aerobically by CP, but only by higher concentrations anaerobically (Fig. 4B, C). In stark contrast to aerobic growth of STM sitA mntH zupT, where no CFUs were recovered, CP only minimally hindered anaerobic growth of the mutant (Fig. 4C). These results demonstrate that CP’s antimicrobial activity is more effective in aerobic conditions. Although CP can bind Fe2+ in strictly anaerobic conditions at the same site it binds Mn (Site I) (Nakashige et al., 2015), our results indicate that Fe2+ binding by CP does not play a role in our model. Of note, we observed maximal CP antimicrobial activity only in the presence of O2, a condition where all Fe2+ is rapidly oxidized to Fe3+, which is not bound by CP. Our data are consistent with earlier work on S. aureus (Kehl-Fie et al., 2011) that suggests CP exacerbates the oxidative stress encountered by bacteria in harsh host environments.
The Mn-dependent superoxide dismutase SodA promotes STM growth in CP-supplemented media
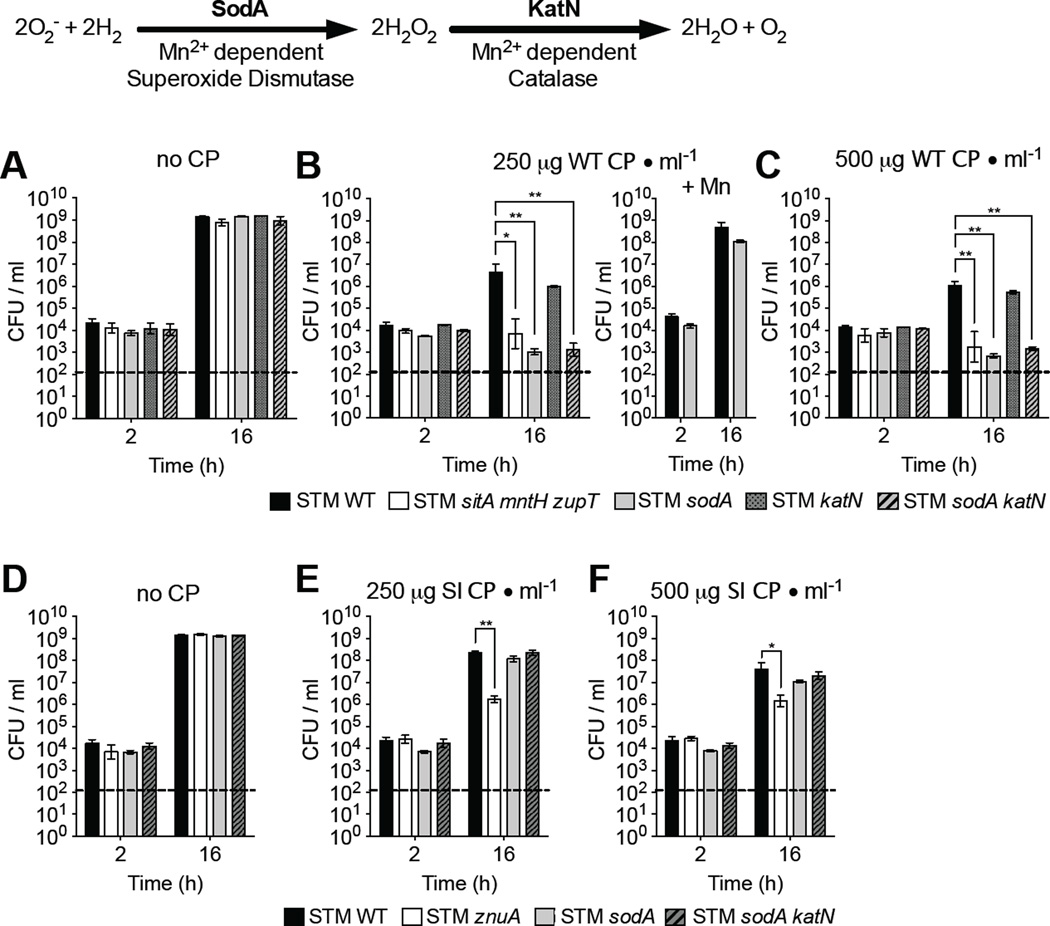
Mn is an essential cofactor for SodA and KatN (Keele et al., 1970; Robbe-Saule et al., 2001), bacterial enzymes that detoxify superoxide and hydrogen peroxide, respectively (Imlay, 2013). As such, Mn acquisition by STM may promote resistance to oxidative stress. We thus investigated whether SodA and KatN help STM resist CP-mediated growth inhibition (Fig. 5). We found that ≥250µg/ml CP appeared to kill STM sodA, with relatively few CFUs recoverable after 16h (Fig. 5A–C). This result was dependent on Mn binding by CP, as adding MnCl2 or using the CP Site I mutant (no Mn binding) rescued STM sodA (Fig. 5B, E, F).
Figure 5. STM utilizes SodA to resist CP-mediated growth inhibition.
