Abstract
Background:
Heidelberg retina tomogram (HRT) and optical coherence tomography (OCT) are two widely used imaging modalities to evaluate the optic nerve head (ONH) in glaucoma.
Purpose:
To compare the ONH parameters of HRT3 and high-definition OCT (HD-OCT) and evaluate their diagnostic abilities in perimetric and preperimetric glaucoma.
Design:
Cross-sectional analysis.
Methods:
35 control eyes (24 subjects), 21 preperimetric glaucoma eyes (15 patients), and 64 perimetric glaucoma eyes (44 patients) from the Longitudinal Glaucoma Evaluation Study underwent HRT3 and HD-OCT examinations.
Statistical Analysis:
Agreement between the ONH parameters of HRT and HD-OCT were assessed using Bland-Altman plots. Diagnostic abilities of ONH parameters were evaluated using area under the receiver operating characteristic curves (AUCs), sensitivity at fixed specificity, and likelihood ratios (LR).
Results:
Optic disc area, vertical cup to disc ratio, and cup volume with HD-OCT were larger than with HRT, while the rim area was smaller with HD-OCT (P < 0.001 for all comparisons). AUCs of all HD-OCT ONH parameters (0.90-0.97 in perimetric and 0.62-0.71 in preperimetric glaucoma) were comparable (P > 0.10) to the corresponding HRT ONH parameters (0.81-0.95 in perimetric and 0.55-0.72 in preperimetric glaucoma). LRs associated with diagnostic categorization of ONH parameters of both HD-OCT and HRT were associated with larger effects on posttest probability of perimetric compared to preperimetric glaucoma.
Conclusions:
ONH measurements of HD-OCT and HRT3 cannot be used interchangeably. Though the diagnostic abilities of ONH parameters of HD-OCT and HRT in glaucoma were comparable, the same were significantly lower in preperimetric compared to perimetric glaucoma.
Keywords: Glaucoma, Heidelberg retina tomogram, optic nerve head, optical coherence tomography
Glaucoma is a progressive optic neuropathy characterized by changes in optic nerve head (ONH) and retinal nerve fiber layer (RNFL) with or without visual field (VF) defects. Characteristic ONH changes in glaucoma consists of focal or diffuse neuroretinal rim (NRR) thinning with enlargement of the ONH cup and notching. Evaluation of simultaneous stereoscopic optic disc photographs by experts is the current standard for documenting the ONH changes in glaucoma and monitoring for structural progression.[1] However, the two most important limitations of disc photograph assessment are the poor agreement among experts in assessing the glaucomatous ONH changes[2,3,4,5] and the lack of quantification of the structural changes. Heidelberg retina tomogram (HRT, Heidelberg Engineering, Dossenheim, Germany) is a confocal scanning ophthalmoscopic technology used to objectively measure the ONH parameters. Earlier studies have reported good test-retest variability[6,7] and good diagnostic abilities of the ONH parameters of HRT in glaucoma.[8,9,10] In addition to the HRT, the other imaging technology that is used to evaluate the ONH objectively is the optical coherence tomography (OCT). Studies using the current version of OCT (high-definition [HD] OCT) have also reported good test-retest variability[11,12,13] and diagnostic ability[14,15,16] of the ONH parameters of HD-OCT in glaucoma. Though there are studies comparing the ONH parameters of HRT and HD-OCT,[13,17,18,19,20,21,22,23] there is limited literature on the comparison of the diagnostic abilities of ONH parameters of HRT and HD-OCT in glaucoma.[22,23] The purpose of our study was to compare the ONH parameters of HD-OCT and HRT in an Indian population and compare their diagnostic abilities in detecting perimetric and preperimetric glaucoma.
Methods
This was a cross-sectional analysis of the baseline evaluations of the participants included in the ongoing Longitudinal Glaucoma Evaluation Study (LOGES). LOGES is a prospective longitudinal study conducted at a tertiary eye care center in South India to evaluate the structure and function in glaucoma longitudinally. Participants in LOGES include normal subjects, patients with glaucoma and glaucoma suspects, who are longitudinally evaluated clinically and with functional and imaging tests. Written informed consent was obtained from all subjects, and the Institute Ethics Committee approved all methodology. All methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects.
The methodology of LOGES has been described earlier.[16] In brief, the inclusion criteria were age ≥ 18 years, best corrected visual acuity of 20/40 or better and refractive error within ± 5 D sphere and ± 3 D cylinder. Exclusion criteria were the presence of any media opacities that prevented good quality optic disc photographs and other imaging tests, and any retinal (including macular) or neurological disease other than glaucoma which could confound the evaluations. All participants underwent a comprehensive ocular examination which included a detailed medical history, best corrected visual acuity measurement, slit-lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy, dilated fundus examination, VF examination, and stereoscopic optic disc photography. OCT imaging was performed with Cirrus HD-OCT (Carl Zeiss MeditecInc, Dublin, CA, USA) and confocal scanning laser ophthalmoscopy with HRT3 (Heidelberg Engineering, Dossenheim, Germany).
VF examination was performed using a Humphrey Field Analyzer, model 750i (Zeiss Humphrey Systems, Dublin, CA, USA), with the Swedish interactive threshold algorithm standard 24-2 program. Reliability criteria for inclusion were fixation losses and false negative response rates of <20% and false positive response rates of <15%. All VFs were graded by a single observer masked to the clinical examination results, optic disc photos, and the results of the imaging tests of the same and the opposite eye. VFs were classified as “glaucomatous” if the pattern standard deviation had a P < 5% and the glaucoma hemifield test result was outside the normal limits.[24] VFs were classified as “normal” otherwise.
