Abstract
Background:
Haemorrhagic shock accounts up to 50% of early trauma deaths. Hematopoietic failure has been observed in experimental animals and human following shock and injury. One of the facets of bone marrow failure is multiple organ dysfunction syndrome and is commonly seen in patients recovering from severe trauma and hemorrhagic shock. Bone Marrow (BM) dysfunction is associated with mobilization of hematopoietic progenitor cells (HPCs) into peripheral blood. Present study explored the association of peripheral blood hematopoietic progenitor cells (HPCs) with mortality in trauma haemorrhagic shock patients (T/HS).
Materials and Methods:
Prospective cohort studies of patients presenting within 8 hrs of injury with T/HS to the Department of Emergency Medicine, Jai Prakash Narayan Apex Trauma Center, All India Institute of Medical Sciences were recruited. Peripheral blood samples were collected in each patient for measurement of peripheral blood HPCs. Peripheral blood progenitor cell (PBPC) quantification was performed by measuring HPCs counts using the haematology analyzer (Sysmex XE-2100). Clinical and laboratory data were prospectively collected after consent. Ethical approval was taken and data was analysed by Stata 11.2.
Results:
39 patients with trauma hemorrhagic shock and 30 normal healthy controls were recruited. HPCs were significantly higher (P < 0.001) in the T/HS as compared to control. Among study group, 14 patients died within 24 h. at the hospital admission, and found HPCs concentrations were highly significant (<0.001) in non-survivors (n = 14) when compared with survivors (n = 25) among T/HS patients.
Conclusions:
Our studies suggest the peripheral blood HPCs may be early prognostic marker for mortality among patients who presented with trauma hemorrhagic shock on admission. But the exact molecular mechanism and signalling pathway involved in the change of the behaviour of bone marrow microenvironment is still unclear.
Keywords: Hematopoietic progenitor cells, outcome, trauma hemorrhagic shock
INTRODUCTION
Severe trauma and hemorrhagic shock is the leading cause of mortality in individuals between 5 and 44 years.[1,2] Hematopoietic failure has been observed in experimental animals and human following shock and injury. One of the facets of bone marrow failure is multiple organ dysfunction syndrome and is commonly seen in patients recovering from severe trauma and haemorrhagic shock.
Bone marrow (BM) is composed of after stromal cells, fibronectin, proteoglycans and hematopoietic stem cells (HSCs) niche in mammals. Previous studies demonstrated that impaired growth of hematopoietic progenitor cells (HPCs) and stromal cells associated with bone marrow failure in T/HS.[3,4] Shah et al. (2009) reported in severe trauma, initial mobilization of HPCs from BM to peripheral blood into injured or inflammatory tissue, is beneficial for wound healing and maintaining immune response.[5] Increased peripheral blood HPCs were associated with BM dysfunction.[6] There is paucity of literature regarding the HSCs behavior and its correlation with outcomes following T/HS.
The present study explored the role of circulating Haematopoietic progenitor cells and its correlation with outcomes in T/HS. It also focuses on technique to study HPCs.
MATERIALS AND METHODS
Study design: Prospective cohort study
Study group
Inclusion criteria
Trauma victims with hemorrhagic-shock
Age group > 18, <60 years
Systolic blood pressure of ≤90 mmHg.
Patients or proxy must be willing to provide informed consent
Patients presenting within 8 h injury to the Emergency Department.
Exclusion criteria include
Age group <18, >60 years
Systolic blood pressure >90 mmHg
Patients already resuscitated with colloids or crystalloids before reporting to the emergency department.
Patients had a history of haematological diseases or pre-existing anaemia, liver or renal failure
Cardiogenic shock
Head injury
Hematologic diseases or preexisting anemia, had active HIV infection, or had a history of renal or liver failure
Septic shock
Neurogenic shock
Control group: Healthy control
Ethical approval
Our study was approved (Ref. no. IEC/NP-278/2010) by a research ethics committee, All India Institute of Medical Sciences, New Delhi, India. Before the sample collection, signed informed consent was obtained from patients or patients' relatives, those who fulfilled the inclusion criteria before the procedure. Sample were collected after filling the patient information sheet (PIS) and patient information consent form (PICF).