(A–C) STM strains grown aerobically in (A) LB, (B) LB + 250µg CP/ml and when indicated (+Mn) + 50µM MnCl2, or (C) LB + 500µg CP/ml. (D-F) STM strains grown aerobically in (D) LB, (E) LB + 250µg Site I CP mutant/ml, or (F) LB + 500µg Site I CP mutant/ml. Bars = mean CFU/ml culture of ≥3 biological replicates ± SEM. * (P ≤ 0.05), ** (P ≤ 0.01). ND = not detected. Dashed line = limit of detection. (See also Fig. S3)
As growth of STM sodA in media + CP resembled that of STM sitA mntH zupT (Fig. 5A–C), CP-mediated Mn sequestration may result in impaired SodA function. To test this, we constructed a sitA mntH zupT sodA mutant and compared its growth to STM sitA mntH zupT in media + CP. Growth inhibition of both mutants by CP was equal (Fig. S3E), indicating that Mn transporter ablation is epistatic to SodA activity when CP sequesters Mn. SodA activity was similarly reduced in a sitA mntH mutant grown in M9 minimal media, but rescued by adding Mn (Fig S6A).
In contrast to the sodA mutant, growth of STM katN was not inhibited by CP, and growth of STM sodA katN was not inhibited more than STM sodA (Fig. 5A–F). The lack of a KatN phenotype in vitro likely results from expression of functionally redundant hydrogen peroxide scavengers by STM (Hebrard et al., 2009). Consistent with this idea, KatN activity was undetectable in STM sitA mntH grown in Mn-deficient media, but the activity of two heme-dependent catalases (KatE and KatG) was maintained (Fig. S6B). Collectively, our findings indicate that Mn sequestration by CP enhances oxidative stress under aerobic conditions and inhibits bacterial growth. STM counteracts this by expressing specialized transporters to acquire Mn, which is essential for the superoxide-detoxifying function of SodA.
The Mn-dependent enzymes SodA and KatN give STM a competitive advantage in the inflamed gut
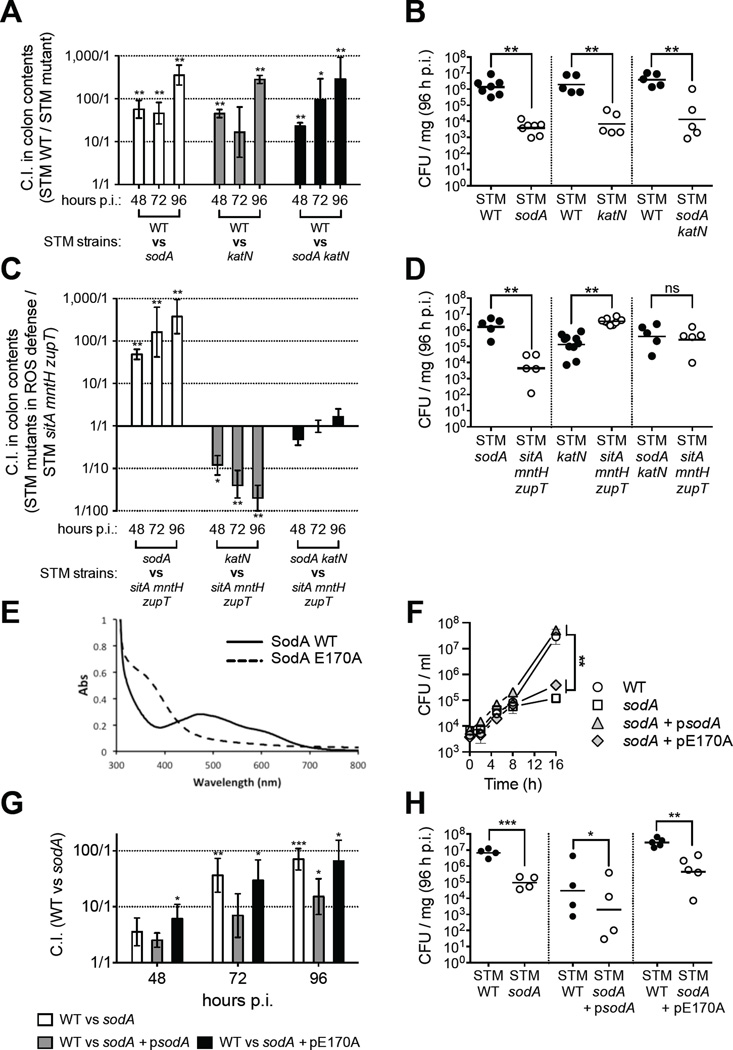
As our in vitro data suggested that Mn acquisition could help STM to detoxify ROS in vivo, we next determined whether SodA and KatN provide a competitive advantage to STM during colitis (Fig. 6). When streptomycin-treated mice were infected 1:1 with STM WT and a mutant (STM sodA, katN or sodA katN), we recovered significantly more WT in the colon content at every time point analyzed (Fig. 6A). Even more, the colonization defect of each mutant was so similar (Fig. 6B) that it suggested SodA and KatN promote growth of STM by a common pathway.
Figure 6. Mn-dependent antioxidant enzymes SodA and KatN promote STM growth in the inflamed gut.