Stereoscopic optic disc photographs were obtained by trained technicians using digital fundus camera (FF 450plus with VISUPAC 4.2.2, Carl Zeiss Meditec Systems GmbH, Pirmasens, Germany). Photographs consisted of a 50° image centered on the optic disc, a similar image centered on the macula, a 30° image centered on the optic disc, and a 20° image centered on the disc. All these images also consisted of one colored and one red-free image each. Each optic disc photograph was evaluated independently by two of the three experts all of whom were masked to the clinical details of the subjects and also the VF, imaging, and other eye examination results. They classified the optic discs into glaucomatous and nonglaucomatous (control) groups based on the presence or absence of characteristic glaucomatous optic disc changes (focal or diffuse NRR thinning, localized notching, or nerve fiber layer defects). Optic discs that could not be definitively classified into glaucoma or control groups were classified as suspects. Discrepancies between the two experts were resolved by consensus.
HD-OCT imaging was performed using the optic disc cube 200 × 200 protocol (software version 6.5.0.772). This protocol has been described in detail previously.[14,17] Scans with a signal strength of <6 or with motion artifacts were excluded. The protocol automatically determines multiple ONH parameters using inbuilt software. The software identifies the termination of Bruch's membrane as the disc margin. The rim width is then determined by measuring the thickness of the neuroretinal tissue in the optic disc as it turns to exit through the opening in the Bruch's membrane.[14] Scans were also manually inspected for the intactness of ONH segmentation algorithm and those with segmentation algorithm failure were excluded. The ONH parameters, except the disc area, are subsequently compared to the internal reference database consisting of 284 subjects[25] and a color-coded, diagnostic categorization is provided. Parameter values falling between the 95th and 100th percentile values of the reference database are coded “white,” between 5th and 95th percentile values are coded “green,” between 1st and 5th are coded “yellow,” and those below the 1st percentile values are coded “red,” As most of the optic discs in the reference database have a disc area between 1.3 and 2.5 mm2, ONH parameters of discs with areas outside this range are not color-coded.
HRT imaging of the ONH was analyzed using the software version 3. Details of the scanning protocol of HRT have been described earlier.[10,26] Three consecutive scans are obtained, aligned, and then averaged by HRTs software to create a single mean topography image for analysis. Images were included if the global pixel standard deviation was <40 microns. Optic disc margin was marked at the inner border of the scleral ring by an experienced operator while viewing the stereo disc images. After marking the disc margin contour line, the HRT software automatically places a standard reference plane 50 μm posterior to the mean retinal height between 350° and 356° along the contour line. The part of the optic disc superficial to the reference plane is considered the NRR and the part deep to the plane is considered the cup. The HRT software automatically determines a number of ONH parameters. Similar to that with HD-OCT, ONH parameters of HRT are compared to an ethnicity-specific internal reference database consisting of 733 Caucasian, 215 African-American, and 100 Indian eyes[27] and a color-coded, diagnostic categorization is provided. Parameter values falling between the 5th and 95th percentile values of the reference database are coded with a green check mark, between 0.1th and 5th percentile values are coded with a yellow exclamation mark, and those below the 0.1th percentile values are coded with a red cross.
Disc area, rim area, vertical cup to disc ratio (VCDR), and cup volume measurements were considered for analysis as these are the common ONH parameters provided both by Cirrus HD-OCT and HRT softwares.
For the current study, control eyes were the ones with nonglaucomatous optic discs and normal VFs. Glaucomatous eyes were divided into perimetric glaucoma group if the optic disc and VF classification were glaucomatous and preperimetric glaucoma group if the optic disc was glaucomatous but the VF was normal. The classification of optic discs into glaucomatous and nonglaucomatous groups was based on the evaluation of stereoscopic optic disc photographs.
Statistical analysis
Descriptive statistics included mean and standard deviation for normally distributed variables and median and interquartile range for nonnormally distributed variables. Shapiro-Wilk test was used to check for the normality of distribution. Bland-Altman plots were used to assess the limits of agreement (LoA) between HD-OCT and HRT for the ONH parameter measurements. In a Bland-Altman plot, the difference between the measurements with the two devices is plotted against their mean.[28] The mean difference between the measurements on the Bland-Altman plot is an estimate of the fixed bias. Bland-Altman plot also detects the proportional bias in the measurements, which is the relationship of the difference in the measurements and the mean of the measurements. The presence of proportional bias indicates that the devices do not agree equally through the range of measurements. Proportional bias was formally evaluated by regressing the difference between the measurements with two devices on the average of the measurements with two devices. Receiver operating characteristic (ROC) curves were used to describe the ability of ONH parameters of HD-OCT and HRT to discriminate perimetric and preperimetric glaucomatous eyes from control eyes. Sensitivities at fixed specificities of 80% and 95% were determined for all the parameters. To obtain confidence intervals for area under the ROC curves (AUCs), a bootstrap re-sampling procedure was used (n = 1000 re-samples). As measurements of both eyes of the same subject are likely to be correlated, the standard statistical methods for parameter estimation lead to underestimation of standard errors and to confidence intervals that are too narrow.[29] Therefore, the cluster of data for the study subject was considered as the unit of resampling and bias corrected standard error was calculated during all estimations. This procedure has been used in literature to adjust for the presence of multiple correlated measurements from the same unit.[30,31] Z-test was used to compare the AUCs[32,33] and Chi-square test to compare the sensitivities at fixed specificities of HD-OCT parameters in diagnosing perimetric and preperimetric glaucoma. Likelihood ratios (LRs) were reported for diagnostic categorization (outside normal limits, borderline, or within normal limits) provided after comparison with the instrument's internal reference database. LR is the probability of a given test result in those with disease divided by the probability of the same test result in those without the disease.[34] The LR for a given test result indicates how much that result will raise or lower the probability of disease. An LR of 1 or close to 1 would mean that the test provides no additional information about the posttest probability of the disease. LRs higher than 10 or lower than 0.1 would be associated with large effects on posttest probability, LRs from 5 to 10 or from 0.1 to 0.2 would be associated with moderate effects, and LRs from 2 to 5 or from 0.2 to 0.5 would be associated with small effects.[34] LRs associated with the parameter results were calculated as conditional LR+, LR−, and LR ± for the results flagged in red, green, and yellow, respectively. The LRs and the 95% confidence interval for them were calculated according to the method proposed by Simel et al.[35] Statistical analyses were performed using commercial software (Stata Version 11.2; StataCorp, College Station, TX, USA). A P ≤ 0.05 was considered statistically significant.
