Abstract
Context:
Analysis of diagnostic ability of macular ganglionic cell complex and retinal nerve fiber layer (RNFL) in glaucoma.
Aim:
To correlate functional and structural parameters and comparing predictive value of each of the structural parameters using Fourier-domain (FD) optical coherence tomography (OCT) among primary open angle glaucoma (POAG) and ocular hypertension (OHT) versus normal population.
Setting and Design:
Single centric, cross-sectional study done in 234 eyes.
Materials and Methods:
Patients were enrolled in three groups: POAG, ocular hypertensive and normal (40 patients in each group). After comprehensive ophthalmological examination, patients underwent standard automated perimetry and FD-OCT scan in optic nerve head and ganglion cell mode. The relationship was assessed by correlating ganglion cell complex (GCC) parameters with mean deviation. Results were compared with RNFL parameters.
Statistical Analysis:
Data were analyzed with SPSS, analysis of variance, t-test, Pearson's coefficient, and receiver operating curve.
Results:
All parameters showed strong correlation with visual field (P < 0.001). Inferior GCC had highest area under curve (AUC) for detecting glaucoma (0.827) in POAG from normal population. However, the difference was not statistically significant (P > 0.5) when compared with other parameters. None of the parameters showed significant diagnostic capability to detect OHT from normal population. In diagnosing early glaucoma from OHT and normal population, only inferior GCC had statistically significant AUC value (0.715).
Conclusion:
In this study, GCC and RNFL parameters showed equal predictive capability in perimetric versus normal group. In early stage, inferior GCC was the best parameter. In OHT population, single day cross-sectional imaging was not valuable.
Keywords: Early diagnosis of glaucoma, Fourier-domain-optical coherence tomography, ganglion cell complex, retinal nerve fiber layer
Glaucoma is a multifactorial optic neuropathy characterized by a loss of retinal ganglion cells (RGCs) with subsequent loss of nerve fibers resulting in functional visual impairment.[1]
Globally, it is the second most common cause of blindness after cataract. It has been estimated that approximately 60.5 million patients will be affected by glaucoma alone in 2010 and it will be increased to 79.6 million by 2020. Asians will have 47% of disease worldwide.[2] In India, glaucoma accounts for 12% of blindness and 11.4% of low vision.[3] The prevention and treatment of glaucoma is complicated by the lack of early warnings for impending vision loss and uncertainties in the diagnosis. Primary open angle glaucoma (POAG) is asymptomatic in its early stages.
Structural changes precede the development of optic nerve head cupping and visual field loss.[4,5,6,7,8,9] Changes in nerve fiber layer thickness of 20 μ may be significant interval changes in glaucoma.[10] Optical coherence tomography (OCT) provides objective, quantitative, and reproducible measurements of the ganglion cell complex (GCC) and retinal nerve fiber layer (RNFL) thickness.[11] Fourier-domain (FD)-OCT has resolution power up to 5 μ in measuring the average RNFL thickness, offering a marked advantage in the early detection of glaucoma and in the objective assessment of progression of glaucomatous damage.[12] Although RNFL correlation with visual field has been well documented, but predictability of macular GCC and its correlation with retinal sensitivity is still unexplored.
There were not enough Indian studies published in the literature evaluating the macular thickness parameters and comparative study with RNFL parameters in glaucoma versus ocular hypertension (OHT) or normal population.
Materials and Methods
Participants were consecutively enrolled from the glaucoma clinic of Tertiary Care Eye Hospital from August 2011 to May 2012. The study was approved by our Institutional Review Board and Ethical Committee. Patients were selected from glaucoma clinic of the institute where they were diagnosed as POAG or OHT and were on treatment or observation. Age- and sex-matched control (normal) population were selected randomly from outpatient department. During the study, all patients in three groups were enrolled and screened on single day cross-sectional basis after written consent from the patient. The authors declare no financial or proprietary interests.
Primary open angle glaucoma
Inclusion criteria
These include glaucomatous optic nerve head changes (diffuse or localized rim thinning and disc hemorrhage, notch, bayonetting, baring or vertical cup-to-disc ratio >0.3 or difference in cup disc ratio of more than 0.2 in the two eyes, in the absence of significant difference in disc size), presence of glaucomatous visual field defects that corresponded with the RNFL defects, optic nerve head abnormalities and gonioscopically open angles, refractive error +4D to −6D.