(A, C, G) Mice coinfected 1:1 with indicated STM strains (n >4/group). Bars = mean C.I. ± SEM. For (A, G) C.I. = CFU STM WT / mutant, for (C) mutant / sitA mntH zupT (B, D, H) CFUs in colon contents of individual mice (B) from A, (D) from C, or (H) from G. Horizontal bars = geometric mean. (E) UV/vis absorption spectra of SodA WT and E170A reveal Mn(III) and Fe(III) coordination, respectively. (F) Growth in M9 by STM strains. Data = mean of 3 replicates ± SEM. * (P ≤ 0.05), ** (P ≤ 0.01). (See also Fig. S6)
Because Mn is an essential cofactor for SodA and KatN, and STM sitA mntH zupT is impaired in Mn uptake, we investigated whether the sitA mntH zupT mutant’s colonization defect in the inflamed gut was a consequence of impaired SodA and KatN function. For this, we infected streptomycin-treated mice 1:1 with STM sitA mntH zupT and mutants lacking SodA, KatN, or both. STM sodA outcompeted STM sitA mntH zupT (Fig. 6C, D), indicating that loss of SodA alone does not account for the growth defect of the Mn transporter mutant. To our surprise, the sitA mntH zupT mutant had a competitive advantage over STM katN (Fig. 6C, D). Although STM sitA mntH zupT and STM katN both lack KatN activity, only STM katN maintains a functional SodA. Therefore, STM katN may accumulate hydrogen peroxide from SodA’s activity, which it cannot promptly detoxify in vivo without KatN. Consistent with this hypothesis, STM sodA katN and STM sitA mntH zupT were recovered at equal levels following competitive infection (Fig. 6C, D). Together, these results indicate that Mn transporters enhance STM’s competitive advantage in the inflamed gut by acquiring a necessary cofactor for SodA and KatN, thus allowing STM to overcome oxidative stress in this hostile environment.
We next tested whether Mn is important for SodA function in vivo. The absorption spectrum of STM’s SodA (STmSodA) reveals a profile similar to that of the previously reported E. coli ortholog (EcSodA) (Whittaker and Whittaker, 1998), suggesting the presence of a Mn center (Fig. 6E). A glutamate bridge is essential for Mn-selective binding by EcSodA (Whittaker and Whittaker, 1998), and an E170A mutation disrupts Mn binding; EcSodA E170A instead binds Fe and lacks superoxide dismutase activity (Whittaker and Whittaker, 1998). We constructed STmSodA E170A (Fig. S6C, D), and spectroscopic characterization showed an absorption spectrum indicative of Fe coordination (Fig. 6E), consistent with EcSodA E170A (Whittaker and Whittaker, 1998). Inductively coupled plasma mass spectrometry analyses confirmed the dominant metal ion constitution (within 2% error) of STmSodA WT and E170A to be Mn and Fe, respectively (data not shown). Consistent with our hypothesis, trans complementation with STmSodA WT rescued the sodA mutant in M9 minimal media (Fig. 6F) and the colon content of mice (Fig. 6G, H), whereas STmSodA E170A did not (Fig. 6F–H). These results indicate that Mn coordination by SodA is crucial for its activity in the inflamed gut.
Mn acquisition boosts STM’s resistance to neutrophil-derived ROS and CP in the inflamed gut
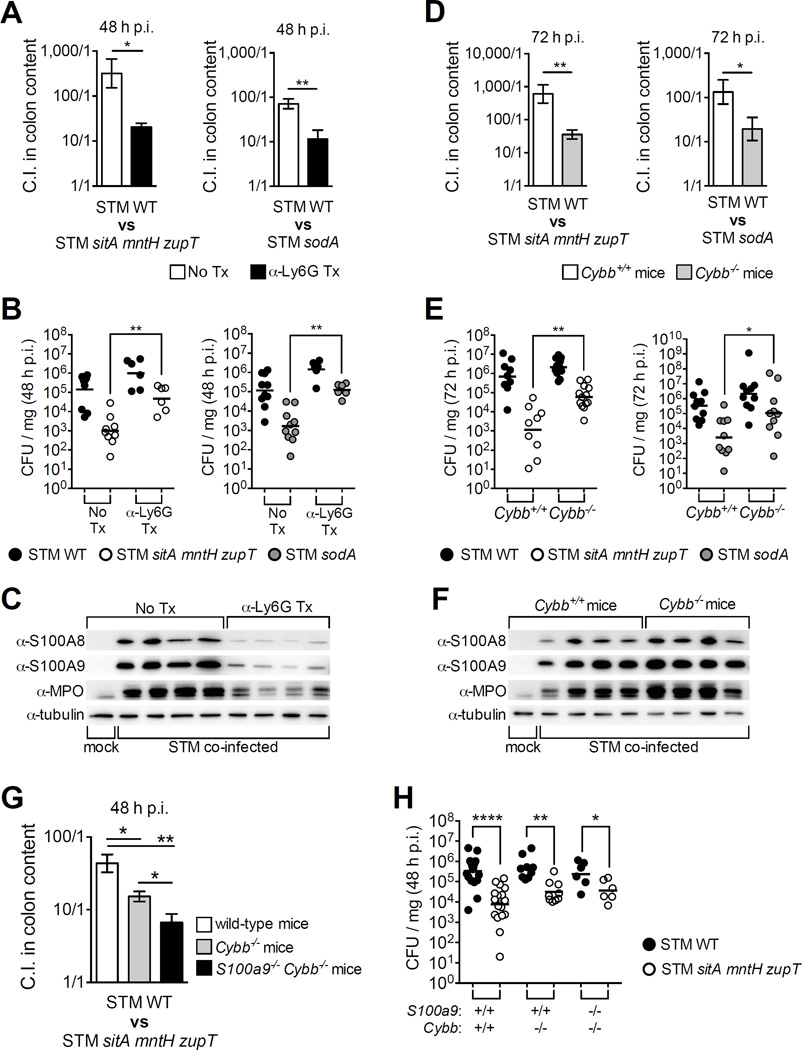
Neutrophil recruitment to the gut is a hallmark response to STM infection. Neutrophils produce ROS, leading to a higher oxidative environment that STM can exploit; e.g., ROS oxidize thiosulfate to tetrathionate, which STM respires to utilize ethanolamine and grow anaerobically (Thiennimitr et al., 2011; Winter et al., 2010). Nevertheless, STM must deal with excess ROS that can suppress its growth. As SodA and KatN promoted STM growth in the inflamed gut (Fig. 6), we investigated whether STM utilizes Mn to mitigate the oxidative burst of neutrophils in the gut (Fig. 7). Mice were depleted of neutrophils by an α-Ly-6G antibody, which was confirmed by flow cytometry (Fig. S7A, B) and by immunoblot detection of myeloperoxidase and CP in the cecum (Fig. 7C, S7C). When neutrophil-depleted mice were infected 1:1 with STM WT and STM sitA mntH zupT or STM sodA, we observed a significant reduction in WT’s competitive advantage (Fig. 7A) due to greater recovery of the mutants (Fig. 7B).
