Abstract
Background:
This study evaluated the visual function after implantation of a multifocal toric intraocular lenses (IOLs).
Materials and Methods:
This study involved 10 eyes from eight cataract patients with corneal astigmatism of 1.0 diopter (D) or higher who had received phacoemulsification with implantation of an AcrySof IQ ReSTOR Toric IOL. Six-month evaluations included visual acuity, spherical equivalent (SE), defocus curve, residual astigmatism, IOL rotation, contrast sensitivity (CS), wavefront aberrations, modulation transfer function (MTF), and patient satisfaction assessments.
Results:
At 6 months postoperatively, uncorrected distance visual acuity (logarithm of the minimum angle of resolution) was 0.09 ± 0.04, corrected distance visual acuity was 0.02 ± 0.11, and uncorrected near visual acuity was 0.12 ± 0.07. The mean SE was −0.095 ± 0.394 D (±0.50 D in 90%). Refractive astigmatism at the 6-month follow-up visit was significantly reduced to 0.35 ± 0.32 D from 1.50 ± 0.41 D presurgery (P < 0.05). The mean IOL axis rotation was 3.20 ± 1.55°. Postoperative CS levels were high. Postoperative total order aberrations (TOAs), lower-order aberrations (LOAs), higher-order aberrations (HOAs), and spherical aberrations were decreased compared with preoperative values (P < 0.05). At 3 months postoperatively, TOAs, LOAs, and HOAs with a 3 mm pupil diameter as well as TOAs, LOAs, and astigmatism aberrations with a 5 mm pupil diameter were statistically lower than those at 1-month post surgery, but without subsequent significant changes (P > 0.05). There was an increase in MTF results between preoperative and postoperative evaluations at all spatial frequencies.
Conclusions:
The diffractive multifocal toric IOL is able to provide a predictable astigmatic correction with apparently outstanding levels of optical quality after implantation.
Keywords: Aberration, astigmatism, cataract, contrast sensitivity, multifocal intraocular lens, toric intraocular lens
Modern cataract surgery has become a refractive surgery with the use of advanced technology intraocular lenses (IOLs). Cataract patients’ desire to postoperative spectacle independence, which can be achieved with implantation of multifocal IOLs, has increased over time. However, a number of such patients also have a preexisting corneal astigmatism. According to a recent study,[1] 20-30% of them have more than 1.25 diopters (D) of corneal astigmatism. Any astigmatism over 1.00 D with multifocal IOLs should be corrected for better results.[2]
Patients with cataract and corneal astigmatism who received traditional multifocal IOLs often required additional corneal refractive procedures, including limbal relaxing incisions,[3] opposite clear corneal incisions,[4] laser refractive surgery,[5] and femtosecond laser-assisted astigmatic keratotomy.[6,7] However, these procedures are associated with some complications, such as lack of precision, wound-healing, and regression problems.[8,9] Although it was considered that toric IOLs could compensate for preexisting corneal astigmatism,[10] they were only able to improve distance visual acuity, with little effect on intermediate and near-distance visual acuity. Recent advances in IOL technology have enabled addition of a toric component to multifocal IOLs, and multifocal toric IOLs are now part of the premium-IOL family. These lenses satisfy the demand for the spectacle independence for distance and near vision while providing outstanding compensation for astigmatism. The AcrySof IQ ReSTOR Multifocal Toric IOL (Alcon Laboratories, Inc., Fort Worth, TX, USA) has become an ideal choice to correct corneal astigmatism during cataract surgery.
The purpose of this study was to assess visual outcomes and patient satisfaction after implanting the aspheric AcrySof IQ ReSTOR Multifocal Toric IOL in patients with corneal astigmatism, and provide referential clinical data for future cataract surgery and new IOL developments.
Materials and Methods
The study protocol was approved by the Medical Ethics Committee of the hospital and adhered to the tenets of the Declaration of Helsinki. All patients were adequately informed and signed consent forms.
This study comprised 10 eyes of eight patients ranging in age from 29 to 87 years, who were recruited within 6 months. Inclusion criteria were patients with visually significant cataract and preexisting regular corneal astigmatism between 1.0 and 2.5 D. Exclusion criteria were irregular corneal astigmatism, corneal disease, macular degeneration or retinopathy, abnormal iris, glaucoma, pseudoexfoliation syndrome, amblyopia, intraocular inflammation, retinal detachment, previous ocular surgery, and a history of eye trauma.