Exclusion criteria
These include any posterior segment pathology, history of accelerated hypertension, coronary artery disease, diabetes and any past cerebrovascular accident, best-corrected visual acuity (BCVA) equal or worse than 6/60, presence of significant cataract.
Ocular hypertension
Inclusion criteria
These include open angle, IOP >21 mmHg in applanation (corrected for central corneal thickness), normal optic nerve head, absence of visual field defect, refractive error +4D to −6D.
Exclusion criteria
These include BCVA <6/6, macular pathology, diabetes, uncontrolled hypertension, refractive error +4D to −6D.
Normal population
Inclusion criteria
These include intraocular pressure of <21 mmHg, a normal appearing optic disc head, no RNFL defect in red free, normal SAP result.
Exclusion criteria
These include BCVA <6/6, chronic ocular disease, systemic diseases that might have affected the eyes, systemic corticosteroid use.
Clinical assessment
Review of medical history, BCVA with any addition on refractive error at presentation, IOP by applanation tonometry, slit-lamp biomicroscopy for anterior segment examination including type of lenticular changes, gonioscopy, direct ophthalmoscopy/disc examination with 90D, central corneal thickness, visual field by static perimetry Humphrey VF 24-2 (Carl zeiss Meditec Inc., Dublin, California, USA), OCT for RNFL, and macular ganglion cell thickness [GCC]). For each patient, all examinations were performed on a single day (cross-sectional study).
Visual field testing: All subjects underwent SITA standard 24-2 perimetry (Carl Zeiss Meditec Inc., Dublin, CA, USA). A reliable visual field test was defined as one with fewer than 20% fixation losses, false positive, or false negatives. A field defect was defined by Anderson criteria as having three or more significant (P < 0.05) noncontiguous points with at least one at the P < 0.01 level on the same side of the horizontal meridian in the pattern standard deviation (SD) plot, classified as outside normal limit in the glaucoma hemifield test and confirmed in two consecutive tests. The patients were classified into three subgroups: Early, moderate, and severe. Early glaucoma was defined by visual field loss with mean deviation (MD) <6 dB, moderate glaucoma MD 6-12 dB, and severe glaucoma >12 dB.
Optical coherence tomography procedure
All subjects were scanned using the RTVue® system Version 6.3 (Optovue, Inc., Fremont, CA, USA). It takes 26,000 A-scans/s with a frame rate of 256-1024 A-scans per frame. It has a depth resolution of 5 μm and a transverse resolution of 10 μm. Scan beam wavelength is 840 ± 10 nm with exposure power at pupil: 750 μW.
The GCC scan covers 7 mm square area centered 0.75 mm temporal to the fovea. It takes 14,928 A-scans in 0.6 s. This scan takes images at 0.5 mm intervals.[13]
RNFL analysis was done in optic nerve head mode. It consists of 12 radial lines and six concentric rings centered on optic disc. Prototype patient information is given in Fig. 1.
Figure 1.
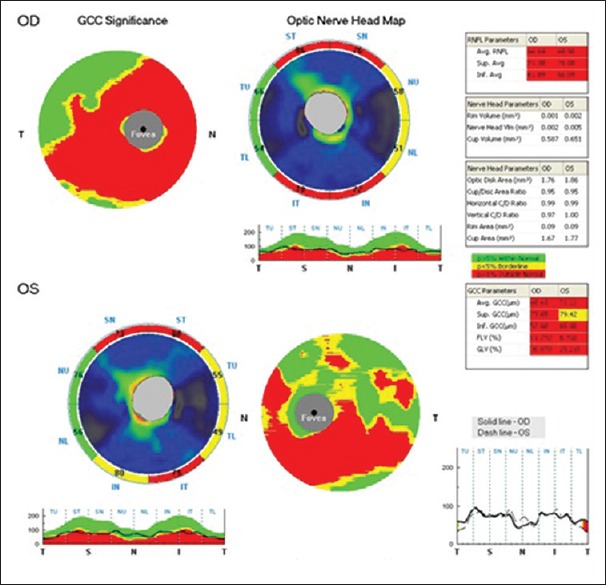
Optical coherence tomography pictures of prototype patient. Optical coherence tomography pictures showing normative values of five parameters of ganglion cell complex and three parameters of retinal nerve fiber layer. This picture shows thinning of superior and inferior retinal nerve fiber layer and corresponding thinning of ganglion cell complex
Statistical analysis
The SPSS program IBM SPSS Statistics (Version 19.0. Armonk, NY) was used for statistical analysis (MedCalc software version 12.2.10, Ostend, Belgium). An analysis of variance (ANOVA) test was used to compare the measured parameter values between the patient groups. Sensitivity and specificity for OCT parameters were determined. P = 0.05 was considered statistically significant. One-way ANOVA and post hoc Tukey honest significant difference test were applied to look for RNFL thickness, and macular thickness measurement differences between glaucomatous, OHT, and healthy eyes. The relationships between mean RNFL/GCC thickness and MD were evaluated with regression analyses. Pearson's correlation coefficients were used to assess the correlations between continuous variables. Receiver operating characteristic (ROC) curves were used to describe the ability to differentiate glaucomatous and OHT from healthy eyes of each of the FD-OCT.
