Abstract
BACKGROUND
Information on actual sodium intake and its relationships with blood pressure (BP) and clinical events in South America is limited. The aim of this cohort study was to assess the relationship of sodium intake with BP, cardiovascular (CV) events, and mortality in South America.
METHODS
We studied 17,033 individuals, aged 35–70 years, from 4 South American countries (Argentina, Brazil, Chile, and Colombia). Measures of sodium excretion, estimated from morning fasting urine, were used as a surrogate for daily sodium intake. We measured BP and monitored the composite outcome of death and major CV events.
RESULTS
Overall mean sodium excretion was 4.70±1.43g/day. A positive, nonuniform association between sodium and BP was detected, with a significant steeper slope for the relationship at higher sodium excretion levels (P < 0.001 for interaction). With a median follow-up of 4.7 years, the primary composite outcome (all-cause death, myocardial infarction, stroke, or heart failure) occurred in 568 participants (3.4%). Compared with sodium excretion of 5–6g/day (reference group), participants who excreted >7g/day had increased risks of the primary outcome (odds ratio (OR) 1.73; 95% confidence interval (CI) 1.24 to 2.40; P < 0.001), as well as death from any cause (OR 1.87; 95% CI 1.23 to 2.83; P = 0.003) and major CV disease (OR 1.77; 95% CI 1.12 to 2.81; P = 0.014). Sodium excretion of <3g/day was associated with a statistically nonsignificant increased risk of the primary outcome (OR 1.20; 95% CI 0.86 to 1.65; P = 0.26) and death from any cause (OR 1.25; 95% CI 0.81 to 1.93; P = 0.29), and a significant increased risk of major CV disease (OR 1.50; 95% CI 1.01 to 2.24; P = 0.048), as compared to the reference group.
CONCLUSIONS
Our results support a positive, nonuniform association between estimated urinary sodium excretion and BP, and a possible J-shaped pattern of association between sodium excretion over the entire range and clinical outcomes.
Keywords: sodium intake, blood pressure, cardiovascular disease, hypertension, mortality.
Hypertension (HTN) is a leading underlying cause of death, stroke, and myocardial infarction.1–3 In South America, the prevalence of HTN varies from 9% to nearly 50%, depending on geographical areas and extents of diagnosis, awareness, treatment, and control of HTN.4,5 Around 13% of deaths and 5.1% of disability-adjusted life years in Latin America have been attributed to HTN.6 Since sodium intake is regarded as a key determinant of blood pressure (BP), reducing sodium intake has been proposed as a compelling strategy for cardiovascular (CV) disease prevention.
Current Latin American guidelines recommend a low sodium intake (less than 2.4g/day) for CV prevention.7–9 However, the optimal range of sodium intake for CV health remains unresolved. In recent years, from 1990 to 2010, numerous countries in Latin America have experienced substantial changes to their diet, including an increase in the consumption of foods that are generally not recommended such as processed and fried food.10 Given that the effect of sodium intake on BP is influenced by the background diet, it is important to assess the association between sodium intake and CV outcomes within specific geographic regions in the context of the local culture and nutritional practices.11 Currently, Latin American guidelines are developed based on extrapolation of data mostly from North America and Europe. Recently, the Prospective Urban and Rural Epidemiology (PURE) study of over 102,000 people in 17 low, middle, and high income countries found a J-shaped relationship between sodium and CV disease, with a <3g/day and >7g/day sodium intake associated with an increased risk compared to a 4 to 6g/day intake, despite a positive association between sodium and BP.12,13 A recent meta-analysis of 25 cohort studies found a similar optimal range.14
To date, the association between sodium intake and CVD events and mortality has not been investigated in a Latin American population. While the participants of the current study were the same individuals who were included in the main overall study, the distinct characteristics of the population in this region of the world warrant that an analysis be conducted separately on this population. The aim of this study therefore was to assess the association of sodium excretion (a surrogate for intake) with BP, CV events and mortality in the PURE study participants living in South America.
METHODS
Study design and participants
The PURE study is an ongoing epidemiologic cohort study which enrolled over 157,000 adults aged 35–70 years from urban and rural communities located in 18 countries around the world, including 23,141 participants from South America (Argentina, Brazil, Chile, and Colombia).15 The study was approved by the ethics committees at all participating centers and at the Hamilton Health Sciences, Hamilton, Ontario, Canada. All participants provided written informed consent.
Procedures
A morning fasting midstream urine sample was collected from each participant and stored frozen at −20 to −70 °C. Samples were shipped in ambient packaging using STP-250 shipping boxes (Saf-T-Pak) for analysis at the Clinical Research and Clinical Trials Laboratory at Hamilton General Hospital in Hamilton, Ontario, Canada. Sodium concentration in each urine specimen was determined by indirect potentiometry using the Beckman Coulter UniCel DxC600 Synchron Clinical System. The mean of duplicate sitting BP was measured by trained research assistants at all centers after at least 3 minutes rest, following a standardized procedure using an Omron digital BP measuring device (Omron HEM-757) provided for all sites.