Results
Two hundred and one eyes of 111 subjects included in LOGES had undergone ONH imaging with HD-OCT and HRT in their baseline visit. Among these, 44 eyes where the disc photographic classification by experts was “disc suspect” were excluded. Fig. 1 is a Venn diagram showing the number of eyes excluded because of poor quality disc photographs, unreliable VFs, and poor quality HD-OCT and HRT images. Thirty-five eyes of 24 control subjects, 21 eyes of 15 patients with preperimetric glaucoma, and 64 eyes of 44 patients with perimetric glaucoma remained for the final analysis. Of the 21 eyes with preperimetric glaucoma, all of them had NRR thinning, one eye had rim notching, and 19 had RNFL defects. Table 1 shows the age, refraction, VF characteristics, and the ONH parameters of the three groups of subjects. The signal strength of the HD-OCT scans was significantly lesser in the perimetric glaucoma eyes compared to the control eyes. Optic disc size as estimated by HD-OCT was also significantly smaller in perimetric glaucoma eyes compared to the control eyes. AUCs and sensitivities at fixed specificities of HD-OCT ONH parameters were therefore calculated after adjusting for the difference in signal strength and disc size between perimetric glaucoma and control groups using covariate-adjustment as proposed by Pepe.[36]
Figure 1.
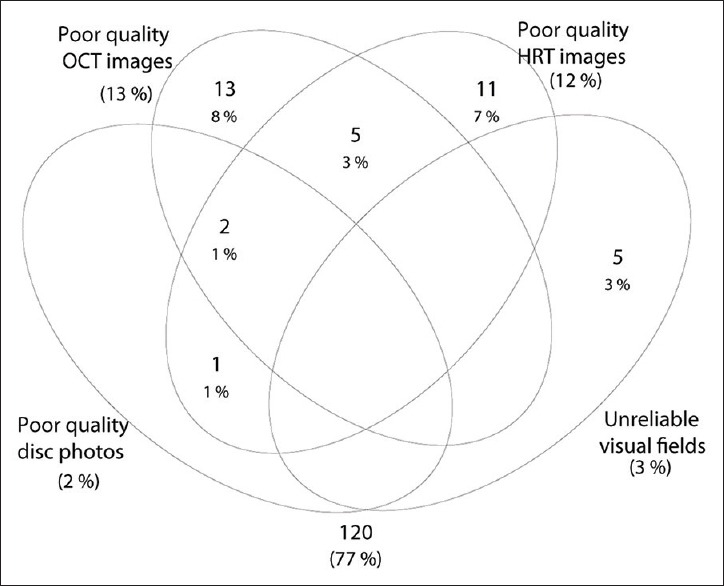
Venn diagram showing the number of eyes excluded from analysis because of poor quality disc photographs, unreliable visual fields, and poor quality high-definition optical coherence tomography and Heidelberg retina tomogram images
Table 1.
Age, visual field, high-definition optical coherence tomography and Heidelberg retina tomography three features of the participants
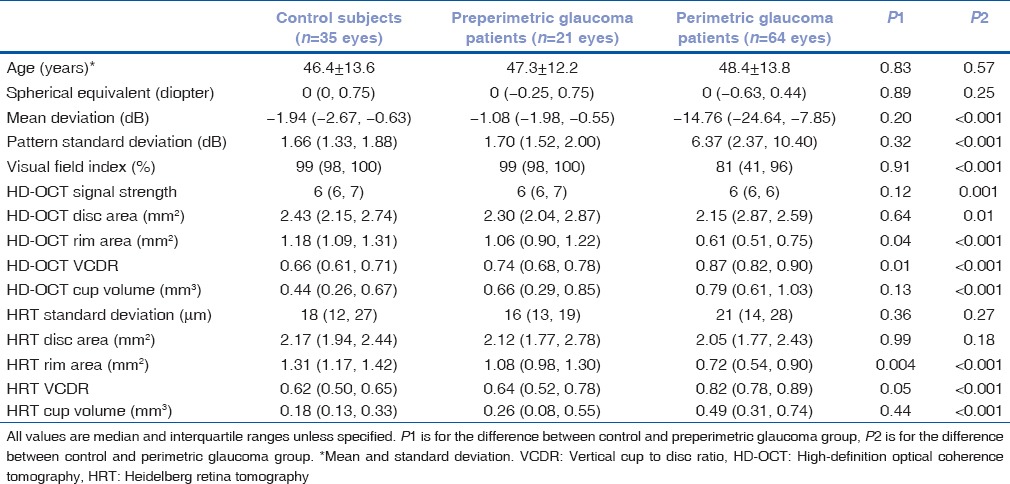
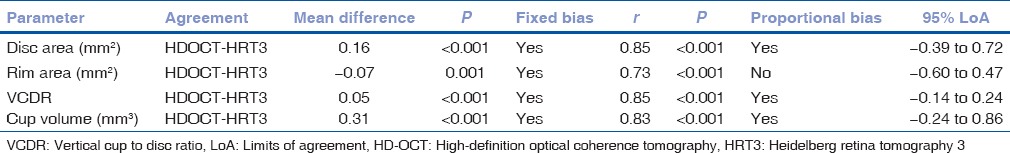
Fig. 2 shows the Bland-Altman plots with mean and 95% LoA between HD-OCT and HRT for disc area, rim area, VCDR, and cup volume measurements. Numerical values associated with the same are shown in Table 2. Disc area, VCDR, and cup volume measurements were significantly greater with HD-OCT compared to HRT while the rim area measurements were significantly lesser with HD-OCT. Significant proportional bias in the agreement was detected for the disc area, VCDR, and cup volume measurements [Fig. 2].