The AcrySof IQ ReSTOR Toric is a single-piece hydrophobic acrylic, diffractive multifocal IOL with a 6.0 mm biconvex optic and an overall diameter of 13.0 mm. It incorporates ultraviolet and blue-light filters. The anterior surface of the IOL bears an aspheric diffractive structure, consisting of nine concentric steps of gradually decreasing heights in the central 3.6 mm optic zone, and providing a near addition of + 3.00 D at the IOL plane. The refractive part of the optic surrounds the diffractive region, directing light to a distant focal point. The posterior surface bears the toric component. The IOL is available in +6.0 to +30.0 D in 0.50 D increments and cylinder correction in the T2-T5 range. The IOL power is calculated using an online calculator with the company-labeled A-constant of 118.9.
Preoperatively, all patients had a full ophthalmologic examination including uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA; international visual testing chart), uncorrected near visual acuity (UNVA) and corrected near visual acuity (CNVA; handheld logarithmic near visual acuity Early Treatment Diabetic Retinopathy Study chart at 40 cm), slit lamp evaluation, fundoscopy, intraocular pressure (IOP; contact Goldman tonometry), laser interference biometry (IOL Master 500, Carl Zeiss Meditec AG, Jena, Germany), endothelial cell density (SP-3000P, Topcon Corp., Tokyo, Japan), corneal topography, aberration measurement (iTrace aberrometer, Tracey Technologies, Houston, TX, USA), and contrast sensitivity (CS) evaluation under photopic (85 cd/m2) conditions (CSV-1000 system, VectorVision, Greenville, OH, USA). The IOL cylinder power and axis placement were calculated using the online AcrySof ReSTOR Toric Calculator. Surgically induced astigmatism (SIA) was assumed to be −0.30 D in all cases. All visual acuities were expressed in logarithm of the minimum angle of resolution (logMAR) notation.
All surgeries were performed by the same surgeon (Zhenping Huang) using a standard technique of sutureless micro coaxial phacoemulsification. Topical anesthesia (proxymetacaine hydrochloride 0.50%) was instilled, and adequate dilation was obtained with intracameral mydriasis in all cases presurgery. Preoperatively, in a supine position, three limbal reference marks were made at 3, 6, and 9 o’clock positions. Intraoperatively, the implantation axis obtained from the IOL calculation was determined according to the corneal reference marks. A 2.2 mm limbal incision at 10 o’clock, and a 1.5 mm side incision at 2 o’clock were made. After a continuous curvilinear capsulorhexis of approximately 5.5 mm and phacoemulsification, the IOL was inserted and rotated into the final position by aligning the corneal axis marks with the reference marks on the IOL. After removing the viscoelastic material, the clear corneal wounds were closed by hydration. Postoperative therapy included a combination of topical antibiotics and steroids.
The postoperative examination protocol at 1, 3, and 6 months was identical to the preoperative protocol, with additional measurement of the axis of internal astigmatism. Rotation of the IOL was also evaluated with the slit lamp to check the positions of the marks locating the flat meridian of the optics and compare them with the targets. In addition, defocus curves were obtained 6 months postoperatively, which were acquired for distance vision by adding positive or negative lenses from +3.00 D to −5.00 D in 0.50 D steps to patients wearing the correction for CDVA. All visual acuities were expressed in logMAR notation.
The root mean square values of total-order aberrations (TOAs), higher-order aberrations (HOAs), lower-order aberrations (LOAs), spherical aberrations (SAs), astigmatism aberrations (AAs), and coma and modulation transfer function (MTF) curves at 5, 10, 15, 20, 25, and 30 cycles per degree (cpd) were calculated with the iTrace aberrometer, which showed good repeatability during the follow-up at pupil diameters of 3.0 mm and 5.0 mm. These pupil sizes were chosen to represent mesopic and scotopic conditions, respectively. The iTrace was a combined ray tracing aberrometer and Placido disk videokeratoscope, which measured the quality of vision using a fundamental thin beam principle of optical ray tracing and sequentially projected 256 near-infrared laser beams into the eye to measure forward aberrations, processing the data point-by-point. All measurements were repeated 3 times.
Evaluations of visual disturbance issues (e.g., glare, halos, starbursts, and diplopia) and visual lifestyle activities (e.g., night time driving, daytime driving, using computer, watching television, cooking, and reading small print) were performed using a questionnaire on a scale of 0-7 (0 = no difficulty, 1-2 = minimal difficulty, 3-5 = moderate difficulty, and 6-7 = severe difficulty) at 6 months postoperatively.[11] All patient-reported outcomes were based on uncorrected vision from patients.