Receiver operating characteristic curve
It is a plot of the true positive rate against the false positive rate for the different possible cut points of a diagnostic test.
This yields diagnostic capability of test under investigation. Area under curve (AUC) nearer to 1 better is the diagnosing capability. AUC <0.6 is usually indicative of poor discriminative power of the test. AUC <0.5 represents discrimination that is no better than results obtained by chance. Differences in the diagnostic ability (AUC) of RNFL and GCC were tested for statistical significance.
Results
In this study, 40 patients were included in each group (total of 234 eyes: 78 normal eyes and 78 open angle glaucoma patients and 78 OHT eyes). Mean age in different group was 56.50 ± 11.69 years in POAG, 52.05 ± 9.31 years in OHT, and 52.7 ± 10.31 years in normal. No statistically significant difference was found in age distribution, sex, eye distribution, and pachymetry distribution between the groups (P > 0.05).
Classification of glaucoma was based on MD: Among POAG, 38 eyes (48.71%) had early glaucoma, 16 eyes (20.51%) had moderate glaucoma, and 26 eyes (33.33%) had advanced glaucoma.
Different parameters of a prototype patient are given in Fig. 1. GCC and RNFL analysis in all patients in different subgroups is given in Table 1.
Table 1.
Ganglion cell complex and retinal nerve fiber layer analysis in different sub-groups
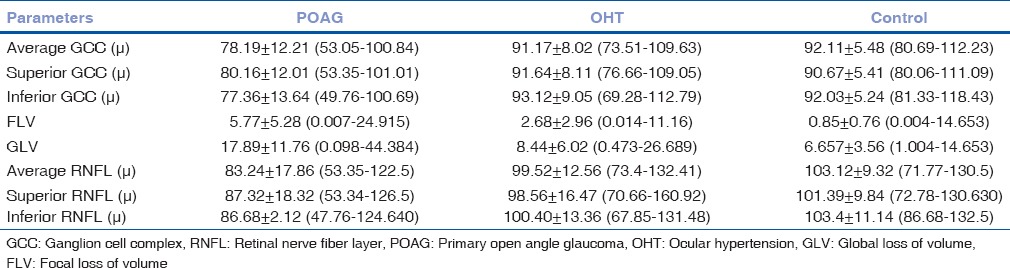
Parameter analysis
As expected, average GCC values are higher in normal and OHT than POAG (92.11 ± 5.48, 91.17 ± 8.02, and 78.19 ± 12.21 μ, respectively). Mean RNFL values are also similar (103.12 ± 9.32, 99.52 ± 12.56, and 83.24 ± 17.86 μ, respectively). Differences in RNFL and GCC parameters between normal and glaucomatous eyes, OHT, and POAG were highly significant (P = 0.00, P < 0.001). Difference of values was not significant between OHT and control (P > 0.05).
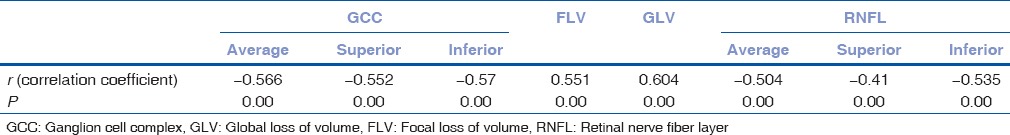
Correlation with visual field sensitivity
The correlation values of different parameters with visual field sensitivity are described in Table 2. There is a strong negative correlation between GCC average, GCC sup, and GCC inferior, RNFL average, RNFL sup, and RNFL inferior with MD except focal loss of volume (FLV) and global loss of volume (GLV) are positively correlated and they are statistically significant (P = 0.00, P < 0.001) [Table 2]. All the values decreased as the disease progresses but FLV and GLV increased as it indicated disease burden.