Figure 7. Mn helps STM resist neutrophil-sourced ROS and CP in the inflamed gut.
(A–C) C57BL/6 mice treated (Tx) with α-Ly-6G antibody to deplete neutrophils were coinfected 1:1 with indicated STM strains. (A, D, G) Bars = mean C.I. (CFU STM WT / mutant) ± SEM. (B, E, H) CFUs in colon contents of individual mice (B) from A, (E) from D, (H) from G. (D–F) Cybb+/+ mice (white bars) and Cybb−/− mice (grey bars) coinfected as in A. (C, F) Immunoblots of cecal tissue extract from STM coinfected mice; MPO = myeloperoxidase. * (P ≤ 0.05), ** (P ≤ 0.01), **** (P ≤ 0.0001). (See also Fig. S7)
To determine if stress in the inflamed gut involved host production of superoxide, we employed Cybb−/− mice, whose phagocytes (macrophages and neutrophils) cannot generate superoxide (Pollock et al., 1995). When Cybb−/− mice were infected 1:1 with STM WT and STM sitA mntH zupT or STM sodA, WT’s competitive advantage was significantly reduced (Fig. 7D) due to greater recovery of the mutants (Fig. 7E). These results indicate that STM evades growth suppression by the neutrophil oxidative burst by acquiring Mn and expressing SodA. Of note, neutrophil influx to the gut was increased in Cybb−/− mice, as shown by elevated myeloperoxidase and CP levels (Fig. 7F, S7D).
Coupling of Mn sequestration and ROS production by neutrophils is thought to enhance host killing of bacteria by inhibiting Mn-dependent defenses; e.g., Mn sequestration by CP disrupts S. aureus superoxide activity, rendering the pathogen susceptible to killing by neutrophils in vitro (Kehl-Fie et al., 2011). As STM utilizes Mn transporters to facilitate oxidative stress resistance in vivo (Fig. 7D), we posited CP enhances oxidative killing in the inflamed gut. To investigate this, we coinfected S100a9−/− Cybb−/− mice along with WT and Cybb−/− littermates with a 1:1 ratio of STM WT and STM sitA mntH zupT. At 48h p.i. we observed a decrease in WT’s competitive advantage in S100a9−/− Cybb−/− mice relative to Cybb−/− mice (Fig. 7G, H), suggesting CP enhances oxidative stress, although STM evades it by employing Mn transporters. Collectively, our findings show that Mn transporters and SodA enable STM to thrive in the inflamed gut by overcoming neutrophil-sourced killing mediated by CP and ROS.
DISCUSSION
Bacterial pathogens must acquire metal ions to replicate and survive in the host. The host, in turn, employs multiple mechanisms to limit pathogen access to metal ions in a process known as nutritional immunity (Hood and Skaar, 2012).
CP is a Zn/Mn-binding host protein abundantly secreted in the inflamed gut; e.g., during Salmonella infection of humans and of mice (Chen et al., 2012; Liu et al., 2012). Previously, we demonstrated that Zn acquisition via the ZnuABC transporter confers resistance to CP in the gut, thus enhancing growth of STM and boosting its competitiveness with the microbiota (Liu et al., 2012). As the role of Mn binding by fecal CP was not known, we dissected the interplay between STM and CP-mediated Mn sequestration.
Our in vitro results show a redundant role for Mn transporters in evading CP, as growth of the sitA mntH zupT mutant, but not the sitA mntH or zupT mutants, was severely attenuated in media + CP (Fig. 1, S3). Furthermore, we demonstrated that Site I (Zn/Mn-binding), but not Site II (Zn-only binding), of CP is primarily responsible for attenuating growth of STM sitA mntH zupT (Fig. 1). These results agree with studies on S. aureus (Damo et al., 2013; Kehl-Fie et al., 2011), further demonstrating that Mn sequestration contributes to CP’s antimicrobial activity.