Statistical analysis was performed with SPSS software (version 17.0, SPSS, Inc., Chicago, IL, USA). Normality of all data samples was first evaluated using the Kolmogorov-Smirnov test. A one-way analysis of variance for repeated measures was used to analyze the data from preoperative examinations, postoperative examinations, and between consecutive postoperative visits. If sphericity could not be assumed, Greenhouse-Geisser estimates were used as a correction factor. Post hoc comparisons were performed using the Bonferroni procedure. A paired-samples t-test was used to compare preoperative and postoperative refractive and keratometric outcomes. The results are expressed as mean ± standard deviation. In all instances, P < 0.05 was considered statistically significant.
Results
Ten eyes (six right [60%], four left [40%]) of eight patients were treated between March and August 2013. The mean age of the seven men (70%) and three women (30%) was 57.33 ± 17.49 years (range, 29-87 years). Preoperatively, the mean axial length was 23.98 ± 1.04 mm (range, 22.12-25.29 mm) and the mean anterior chamber depth was 3.34 ± 0.61 mm (range, 2.84-4.35 mm). The mean spherical power of implanted IOLs was 18.80 ± 4.02 D (range, 10.00-23.50 D) and the mean IOL cylindrical power was 2.25 ± 0.61 D (range, 1.50-3.00 D). All 10 eyes were assessed during at least 6 months of follow-up, and the longest follow-up was about 1 year.
Table 1 shows the preoperative and postoperative visual outcomes. These visual changes were consistent with significant postoperative reduction of manifest cylinders in absolute terms (P < 0.01).
Table 1.
Pre- and post-operative visual acuity with multifocal toric intraocular lens (x±s, n=10)
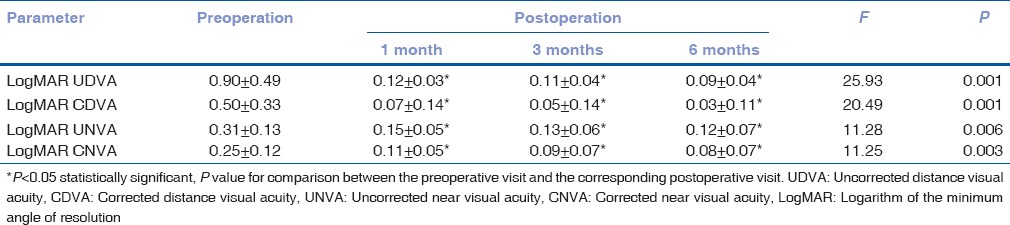
The UDVA was 0.3 logMAR or better in nine eyes (90%) and 0.1 logMAR or better in seven eyes (70%). The UNVA at 40 cm was 0.3 logMAR or better in all eyes (100%) and 0.1 logMAR or better in five eyes (50%). There was a statistically significant improvement in UDVA and CDVA after surgery, without significant changes subsequently (1-6 months). The UNVA (P < 0.01) and CNVA (P < 0.01) showed a remarkable postoperative improvement during the 1st month, but remained constant during the remaining follow-up [Table 1].
The mean defocus curve 6 months after surgery is shown in Fig. 1. Two clear peaks of optimum CDVA at 0.00 D and −2.50 D corresponded to the foci for near and distance vision, respectively. The intermediate-vision results obtained at −1.50 D (67 cm from the eye) was 0.18 ± 0.08 logMAR.
Figure 1.