Sample collection
T/HS patients admitted to Emergency Department, Jai Prakash Narayan Apex Trauma Centre, All India Institute of Medical Sciences from October 2011 to November 2014 were enrolled. Peripheral blood samples (PBS) were collected from T/HS and controls. PBS (n = 39) were prospectively collected from T/HS patients admitted to the Emergency Medicine Department, with in 8h of injuries. PBS were collected in plain vial and Ethylene diamine tetra acetic acid (EDTA) collection tubes via direct venepuncture. Samples were processed within 2 hours of collection.
Hematopoietic progenitor cells enumeration
Enumeration of the HPCs were performed in the immature myeloid information (IMI) of the Sysmex XE 2100, which identifies a population of hematopoietic progenitor cells based on the cell size, complexity and differential cell lysis characteristics.[7]
Clinical data
The clinical details were recorded in a pre-tested format (attached supplementary 1) by research officer. In addition; initial laboratory values including haemoglobin (Hb), total leukocyte count (TLC), platelets, prothrombin time (PT), Activated partial thromboplastin (APTT), urea, creatinine, sodium, potassium, and arterial blood gas parameter (partial pressure of oxygen (pO2) partial pressure of carbon dioxide (pCO2), pH and base excess (BE) were collected. Patients were observed for outcomes in terms of survival or death during the course of hospital stay. These data were analysed to explore the correlation between clinical severity score, survival or death with baseline HPCs.
Statistical analysis
Data analysis
Categorical and continuous data were expressed in frequency (%) and mean ± sd/median (minimum, maximum), respectively. The associations between two categorical variables were seen by using Chi-square/Fisher's exact test. For normally distributed continuous variables, the mean differences were compared by using Students's t-test for two independent groups and one-way analysis of variance for more than two independent groups. For skewed data (non-normal distribution), the differences between two independent groups were seen by using Mann-Whitney test and the difference among more than two independent groups were seen by using Kruskal-Wallis test. All the P values less than 0.05 were taken as significant. Statistical analysis was done by using statistical software stata 11.2.
RESULTS
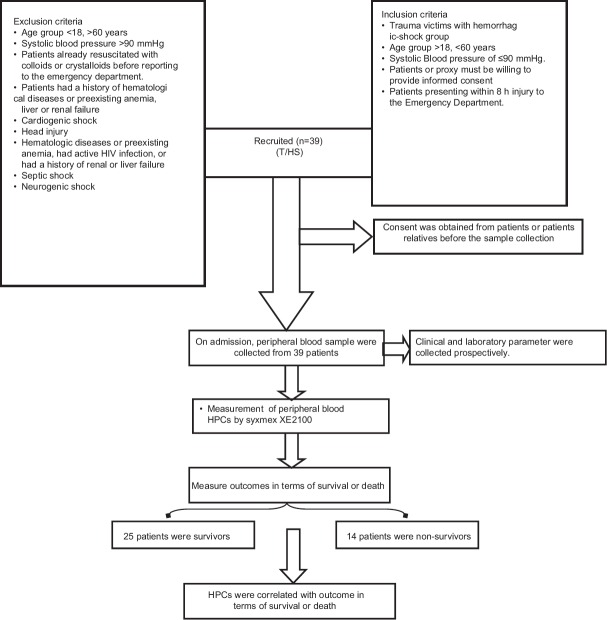
39 T/HS patients, between 18-60 yrs, with 31 males and 8 females and 30 controls were recruited for the study. Results from the samples collected from 25 T/HS who had survived were compared with the data from the non-survivors of T/HS. Registries of the patients were shown in Figure 1.
Figure 1.
Recruitment of T/HS patients
In T/HS, the levels of peripheral blood HPCs were elevated when compared to normal healthy volunteers (P < 0.001) [Table 1]. Subgroup analysis showed increased peripheral blood HPCs levels among non-survivors (n = 14) compared to survivors (n = 25, P < 0.001) [Table 2].
Table 1.
Comparison of circulating haematopoietic progenitor cells among trauma haemorrhagic shock patients and healthy volunteers

Table 2.