The Kawasaki formula was employed for estimating 24-hour sodium excretion estimation,16 which was used as a surrogate for daily sodium intake. A validation study using the Kawasaki formula with actual 24-hour urine collection in 1,083 people from 11 countries showed an intraclass correlation coefficient of 0.71 (95% confidence interval (CI), 0.65 to 0.76).17 Past medical history and physical measurements at baseline were obtained from every participant with standardized procedures and questionnaires. Standardized case-report forms were used to capture data on major CV events and death during follow-up, which were adjudicated centrally in each country by trained physicians using standardized definitions. For the current analysis, we included all adjudicated outcome events in the PURE study database through December 2014.
Statistical analysis
Continuous variables were expressed as mean (±SD) and categorical variables as percentage. Multivariable linear regression was used to assess the relationship between electrolyte excretion and BP, including age, sex, body mass index, educational level, alcohol consumption, and country as covariates. The difference in systolic and diastolic BP per 1g (43.5 mmol) of urinary sodium excretion was calculated. Analysis of covariance was performed to calculate mean BP among sodium excretion levels adjusted by the same covariates. Participants were categorized into urinary sodium excretion groups, based on 1g/day increments of excretion. Since few individuals had excretion values <2 or >8g/day, we truncated excretion values at <3 and >7g/day to avoid small numbers of individuals at the extreme ends of the distribution (no fewer than 2.5% of participants in the lowest and highest excretion categories). On the basis of our restricted-cubic-spline plots for the primary outcome, we selected 5–5.99g/day as the reference category because this was the range associated with the lowest risk of all CV events in multivariable analysis. Secondary analyses using sodium creatinine ratio quintile groups, as a free-formula approach of urinary sodium excretion, and usual sodium excretion were performed. Usual excretion was calculated using regression dilution bias on the basis of baseline measurement and remeasurement at 30–90 days in 448 participants from the overall PURE cohort.18 Interaction tests were performed for estimated sodium excretion level (<3g, 3–5g, and >5g) and country.
A composite of all-cause death, myocardial infarction, stroke, and heart failure was the primary clinical outcome. To account for the clustering nature of the data, a multivariable logistic-regression analysis with generalized estimating equations was performed. The primary model included age, sex, body mass index, smoking status, diabetes, educational level, alcohol consumption, past CV events, and country in the model. Secondary multivariable models included the primary model plus ratio of low-density lipoprotein cholesterol to high-density lipoprotein cholesterol, dietary factors (energy intake, fruit, and vegetable intake) and BP related variables (systolic BP, history of HTN, and use of antihypertensive therapy at baseline). Restricted cubic splines with 4 knots were used to plot the association between sodium excretion and clinical outcomes. Sensitivity analyses were performed excluding those with previous CV events, those with cancer (diagnosed before or during the study), and had major CV events during the first 2 years of study. All analyses were performed with the use of IBM SPSS Statistics for Windows (IBM, Version 22.0.; Armonk, NY) and SAS software version 9.4, for the UNIX operating system (SAS Institute, Cary, NC).
RESULTS
A total of 17,033 (73.6 %) individuals who had provided a urine sample out of the 23,141 participants were included in the study. Baseline characteristic of the study participants are shown in Table 1. Mean age was 51.5 (±9.6) and 59.7% were female. Overall mean sodium excretion was 4.70±1.43g/day. Estimated sodium excretion was higher in males than females (5.14±1.46 vs. 4.40±1.34g/day; P < 0.001) and in rural areas than urban areas (4.94±1.45 vs. 4.5±1.39g/day; P < 0.001).
