Abstract
BACKGROUND
Revascularization of a stenotic renal artery improves kidney function only in select patients with renovascular hypertension (HT) secondary to atherosclerosis. However, the effects of reversal of renovascular HT (RRHT) on the nonstenotic kidney are unclear. We hypothesized that concurrent hypercholesterolemia (HC) attenuates nonstenotic kidney recovery.
METHODS
Female domestic pigs were randomized as Normal, renovascular HT, HT+RRHT, HTC (renovascular HT and HC), and HTC+RHT (n = 7 each). RRHT or sham was performed after 6 weeks of HT. Nonstenotic renal blood flow, glomerular filtration rate, and injurious pathways were studied 4 weeks later.
RESULTS
Mean arterial pressure increased similarly in HT and HTC and decreased after RRHT. Oxidative stress increased in HT and HTC kidneys, and decreased in HT+RRHT, but remained elevated in HTC+RRHT. Renal interstitial fibrosis, glomerulosclerosis, and tubular injury were all attenuated in HT+RRHT, but not HTC+RRHT. Endothelin-1 signaling and PGF2α isoprostane levels were elevated in both HTC and HTC+RRHT pigs.
CONCLUSIONS
RRHT reverses nonstenotic kidney injury in experimental renovascular HT, but concurrent HC blunts regression of kidney injury, possibly due to predominant vasoconstrictors and oxidative stress. These findings reinforce the contribution of the nonstenotic kidney and of prevailing cardiovascular risk factors to irreversibility of kidney dysfunction after revascularization.
Keywords: blood pressure, endothelin-1, hypercholesterolemia, hypertension, oxidative stress, renovascular hypertension.
Renovascular disease is the major cause of secondary hypertension (HT),1 which is most commonly caused by atherosclerosis. The prevalence of renovascular HT as an underlying cause for end-stage renal disease is on the rise, especially in the elderly population.2–4 Hypercholesterolemia (HC), a surrogate for early atherosclerosis, is a risk factor for renal functional abnormalities.3 In both animal models and humans, HT3 and HC2 may induce renal vascular dysfunction, glomerulosclerosis,4,5 and tubular damage,5 largely increasing production of oxygen radical species (ROS),6 which may in turn decrease bioavailability of nitric oxide (NO) and increase activity of vasoconstrictors like endothelin (ET)-1 or thromboxane-A2. Furthermore, similar to patients with chronic kidney disease, in whom comorbidities are important independent drivers of the adverse outcomes,7 concurrent HT and HC (HTC) modulate deterioration of renal function in pigs.2,8
Both clinical trials and experimental studies have shown that revascularization of a stenotic renal artery is often unsuccessful in restoring renal function,9 particularly when associated with atherosclerosis,10,11 which is often attributed to persistent damage in the stenotic kidney. For example, reversal of renovascular HT (RRHT) partially restored renal blood flow (RBF) and tissue oxygenation within the poststenotic human kidneys, but not total glomerular filtration rate (GFR) or systemic inflammation.12 Yet, inadequate regression of damage in the nonstenotic contralateral kidney (CLK) may also contribute to incomplete restoration of renal function after RRHT.
In particular, persistent exposure to enduring cardiovascular risk factors like HC after resolution of HT may continue fueling intrarenal damage. Furthermore, a fall in CLK GFR due to RRHT, without corresponding elevation of stenotic-kidney GFR, might blunt improvement in GFR of total renal mass. However, the fate of the nonstenotic HTC kidney after RRHT remains unclear, partly because of the challenge in assessing single-kidney function. The present study was designed to test the hypothesis that swine CLK damage, exacerbated by HTC compared with HT, persists after RRHT.