Table 2.
Correlation with mean deviation and ganglion cell complex and retinal nerve fiber layer parameters
Diagnostic value of ganglion cell complex and retinal nerve fiber layer thickness among different groups
For calculating diagnostic value, receiver operating curves were analyzed. Patients were divided into five groups for ROC calculation: POAG/normal, POAG/OHT, OHT/normal, early POAG/normal, and early POAG/OHT. Results are given in Table 3.
Table 3.
Receiver operating curve
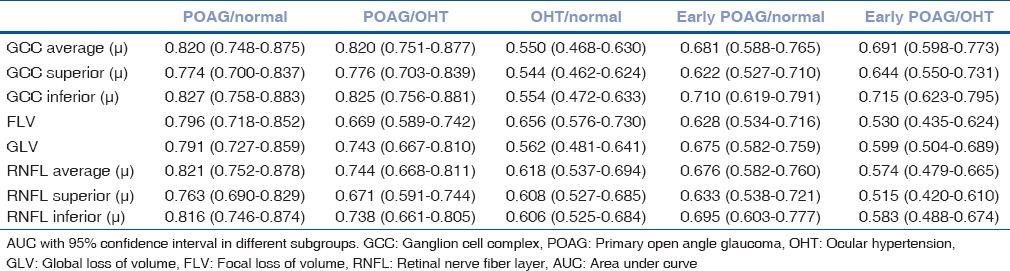
The diagnostic values of different GCC parameters (average, superior, inferior, FLV, and GLV) and RNFL parameters (average, superior, and inferior) were compared with ROC curves [Table 4]. None of the GCC parameters was found having statistically more AUC than RNFL parameters. Among all eight parameters, inferior GCC thickness was the best indicator to discriminate diseased population between glaucoma and normal eyes (AUC: 0.827) [Fig. 2]. GCC inferior and average had statistically better predictability than GCC superior (P = 0.0329 and P = 0.0351, respectively). Diagnostic value of FLV and GLV did not show much difference (P = 0.3787 and P = 0.2241, respectively). RNFL average had significantly better predictability than RNFL superior (P = 0.0277). Inferior GCC had better diagnostic value than that of inferior RNFL but it was not significant (P = 0.6566).
Table 4.
Sensitivity and specificity with inferior ganglion cell complex
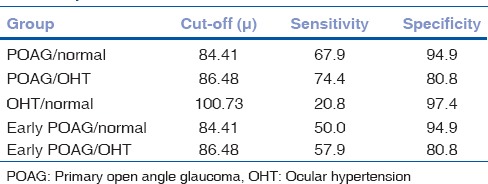
Figure 2.
Receiver operating curve in primary open angle glaucoma/control. At cut-off point of 84.41 μ, sensitivity of inferior ganglion cell complex is 67.9% sensitive and 94.9% specific in discriminating glaucoma from normal population
The RNFL and GCC parameters were similar in ability (P > 0.05) to diagnose diseased population in glaucoma versus OHT group with inferior GCC having the highest diagnostic value (0.825) [Fig. 3].
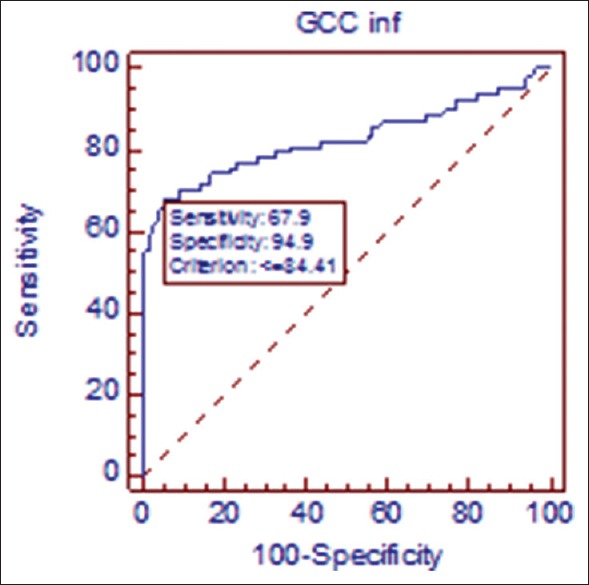
Figure 3.