Table 1.
Baseline characteristics according to country
| Characteristic | Argentina N = 6,529 (38.3) |
Brazil N = 5,323 (31.2) |
Chile N = 668 (3.9) |
Colombia N = 4,513 (26.6) |
Total N = 17,033 |
|---|---|---|---|---|---|
| Sodium excretion, g ± SDa | 4.66±1.35 | 4.57±1.47 | 4.88±1.45 | 4.89±1.48 | 4.70±1.43 |
| Potassium excretion, g ± SDa | 1.74±0.40 | 2.32±0.53 | 2.06±0.60 | 2.33±0.60 | 2.09±0.58 |
| Creatinine excretion, g ± SDb | 1.41±0.42 | 1.35±0.39 | 1.26±0.35 | 1.22±0.32 | 1.34±0.39 |
| Age, mean ± SD | 51.1±9.9 | 52.2±9.4 | 52.0±9.4 | 50.8±9.8 | 51.4±9.6 |
| Female sex, n (%) | 3,980 (61.0) | 2,916 (54.8) | 443 (66.3) | 2,838 (62.9) | 10,177 (59.7) |
| Educational level, n (%) |
|||||
| Less than high-school | 4,740 (72.7) | 2,531 (47.5) | 444 (66.9) | 2,924 (64.9) | 10,639 (62.6) |
| High-school | 1,472 (22.6) | 1,105 (20.8) | 172 (25.9) | 890 (19.8) | 3,639 (21.4) |
| Some college or more | 305 (4.7) | 1,687 (31.7) | 48 (7.2) | 688 (15.3) | 2,728 (16.0) |
| BMI, mean ± SD | 29.4±6.0 | 27.8±5.0 | 29.8±5.2 | 26.4±4.7 | 28.1±5.5 |
| Waist-to-hip ratio, mean ± SD |
0.89±0.082 | 0.92±0.081 | 0.91±0.07 | 0.89±0.08 | 0.90±0.082 |
| Tobacco use, n (%) | |||||
| Never | 3,346 (51.2) | 2,834 (53.3) | 443 (67.3) | 3,006 (66.8) | 9,629 (56.6) |
| Former | 1,487/6529 (22.8) | 1,467/5322 (27.6) | 94/658 (14.3) | 929/4499 (20.6) | 3,977 (23.4) |
| Current | 1,696 (26.0) | 1,021 (19.2) | 121 (18.4) | 564 (12.5) | 3,402 (20.0) |
| Physical activity, n (%) | |||||
| Low | 587 (9.2) | 518 (10.3) | 65 (10.5) | 420 (11.2) | 1,590 (10.1) |
| Medium | 1769 (27.8) | 1577 (31.4) | 198 (32.0) | 1,263 (33.6) | 4,807 (30.5) |
| High | 4,011 (63.0) | 2,933 (58.3) | 356 (57.5) | 2,080 (55.3) | 9,380 (59.5) |
| Alcohol consumption, n (%) | |||||
| Never drank | 2,064 (31.6) | 2,784 (52.3) | 418 (62.7) | 2,507 (55.6) | 7,773 (45.7) |
| Former drinker | 374 (5.7) | 443 (8.3) | 24 (3.6) | 716 (15.9) | 1,557 (9.1) |
| Current | 4,090 (62.7) | 2,096 (39.4) | 225 (33.7) | 1,286 (28.5) | 7,697 (45.2) |
| Diabetes, n (%) | 449 (6.9) | 450 (8.5) | 68 (10.2) | 263 (5.8) | 1,230 (7.2) |
| Blood pressure mm Hg mean ± SD | |||||
| Systolic | 135.2±21.7 | 132.2±21.2 | 125.9±20.0 | 127.8±20.9 | 132.0±21.5 |
| Diastolic | 82.6±12.5 | 84.5±12.4 | 79.2±11.7 | 79.9±12.0 | 82.4±12.5 |
| Self-reported HTN or BP ≥140/90mm Hg, n (%) | 3,369 (51.6) | 2,792 (52.5) | 276 (41.3) | 1,703 (37.7) | 8140 (47.8) |
| BP ≥140/90mm Hg, n (%) | 2,766 (42.4) | 2,046 (38.4) | 174 (26.0) | 1,259 (27.9) | 6,245 (36.7) |
| Coronary heart disease, n (%) | 176 (2.7) | 285 (5.4) | 11 (1.6) | 114 (2.5) | 586 (3.4) |
| Stroke, n (%) | 97 (1.5) | 97 (1.8) | 16 (2.4) | 88 (2.0) | 298 (1.8) |
| Heart failure, n (%) | 40 (.6) | 85 (1.6) | 6 (.9) | 84 (1.9) | 215 (1.3) |
| Cardiovascular disease, n (%) | 506 (7.8) | 713 (13.4) | 48 (7.2) | 289 (6.4) | 1,556 (9.1) |
| On BP medications, n (%) | 1,672 (25.7) | 1,766 (33.2) | 162 (24.3) | 596 (14.7) | 4,196 (25.3) |
| On statin medication, n (%) | 210 (3.2) | 407 (7.6) | 42 (6.3) | 35 (0.9) | 694 (4.2) |
Abbreviations: BP, blood pressure; HTN, hypertension; BMI, body mass index.
aEstimated daily sodium excretion was calculated using the Kawasaki formula. bCreatinine excretion was estimated according to a formula that includes age, sex, weight, and height.
Overall, 2.8% of participants had an estimated sodium excretion of less than 2.3g/day, 0.5% below 1.5g/day, 17% more than 6g/day, and 6.5% more than 7g/day. A majority of participants (73.1%) had sodium excretion between 3–6g per day. After adjustment for regression dilution bias, 0.1% of participants had an estimated sodium excretion of less than 2.3g/day, none had less than 1.5g/day, 11.5% more than 6g/day, and 2.5% more than 7g/day.