Comparison of HPCs and ISS between survivor and non-survivors in T/HS

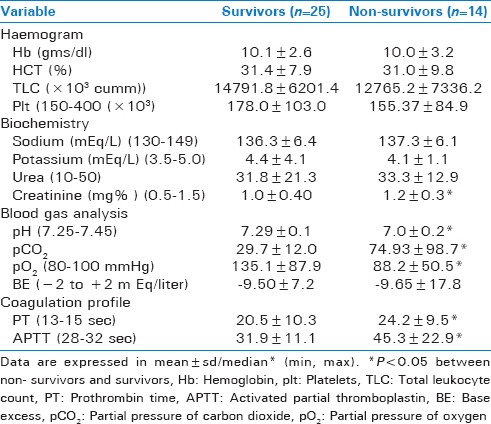
Elevation in peripheral blood HPCs is also observed in non-survivors patients with ISS score 17.0 (9, 50) vs. 12 (4, 50) P < 0.05) [Table 2], creatinine (1.2 ± 0.3 vs. 1.0 ± 0.40), P < 0.05), PT (24.2 ± 9.5 vs. 20.5 ± 10.3) P < 0.05) APTT (45.3 ± 22.9 vs. 31.9 ± 11.1), P < 0.05), pH (7.0 ± 0.2 vs. 7.29 ± 0.1), P < 0.05, pO2 (88.2 ± 5 vs. 135.1 ± 8), P < 0.05, pCO2 (74.93 ± 9 vs. 29.7 ± 12), P < 0.05 when compared to the non-survivors [Table 3].
Table 3.
Baseline laboratory parameters among survivor and non-survivors in T/HS
DISCUSSION
Trauma hemorrhagic shock cause tissues hypo perfusion and consequent cellular hypoxia, hypoglycemia and metabolic damage. It leads to organ dysfunction by suppressing the immune system and elevation of inflammatory response.[8] Previous studies reported that in severe injury and HS, mobilization of HPCs from BM to peripheral blood are associated with BM dysfunction.[6] Therefore, we evaluated the circulating peripheral blood HPCs and its correlation with outcome.
We found increase in peripheral blood HPCs with T/HS when compared to healthy control. In study group, peripheral bloods HPCs were higher among non-survivors as compared to survivors. Previous study showed that when peripheral bloods HPCs were grown in methylcellulose media; it increased in severely injured patients versus control (15 ± 26 vs. 3 ± 1, <0.05).[3] This study only recruited severe injury group and did not correlate with outcomes. Our study correlated with ISS and outcomes in terms of survival or death.
Baranski et al. showed two times increase in HPCs mobilization into peripheral blood and plasma G-CSF levels at three hours following lung contusion and hemorrhagic shock (LC/HS).[9] Kollet et al. reported T/HS induced stress condition and activation of osteoclast results in mobilization of progenitor cells into peripheral blood, when compared to myocardial infarction and stable angina.[10]
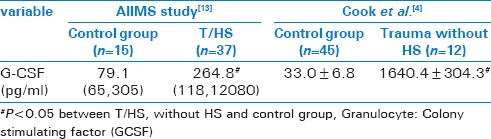
In severe trauma, initial mobilization of hematopoietic progenitor cells from BM to peripheral blood, is beneficial for wound healing and maintaining immune response.[5] Fonseca et al. reported that in severe trauma induced stress condition, increased urine nor-epinepherine level versus control (139 ± 59 mcg/day vs. 35 ± 9 mcg/day) promoted BM dysfunction and hematopoietic failure in human.[11] Excessive release of inflammatory cytokines leads to sustained elevation of catecholamine concentrations.[4] Elevated catecholamine levels alters the regulation of signaling pathways (CXCR4 and SDF1) resulting in suppression of bone marrow HPCs and continued mobilization of HPCs into peripheral blood leading to persistent anemia.[12,13,14] Similarly, stress condition induced by catecholamine is also seen in burn injuries.[15] Previous studies showed Granulocyte colony stimulating factor (G-CSF) as one of potent stimulator of hematopoietic mobilization, in BM dysfunction following severe trauma and haemorrhagic shock [16,17] [Table 4].
Table 4.