Receiver operating curve in primary open angle glaucoma/ocular hypertension. At cutoff point of 86.48 μ, sensitivity of inferior ganglion cell complex is 74.4% sensitive and 80.8% specific in discriminating glaucoma from ocular hypertensive population
None of the parameters were significant in diagnosing OHT from normal. AUC values of all parameters in this group were on the lower side (0.5-0.6). For example, ROC of inferior GCC is given in Fig. 4. In early glaucoma/normal and early glaucoma/OHT group, the only parameter which was statistically significant was inferior GCC (AUC: 0.710 and 0.715, respectively) [Figs. 5 and 6]. All the other parameters in this study failed to detect differences between early POAG versus normal or OHT.
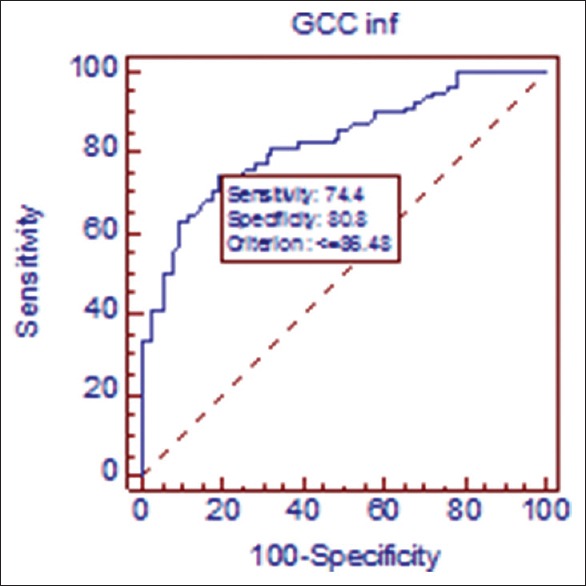
Figure 4.
Receiver operating curve in ocular hypertension/normal. At cutoff point of 100.73 μ, sensitivity of inferior ganglion cell complex is 20.8% sensitive and 97.4% specific in discriminating ocular hypertensive from normal population
Figure 5.
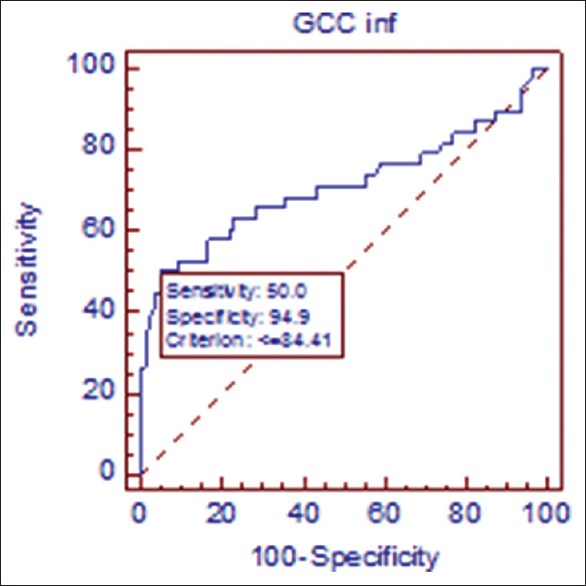
Receiver operating curve in early primary open angle glaucoma/normal. At cutoff point of 84.41 μ, sensitivity of inferior ganglion cell complex is 50.0% sensitive and 94.9% specific in discriminating early glaucoma from normal population
Figure 6.
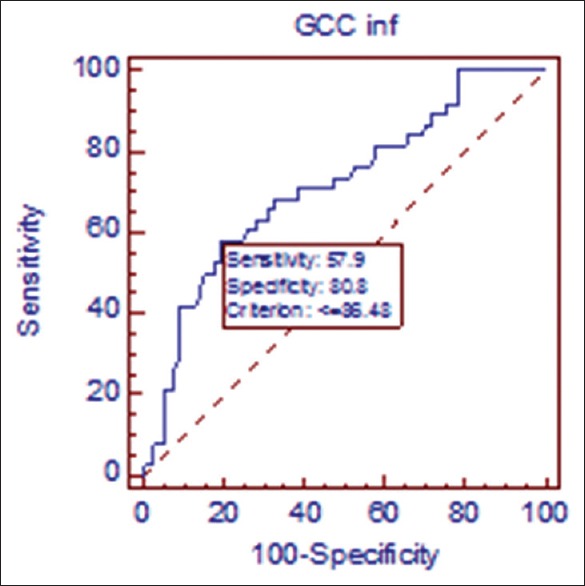
Receiver operating curve in early primary open angle glaucoma/ocular hypertension. At cutoff point of 86.48 μ, sensitivity of inferior ganglion cell complex is 57.9% sensitive and 80.8% specific in discriminating early glaucoma from ocular hypertensive population
We have calculated sensitivity and specificity of all parameters and their cutoff. Table 4 and Figs. 2–5 show values of inferior GCC only. While the cutoff specificities were high (80-95%), sensitivities were quite variable (20-75%).