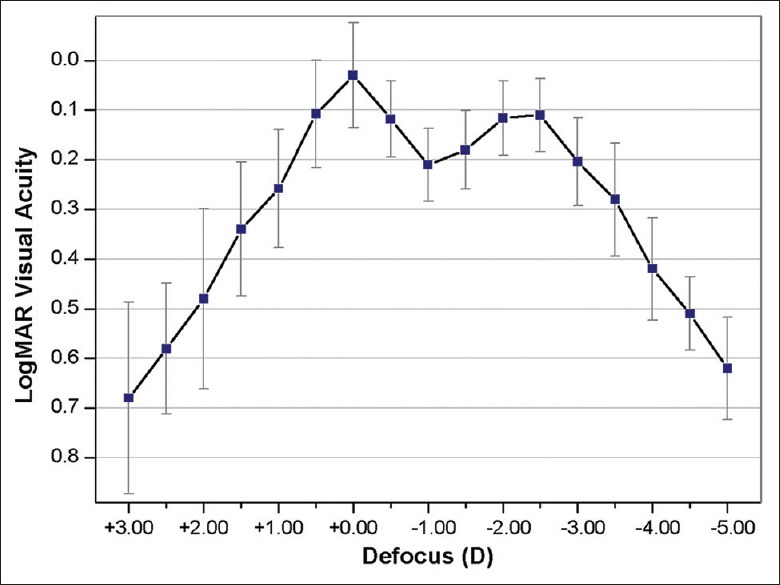
Mean defocus curve at 6-month follow-up visit (n = 10 eyes)
At 6 months, the postoperative spherical equivalent refraction (−0.095 ± 0.394 D) was within ± 0.50 D of the attempted correction in nine eyes (90%) and within ± 1.00 D in 10 eyes (100%), which was significantly reduced from the preoperative visit (−1.138 ± 2.293 D; P = 0.032). Spherical refraction was within ± 0.50 D of the attempted spherical correction in all eyes. The refractive cylinder was within ± 0.50 D in seven eyes (70%) and ± 1.00 D in 10 eyes (100%).
The refractive astigmatism at the 6-month visit (0.35 ± 0.32 D) decreased significantly compared with preoperative values (1.50 ± 0.41 D; P < 0.01) whereas there was no statistical significance with the anticipated residual astigmatism (0.13 ± 0.09 D; P = 0.095). The magnitude of corneal astigmatism was not modified significantly between the preoperative visit (1.62 ± 0.63 D) and the 6-month visit (1.71 ± 0.69 D; P = 0.133).
The mean toric IOL axis rotation was 2.50 ± 1.27°, 3.20 ± 1.55°, and 3.50 ± 1.65° at 1, 3, and 6 months, respectively. Although there was a trend toward a small increase in degree over time, no statistically significant differences were found in IOL rotation (F = 3.390, P = 0.086), and no IOL required secondary repositioning because of excessive rotation during follow-up. All eyes were within ±10° of the intended axis.
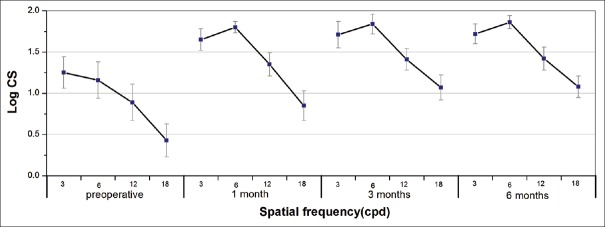
Fig. 2 shows the CS function under monocular photopic testing conditions. Postoperative CS increased significantly compared with preoperative measurements (3, 6, 12, and 18 cpd, all P < 0.05), and no statistically significant changes were found in CS at any spatial frequency during follow-up (P > 0.05), except for the highest spatial frequency (18 cpd), at which the trend toward improvement was statistically significant between 1 and 3 months postsurgery (P = 0.026).
Figure 2.
Mean contrast sensitivity function (in logarithmic scale) under photopic conditions (85 cd/m2) at preoperative and postoperative visits
Table 2 shows the aberrometric outcomes at preoperative and postoperative visits. For a 3.0 mm pupil, there were statistically significant decreases in TOAs, LOAs, HOAs, SAs, and AAs between preoperative and postoperative measurements (P < 0.05) except for coma (P > 0.05). In addition, TOAs, LOAs, and HOAs at the postoperative 3-month visit were statistically lower than those at the postoperative 1-month visit (P = 0.034, P = 0.044, and P = 0.005, respectively), although no statistically significant differences were found in other aberrations during the follow-up (P > 0.05).
Table 2.