G-CSF levels in T/HS vs. Trauma without hemorrhagic shock
Sifri et al. showed BM suppression among GM-CFU and BFU-E colony in castrated male and male rats after T/HS.[18,19] There were 60% decrease in all BM HPCs colony growth, including CFU-GEMM, BFU-E, and CFU-E, when compared to unmanipulated control (UC) at 3h (12 ± 1*, 26 ± 1*, 31 ± 1* vs. 36 ± 1, 65 ± 1, 73 ± 1*P < 0.05) in lung contusion following by hemorrhagic shock.[20]
BM derived stem and progenitor cells have a capacity for self-renewal, differentiation, survival, migration, proliferation and mobilization; which are regulated by extrinsic and intrinsic signal provided by their microenvironment. BM HPCs are thought to be located within specific stroma niches.[21] This specific microenvironment provides soluble factors and cellular interaction required for HPCs proliferation and differentiation. As HPCs differentiate, they may move for one niche to another.[14]
Peripheral blood HPCs quantification was done by sysmex XE 2100 automated haematology analyser in the present study. This is a quick (90 second), user friendly, low cost alternative method for quantification of peripheral blood HPCs. Flow cytometry is the recommended for hematopoietic stem cell count with (monoclonal antibody) MoAb anti – CD34: This however requires skilled personal and expensive procedure and is a time consuming. Sysmex XE2100 does not require MoABs and it can also be used for measurement of complete blood count (CBC) count and leukocyte differential (LDS) count.[22,23] Basic principle of Sysmex XE-2100 automated haematology analyzer has immature myeloid information (IMI) channel which are used for quantification of HPCs per microliter of blood. The IMI channel of the Sysmex system lyses mature and erythropoietic cells by means of a special lyse reagent. Cells in the area of low volume and a reduced plasma/nucleus relation are detected as HPCs and analyzed using a special HPCs software program. HPCs like all immature cells, are resistant to the lytic reagent and are located within a specific gated area of the scattergram.[24,25]
Limitations
HPCs were assessed only once in T/HS. HPCs were not measured in trauma patients without HS. Further studies may evaluate the peripheral blood HPCs at different time point and its correlation with outcomes following T/HS. The role of signaling pathways involved in HPCs mobilization needs attention and design new therapeutic target to reactivate BM.
CONCLUSION
Peripheral blood haematopoietic progenitor cells increased after trauma hemorrhagic shock. The peripheral blood HPCs were elevated among non-survivors. Peripheral blood HPCs may be explored as an early marker for mortality among T/HS patients.
Financial support and sponsorship
Done as a part of ICMR project.
Conflicts of interest
Tbhere are no conflicts of interest.
Acknowledgement
This study was financially supported by Indian Council of Medical Research (ICMR), New Delhi, India.
REFERENCES
- 1.Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: Hemorrhagic shock. Crit Care. 2004;8:373–81. doi: 10.1186/cc2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: An overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(Suppl):S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 3.Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA, et al. Bone marrow failure following severe injury in human. Ann Surg. 2003;238:748–53. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson Y, Hostmann A, Matenov A, Ertel W, Oberholzer A. Erythropoiesis in multiple injured patients. J Trauma. 2006;61:1285–91. doi: 10.1097/01.ta.0000240969.13891.9b. [DOI] [PubMed] [Google Scholar]
- 5.Shah S, Ulm J, Sifri ZC, Mohr AM, Livingston DH. Mobilization of bone marrow cells to the site of injury is necessary for wound healing. J Trauma. 2009;67:315–22. doi: 10.1097/TA.0b013e3181a5c9c7. [DOI] [PubMed] [Google Scholar]
- 6.Badami CD, Livingston DH, Sifri ZC, Caputo FJ, Bonilla L, Mohr AM, et al. Hematopoietic progenitor cells mobilize to the site of injury after trauma and hemorrhagic shock in rats. J Trauma. 2007;63:596–602. doi: 10.1097/TA.0b013e318142d231. [DOI] [PubMed] [Google Scholar]