Discussion
RGCs are selectively lost early in glaucoma. Imaging macular ganglionic cell is of special importance as approximately 50% of RGCs are located in the macular region. Exact location is 4-5 mm from the center of the fovea.[14] The density reaches its peak at 750-1100 μm from the foveal center. The cell density may be 4-6 cell bodies thick.[15] It is the site for initial changes of glaucomatous damage.[16] Zeimer et al. hypothesized quantitative detection of glaucomatous damage at the posterior pole using retinal thickness mapping may provide a unique method for the early detection and monitoring of early glaucomatous tissue loss.[17]
In the present study, macular and RNFL thickness showed similar diagnostic value to detect glaucoma in different subgroups. The GCC parameters readily diagnosed glaucomatous patients in ocular hypertensive and normal population. In subgroup analysis also, GCC parameter (inferior GCC) was a better analytic tool to diagnose of early glaucoma from ocular hypertensive and normal population. Our study also revealed strong structure-function correlation of macular and RNFL parameters with visual field sensitivity.
We conducted the study with three groups: POAG, OHT, and normal. In each group, 40 patients (78 eyes – 2 one-eyed in each group) were included. All three groups were age and sex matched.
Normative value analysis
All OCT parameters were significantly different (P < 0.001) in POAG versus normal and OHT group. This findings correlated with previous various studies.[13,14,15,16,17,18,19,20,21]
No statistical difference was found between OHT and control. The reason could be OHT patients were not classified into preperimetric and perimetric group by doing another preperimetric evaluation such as frequency-doubling perimetry (FDT). Smaller sample size could also be a cause. This result though matched with previous study of Schulze et al.,[22] the observation revealed glaucoma patients showed a significant reduction in GCC and macular retinal thickness compared to patients with OHT and normal subjects. No differences in GCC were found between the patients with OHT and normal subjects.
Visual field correlation
All RNFL and GCC parameters are strong correlated. In our study as the disease progresses, as expected, retinal sensitivity decreases with thinning of GCC and RNFL parameters, FLV and GLV increased. This correlated with previous studies.[23,24]
Diagnostics values in different group
The OCT RTVue directly measures GCC thickness which is the initial target of glaucoma. Few study states that macular GCC parameters are comparable with circumpapillary RNFL measurements using FD-OCT.[13,18,19] In the present study, we observed similar AUC values of GCC and RNFL thickness for glaucoma detection in different subgroups.
Inferior GCC thickness appeared to be a better discriminative marker for early glaucoma compared with RNFL thickness, although the AUC difference was not significant. This finding can have two explanations. First, GCC is a direct measure of RGC integrity. As cell body (RGC) loss can be observed earlier than axonal loss, theoretically, macular GCC parameters may prove to be an early indicator than RNFL parameters. Second, as macular GCC scan is done with 7-mm × 7-mm grid centered on the central macula, early glaucomatous damage that starts in the paracentral region (10°-20°) can easily be detected with this technique.[13] However, a longitudinal study with this cohort can be conducted to find out the reproducibility and predictability of this parameter. We have not analyzed the data in moderate to severe glaucoma because of less number of patients. GCC thickness measurement is less reliable in severe disease as only 50% of the RGCs are present in the macula.[14] In contrast, 100% of the axons of RGCs are assessed in a peripapillary OCT RNFL scan. Hence, measurement of measurement of RNFL loss can be more accurate at this stage.