Urinary sodium excretion and blood pressure
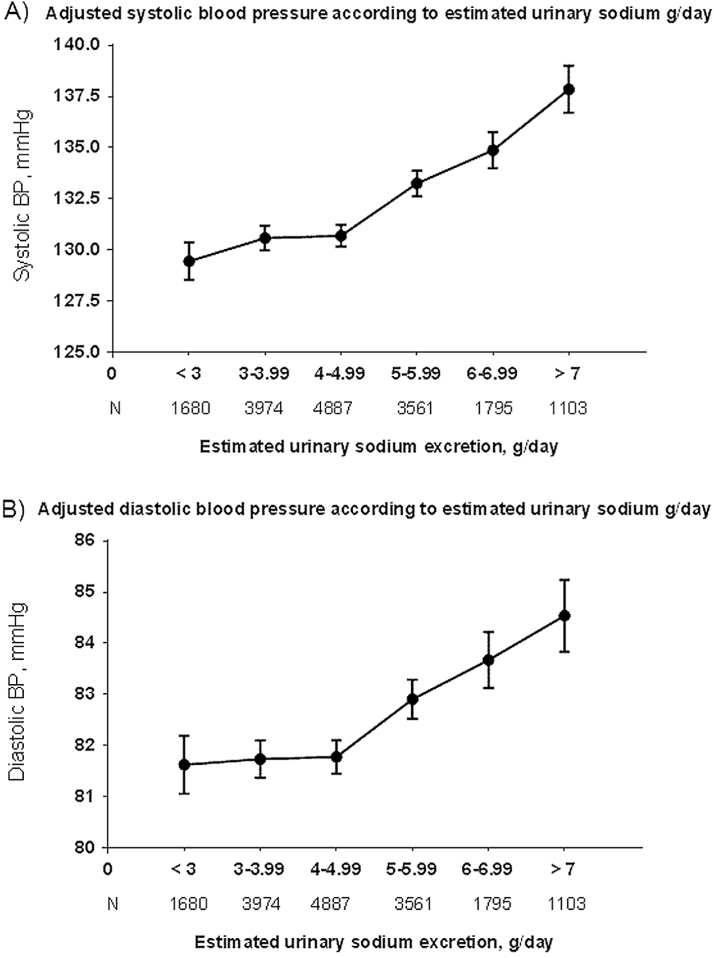
After adjusting for covariates, we found a significant positive association between estimated sodium excretion with systolic (P < 0.001 for trend) and diastolic (P < 0.001 for trend) BPs (Figure 1A,B). Secondary analysis using urinary sodium to creatinine ratio shows a similar pattern (see Supplementary Figure S1). Multivariable linear regression showed that overall for each gram of estimated sodium excretion there were increments of 1.52mm Hg in systolic (P < 0.001) and 0.58mm Hg in diastolic BPs (P < 0.001). After applying regression dilution bias correction, for each gram of estimated sodium excretion there was an increment of 2.21mm Hg in systolic (P < 0.001) and an increment of 0.83mm Hg in diastolic BPs (P < 0.001) (see Supplementary Table S1 for country specific slopes).
Figure 1.
Systolic (A) and diastolic (B) adjusted BPs means according to the estimated urinary sodium excretion using analysis of covariance (ANCOVA). Covariates: age, sex, body mass index, educational level, alcohol consumption, and country.
The relationship over the whole range of estimated sodium excretion with systolic BP was nonlinear, with a significantly steeper slope for the association at a higher sodium excretion of more than 5g/day (1.90mm Hg per gram of sodium; 95% CI 1.42 to 2.38; P < 0.001) than at a level of excretion of 3–5g/day (0.41mm Hg per gram; 95% CI −0.30 to 1.12; P = 0.26) or less than 3g per day (0.39mm Hg per gram; 95% CI −1.60 to 2.39; P = 0.69) (P = 0.002 for interaction). Similar results were observed for diastolic BP (P < 0.001 for interaction). The slope of the association between sodium excretion and systolic (P = 0.24 for interaction) and diastolic (P = 0.09 for interaction) BPs was similar across countries, after adjusting for covariates and regional differences in potassium excretion.
Urinary sodium excretion and clinical outcomes
A total of 16,549 (97.2%) participants had available follow-up clinical data, with a median follow-up of 4.7 years. The primary composite outcome of all-cause death or a major CV event in these participants occurred in 568 individuals (3.4%); 417 participants had died (143 from CV deaths), 148 had myocardial infarction, 102 had stroke, and 41 had heart failure. The group with 5–6g estimated urinary sodium excretion was in the lowest risk category with multivariable analysis and this was used as the reference category.
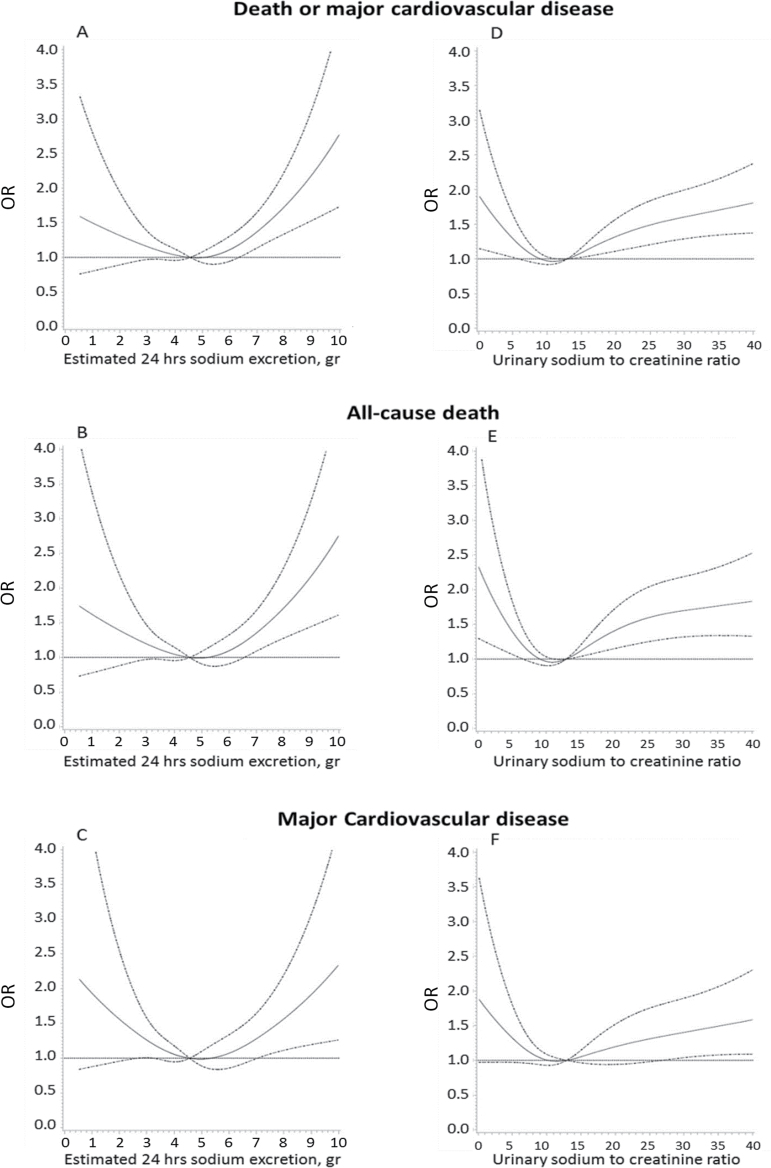