- 7.Padmanabhan A, Reich-Slotky R, Jhang JS, Dael S, Crowder T, Colovai AI, et al. Use of the haematopoietic progenitor cell parameter in optimizing timing of peripheral blood stem cell harvest. Vox Sang. 2009;97:153–9. doi: 10.1111/j.1423-0410.2009.01183.x. [DOI] [PubMed] [Google Scholar]
- 8.Molina PE. Neurobiology of the stress response: Contribution of the sympathetic nervous system to the neuroimmune axis in traumatic injury. Shock. 2005;24:3–10. doi: 10.1097/01.shk.0000167112.18871.5c. [DOI] [PubMed] [Google Scholar]
- 9.Baranski GM, Offin MD, Sifri ZC, Elhassan IO, Hannoush EJ, Alzate WD, et al. β-blockade protection of bone marrow following trauma: The role of G-CSF. J Surg Res. 2011;170:325–31. doi: 10.1016/j.jss.2011.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca RB, Mohr AM, Wang L, Clinton E, Sifri ZC, Rameshwar P, et al. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect (Larchmt) 2004;5:385–93. doi: 10.1089/sur.2004.5.385. [DOI] [PubMed] [Google Scholar]
- 12.Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 13.Hoggatt J, Pelus LM. Hematopoietic stem cell mobilization with agents other than G-CSF. Methods Mol Biol. 2012;904:49–67. doi: 10.1007/978-1-61779-943-3_4. [DOI] [PubMed] [Google Scholar]
- 14.Hoggatt J, Pelus LM. Mobilization of hematopoietic stem cells from the bone marrow niche to the blood compartment. Stem Cell Res Ther. 2011;2:13. doi: 10.1186/scrt54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulp GA, Herndon DN, Lee JO, Suman OE, Jeschke MG. Extent and magnitude of catecholamine surge in paediatric burn patients. Shock. 2010;33:369–74. doi: 10.1097/SHK.0b013e3181b92340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook KM, Sifri ZC, Baranski GM, Mohr AM, Livingston DH. The role of plasma granulocyte colony stimulating factor and bone marrow dysfunction after severe trauma. J Am Coll Surg. 2013;216:57–64. doi: 10.1016/j.jamcollsurg.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar M, Bhoi S, Subramanian A, Kamal VK, Mohanty S, Rao DN. Evaluation of serum granulocyte colony stimulating factor in patients admitted with trauma haemorrhagic shock. Int J Adv Res Biol Sci. 2015;2:107–14. [Google Scholar]
- 18.Sifri ZC, Kaiser VL, Ananthakrishnan P, Wang L, Mohr AM, Hauser CJ, et al. Bone marrow failure in male rats following trauma/hemorrhagic shock (T/HS) is mediated by mesenteric lymph and modulated by castration. Shock. 2006;25:12–6. doi: 10.1097/01.shk.0000188708.97153.ce. [DOI] [PubMed] [Google Scholar]
- 19.Sifri ZC, Cohen D, Ananthakrishnan P, Wang L, Kaiser VL, Mohr AM, et al. Sex hormones affect bone marrow dysfunction after trauma and hemorrhagic shock. Crit Care Med. 2007;35:864–9. doi: 10.1097/01.CCM.0000256839.50053.1D. [DOI] [PubMed] [Google Scholar]
- 20.Pasupuleti LV, Cook KM, Sifri ZC, Kotamarti S, Calderon GM, Alzate WD, et al. Does selective beta 1 blockade provide bone marrow protection after trauma/hemorrhagic shock? Surgery. 2012;152:322–30. doi: 10.1016/j.surg.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurudutta GU, Satija NK, Singh VK, Verma YK, Gupta P, Tripathi RP. Stem cell therapy: A novel and futuristic treatment modality for disaster injuries. Indian J Med Res. 2012;135:15–25. doi: 10.4103/0971-5916.93419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Letestu R, Marzac C, Audat F, Belhocine R, Tondeur S, Baccini V, et al. Use of hematopoietic progenitor cell count on the Sysmex XE-2100 for peripheral blood stem cell harvest monitoring. Leuk Lymphoma. 2007;48:89–96. doi: 10.1080/10428190600886149. [DOI] [PubMed] [Google Scholar]
- 23.Briggs C, Harrison P, Grant D, Staves J, Chavada N, Machin SJ. Performance evaluation of the sysmex XE-2100™ automated haematology analyser. Sysmex J Int. 1999;9:113–9. [Google Scholar]
- 24.Stolzel F, Oelschlagel U, Holig K, Ehninger G, Bornhauser M, Thiede C. Increased accuracy of HPC quantification using the XE-HPC master technology. Sysmex J Int. 2003;13:83–6. [Google Scholar]
- 25.Cymbalista F, Letestu R. Haemopoietic progenitor cell (HPC) counts on the Sysmex XE-2100: A new toll for peripheral blood stem cell (PBSC) harvest monitoring. Sysmex J Int. 2005;15:21–6. [Google Scholar]