Macular GCC definitely plays an important role in patients with peripapillary atrophy such as high myopes, where RNFL analysis may yield fallacious results. We have excluded high myopes from the study. However, it has been proven even in high myopes the ability to diagnose glaucoma with macular GCC thickness was comparable with that of peripapillary RNFL thickness by Kim et al.[18] Observation of Lee et al. has shown the macular thickness is positively correlated with the peripapillary RNFL thickness in healthy Chinese children. They have also proved macular thickness is independent of the axial length and refractive status of the nonglaucomatous healthy child.[25]
We had lesser AUC values in early POAG/normal, early POAG/OHT group than previous studies, highest AUC was that of inferior GCC (0.710 and 0.715, respectively). In a study by Kim et al., AUC values were 0.907, 0.847, and 0.893 for average, superior, and inferior GCC, respectively, in early glaucoma/normal group.[13] This difference can be explained due difference in sample size. They observed that macular GCC thickness and RNFL thickness showed similar diagnostic performance for detecting early, moderate, and severe glaucoma. We had less number of patients in moderate and severe glaucoma. Hence, same study in larger population may yield higher AUC in all stages of glaucoma.
Sensitivity and specificity of Inferior GCC in different groups are demonstrated in Table 4. The table shows in this cutoff though specificities were high (80-95%), sensitivities were low (20-75%). This also resembles study outcomes of Rolle et al., where they concluded that AUCs did not significantly differ in macular and peripapillary RNFL values. Specificities were high at both the fifth and first percentiles (up to 97%), but sensitivities were low, especially at the first percentile (55-27%).[19]
There are several limitations in our study. Larger sample size will help to differentiate the better predictive value of the each parameter. Longitudinal study with OHT patients for further classification with pre-perimetric tests (e.g., frequency doubling perimetry), followed by OCT correlation can better predict likelihood of the disease. Normal intraobserver variation of RNFL is well known. More than 2 SD, that is, more than 20 μ change is suggestive of RNFL progression. Intra and inter-observer variation of GCC is not known. Repeated OCT sampling in the same patient for GCC may determine normal variation beyond which can be termed as GCC progression.
Conclusion
In our study, GCC parameters had statistically equal predictive value as that of the RNFL in detecting glaucoma from normal population. We found only inferior GCC had the best discrimination power to detect early glaucoma from ocular hypertensive and normal population. There was no statistically significant difference in OCT parameter between OHT and normal population. GCC and RNFL can be complimentary to each other for diagnosis of glaucoma. In special situation where RNFL determination is tricky, GCC analysis may aid to the diagnosis. GCC and RNFL showed strong correlation with its functional component (visual field sensitivity).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dielemans I, Vingerling JR, Wolfs RC, Hofman A, Grobbee DE, de Jong PT. The prevalence of primary open-angle glaucoma in a population-based study in The Netherlands. The Rotterdam study. Ophthalmology. 1994;101:1851–5. doi: 10.1016/s0161-6420(94)31090-6. [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramakrishnan R, Nirmalan PK, Krishnadas R, Thulasiraj RD, Tielsch JM, Katz J, et al. Glaucoma in a rural population of Southern India: The Aravind comprehensive eye survey. Ophthalmology. 2003;110:1484–90. doi: 10.1016/S0161-6420(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 4.Cvenkel B, Kontestabile AS. Correlation between nerve fibre layer thickness measured with spectral domain OCT and visual field in patients with different stages of glaucoma. Graefes Arch Clin Exp Ophthalmol. 2011;249:575–84. doi: 10.1007/s00417-010-1538-z. [DOI] [PubMed] [Google Scholar]
- 5.Quigley HA, Miller NR, George T. Clinical evaluation of nerve fiber layer atrophy as an indicator of glaucomatous optic nerve damage. Arch Ophthalmol. 1980;98:1564–71. doi: 10.1001/archopht.1980.01020040416003. [DOI] [PubMed] [Google Scholar]