A possible J-shaped association between sodium excretion and CV events and mortality was apparent (Figure 2 and Supplementary Figure S2). Compared with sodium excretion of 5–6g/day (reference category), participants with sodium excretion of greater than 7g/day was associated with increased risks of the primary composite outcome (odds ratio (OR) 1.73; 95% CI 1.24 to 2.40; P < 0.001), death from any cause (OR 1.87; 95% CI 1.23 to 2.83; P = 0.003), and major CV disease (OR 1.77; 95% CI 1.12 to 2.81; P = 0.014) on multivariable analyses. After adjusting for BP or prior diagnosis of HTN, the association between high sodium excretion and each of the outcomes was attenuated but remained statistically significant (Table 2, Supplementary Tables S2 and S3). On multivariable analyses, compared with sodium excretion of 5–6g/day, sodium excretion of less than 3g/day was associated with a statistically nonsignificant increased risk of the primary composite outcome (OR 1.20; 95% CI 0.86 to 1.65; P = 0.26) and death from any cause (OR 1.25; 95% CI 0.81 to 1.93; P = 0.29), and a significant increase in major CV disease (OR 1.50; 95% CI: 1.01 to 2.24; P = 0.048) (Figure 2).
Figure 2.
Estimated urinary sodium excretion and death or major cardiovascular disease (A), all-cause death (B) and major cardiovascular disease alone (C). Urinary sodium to creatinine concentration ratio and death or major cardiovascular disease (D), all-cause death (E), and major cardiovascular disease alone (F). Results are from restricted cubic splines logistic-regression modeling, including age, sex, body mass index (BMI), smoking status, diabetes, educational level, alcohol consumption, past cardiovascular events, and country. Major cardiovascular disease refers to myocardial infarction, stroke, or heart failure.
Table 2.
Association of estimated urinary sodium excretion with the primary outcome
| <3g/day (N = 1,638) | 3–3.99g/day (N = 3,885) | 4–4.99g/day (N = 4,758) | 5–5.99g/day (N = 3,457) | 6–6.99g/day (N = 1,748) | >7g/day (N = 1,063) | ||
|---|---|---|---|---|---|---|---|
| Death or major cardiovascular disease, N (%)a | 50 (3.1) | 115 (3.0) | 161 (3.4) | 110 (3.2) | 73 (4.2) | 59 (5.6) | |
| Analysis—OR (95% CI) | |||||||
| Univariate analysisb | 0.95 (0.69–1.31) | 0.92 (0.70–1.21) | 1.06 (0.85–1.32) | 1 | 1.32 (1.01–1.74) | 1.78 (1.26–2.52) | |
| Multivariable analysis | |||||||
| Primary analysis | 1.20 (0.86–1.65) | 1.07 (0.81–1.40) | 1.11 (0.88–1.39) | 1 | 1.30 (0.97–1.73) | 1.73 (1.24–2.40) | |
| Including LDL to HDL ratio | 1.14 (0.81–1.60) | 1.10 (0.84–1.43) | 1.14 (0.91–1.43) | 1 | 1.30 (0.97–1.75) | 1.94 (1.39–2.70) | |
| Including dietary factorsc | 1.18 (0.86–1.62) | 1.09 (0.81–1.45) | 1.10 (0.87–1.39) | 1 | 1.25 (0.93–1.68) | 1.66 (1.17–2.35) | |
| Including dietary factors and hypertensiond | 1.18 (0.86–1.62) | 1.10 (0.82–1.46) | 1.12 (0.88–1.43) | 1 | 1.23 (0.91–1.65) | 1.55 (1.07–2.24) | |
| Excluding cardiovascular disease and cancere | 1.14 (0.73–1.78) | 1.14 (0.82–1.59) | 1.05 (0.77–1.43) | 1 | 1.46 (0.95–2.26) | 1.93 (1.20–3.09) | |
| Excluding events in year 1 | 1.13 (0.72–1.75) | 1.18 (0.82–1.70) | 1.00 (0.74–1.34) | 1 | 1.74 (1.12–2.71) | 2.05 (1.29–3.25) | |
| Excluding events in years 1 and 2 | 1.07 (0.63–1.84) | 1.04 (0.71–1.52) | 0.87 (0.64–1.19) | 1 | 1.60 (1.03–2.48) | 1.86 (1.18–2.94) |
Results from generalized estimating equations (GEE).