In the inflamed mouse gut, STM mutants in two or more Mn transporters (e.g. STM sitA mntH) are severely attenuated when competing with WT (Fig. 2). Moreover, in agreement with our previous findings (Cerasi et al., 2014), ZupT was dispensable for colonization of the inflamed intestine as no further growth inhibition was observed in vivo for the sitA mntH zupT mutant (Fig. 2). Even so, Mn transporter mutants colonized the inflamed gut to WT levels when no close competitor was present (Fig. S4). As metal transporters can exhibit low affinity for additional metals, it is plausible that other metal transporters maintained STM’s growth in the absence of a competitor by importing sufficient amounts of Mn. Nevertheless, the strong attenuation of Mn transporter mutants in competition with WT indicates that low affinity transporters are not as efficient at acquiring Mn in the inflamed gut as are SitABCD and MntH. Moreover, Mn transporters are essential for STM to outgrow E. coli (Fig. 2), indicating that Mn acquisition is important for competition among the Enterobacteriaceae.
Although CP efficiently sequesters Mn, it is not the only means of Mn starvation in the gut as Mn transporter mutants were not rescued in CP-deficient mice (Fig. 3). Instead, these mutants were partially rescued in Il22−/− mice. IL-22 is highly induced in the cecum of STM-infected mice, where it promotes expression of antimicrobial proteins (Behnsen et al., 2014). Our data suggest that, in addition to CP, IL-22 induces production of additional Mn chelators. In the absence of IL-22, expression of these chelators is apparently lowered, which results in better growth of STM Mn transporter mutants (Fig. 3). This is consistent with our findings that IL-22 promotes expression of metal-sequestering antimicrobials (e.g., LCN2 and CP) in the inflamed gut (Behnsen et al., 2014).
Mn is an essential cofactor for many bacterial stress response enzymes, including the superoxide dismutase SodA (Kehres and Maguire, 2003). As the sodA, sitA mntH zupT, and sitA mntH zupT sodA mutants exhibited a similar growth defect under Mn-starved aerobic conditions (Fig. 5, S3), Mn sequestration appears to enhance oxidative stress. Consistent with this, CP only minimally inhibited growth of STM sitA mntH zupT anaerobically, where levels of free radical species are greatly reduced (Fig. 4). Mn transporters may thus promote the growth of STM over susceptible bacteria in the inflamed gut, where both Mn starvation and free radical production are hindrances to microbial survival.
Neutrophils generate ROS during intestinal inflammation. Furthermore, elevated O2 tension in the inflamed gut is postulated to kill obligate anaerobes and increase facultative anaerobes such as the Enterobacteriaceae (Rigottier-Gois, 2013). Together, these suggest an environment favoring growth of facultative anaerobes that can mitigate oxidative stress; in fact, Salmonella utilizes both aerobic and anaerobic respiration for growth in the inflamed gut (Rivera-Chavez et al., 2016). As study of Salmonella superoxide dismutases had not been carried out in a model of colitis (De Groote et al., 1997; Tsolis et al., 1995), SodA previously appeared to only play a role in Salmonella survival within macrophages (Tsolis et al., 1995). Thus, our finding that SodA promotes growth of STM in the inflamed gut (Fig. 6) demonstrates that enzymatic, Mn-dependent oxidative stress mitigation is an important mechanism for STM to thrive in this environment. We also show that the Mn-dependent catalase KatN helps STM colonize the inflamed gut (Fig. 6), suggesting that the Fe-dependent catalases KatE and KatG do not compensate for its absence in vivo, whereas they appear to do so in vitro (Fig. S6). Collectively, these results indicate that the Mn-containing enzymes SodA and KatN, and the Fe-containing enzymes SodB, KatE and KatG, play non-redundant roles in infection, as previously hypothesized (Pacello et al., 2012).
By depleting neutrophils or ablating their ability to produce ROS (Cybb−/−), we reduced STM WT’s advantage over STM sitA mntH zupT and STM sodA (Fig. 7). Consistent with CP’s ability to enhance oxidative stress in vitro (Fig. 5 and (Damo et al., 2013; Kehl-Fie et al., 2011)), STM WT’s competitive advantage was further reduced in mice lacking both phagocyte ROS production and CP (Fig. 7). Nevertheless, WT’s remaining 6-fold advantage in S100a9−/− Cybb−/− mice may indicate other important sources of ROS during infection, such as dual oxidase 2 (DUOX2). Produced by intestinal epithelial cells and highly induced in an IL-22-dependent fashion during STM infection (Behnsen et al., 2014), DUOX2 generates ROS independent of NADPH oxidase and regulates the gut microbiota (Grasberger et al., 2015). Additionally, our finding that STM sodA outcompetes STM sitA mntH zupT (Fig. 6C) suggests additional roles for Mn in Salmonella pathogenesis of the inflamed gut beyond mitigating oxidative stress. In line with this, Fe-dependent enzymes are primary targets of mismetallation under high oxidative stress, and bacterial cells can adapt by replacing Fe with Mn in some enzymes (Imlay, 2014).
Altogether, we show that Mn acquisition is important for STM to thrive in the inflamed gut, a highly oxidative environment where metal ions are limited by the host whilst being needed in greater abundance by the pathogen. Although limiting gut inflammation seems like an attractive avenue to limit STM growth in the lumen, such broad actions can compromise the host’s ability to restrict Salmonella’s dissemination to extraintestinal sites. Instead, as Mn, Fe, and Zn uptake each promotes STM growth in the inflamed gut, targeting mechanisms of metal acquisition may be a more practical approach to limit infection.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Culture Conditions
IR715 is a fully virulent, nalidixic acid-resistant derivative of S. Typhimurium (STM) WT isolate ATCC 14028. E. coli JB2 is a mouse commensal strain isolated from the feces of mice bred at UC Irvine. Construction of STM mutants is described in Supplemental Experimental Procedures. Strains were grown aerobically at 37°C in Luria-Bertani (LB) broth unless otherwise noted. Bacterial strains and primers employed are listed in Tables S1 and S2.