METHODS
Experimental design
All procedures were approved by the Institutional Animal Care and Use Committee. Thirty-five juvenile female domestic pigs (initially 3 months old and weighing 25–35kg) were randomized as Normal (n = 7), renovascular HT (n = 14), and HTC (n = 14). Normal and HT animals were fed normal pig chow, and HTC pigs a high-cholesterol diet (2% cholesterol and 15% lard, TD-93926, Harlan-Teklad).13
Six weeks after initiation of diet (Figure 1), unilateral renal artery stenosis was induced in HT and HTC pigs by placing a local-irritant coil in the main renal artery, whereas normal animals underwent sham angiography without coil placement. Mean arterial pressure was subsequently measured by a PhysioTel telemetry system (Data Sciences International, St. Paul, MN) implanted at baseline in the left femoral artery and averaged over the last few days before study completion.14
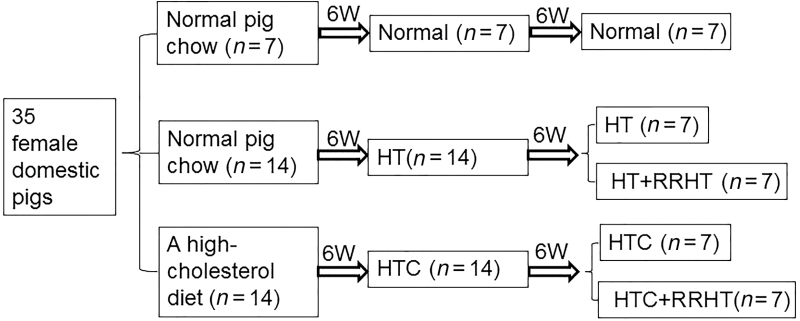
Figure 1.
Schematic of the experimental design. Abbreviations: HT: hypertension; RRHT, reversal of renovascular HT; HTC, HT and hypercholesterolemia.
Six weeks after induction of renal artery stenosis, animals were anesthetized, the degree of stenosis determined by angiography, and half the pigs in the HT and HTC groups randomly selected for RRHT or sham. This protocol resulted in 5 experimental groups (n = 7 each): Normal, HT, HT+RRHT, HTC, and HTC+RRHT (Figure 1).
Four weeks later, CLK function was determined using multidetector computed tomography. Prior to each in vivo study, animals were anesthetized with intramuscular Telazol (Fort Dodge Animal Health, New York, NY) (5mg/kg) and xylazine (2mg/kg), intubated, and anesthesia maintained with intravenous ketamine (0.2mg/kg/min) and xylazine (0.03mg/kg/min).
In vitro studies were subsequently performed to measure total and low-density lipoprotein cholesterol in plasma (Roche), plasma renin activity (Radioimmunoassay; DiaSorin, Stillwater, MN), serum creatinine (Arbor Assays, Ann Arbor, MI), ET-1 (ELISA; R&D Systems), PGF2α isoprostane (ELISA; Cayman, Ann Arbor, MI), and circulating thromboxane-A2 levels (ELISA; R&D Systems, Cat# KGE011). After completion of all studies, the pigs were euthanized with sodium pentobarbital (100mg/kg IV, Fort Dodge Laboratories). Kidneys were sectioned and immediately shock-frozen in liquid nitrogen and stored at −80 °C, or preserved in formalin.
Multidetector computed tomography studies
In anesthetized animals, the femoral artery was catheterized, followed by a heparin bolus (5,000U). Under fluoroscopic guidance, local-irritant coils were implanted in the proximal-middle right renal artery, as previously described.11,15 Using contrast-enhanced multidetector computed tomography, nonstenotic regional RBF and GFR were evaluated from time-intensity curves generated consequent to a bolus of contrast media (iopamidol, 0.5 cc/kg, Omnipaque; Novalplus, Princeton, NJ). Multidetector computed tomography images were analyzed with ANALYZE (Biomedical Imaging Resource, Mayo Clinic, MN). In each region of the kidney, the parameters obtained from the vascular curve secondary to transit of a contrast bolus were used to calculate cortical and medullary perfusion. RBF was calculated as the sum of cortical and medullary blood flows (product of cortical and medullary perfusion and volumes), and GFR from the cortical proximal-tubular curve.16 The degree of stenosis was calculated as the decrease in renal arterial luminal area.17
Kidney tissue studies
Renal morphology in each pig was examined in representative 5-µm-thick formalin-embedded sections stained with trichrome. Interstitial and perivascular fibrosis were assessed by a computer-aided image analysis program (AxioVision 4.8.2; Carl Zeiss Microscopy, Thornwood, NY). In each slide, trichrome staining was semiautomatically quantified in 6–10 fields, expressed as fraction of kidney surface area, and the results from all fields averaged. Tubular injury and glomerular scores were measured as described previously.16,18,19 Oxidative stress indicated by in situ production of superoxide anion was quantified in 30-µm dihydroethidium-stained slides.18
Western blotting
Standard western blotting protocols were followed using specific antibodies against total endothelial NO synthase (eNOS; 1:1,000, Abcam), phosphorylated eNOS (peNOS; 1:1,000, Cell Signaling), ET-1 (1:200, Santa Cruz), Endothelin-1 ETA (Abcam, 1:5,000 ab117521) and ETB (Abcam, 1:5,000 ab117529) receptors, and caveolin-1 (CAV-1; 1:1,000, Cell Signaling Technology). Protein expression was determined in each animal, and the intensities of the protein bands quantified and normalized for a GAPDH (1:5,000, Abcam) loading control (except for peNOS/total eNOS).