Figure 2.
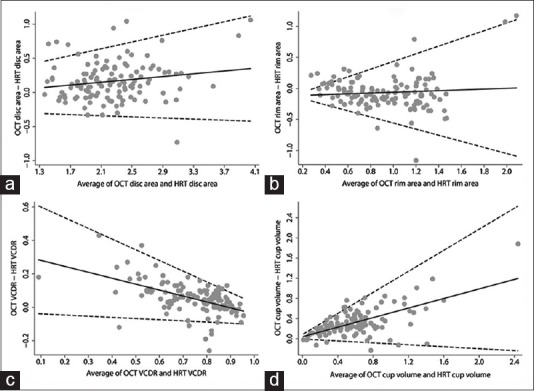
Bland–Altman plots showing the agreement between high-definition optical coherence tomography and Heidelberg retina tomography for (a) disc area, (b) rim area, (c) vertical cup-disc ratio, and (d) cup volume parameters
Table 2.
Agreement between high-definition optical coherence tomography and Heidelberg retina tomogram for various optic nerve head parameters
The AUCs and sensitivities at fixed specificities of the ONH parameters with HD-OCT and HRT to differentiate perimetric and preperimetric glaucoma from control eyes are shown in Table 3. AUCs and sensitivities at fixed specificities of all HD-OCT and HRT parameters were significantly greater in perimetric compared to preperimetric glaucoma. AUCs and sensitivities at fixed specificities of all HD-OCT ONH parameters were comparable to the respective HRT ONH parameters (P > 0.10 for all comparisons) both in perimetric and preperimetric glaucoma [Fig. 3].
Table 3.
Areas under the receiver operating characteristic curves and sensitivities at fixed specificities of optic nerve head parameters of high-definition optical coherence tomography and Heidelberg retina tomography 3 in glaucoma
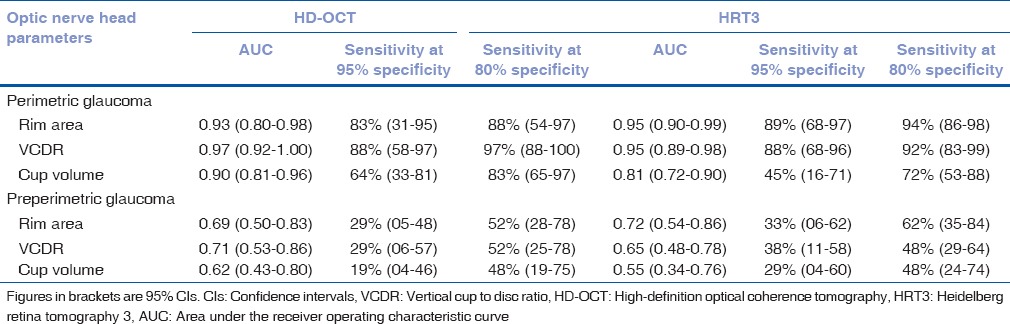
Figure 3.
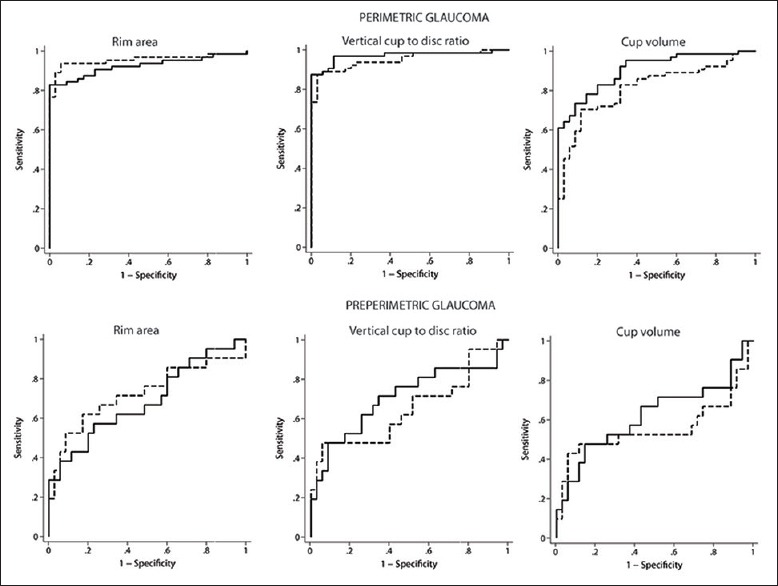
Receiver operating characteristic curves of the optic nerve head parameters of high-definition optical coherence tomography (solid lines) and Heidelberg retina tomography (dashed lines) in perimetric and preperimetric glaucoma
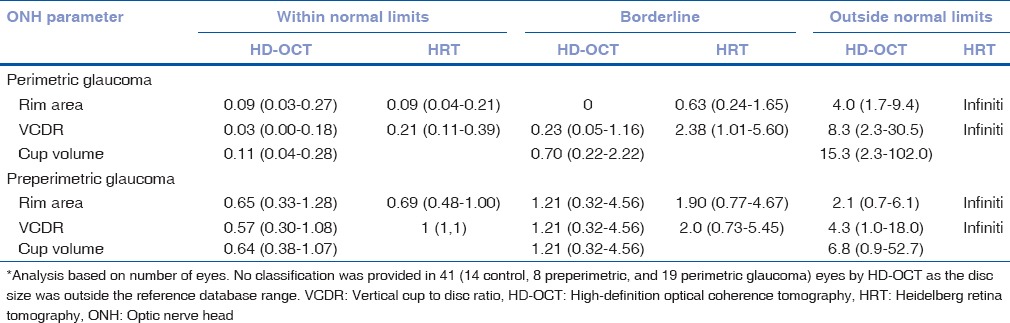
Table 4 shows the LRs associated with the reference database classification of the ONH parameters of HD-OCT and HRT to discriminate perimetric and preperimetric glaucoma from control eyes. Outside normal limits classification of all HD-OCT and HRT parameters were associated with moderate effects on the posttest probability of perimetric glaucoma while the same were associated with small effects on the posttest probability of preperimetric glaucoma. Within normal limits, classification of all HD-OCT and HRT parameters was associated with moderate to large effects on the posttest probability of perimetric glaucoma while the same were associated with no effects on the posttest probability of preperimetric glaucoma. Borderline classification of most HD-OCT and HRT parameters was associated with no effects on the posttest probability of both perimetric and preperimetric glaucoma.