Comparison of pre- and post-operative aberrations with 3 mm and 5 mm pupils (x±s, n=10)
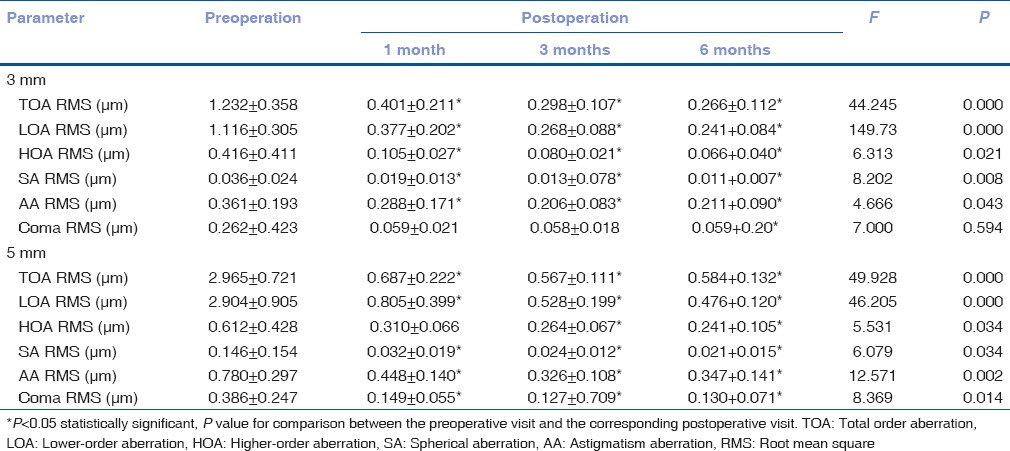
For a 5.0 mm pupil, all the aberrometric values decreased more clearly. Postoperatively, there were no statistically significant changes in HOAs, SAs, and coma (P > 0.05). However, some statistically significant downtrends were found in TOAs, LOAs, and AAs between the postoperative 1-month and 3-month visits (P = 0.048, P = 0.033, and P = 0.046, respectively).
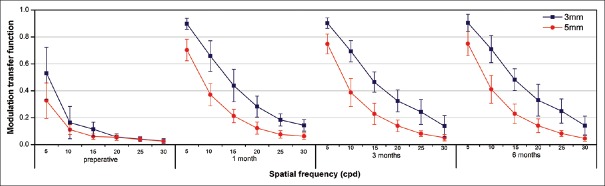
Fig. 3 shows the mean MTF at 5, 10, 15, 20, 25, and 30 cpd. There was an obvious increase in the MTF between preoperation and postoperation values at any spatial frequency with 3.0 mm pupils (5, 10, 15, 20, 25, and 30 cpd; P = 0.000, P = 0.000, P = 0.000, P = 0.000, P = 0.001, and P = 0.002, respectively) and 5.0 mm pupils (P = 0.000, P = 0.000, P = 0.000, P = 0.004, P = 0.001, and P = 0.004, respectively). Moreover, no significantly higher values with 3.0 or 5.0 mm pupils were detected during the postoperative follow-up for all spatial frequencies (P > 0.05).
Figure 3.
The modulation transfer function curve at preoperative and postoperative visits
All patients with the AcrySof IQ ReSTOR diffractive multifocal toric implantation completed the questionnaire. Fig. 4 shows the scores for the postoperative subjective questionnaire. Mild symptoms of glare and halos were found in our study. Only one patient with implantation of a toric multifocal IOL in one eye and a multifocal IOL in the other reported a score of 3 for halos. No cases of perception of diplopia were detected. Minimal difficulties were found for activities such as watching television, daytime driving, using computer and cooking. However, moderate difficulty (a score of 3) in night driving was reported by two patients with implantation of a multifocal IOL in the fellow eye. The majority of patients were satisfied with their distance, intermediate, and near visual lifestyle activities.
Figure 4.
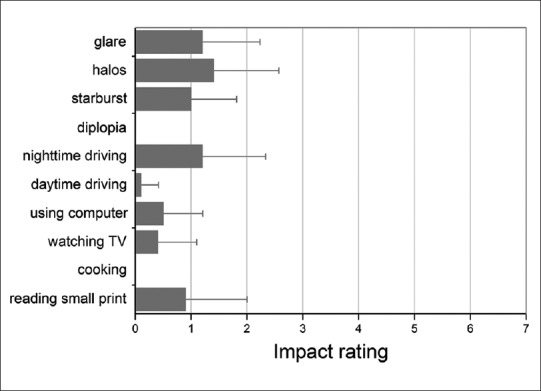
Evaluation scores of visual phenomena questionnaire 6 months postoperatively
All patients (10/10) reported complete spectacle independence for distance vision. One patient (1/10) reported occasional use of spectacles for reading.
No adverse events occurred, and no secondary procedure was required during the follow-up. Transient macular edema occurred in one eye after cataract surgery, which was resolved with corticosteroid eye drops. There was no significant posterior capsule opacification requiring neodymium: yttrium aluminum garnet laser capsulotomy up to the last postoperative visit.