Abbreviations: CI, confidence interval; OR, odds ratio; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
aDeath, myocardial infarction, stroke, or heart failure. bPerformed using generalized estimating equation model to address clustering of data. cCaloric, fruit, vegetable, and potassium intake. dHypertension included baseline systolic blood pressure, history of hypertension, and use of antihypertensive therapy. eExcluding patients with previous cardiovascular disease and those with previous or new cancer during the study follow-up.
A similar pattern of results was found for sodium to creatinine concentration ratio vs. each of the outcomes. The lowest quintile showed a statistically significant increased risk of the primary composite outcome (OR 1.30; 95% CI 1.01 to 1.68; P = 0.04) and death from any cause (OR 1.59; 95% CI 1.17 to 2.17; P = 0.003), while the highest quintile had an increased risk of the primary outcome (OR 1.52; 95% CI 1.10 to 2.13; P = 0.013) and death from any cause (OR 1.67; 95% CI 1.12 to 2.50; P = 0.012), as compared to the middle quintile group (Figure 2D,E). Further analysis excluding individuals with CV disease or cancer at baseline or those who had events in the first or 2 years of follow-up did not materially affect the estimates (Table 2).
DISCUSSION
In this cohort of 17,033 individuals in 4 South American countries, we detected a positive association between estimated daily sodium excretion and BP, which was consistent in all 4 countries. We also observed a possible J-shaped pattern of association between estimated sodium excretion and clinical outcomes, where sodium excretion of greater than 7g/day was associated with an increased risk of CV events compared to 5–6g/day, and sodium excretion below 3g/day was associated with an apparent increased risk or a trend for an increased risk of CV events or mortality. To date, this is the largest study of sodium intake and clinical events in South America.
In general, current guidelines in South American countries recommend maintaining daily salt intake less than 6g (2.4g of sodium).19–22 The overall daily mean level of sodium excretion in our analysis was 4.70±1.43g, which is generally consistent with previous assessments in these South American countries. The INTERSALT study found a mean sodium excretion of 3.58g in Argentina (200 participants) and 4.63 in Colombia (191 participants).23 The participants from Brazil comprised of 393 individuals from native tribes with extremely low sodium intake.23,24 In a national study of sodium intake in Brazil, the mean sodium consumption was 4.5g/day, and similar in rural and urban areas and across income strata.25 In the 2010 Chilean National Health Survey, sodium consumption was estimated to be 3.94g/day.26 In our study, we found that less than 2.8% of the population had a sodium excretion of below 2.3g/day, which suggests that prolonged consumption of a low sodium diet is rare, which is similarly observed in other parts of the world.13 Conversely, only 17% had a sodium excretion over 6g daily for which a definite excess harm was observed.
In our study, the overall slope of the association between sodium and BP was 1.52/0.58mm Hg (systolic/diastolic) per gram increment of sodium in 24 hours, which is steeper than the 0.94/0.03mm Hg reported in INTERSALT.23 When we excluded individuals older than 59 years (the upper age limit of INTERSALT), the estimate did not change appreciably (1.34/0.57mm Hg per gram of sodium). After adjusting for regression dilution bias, the overall slope of the association was 2.21/0.83mm Hg per gram increment of sodium, which is in keeping with findings from a recent meta-analysis of sodium reduction trials.27 Further we found a positive but nonlinear association between sodium excretion and BP, consistent with the overall PURE cohort.13 Specifically a steep slope for this association was found among individuals with higher sodium excretion (>5g/day), but a weak, nonsignificant association was found among those with 3 to 5g/day or <3g/day sodium excretion. These findings are aligned with a recent meta-analysis of randomized trials.28 Our findings suggest that in a South American population, lowering sodium intake at the highest levels of intake may result in a marked reduction of BP in the population, whereas lowering sodium intake from average levels to lower levels of intake is more likely to produce negligible BP lowering.
Our present analysis supported a nonlinear association between sodium excretion and clinical outcomes and most closely resembled a possible J-shaped relationship. For each outcome, the estimates for lower sodium intake (<3g/day) compared to 5–6g/day showed a harmful association or a nonsignificant trend for harm. Sensitivity analysis using sodium to creatinine ratio, a formula-free approach, showed similar results. When excluding clinical events in the first 2 years, the increased risk of clinical events with lower sodium (<3g/day) compared to 5–6g/day is attenuated but remains in the harmful direction. Our findings were consistent with the overall PURE cohort, other recent cohort studies of North American/European populations29–33 and a recent meta-analysis of cohort studies involving 274,683 individuals, which showed a clear degree of excess harm with lower levels of sodium excretion.14 We are not aware of any previous data on the association between sodium excretion and CV clinical events in South American countries. Our findings indicate that in a South American population, sodium excretion of >7g/day is associated with higher risk of CV disease/mortality compared to 5–6g/day, and sodium excretion of <3g/day is also related to an increased risk or no lower risk of CV disease or mortality.