Bacterial Growth Assays in LB + CP
Recombinant WT calprotectin (CP) and mutants were produced as described by (Damo et al., 2013). Growth in media + CP was performed as described by (Kehl-Fie et al., 2011; Liu et al., 2012) with minor modifications (see Supplemental Experimental Procedures). Anaerobic (3% H2, 5% CO2, 92% N2) growth was performed in a Bactron II Anaerobic Chamber (Shellab).
Measurement of Mn in Fecal Samples by ICP-MS
Manganese (Mn) in mouse feces was quantified by ICP-MS as described previously (Corbin et al., 2008; Liu et al., 2012) and detailed in Supplemental Experimental Procedures.
Mouse Experiments
C57BL/6 mice, S100a9−/− mice (Manitz et al., 2003), Il22−/− mice (Zheng et al., 2007), Cybb−/− mice (Pollock et al., 1995), and S100a9−/− Cybb−/− mice were used in our study. C57BL/6 mice were purchased from Taconic Farms, Charles River, or Jackson laboratory (WT control for Cybb−/− mice), or bred in house (WT controls for S100a9−/−mice, Il22−/− mice, Cybb−/− mice and S100a9−/− Cybb−/− mice). Mice were infected as previously described (Liu et al., 2012). All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee at UC Irvine.
Superoxide Dismutase and Catalase Activity Assays
Soluble protein extracts of STM strains grown in either M9 or Vogel Bonner media (SOD activity assay) or LB (catalase activity assay) were separated by electrophoresis in a non-denaturing polyacrylamide gel. SOD and catalase activity were visualized as described (Beauchamp and Fridovich, 1971; Woodbury et al., 1971).
Neutrophil Depletion
Mice received 500µg (200µl) anti-Ly-6G antibody clone 1A8 (BioXCell; diluted in sterile PBS) via intraperitoneal injection 24h pre-infection and again 24h p.i. to maintain neutrophil depletion. Depletion was confirmed by flow cytometry (see Supplemental Experimental Procedures).
Western Blotting
Total protein was extracted from mouse cecal tissue using Tri-Reagent (Molecular Research Center), resolved by SDS-PAGE and transferred to a PVDF membrane. Detection of mouse tubulin, S100A8, S100A9 and myeloperoxidase was performed as described by (Liu et al., 2012).
Quantitative Real-Time PCR
Total RNA was extracted from mouse cecal tissue using Tri-Reagent (Molecular Research Center). Reverse transcription of 1µg total RNA was performed using the Transcription First Strand cDNA synthesis kit (Roche). Quantitative real-time PCR was performed with primers listed in Table S3.
Histopathology
Tissue samples were fixed in formalin, processed with standard procedures for paraffin embedding, sectioned at 5µm, and stained with hematoxylin and eosin. A board-certified pathologist scored pathology of cecal samples by blinded examination of cecal sections as previously described (Barthel et al., 2003; Liu et al., 2012; Raffatellu et al., 2009).
Statistical Analysis
Differences between treatment groups were analyzed by ANOVA followed by Student’s t test. A P value ≤ 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Russell Gerards for help with ICP-MS. MR’s lab is supported by Public Health Service Grant (PHSG) AI83663, AI101784, AI105374, AI114625, DK058057. MR holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. Other MR lab support: VDO by NIH-MBRS-IMSD GM055246 and a UCI President’s Dissertation Year Award; SK by an American Heart Association (AHA) predoctoral fellowship; JZL by NIH T32 AI60573 and an AHA predoctoral fellowship; JB by an AHA postdoctoral fellowship. EPS and WJC labs are supported by PHSG AI101171. EPS holds a Merit Review Grant BX002482 from the Office of Medical Research, Department of Veterans Affairs. CWG lab is supported by PHSG AI099687 and AI095208.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental information includes 7 figures, 3 tables, Supplemental Experimental Procedures, and Supplemental References.
AUTHOR CONTRIBUTIONS
VDO and MR conceived the study. VDO, SK, JB, JZL, NC, SPN, SGR, JRM, MC, RAE, AB, AJO, CWG, WJC, EPS, and MR designed the experiments and analyzed data. VDO, DL, CSL, SK, JB, JZL, SGR, NC, RAE, CMJ, MC performed experiments. VDO, SPN and MR wrote the paper.