Discussion
In the area of refractive lens exchange, there is a high demand for individualized procedures. Multifocal toric IOLs combining a diffractive anterior surface with a toric posterior surface have been introduced into clinical practice. They can provide a broad range of vision ability and correct preoperative corneal astigmatism within a single surgical procedure. Previous studies on the AT LISA toric 909M IOL (Carl Zeiss Meditec AG, Jena, Germany)[11,12] and the LENTIS Mplus Toric IOL (Oculentis GmbH, Berlin, Germany)[13] have shown the ability of this diffractive toric technology to restore distance and near visual function as well as good postoperative optical and visual quality. To the best of our knowledge, to date, there are few peer-reviewed studies on the diffractive ReSTOR IQ Toric IOL.
In our study, the AcrySof IQ ReSTOR Toric IOL offered excellent visual outcomes. Six months postoperatively, 80% of the eyes had residual refractive astigmatism of 0.50 D or less, indicating the ability of this IOL to correct corneal cylinder variations after cataract extraction in eyes with astigmatism. The significant improvement in UDVA could be related to a large reduction in the refractive cylinder. CDVA was also slightly better than UNVA at most follow-up time points, probably due to the correction of small remaining refractive errors. The postoperative visual acuities showed no difference between the 1-month and 6-month values, suggesting that visual rehabilitation was completed within 1 month after surgery. The finding was consistent with the visual improvement reported by other authors using other modalities of toric IOLs[11,12,13] as well as the same IOL model.[14] The latter showed, postoperatively, 87% of eyes achieved UDVA of 0.1 logMAR or better, ranging from 0.30 to −0.10 logMAR whereas the mean UDVA of our study was 0.10 ± 0.14, and 70% of eyes in our study achieved that goal. Regarding near vision, a significant improvement in UNVA was found as well, with all eyes reaching 0.3 logMAR or better in our study versus 100% in the previous study.
The defocus curve showed two peaks of maximum vision and the emerging depth of field was 4.50 D (range, +1.00 D to −3.50 D), giving patients acceptable intermediate vision. Such a good defocus curve might depend on IOLs with +3.00 D add power and a small residual astigmatism. It is also helpful to explain the few difficulties in performing tasks at intermediate distances on the questionnaires. The results of a previous study by Visser et al.[11] were slightly better than ours because they might have obtained a binocular defocus curve while we obtained a monocular defocus curve with a different toric multifocal IOL.
In our study, all mini-incisions (2.2 mm) were performed to reduce surgical trauma, and SIA was assumed to be 0.30 D in all cases. Residual astigmatism was 0.35 ± 0.32 D, which was decreased obviously compared with preoperative values. The results indicated that toric multifocal IOLs are effective in correcting astigmatism.
The achievement of a perfect astigmatic correction with this IOL also requires long-term rotational stability in the capsular bag. AcrySof Toric IOLs have shown excellent rotational stability with a mean postoperative IOL misalignment of 3.67 ± 2.29° at 3 months postsurgery.[15] In this study, the low level of rotation (3.50 ± 1.65°) was incomparable with the data of the same multifocal toric IOLs (2.97 ± 2.33°)[14] and AcrySof Toric IOLs,[15] indicating that a diffractive optic part seemed not to have an impact on the postoperative rotational stability of the IOLs.
CS was measured in this study to evaluate the optical performance of this toric multifocal IOLs. The level of CS is closely related to the efficiency of the diffractive design of the IOL and also depends on the potential visual acuity. Although multifocal IOLs have been reported to cause up to a 50% reduction in CS,[16] our CS values were comparable to the normal range for older adults that Pomerance and Evans[17] obtained with the CSV-1000. There was no significant trend toward improvement in CS during the postoperative follow-up, except for the highest spatial frequency evaluated (18 cpd). The changes in 18 cpd may be related to misalignment of the astigmatic correction and the small improvement in CDVA between the first and sixth postoperative months.
MTF describes the fidelity with which contrast is transferred from the object to the image through the optical system.[18] It is more objective and rarely affected by retinal nerve pathways compared with CS so that it can provide quality of vision information more accurately.[19] In our study, no differences in values of MTF were found during the postoperative follow-up for all spatial frequencies. However, the MTF of 5.0 mm pupils was lower compared with that of 3.0 mm pupils because of the distribution of light energy between the distance and near focal points and light scatter.