Table 4.
Likelihood ratios (with 95% confidence interval)* of the reference database classification of high-definition optical coherence tomography and Heidelberg retina tomography optic nerve head parameters to discriminate glaucoma from control eyes
Discussion
In this study to compare the ONH parameters of HD-OCT and HRT, we found that the HD-OCT measurements of disc area, cup to disc ratio, and cup volume were significantly greater than those of HRT. Rim area measurement of HD-OCT was significantly smaller than that of HRT. Similar results have been reported previously by multiple studies[13,17,18,19,20,21,22,23] except for the results relating to the disc area measurements. Few of the previously mentioned studies have found the disc area measurements to be greater with HRT compared to HD-OCT[13,18,20,21] while a few have reported comparable disc area measurements between the two.[17,19] Contrary to all these results, we found that the disc area measurements were significantly greater with HD-OCT compared to HRT. This is probably related to the subjectivity involved in the disc area measurement of HRT. HRT requires the operator to mark the disc margins using multiple points, and previous studies have reported the variability this brings into the HRT measurements.[37,38] Evaluating the agreement between HD-OCT and HRT for ONH measurements, we found that the 95% LoA for all ONH parameters were wide. Bland-Altman plots demonstrated both a significant fixed and proportional bias between HD-OCT and HRT for all ONH measurements. Significant proportional bias in ONH parameter measurements has also been reported by previous studies.[13,17,23] This result demonstrates that the ONH measurements of HD-OCT and HRT cannot be used interchangeably. Similar results are reported previously.[13,17,18,19,20,21,22,23] The reason for the differences in the ONH measurements between HD-OCT and HRT are due to the difference in the method of disc margin and cup margin detection by the two instruments. In HD-OCT, disc margin corresponds to the Bruch's membrane opening and is determined automatically, while HRT requires the disc margins to be marked manually by an operator. The demarcation between the NRR and the cup happens automatically in HD-OCT by proprietary software while the same in HRT happens with respect to a standard reference plane placed 50 μm below the retinal surface.
Evaluating the ability of ONH parameters of HD-OCT and HRT in diagnosing glaucoma, we found that AUCs and sensitivities at fixed specificities of HD-OCT ONH parameters were comparable to that of HRT ONH parameters. Though there are multiple studies evaluating the agreement between the ONH parameters of HD-OCT and HRT as discussed before, studies comparing the diagnostic abilities of ONH parameters of HD-OCT and HRT in glaucoma are limited. Shin et al. compared the diagnostic abilities of the ONH parameters of HD-OCT and HRT in glaucoma and found that the AUCs of HD-OCT ONH parameters were significantly greater than those of the HRT ONH parameters.[21] However, it is important to note that the control group in the study by Shin et al. consisted of glaucoma suspect eyes and not normal eyes as in our study. Glaucoma suspect eyes in their study had diffuse or localized thinning of the NRR, VCDR greater than the fellow eye by 0.2, and/or RNFL defect but with intraocular pressures <22 mmHg and normal VFs. The control group in our study consisted of eyes with no suspicious findings for glaucoma. We have previously demonstrated how the control group affects the diagnostic abilities of OCT in glaucoma.[39,40] However, more recent studies comparing the diagnostic abilities of the ONH parameters of HD-OCT and HRT have found comparable AUCs and sensitivities.[22,23] In both these studies, the definition of glaucoma was based on the presence of a VF defect and the optic disc features were not considered for the diagnosis. Though it is preferable to use a functional criterion for defining glaucoma while evaluating structural abnormalities like ONH changes,[41] we chose a combination of structural and functional criteria to be more “specific” with the diagnosis and so could separate out a group of eyes with preperimetric glaucoma, which is more challenging to diagnose for the analysis. Preperimetric glaucoma in our study was diagnosed based on a single evaluation of the disc photographs. There is a possibility of a few optic discs diagnosed as preperimetric glaucoma actually being normal physiological variants. This is, however, less likely as two experts independently classified the optic discs as glaucomatous. AUCs and sensitivities of ONH parameters in our study were significantly greater in diagnosing perimetric compared to preperimetric glaucoma. This is in agreement with the previous studies which have reported the diagnostic abilities of imaging tests to be significantly affected by the severity of glaucoma with the diagnostic abilities being greater in eyes with severe disease.[42,43,44,45,46]
In addition to sensitivity, specificity, and AUC, diagnostic tests are also summarized in terms of LR, which is higher than the previous measures in the hierarchy, as it expresses the magnitude by which the probability of a diagnosis in a given patient is modified by the results of the test.[47,48] In other words, the LR indicates how much a given diagnostic test result will raise or lower the pretest probability of the disease in question. We therefore evaluated the LRs associated with the diagnostic categorization of ONH parameters of HD-OCT and HRT. Reference database classification of ONH parameters of both HD-OCT and HRT were associated with a larger effect on the posttest probability of perimetric glaucoma compared to that on preperimetric glaucoma. While interpreting the LRs it is important to note that the HRT has an Indian ethnicity-specific internal reference database of 100 eyes of Indian subjects,[27] while the reference database of Cirrus HD-OCT used in this study comprised only three Indian subjects, that is, 1% of the 284 subjects in the database (Cirrus HD-OCT User manual, available at www.meditec.zeiss.com). No classification was in fact provided in 41 eyes by HD-OCT as the disc size was outside the range of reference database. Moreover, the consideration for diagnostic categorization is slightly different between the two instruments with red representing values lesser than the lower 1% of the reference database values in HD-OCT and <0.1% of the reference database values in HRT.