The main limitations of the study are analogous to previous PURE overall cohort studies.12,13 First, the 24-hour urinary sodium excretion is calculated by applying the Kawasaki formula to an overnight urine sample. Although using repeated 24-hour urine samples can be considered the reference method for estimating sodium intake, this would be impractical for large studies. Further, less frequent measurements introduce mainly random error and reduce statistical precision and most likely to bias relationships toward the null, but would not be expected to change the overall pattern of association. The Kawasaki formula was evaluated in a previous validation study within the PURE cohort, in which this formula had better correlation and agreement with 24-hour urinary sodium excretion compared with other formulas.17 In our formula-free secondary analyses using sodium to creatinine concentration ratio, we found a similar nonlinear pattern between sodium level and CV events and mortality, despite a positive association with BP. Therefore, the use of formulae approaches or sodium to creatinine concentration ratio does not alter the primary conclusion of our findings. Second, this cohort used a non-probabilistic sampling approach and not all South American countries were included, raising a concern of lack of representativeness. However, as mentioned, our mean sodium excretion levels are similar to other previous assessments in these countries. Given the homogeneity of the associations across countries, it is likely that our findings are applicable to other parts of South America. Third, residual confounding cannot be completely ruled out in any epidemiologic study. However, we measured and controlled for known risk factors in extensive multivariable analyses and the parameter estimates remained consistent, which suggests that confounding is unlikely to have been a factor on our study. Finally, a limited number of events at both the low and high ends of sodium excretion resulted in reduced precision of our estimates. Nevertheless, the possible J-shaped pattern was apparent across different outcomes, in secondary multivariable models and sensitivity analyses and is consistent with the findings of the global cohort which included over 102,000 people.
In conclusion, our results support the evidence of a positive nonlinear association between estimated urinary sodium excretion and BP and a possible J-shaped pattern of association between estimated sodium excretion and clinical outcomes in South American countries. These findings are consistent with previous findings in other geographical regions, in which lower sodium consumption is associated with increased risk, or no lower risk, of CV events/mortality compared to a 3–6g/day sodium intake. With three-quarters of the population consuming 3–6g/day, an intake range related with the lowest risk of clinical outcomes, and less than 20% of the population consuming more than 6g/day, our results are not consistent with a universal sodium reduction policy.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
S.Y. designed the work and developed the protocol. P.L., A.M., S.R., K.T., and S.Y. were involved in data management and statistical analyses. P.L. wrote the first and subsequent drafts of the article and is the primary responsible for final content. R.D., A.O., A.A., G.O., F.L., P.S., P.L.J., P.C.L., M.J.O., S.R., K.T., and S.Y. were also involved in investigator training, data collection, and supervised the whole study. All the authors provided inputs for the article and critically reviewed the manuscript and provided suggestions. All the authors have read the manuscript and agree to its contents.
Supplementary Material
ACKNOWLEDGMENTS
S.Y. is funded by the Marion Burke Chair of the Heart and Stroke Foundation of Canada. The main PURE study and its components are funded by the Population Health Research Institute, the Canadian Institutes of Health Research, Heart and Stroke Foundation of Ontario and through unrestricted grants from several pharmaceutical companies (with major contributions from Astra Zeneca (Canada), Sanofi-Aventis (France and Canada), Boehringer Ingelheim (Germany and Canada), Servier, and GSK), and additional contributions from Novartis and King Pharma and from various national or local organizations in participating countries. These include: Argentina: Fundacion ECLA; Bangladesh: Independent University, Bangladesh and Mitra and Associates; Brazil: Unilever Health Institute; Canada: Public Health Agency of Canada and Champlain Cardiovascular Disease Prevention Network; Chile: Universidad de la Frontera; China: National Center for Cardiovascular Diseases; Colombia: Colciencias, grant number: 6566-04-18062; India: Indian Council of Medical Research; Malaysia: Ministry of Science, Technology and Innovation of Malaysia, grant number: 100 - IRDC/BIOTEK 16/6/21 (13/2007), Grant number 07-05-IFN-BPH 010, Ministry of Higher Education of Malaysia, grant number: 600 - RMI/LRGS/5/3 (2/2011), Universiti Teknologi MARA, Universiti Kebangsaan Malaysia (UKM-Hejim-Komuniti-15–2010); Poland: Polish Ministry of Science and Higher Education, grant number: 290/W-PURE/2008/0, Wroclaw Medical University; South Africa: The North-West University, SANPAD (SA and Netherlands Programme for Alternative Development), National Research Foundation, Medical Research Council of SA, The SA Sugar Association (SASA), Faculty of Community and Health Sciences (UWC); Sweden: AFA Insurance, Swedish Council for Working Life and Social Research, Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning, Swedish Heart and Lung Foundation, Swedish Research Council, Grant from the Swedish State under (LäkarUtbildningsAvtalet), Agreement, Grant from the Västra Götaland Region (FOUU); Turkey: Metabolic Syndrome Society, Astra Zeneca, Sanofi Aventis; UAE: Sheikh Hamdan Bin Rashid Al Maktoum Award For Medical Sciences and Dubai Health Authority, Dubai UAE. R.D., A.O., A.A., G.O., F.L., P.S., P.L.-J., P.C.-L., K.T., and S.Y. were involved in obtaining funding for the study.
REFERENCES
- 1. World Health Organization. Global status report on noncommunicable diseases 2010. Geneva. 2010.