With regard to the analysis of aberrations, significant improvements in the values of TOAs, HOAs and SAs were related to the aspherical surface of the IOLs, which would compensate for the corneal aberrations.[20,21] The additional toric rear surface of the IOLs can effectively reduce LOAs and AAs, thus reducing the TOAs. Moreover, no differences in coma aberrations were found during the postoperative follow-up, indicating the centrality of the IOL in the capsular bag. It is known that better optical performance with an aspheric IOL exists when the IOL is centered within ±0.4 mm and tilted fewer than 7°.[22] A long-term follow-up would be appropriate to analyze the biological behavior because an asymmetric contraction of a capsule fibrosis could develop and decenter the IOL.[19]
Good stability of the IOLs and stabilization of corneal optics after surgery would account for the early change in postoperative visual outcomes. The HOAs, coma, and SAs of our study were in concordance with the data for the +3.00 D ReSTOR IOL published by de Vries et al.,[23] indicating that the added toricity of the AcrySof IQ ReSTOR Toric IOL did not worsen the visual acuity outcomes over those with the nontoric version of the same multifocal IOLs.
Minimal difficulties in patients’ subjective experiences with photic phenomena and visual tasks at all distances were found in our study. However, a score of 3 for halos and night driving was found in individual patients because of the diffractive multifocal component of the IOL. Furthermore, the study evaluated eight patients, and only two had bilateral implantation of multifocal toric IOLs. Among the other six, four had the nontoric model of multifocal IOLs in the fellow eye, one had a toric IOL, and one had not undergone a cataract operation in the fellow eye, as the crystalline lens did not meet the operation timing. The situation of the fellow eye may also have an influence on visual disturbance to some extent. All patients achieved spectacle independence for distance and near vision except for one with a toric IOL, who still needed spectacles for near vision. In any case, questionnaire results were limited because of the variability of the fellow eyes. Future studies on patients’ subjective experiences with bilateral implantation of multifocal toric IOLs should be performed.
Conclusion
The outstanding predictability, stability, and optical quality suggest that the AcrySof IQ ReSTOR Toric IOL is a good option to compensate for both presbyopia and astigmatism. Although the results are limited by a small sample size (10 eyes), a short-term follow-up as well as the lack of a comparison group of patients with other IOLs, randomized comparative studies with a larger cohort, and a longer follow-up are needed to address these limitations in the further work.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hoffmann PC, Hütz WW. Analysis of biometry and prevalence data for corneal astigmatism in 23,239 eyes. J Cataract Refract Surg. 2010;36:1479–85. doi: 10.1016/j.jcrs.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 2.de Vries NE, Webers CA, Touwslager WR, Bauer NJ, de Brabander J, Berendschot TT, et al. Dissatisfaction after implantation of multifocal intraocular lenses. J Cataract Refract Surg. 2011;37:859–65. doi: 10.1016/j.jcrs.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 3.Ouchi M, Kinoshita S. AcrySof IQ toric IOL implantation combined with limbal relaxing incision during cataract surgery for eyes with astigmatism >2.50 D. J Refract Surg. 2011;27:643–7. doi: 10.3928/1081597X-20110317-03. [DOI] [PubMed] [Google Scholar]
- 4.Qammar A, Mullaney P. Paired opposite clear corneal incisions to correct preexisting astigmatism in cataract patients. J Cataract Refract Surg. 2005;31:1167–70. doi: 10.1016/j.jcrs.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 5.Norouzi H, Rahmati-Kamel M. Laser in situ keratomileusis for correction of induced astigmatism from cataract surgery. J Refract Surg. 2003;19:416–24. doi: 10.3928/1081-597X-20030701-07. [DOI] [PubMed] [Google Scholar]
- 6.Kim P, Sutton GL, Rootman DS. Applications of the femtosecond laser in corneal refractive surgery. Curr Opin Ophthalmol. 2011;22:238–44. doi: 10.1097/ICU.0b013e3283477c9c. [DOI] [PubMed] [Google Scholar]