Most diagnostic studies in glaucoma have employed a case-control design similar to the one used in our study, including glaucoma patients (cases), defined based on the presence of characteristic glaucomatous optic disc, and RNFL changes with or without VF defects; and normal subjects (controls), usually recruited from the general population. However, in clinical practice, a diagnostic test is used to detect disease in subjects suspected of having disease and not in subjects with either clear-cut evidence of the disease or with no suspicious findings of the disease. Multiple studies have evaluated the bias introduced in such situations.[39,40,49] Therefore, caution should be exercised when interpreting estimates of diagnostic ability provided in our study. These estimates should not be extrapolated to the situation of detecting disease in glaucoma suspects. Longitudinal investigations of suspect eyes using HD-OCT and HRT in LOGES should be able to clarify their role in providing definitive diagnosis in glaucoma suspect eyes. The other limitation of our study was the small sample size, especially for the diagnostic ability evaluations. The number of eyes available for LR calculation on HD-OCT reduced further due to the disc size being outside the reference database range. This may have led to the LRs associated with the borderline categorization of HD-OCT to be greater in preperimetric compared to the perimetric glaucoma.
We did not correct the HD-OCT ONH measurements for the ocular magnification. Previous studies have corrected the ONH measurements of OCT for ocular magnification using Littman's formula to avoid overestimation of disc size in myopic eyes and underestimation in hyperopic eyes.[13,50] We, however, feel that the refractive error in eyes of our study was small to affect the ONH measurements of HD-OCT.
Evaluating the ONH parameters in different ethnicities separately is important because the ONH characteristics depend on the ethnicity of the subjects.[51,52] Though multiple studies have compared the ONH parameters of HRT and HD-OCT, and a few have evaluated their diagnostic abilities in glaucoma, our study is the first one to do this in an Indian population. The results of our study in the Indian population were to a large extent comparable to that found in other ethnicities. Some of the differences in the findings of our study and that of the previous studies may be related to the ethnic differences in the included subjects.
Conclusion
ONH measurements of HD-OCT and HRT cannot be used interchangeably. Diagnostic abilities of the ONH parameters of HD-OCT and HRT in glaucoma were comparable. However, the diagnostic abilities were significantly lower in preperimetric compared to perimetric glaucoma.
Financial support and sponsorship
Hyderabad Eye Research Foundation.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Preferred practice pattern for primary open-angle glaucoma. San Francisco, California: American Academy of Ophthalmology; 2005. [Google Scholar]
- 2.Varma R, Steinmann WC, Scott IU. Expert agreement in evaluating the optic disc for glaucoma. Ophthalmology. 1992;99:215–21. doi: 10.1016/s0161-6420(92)31990-6. [DOI] [PubMed] [Google Scholar]
- 3.Abrams LS, Scott IU, Spaeth GL, Quigley HA, Varma R. Agreement among optometrists, ophthalmologists, and residents in evaluating the optic disc for glaucoma. Ophthalmology. 1994;101:1662–7. doi: 10.1016/s0161-6420(94)31118-3. [DOI] [PubMed] [Google Scholar]
- 4.Azuara-Blanco A, Katz LJ, Spaeth GL, Vernon SA, Spencer F, Lanzl IM. Clinical agreement among glaucoma experts in the detection of glaucomatous changes of the optic disk using simultaneous stereoscopic photographs. Am J Ophthalmol. 2003;136:949–50. doi: 10.1016/s0002-9394(03)00480-x. [DOI] [PubMed] [Google Scholar]
- 5.Jampel HD, Friedman D, Quigley H, Vitale S, Miller R, Knezevich F, et al. Agreement among glaucoma specialists in assessing progressive disc changes from photographs in open-angle glaucoma patients. Am J Ophthalmol. 2009;147:39–44.e1. doi: 10.1016/j.ajo.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatch WV, Flanagan JG, Williams-Lyn DE, Buys YM, Farra T, Trope GE. Interobserver agreement of Heidelberg retina tomograph parameters. J Glaucoma. 1999;8:232–7. [PubMed] [Google Scholar]
- 7.Miglior S, Albé E, Guareschi M, Rossetti L, Orzalesi N. Intraobserver and interobserver reproducibility in the evaluation of optic disc stereometric parameters by Heidelberg retina tomograph. Ophthalmology. 2002;109:1072–7. doi: 10.1016/s0161-6420(02)01032-1. [DOI] [PubMed] [Google Scholar]
- 8.Mikelberg FS, Parfitt CM, Swindale NV, Graham SL, Drance SM, Gosine R. Ability of the Heidelberg retina tomograph to detect early glaucomatous visual field loss. J Glaucoma. 1995;4:242–7. [PubMed] [Google Scholar]
- 9.Bathija R, Zangwill L, Berry CC, Sample PA, Weinreb RN. Detection of early glaucomatous structural damage with confocal scanning laser tomography. J Glaucoma. 1998;7:121–7. [PubMed] [Google Scholar]
- 10.Wollstein G, Garway-Heath DF, Hitchings RA. Identification of early glaucoma cases with the scanning laser ophthalmoscope. Ophthalmology. 1998;105:1557–63. doi: 10.1016/S0161-6420(98)98047-2. [DOI] [PubMed] [Google Scholar]