REFERENCES
- Aguirre JD, Culotta VC. Battles with iron: manganese in oxidative stress protection. J Biol Chem. 2012;287:13541–13548. doi: 10.1074/jbc.R111.312181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MM, Newsom DL, Gonzalez JF, Sabag-Daigle A, Stahl C, Steidley B, Dubena J, Dyszel JL, Smith JN, Dieye Y, et al. Fructose-asparagine is a primary nutrient during growth of Salmonella in the inflamed intestine. PLoS Pathog. 2014;10:e1004209. doi: 10.1371/journal.ppat.1004209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, Salzman N. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 2008;76:907–915. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Behnsen J, Jellbauer S, Wong CP, Edwards RA, George MD, Ouyang W, Raffatellu M. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity. 2014;40:262–273. doi: 10.1016/j.immuni.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer E, Bergevin I, Malo D, Gros P, Cellier MF. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2002;70:6032–6042. doi: 10.1128/IAI.70.11.6032-6042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy MB, Hayden JA, Nolan EM. Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J Am Chem Soc. 2012;134:18089–18100. doi: 10.1021/ja307974e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy MB, Nakashige TG, Gaillard A, Nolan EM. Contributions of the S100A9 C-terminal tail to high-affinity Mn(II) chelation by the host-defense protein human calprotectin. J Am Chem Soc. 2013;135:17804–17817. doi: 10.1021/ja407147d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellier MF, Bergevin I, Boyer E, Richer E. Polyphyletic origins of bacterial Nramp transporters. Trends Genet. 2001;17:365–370. doi: 10.1016/s0168-9525(01)02364-2. [DOI] [PubMed] [Google Scholar]
- Cerasi M, Liu JZ, Ammendola S, Poe AJ, Petrarca P, Pesciaroli M, Pasquali P, Raffatellu M, Battistoni A. The ZupT transporter plays an important role in zinc homeostasis and contributes to Salmonella enterica virulence. Metallomics. 2014;6:845–853. doi: 10.1039/c3mt00352c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Huang JL, Chang CJ, Kong MS. Fecal calprotectin as a correlative marker in clinical severity of infectious diarrhea and usefulness in evaluating bacterial or viral pathogens in children. J Pediatr Gastroenterol Nutr. 2012;55:541–547. doi: 10.1097/MPG.0b013e318262a718. [DOI] [PubMed] [Google Scholar]
- Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, Murphy WJ, Zhang Y, Betz C, Hench L, Fritz G, et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc Natl Acad Sci U S A. 2013;110:3841–3846. doi: 10.1073/pnas.1220341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groote MA, Ochsner UA, Shiloh MU, Nathan C, McCord JM, Dinauer MC, Libby SJ, Vazquez-Torres A, Xu Y, Fang FC. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc Natl Acad Sci U S A. 1997;94:13997–14001. doi: 10.1073/pnas.94.25.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriu E, Liu JZ, Pezeshki M, Edwards RA, Ochoa RJ, Contreras H, Libby SJ, Fang FC, Raffatellu M. Probiotic bacteria reduce Salmonella Typhimurium intestinal colonization by competing for iron. Cell Host Microbe. 2013;14:26–37. doi: 10.1016/j.chom.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- Grasberger H, Gao J, Nagao-Kitamoto H, Kitamoto S, Zhang M, Kamada N, Eaton KA, El-Zaatari M, Shreiner AB, Merchant JL, et al. Increased Expression of DUOX2 Is an Epithelial Response to Mucosal Dysbiosis Required for Immune Homeostasis in Mouse Intestine. Gastroenterology. 2015;149:1849–1859. doi: 10.1053/j.gastro.2015.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass G, Franke S, Taudte N, Nies DH, Kucharski LM, Maguire ME, Rensing C. The metal permease ZupT from Escherichia coli is a transporter with a broad substrate spectrum. J Bacteriol. 2005;187:1604–1611. doi: 10.1128/JB.187.5.1604-1611.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden JA, Brophy MB, Cunden LS, Nolan EM. High-affinity manganese coordination by human calprotectin is calcium-dependent and requires the histidine-rich site formed at the dimer interface. J Am Chem Soc. 2013;135:775–787. doi: 10.1021/ja3096416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebrard M, Viala JP, Meresse S, Barras F, Aussel L. Redundant hydrogen peroxide scavengers contribute to Salmonella virulence and oxidative stress resistance. J Bacteriol. 2009;191:4605–4614. doi: 10.1128/JB.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, Sugitani N, Chazin WJ, Caprioli RM, Skaar EP. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog. 2012;8:e1003068. doi: 10.1371/journal.ppat.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol. 2013;11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA. The mismetallation of enzymes during oxidative stress. J Biol Chem. 2014;289:28121–28128. doi: 10.1074/jbc.R114.588814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiraman A, Slauch JM. The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium. Mol Microbiol. 2000;35:1146–1155. doi: 10.1046/j.1365-2958.2000.01783.x. [DOI] [PubMed] [Google Scholar]
- Karlinsey JE, Maguire ME, Becker LA, Crouch ML, Fang FC. The phage shock protein PspA facilitates divalent metal transport and is required for virulence of Salmonella enterica sv. Typhimurium. Mol Microbiol. 2010;78:669–685. doi: 10.1111/j.1365-2958.2010.07357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BB, Jr, McCord JM, Fridovich I. Superoxide dismutase from Escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970;245:6176–6181. [PubMed] [Google Scholar]
- Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, Munro KA, Chazin WJ, Skaar EP. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe. 2011;10:158–164. doi: 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres DG, Janakiraman A, Slauch JM, Maguire ME. SitABCD is the alkaline Mn(2+) transporter of Salmonella enterica serovar Typhimurium. J Bacteriol. 2002;184:3159–3166. doi: 10.1128/JB.184.12.3159-3166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres DG, Maguire ME. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev. 2003;27:263–290. doi: 10.1016/S0168-6445(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Kehres DG, Zaharik ML, Finlay BB, Maguire ME. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol Microbiol. 2000;36:1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- Lawley TD, Bouley DM, Hoy YE, Gerke C, Relman DA, Monack DM. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect Immun. 2008;76:403–416. doi: 10.1128/IAI.01189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Jellbauer S, Poe AJ, Ton V, Pesciaroli M, Kehl-Fie TE, Restrepo NA, Hosking MP, Edwards RA, Battistoni A, et al. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe. 2012;11:227–239. doi: 10.1016/j.chom.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Lusitani D, Malawista SE, Montgomery RR. Calprotectin, an abundant cytosolic protein from human polymorphonuclear leukocytes, inhibits the growth of Borrelia burgdorferi. Infect Immun. 2003;71:4711–4716. doi: 10.1128/IAI.71.8.4711-4716.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier L, Vyas R, Cordova CD, Lindsay H, Schmidt TS, Brugiroux S, Periaswamy B, Bauer R, Sturm A, Schreiber F, et al. Microbiota-derived hydrogen fuels Salmonella Typhimurium invasion of the gut ecosystem. Cell Host Microbe. 2013;14:641–651. doi: 10.1016/j.chom.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Manitz MP, Horst B, Seeliger S, Strey A, Skryabin BV, Gunzer M, Frings W, Schonlau F, Roth J, Sorg C, et al. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol. 2003;23:1034–1043. doi: 10.1128/MCB.23.3.1034-1043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashige TG, Zhang B, Krebs C, Nolan EM. Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol. 2015;11:765–771. doi: 10.1038/nchembio.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccio SP, Bäumler AJ. Comparative analysis of Salmonella genomes identifies a metabolic network for escalating growth in the inflamed gut. MBio. 2014;5:e00929–e00914. doi: 10.1128/mBio.00929-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacello F, Rotilio G, Battistoni A. Low-shear modeled microgravity enhances Salmonella enterica resistance to hydrogen peroxide through a mechanism involving KatG and KatN. Open Microbiol J. 2012;6:53–64. doi: 10.2174/1874285801206010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp-Wallace KM, Maguire ME. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol. 2006;60:187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476–486. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigottier-Gois L. Dysbiosis in inflammatory bowel diseases: the oxygen hypothesis. ISME J. 2013;7:1256–1261. doi: 10.1038/ismej.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Chavez F, Zhang LF, Faber F, Lopez CA, Byndloss MX, Olsan EE, Xu G, Velazquez EM, Lebrilla CB, Winter SE, et al. Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella. Cell Host Microbe. 2016;19:443–454. doi: 10.1016/j.chom.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe-Saule V, Coynault C, Ibanez-Ruiz M, Hermant D, Norel F. Identification of a non-haem catalase in Salmonella and its regulation by RpoS (sigmaS) Mol Microbiol. 2001;39:1533–1545. doi: 10.1046/j.1365-2958.2001.02340.x. [DOI] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohnle PG, Hunter MJ, Hahn B, Chazin WJ. Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14) J Infect Dis. 2000;182:1272–1275. doi: 10.1086/315810. [DOI] [PubMed] [Google Scholar]
- Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, et al. Salmonella enterica serovar Typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbakk M, Naess-Andresen CF, Lingaas E, Dale I, Brandtzaeg P, Fagerhol MK. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336:763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- Stork M, Grijpstra J, Bos MP, Manas Torres C, Devos N, Poolman JT, Chazin WJ, Tommassen J. Zinc piracy as a mechanism of Neisseria meningitidis for evasion of nutritional immunity. PLoS Pathog. 2013;9:e1003733. doi: 10.1371/journal.ppat.1003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teigelkamp S, Bhardwaj RS, Roth J, Meinardus-Hager G, Karas M, Sorg C. Calcium-dependent complex assembly of the myeloic differentiation proteins MRP-8 and MRP-14. J Biol Chem. 1991;266:13462–13467. [PubMed] [Google Scholar]
- Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Bäumler AJ. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci U S A. 2011;108:17480–17485. doi: 10.1073/pnas.1107857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolis RM, Bäumler AJ, Heffron F. Role of Salmonella Typhimurium Mn-superoxide dismutase (SodA) in protection against early killing by J774 macrophages. Infect Immun. 1995;63:1739–1744. doi: 10.1128/iai.63.5.1739-1744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolis RM, Xavier MN, Santos RL, Bäumler AJ. How to become a top model: impact of animal experimentation on human Salmonella disease research. Infect Immun. 2011;79:1806–1814. doi: 10.1128/IAI.01369-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker MM, Whittaker JW. A glutamate bridge is essential for dimer stability and metal selectivity in manganese superoxide dismutase. J Biol Chem. 1998;273:22188–22193. doi: 10.1074/jbc.273.35.22188. [DOI] [PubMed] [Google Scholar]
- Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339:708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury W, Spencer AK, Stahmann MA. An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem. 1971;44:301–305. doi: 10.1016/0003-2697(71)90375-7. [DOI] [PubMed] [Google Scholar]
- Zaharik ML, Cullen VL, Fung AM, Libby SJ, Kujat Choy SL, Coburn B, Kehres DG, Maguire ME, Fang FC, Finlay BB. The Salmonella enterica serovar Typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infect Immun. 2004;72:5522–5525. doi: 10.1128/IAI.72.9.5522-5525.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Zhou D, Hardt WD, Galan JE. Salmonella typhimurium encodes a putative iron transport system within the centisome 63 pathogenicity island. Infect Immun. 1999;67:1974–1981. doi: 10.1128/iai.67.4.1974-1981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.