- 7.Rückl T, Dexl AK, Bachernegg A, Reischl V, Riha W, Ruckhofer J, et al. Femtosecond laser-assisted intrastromal arcuate keratotomy to reduce corneal astigmatism. J Cataract Refract Surg. 2013;39:528–38. doi: 10.1016/j.jcrs.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 8.Mendicute J, Irigoyen C, Ruiz M, Illarramendi I, Ferrer-Blasco T, Montés-Micó R. Toric intraocular lens versus opposite clear corneal incisions to correct astigmatism in eyes having cataract surgery. J Cataract Refract Surg. 2009;35:451–8. doi: 10.1016/j.jcrs.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 9.Schallhorn SC, Venter JA. One-month outcomes of wavefront-guided LASIK for low to moderate myopia with the VISX STAR S4 laser in 32,569 eyes. J Refract Surg. 2009;25(7 Suppl):S634–41. doi: 10.3928/1081597X-20090611-02. [DOI] [PubMed] [Google Scholar]
- 10.Holland E, Lane S, Horn JD, Ernest P, Arleo R, Miller KM. The AcrySof Toric intraocular lens in subjects with cataracts and corneal astigmatism: A randomized, subject-masked, parallel-group, 1-year study. Ophthalmology. 2010;117:2104–11. doi: 10.1016/j.ophtha.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 11.Visser N, Nuijts RM, de Vries NE, Bauer NJ. Visual outcomes and patient satisfaction after cataract surgery with toric multifocal intraocular lens implantation. J Cataract Refract Surg. 2011;37:2034–42. doi: 10.1016/j.jcrs.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 12.Bellucci R, Bauer NJ, Daya SM, Visser N, Santin G, Cargnoni M, et al. Visual acuity and refraction with a diffractive multifocal toric intraocular lens. J Cataract Refract Surg. 2013;39:1507–18. doi: 10.1016/j.jcrs.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Venter J, Pelouskova M. Outcomes and complications of a multifocal toric intraocular lens with a surface-embedded near section. J Cataract Refract Surg. 2013;39:859–66. doi: 10.1016/j.jcrs.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira TB, Marques EF, Rodrigues A, Montés-Micó R. Visual and optical outcomes of a diffractive multifocal toric intraocular lens. J Cataract Refract Surg. 2013;39:1029–35. doi: 10.1016/j.jcrs.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 15.Scialdone A, De Gaetano F, Monaco G. Visual performance of 2 aspheric toric intraocular lenses: Comparative study. J Cataract Refract Surg. 2013;39:906–14. doi: 10.1016/j.jcrs.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 16.Pieh S, Weghaupt H, Skorpik C. Contrast sensitivity and glare disability with diffractive and refractive multifocal intraocular lenses. J Cataract Refract Surg. 1998;24:659–62. doi: 10.1016/s0886-3350(98)80261-7. [DOI] [PubMed] [Google Scholar]
- 17.Pomerance GN, Evans DW. Test-retest reliability of the CSV-1000 contrast test and its relationship to glaucoma therapy. Invest Ophthalmol Vis Sci. 1994;35:3357–61. [PubMed] [Google Scholar]
- 18.Mencucci R, Giordano C, Favuzza E, Gicquel JJ, Spadea L, Menchini U. Astigmatism correction with toric intraocular lenses: Wavefront aberrometry and quality of life. Br J Ophthalmol. 2013;97:578–82. doi: 10.1136/bjophthalmol-2013-303094. [DOI] [PubMed] [Google Scholar]
- 19.Peng C, Zhao J, Ma L, Qu B, Sun Q, Zhang J. Optical performance after bilateral implantation of apodized aspheric diffractive multifocal intraocular lenses with +3.00-D addition power. Acta Ophthalmol. 2012;90:e586–93. doi: 10.1111/j.1755-3768.2012.02497.x. [DOI] [PubMed] [Google Scholar]
- 20.Rocha KM, Soriano ES, Chamon W, Chalita MR, Nosé W. Spherical aberration and depth of focus in eyes implanted with aspheric and spherical intraocular lenses: A prospective randomized study. Ophthalmology. 2007;114:2050–4. doi: 10.1016/j.ophtha.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Schuster AK, Tesarz J, Vossmerbaeumer U. The impact on vision of aspheric to spherical monofocal intraocular lenses in cataract surgery: A systematic review with meta-analysis. Ophthalmology. 2013;120:2166–75. doi: 10.1016/j.ophtha.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Holladay JT, Piers PA, Koranyi G, van der Mooren M, Norrby NE. A new intraocular lens design to reduce spherical aberration of pseudophakic eyes. J Refract Surg. 2002;18:683–91. doi: 10.3928/1081-597X-20021101-04. [DOI] [PubMed] [Google Scholar]
- 23.de Vries NE, Webers CA, Montés-Micó R, Ferrer-Blasco T, Nuijts RM. Visual outcomes after cataract surgery with implantation of a +3.00 D or +4.00 D aspheric diffractive multifocal intraocular lens: Comparative study. J Cataract Refract Surg. 2010;36:1316–22. doi: 10.1016/j.jcrs.2010.01.036. [DOI] [PubMed] [Google Scholar]