- 11.Savini G, Carbonelli M, Parisi V, Barboni P. Repeatability of optic nerve head parameters measured by spectral-domain OCT in healthy eyes. Ophthalmic Surg Lasers Imaging. 2011;42:209–15. doi: 10.3928/15428877-20110224-02. [DOI] [PubMed] [Google Scholar]
- 12.Mwanza JC, Chang RT, Budenz DL, Durbin MK, Gendy MG, Shi W, et al. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2010;51:5724–30. doi: 10.1167/iovs.10-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang B, Ye C, Yu M, Liu S, Lam DS, Leung CK. Optic disc imaging with spectral-domain optical coherence tomography: Variability and agreement study with Heidelberg retinal tomograph. Ophthalmology. 2012;119:1852–7. doi: 10.1016/j.ophtha.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 14.Mwanza JC, Oakley JD, Budenz DL, Anderson DR. Cirrus Optical Coherence Tomography Normative Database Study Group. Ability of cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology. 2011;118:241–8.e1. doi: 10.1016/j.ophtha.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeoung JW, Choi YJ, Park KH, Kim DM. Macular ganglion cell imaging study: Glaucoma diagnostic accuracy of spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:4422–9. doi: 10.1167/iovs.12-11273. [DOI] [PubMed] [Google Scholar]
- 16.Begum VU, Addepalli UK, Yadav RK, Shankar K, Senthil S, Garudadri CS, et al. Ganglion cell-inner plexiform layer thickness of high definition optical coherence tomography in perimetric and preperimetric glaucoma. Invest Ophthalmol Vis Sci. 2014;55:4768–75. doi: 10.1167/iovs.14-14598. [DOI] [PubMed] [Google Scholar]
- 17.Foo LL, Perera SA, Cheung CY, Allen JC, Zheng Y, Loon SC, et al. Comparison of scanning laser ophthalmoscopy and high-definition optical coherence tomography measurements of optic disc parameters. Br J Ophthalmol. 2012;96:576–80. doi: 10.1136/bjophthalmol-2011-300835. [DOI] [PubMed] [Google Scholar]
- 18.Moghimi S, Hosseini H, Riddle J, Lee GY, Bitrian E, Giaconi J, et al. Measurement of optic disc size and rim area with spectral-domain OCT and scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2012;53:4519–30. doi: 10.1167/iovs.11-8362. [DOI] [PubMed] [Google Scholar]
- 19.Resch H, Deak G, Pereira I, Vass C. Comparison of optic disc parameters using spectral domain cirrus high-definition optical coherence tomography and confocal scanning laser ophthalmoscopy in normal eyes. Acta Ophthalmol. 2012;90:e225–9. doi: 10.1111/j.1755-3768.2012.02385.x. [DOI] [PubMed] [Google Scholar]
- 20.Sato S, Hirooka K, Baba T, Shiraga F. Comparison of optic nerve head parameters using Heidelberg retina tomograph 3 and spectral-domain optical coherence tomography. Clin Experiment Ophthalmol. 2012;40:721–6. doi: 10.1111/j.1442-9071.2012.02782.x. [DOI] [PubMed] [Google Scholar]
- 21.Shin HY, Park HY, Jung KI, Park CK. Glaucoma diagnosis optic disc analysis comparing Cirrus spectral domain optical coherence tomography and Heidelberg retina tomograph II. Jpn J Ophthalmol. 2013;57:41–6. doi: 10.1007/s10384-012-0205-9. [DOI] [PubMed] [Google Scholar]
- 22.Calvo P, Ferreras A, Abadia B, Ara M, Figus M, Pablo LE, et al. Assessment of the optic disc morphology using spectral-domain optical coherence tomography and scanning laser ophthalmoscopy. Biomed Res Int. 2014;2014:275654. doi: 10.1155/2014/275654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kratz A, Lim R, Goldberg I. Optic nerve head assessment: Comparison of Cirrus optic coherence tomography and Heidelberg retinal tomograph 3. Clin Experiment Ophthalmol. 2014;42:734–44. doi: 10.1111/ceo.12344. [DOI] [PubMed] [Google Scholar]
- 24.Anderson DR, Patella VM. Automated Static Perimetry. 2nd ed. St. Louis: Mosby; 1999. p. 152. [Google Scholar]
- 25.Knight OJ, Girkin CA, Budenz DL, Durbin MK, Feuer WJ. Cirrus OCT Normative Database Study Group. Effect of race, age, and axial length on optic nerve head parameters and retinal nerve fiber layer thickness measured by Cirrus HD-OCT. Arch Ophthalmol. 2012;130:312–8. doi: 10.1001/archopthalmol.2011.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao HL, Babu GJ, Sekhar GC. Comparison of the diagnostic capability of the Heidelberg retina tomographs 2 and 3 for glaucoma in the Indian population. Ophthalmology. 2010;117:275–81. doi: 10.1016/j.ophtha.2009.06.062. [DOI] [PubMed] [Google Scholar]
- 27.Strouthidis NG, Garway-Heath DF. New developments in Heidelberg retina tomograph for glaucoma. Curr Opin Ophthalmol. 2008;19:141–8. doi: 10.1097/ICU.0b013e3282f4450b. [DOI] [PubMed] [Google Scholar]
- 28.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 29.Glynn RJ, Rosner B. Accounting for the correlation between fellow eyes in regression analysis. Arch Ophthalmol. 1992;110:381–7. doi: 10.1001/archopht.1992.01080150079033. [DOI] [PubMed] [Google Scholar]
- 30.Zhou XH, Obuchowski NA, McClish DK, editors. Statistical Methods in Diagnostic Medicine. New York: John Wiley & Sons, Inc.; 2002. Analysis of correlated ROC data; pp. 274–306. [Google Scholar]
- 31.Alonzo TA, Pepe MS. Distribution-free ROC analysis using binary regression techniques. Biostatistics. 2002;3:421–32. doi: 10.1093/biostatistics/3.3.421. [DOI] [PubMed] [Google Scholar]