- 6.Sommer A, Katz J, Quigley HA, Miller NR, Robin AL, Richter RC, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 7.Quigley HA, Katz J, Derick RJ, Gilbert D, Sommer A. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology. 1992;99:19–28. doi: 10.1016/s0161-6420(92)32018-4. [DOI] [PubMed] [Google Scholar]
- 8.Wollstein G, Schuman JS, Price LL, Aydin A, Stark PC, Hertzmark E, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123:464–70. doi: 10.1001/archopht.123.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Airaksinen PJ, Drance SM, Douglas GR, Mawson DK, Nieminen H. Diffuse and localized nerve fiber loss in glaucoma. Am J Ophthalmol. 1984;98:566–71. doi: 10.1016/0002-9394(84)90242-3. [DOI] [PubMed] [Google Scholar]
- 10.Harwerth RS, Carter-Dawson L, Shen F, Smith EL, 3rd, Crawford ML. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2242–50. [PubMed] [Google Scholar]
- 11.Glovinsky Y, Quigley HA, Dunkelberger GR. Retinal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci. 1991;32:484–91. [PubMed] [Google Scholar]
- 12.Ishikawa H, Stein DM, Wollstein G, Beaton S, Fujimoto JG, Schuman JS. Macular segmentation with optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46:2012–7. doi: 10.1167/iovs.04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim NR, Lee ES, Seong GJ, Kim JH, An HG, Kim CY. Structure-function relationship and diagnostic value of macular ganglion cell complex measurement using Fourier-domain OCT in glaucoma. Invest Ophthalmol Vis Sci. 2010;51:4646–51. doi: 10.1167/iovs.09-5053. [DOI] [PubMed] [Google Scholar]
- 14.Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- 15.Wässle H, Grünert U, Röhrenbeck J, Boycott BB. Cortical magnification factor and the ganglion cell density of the primate retina. Nature. 1989;341:643–6. doi: 10.1038/341643a0. [DOI] [PubMed] [Google Scholar]
- 16.Meira-Freitas D, Lisboa R, Medeiros FA. Advances in the structural evaluation of glaucoma with optical coherence tomography. Curr Ophthalmol Rep. 2013;1:98–105. doi: 10.1007/s40135-013-0014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeimer R, Asrani S, Zou S, Quigley H, Jampel H. Quantitative detection of glaucomatous damage at the posterior pole by retinal thickness mapping. A pilot study. Ophthalmology. 1998;105:224–31. doi: 10.1016/s0161-6420(98)92743-9. [DOI] [PubMed] [Google Scholar]
- 18.Kim NR, Lee ES, Seong GJ, Kang SY, Kim JH, Hong S, et al. Comparing the ganglion cell complex and retinal nerve fibre layer measurements by Fourier domain OCT to detect glaucoma in high myopia. Br J Ophthalmol. 2011;95:1115–21. doi: 10.1136/bjo.2010.182493. [DOI] [PubMed] [Google Scholar]
- 19.Rolle T, Briamonte C, Curto D, Grignolo FM. Ganglion cell complex and retinal nerve fiber layer measured by Fourier-domain optical coherence tomography for early detection of structural damage in patients with preperimetric glaucoma. Clin Ophthalmol. 2011;5:961–9. doi: 10.2147/OPTH.S20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Huang H, Wang M, Sun X, Qian S. Fourier domain OCT measurement of macular, macular ganglion cell complex, and peripapillary RNFL thickness in glaucomatous Chinese eyes. Eur J Ophthalmol. 2012;22:972–9. doi: 10.5301/ejo.5000131. [DOI] [PubMed] [Google Scholar]
- 21.Greenfield DS, Bagga H, Knighton RW. Macular thickness changes in glaucomatous optic neuropathy detected using optical coherence tomography. Arch Ophthalmol. 2003;121:41–6. doi: 10.1001/archopht.121.1.41. [DOI] [PubMed] [Google Scholar]
- 22.Schulze A, Lamparter J, Pfeiffer N, Berisha F, Schmidtmann I, Hoffmann EM. Diagnostic ability of retinal ganglion cell complex, retinal nerve fiber layer, and optic nerve head measurements by Fourier-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2011;249:1039–45. doi: 10.1007/s00417-010-1585-5. [DOI] [PubMed] [Google Scholar]
- 23.Sihota R, Sony P, Gupta V, Dada T, Singh R. Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. Invest Ophthalmol Vis Sci. 2006;47:2006–10. doi: 10.1167/iovs.05-1102. [DOI] [PubMed] [Google Scholar]
- 24.Cho JW, Sung KR, Lee S, Yun SC, Kang SY, Choi J, et al. Relationship between visual field sensitivity and macular ganglion cell complex thickness as measured by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51:6401–7. doi: 10.1167/iovs.09-5035. [DOI] [PubMed] [Google Scholar]
- 25.Lee JW, Yau GS, Woo TT, Lai JS. The association between macular thickness and peripapillary retinal nerve fiber layer thickness in Chinese children. Medicine (Baltimore) 2015;94:e567. doi: 10.1097/MD.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]