- 2. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364:937–952. [DOI] [PubMed] [Google Scholar]
- 3. O’Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010; 376:112–123. [DOI] [PubMed] [Google Scholar]
- 4. Hernandez-Hernandez R, Silva H, Velasco M, et al. Hypertension in seven Latin American cities: the Cardiovascular Risk Factor Multiple Evaluation in Latin America (CARMELA) study. J Hypertens 2010; 28:24–34. [DOI] [PubMed] [Google Scholar]
- 5. Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 2013; 310:959–968. [DOI] [PubMed] [Google Scholar]
- 6. Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet 2008; 371:1513–1518. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization and Pan Ametican Health Organization expert group. Preventing Cardiovascular Disease in the Americas by Reducing Dietary Salt Intake Population-Wide. http://www.paho.org/hq/index.php?option=com_docman&task=doc_download&gid=16058&Itemid=. Accessed 15 February 2015. [Google Scholar]
- 8. Sanchez RA, Ayala M, Baglivo H, et al. Latin American guidelines on hypertension. Latin American Expert Group. J Hypertens 2009; 27:905–922. [DOI] [PubMed] [Google Scholar]
- 9. Lopez-Jaramillo P, Sanchez RA, Diaz M, et al. Latin American consensus on hypertension in patients with diabetes type 2 and metabolic syndrome. J Hypertens 2013; 31:223–238. [DOI] [PubMed] [Google Scholar]
- 10. Imamura F, Micha R, Khatibzadeh S, et al. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. Lancet Glob Health 2015; 3:e132–e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 12. O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371:612–623. [DOI] [PubMed] [Google Scholar]
- 13. Mente A, O’Donnell MJ, Rangarajan S, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 2014; 371:601–611. [DOI] [PubMed] [Google Scholar]
- 14. Graudal N, Jurgens G, Baslund B, Alderman MH. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am J Hypertens 2014; 27:1129–1137. [DOI] [PubMed] [Google Scholar]
- 15. Teo K, Chow CK, Vaz M, Rangarajan S, Yusuf S. The Prospective Urban Rural Epidemiology (PURE) study: examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am Heart J 2009; 158:1–7 e1. [DOI] [PubMed] [Google Scholar]
- 16. Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol 1993; 20:7–14. [DOI] [PubMed] [Google Scholar]
- 17. Mente A, O’Donnell MJ, Dagenais G, et al. Validation and comparison of three formulae to estimate sodium and potassium excretion from a single morning fasting urine compared to 24-h measures in 11 countries. J Hypertens 2014; 32:1005–1014; discussion 15. [DOI] [PubMed] [Google Scholar]
- 18. Lewington S, Whitlock G, Clarke R, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007; 370:1829–1839. [DOI] [PubMed] [Google Scholar]
- 19. VI Diretrizes Brasileiras de Hipertensão. (VI Brazilean Guidelines of Hypertension), in Portuguese. Arq Bras Cardiol 2010; 95:1–51. [PubMed] [Google Scholar]
- 20. Guías de la sociedad argentina de hipertensión para el diagnóstico, estudio, tratamiento y seguimiento de la hipertensión arterial (in Spanish). (Argentinean society of hypertension guidelines for the diagnosis, study, treatment and follow-up of arterial hypertension). http://www.saha.org.ar/pdf/GUIA_SAHA_VERSION_COMPLETA.pdf Accessed 15 February 2015.
- 21. Guía clínica hipertensión arterial primaria o esencial en personas de 15 años y más. Ministerio de Salud de Chile (in Spanish) (Clinical guidelines for primary or essencial hypertension in people with 15 or more years old. Chilean Ministry of Health). http://web.minsal.cl/portal/url/item/7220fdc4341c44a9e04001011f0113b9.pdf Accessed 15 February 2015.
- 22. Guía de práctica clínica Hipertensión arterial primaria. Sistema General de Seguridad Social en Salud, Colombia (in Spanish) (Clinical practice guideline of primary hypertension. General social securiry of health, Colombia). http://gpc.minsalud.gov.co/Documents/Guias-PDF-Recursos/HTA/GPC_Prof_Sal_HTA.pdf Accessed 15 February 2015.
- 23. Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 1988; 297:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. The INTERSALT Co-operative Research Group. Appendix tables. Centre-specific results by age and sex. J Hum Hypertens 1989; 3:331–407. [PubMed] [Google Scholar]
- 25. Sarno F, Claro RM, Levy RB, Bandoni DH, Ferreira SR, Monteiro CA. Estimated sodium intake by the Brazilian population, 2002–2003. Rev Saude Publica 2009; 43:219–225. [DOI] [PubMed] [Google Scholar]
- 26. Encuesta Nacional de Salud ENS Chile. Ministerio de Salud de Chile. Article in Spanish (National Health Survey, Chile. Chilean Ministry of Health). 2009. –2010.
- 27. He FJ, Li J, Macgregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2013; 4:CD004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graudal N, Hubeck-Graudal T, Jürgens G, McCarron DA. The significance of duration and amount of sodium reduction intervention in normotensive and hypertensive individuals: a meta-analysis. Adv Nutr 2015; 6:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O’Donnell MJ, Yusuf S, Mente A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA 2011; 306:2229–2238. [DOI] [PubMed] [Google Scholar]
- 30. Stolarz-Skrzypek K, Kuznetsova T, Thijs L, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA 2011; 305:1777–1785. [DOI] [PubMed] [Google Scholar]
- 31. Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Estimated urinary sodium excretion and risk of heart failure in men and women in the EPIC-Norfolk study. Eur J Heart Fail 2014; 16:394–402. [DOI] [PubMed] [Google Scholar]
- 32. Ekinci EI, Clarke S, Thomas MC, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care 2011; 34:703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas MC, Moran J, Forsblom C, et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 2011; 34:861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.