- 32.Zhou XH, Obuchowski NA, McClish DK, editors. Statistical Methods in Diagnostic Medicine. New York: John Wiley & Sons; 2002. Comparing the accuracy of two diagnostic tests; pp. 165–94. [Google Scholar]
- 33.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 34.Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA. 1994;271:703–7. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 35.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: Sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44:763–70. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- 36.Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. Oxford, UK: Oxford University Press; 2003. pp. 130–67. [Google Scholar]
- 37.Iester M, Mikelberg FS, Courtright P, Burk RO, Caprioli J, Jonas JB, et al. Interobserver variability of optic disk variables measured by confocal scanning laser tomography. Am J Ophthalmol. 2001;132:57–62. doi: 10.1016/s0002-9394(01)00938-2. [DOI] [PubMed] [Google Scholar]
- 38.Watkins RJ, Broadway DC. Intraobserver and interobserver reliability indices for drawing scanning laser ophthalmoscope optic disc contour lines with and without the aid of optic disc photographs. J Glaucoma. 2005;14:351–7. doi: 10.1097/01.ijg.0000176938.00768.0d. [DOI] [PubMed] [Google Scholar]
- 39.Rao HL, Kumbar T, Addepalli UK, Bharti N, Senthil S, Choudhari NS, et al. Effect of spectrum bias on the diagnostic accuracy of spectral-domain optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci. 2012;53:1058–65. doi: 10.1167/iovs.11-8463. [DOI] [PubMed] [Google Scholar]
- 40.Rao HL, Addepalli UK, Chaudhary S, Kumbar T, Senthil S, Choudhari NS, et al. Ability of different scanning protocols of spectral domain optical coherence tomography to diagnose preperimetric glaucoma. Invest Ophthalmol Vis Sci. 2013;54:7252–7. doi: 10.1167/iovs.13-12731. [DOI] [PubMed] [Google Scholar]
- 41.Garway-Heath DF, Hitchings RA. Sources of bias in studies of optic disc and retinal nerve fibre layer morphology. Br J Ophthalmol. 1998;82:986. doi: 10.1136/bjo.82.9.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medeiros FA, Zangwill LM, Bowd C, Sample PA, Weinreb RN. Influence of disease severity and optic disc size on the diagnostic performance of imaging instruments in glaucoma. Invest Ophthalmol Vis Sci. 2006;47:1008–15. doi: 10.1167/iovs.05-1133. [DOI] [PubMed] [Google Scholar]
- 43.Zangwill LM, Jain S, Racette L, Ernstrom KB, Bowd C, Medeiros FA, et al. The effect of disc size and severity of disease on the diagnostic accuracy of the Heidelberg retina tomograph glaucoma probability score. Invest Ophthalmol Vis Sci. 2007;48:2653–60. doi: 10.1167/iovs.06-1314. [DOI] [PubMed] [Google Scholar]
- 44.Garudadri CS, Rao HL, Parikh RS, Jonnadula GB, Selvaraj P, Nutheti R, et al. Effect of optic disc size and disease severity on the diagnostic capability of glaucoma imaging technologies in an Indian population. J Glaucoma. 2012;21:475–80. doi: 10.1097/IJG.0b013e31821829f1. [DOI] [PubMed] [Google Scholar]
- 45.Leite MT, Zangwill LM, Weinreb RN, Rao HL, Alencar LM, Sample PA, et al. Effect of disease severity on the performance of Cirrus spectral-domain OCT for glaucoma diagnosis. Invest Ophthalmol Vis Sci. 2010;51:4104–9. doi: 10.1167/iovs.09-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao HL, Leite MT, Weinreb RN, Zangwill LM, Alencar LM, Sample PA, et al. Effect of disease severity and optic disc size on diagnostic accuracy of RTVue spectral domain optical coherence tomograph in glaucoma. Invest Ophthalmol Vis Sci. 2011;52:1290–6. doi: 10.1167/iovs.10-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langlotz CP. Fundamental measures of diagnostic examination performance: Usefulness for clinical decision making and research. Radiology. 2003;228:3–9. doi: 10.1148/radiol.2281011106. [DOI] [PubMed] [Google Scholar]
- 48.Medeiros FA, Zangwill LM, Bowd C, Weinreb RN. Comparison of the GDx VCC scanning laser polarimeter, HRT II confocal scanning laser ophthalmoscope, and stratus OCT optical coherence tomograph for the detection of glaucoma. Arch Ophthalmol. 2004;122:827–37. doi: 10.1001/archopht.122.6.827. [DOI] [PubMed] [Google Scholar]
- 49.Medeiros FA, Ng D, Zangwill LM, Sample PA, Bowd C, Weinreb RN. The effects of study design and spectrum bias on the evaluation of diagnostic accuracy of confocal scanning laser ophthalmoscopy in glaucoma. Invest Ophthalmol Vis Sci. 2007;48:214–22. doi: 10.1167/iovs.06-0618. [DOI] [PubMed] [Google Scholar]
- 50.Leung CK, Cheng AC, Chong KK, Leung KS, Mohamed S, Lau CS, et al. Optic disc measurements in myopia with optical coherence tomography and confocal scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2007;48:3178–83. doi: 10.1167/iovs.06-1315. [DOI] [PubMed] [Google Scholar]
- 51.Mansour AM. Racial variation of optic disc size. Ophthalmic Res. 1991;23:67–72. doi: 10.1159/000267091. [DOI] [PubMed] [Google Scholar]
- 52.Seider MI, Lee RY, Wang D, Pekmezci M, Porco TC, Lin SC. Optic disk size variability between African, Asian, white, Hispanic, and Filipino Americans using Heidelberg retinal tomography. J Glaucoma. 2009;18:595–600. doi: 10.1097/IJG.0b013e3181996f05. [DOI] [PMC free article] [PubMed] [Google Scholar]