Statistical methods
Statistical analysis was performed using JMP software package version 9.0 (SAS Institute, Cary, NC). The Shapiro–Wilk test was used to test for deviation from normality. Normally distributed variables were expressed as mean ± SD. Comparisons within groups were performed using the paired Student’s t-test and among groups using analysis of variance and unpaired t-test with Tukey’s post hoc. For data that did not show a Gaussian distribution, results were expressed as median (range) and comparisons within and among the groups performed using nonparametric tests (Wilcoxon and Kruskal–Wallis, respectively) with Steel–Dwass post hoc. All tests were 2-tailed, and P values ≤ 0.05 were considered statistically significant.
RESULTS
Mean arterial pressure increased similarly in the HT and HTC groups (Table 1, P < 0.05 both), but decreased in both groups after RRHT, and was no longer significantly different from Normal. Body weight and plasma renin activity levels in the 5 groups were not significantly different, and serum cholesterol and low-density lipoprotein levels were elevated only in HTC and HTC+RRHT pigs (P ≤ 0.05 each). Serum creatinine levels did not differ among the groups (P = 0.131, analysis of variance). ET-1 levels increased only in HTC and HTC+RRHT pigs (P ≤ 0.05 each). Circulating PGF2α isoprostane levels increased in HT and HTC (both P ≤ 0.05 vs. Normal), were not different than normal in HT+RRHT, but remained elevated in HTC+RRHT (P = 0.04), whereas circulating thromboxane-A2 levels increased only in HTC (Figure 2C, P = 0.002).
Table 1.
Kidney function assessed in renovascular hypertensive and hypercholesterolemic pigs 4 weeks after reversal of HT
| Normal | HT | HT+RRHT | HTC | HTC+RRHT | P value (Shapiro– Wilk test) | P value (1-way ANOVA or Wilcoxon) | |
|---|---|---|---|---|---|---|---|
| Body weight, kg | 46.7±0.6 | 47.0±3.9 | 52.5±2.0 | 46.9±1.8 | 48.2±3.7 | 0.130 | 0.414 |
| Degree of stenosis, % | 0 (0–0) | 99 (90–100)* | 0 (0–0)# | 100 (95–100)*,$ | 0 (0–0)#,& | <0.001 | <0.0001 |
| MAP, mm Hg | 93.7 (89.3–99.0) | 141.7 (112.3–154.0)* | 80.7 (69.0–102.0)# | 146.5 (105.3–170.7)*,$ | 96.7 (80.0–102.3)#,& | 0.005 | 0.0005 |
| Total cholesterol, mg/dl | 85 (68–89) | 96 (58–125) | 77.3 (70.0–87.4) | 392.0 (104.0–1,043.0)*,#,$ | 509 (229–939)*,#,$ | <0.0001 | 0.0003 |
| LDL, mg/dl | 32 (25–50) | 37 (34–44.6) | 39.9 (33.8–44.2)$,& | 213.0 (50.8–746.2)*,#,$ | 387 (113.8–733.6)*,#,$ | <0.0001 | 0.0005 |
| Creatinine, mg/dl | 1.44±0.09 | 1.81±0.07 | 1.62±0.04 | 1.85±0.13 | 1.96±0.10 | 0.667 | 0.131 |
| PGF2α isoprostane, pg/ml | 95.9 (85.9–117) | 168 (95.2–312.3) | 116.4 (84.4–209.6) | 196.8 (127.6–364.3)* | 198.7 (147.5–250.0)* | 0.004 | 0.027 |
| Endothelin-1, pg/ml | 1.41±0.24 | 1.38±029 | 1.48±0.14 | 2.05±0.17*,#,$ | 1.84±0.11* | 0.708 | 0.003 |
| Thromboxane-A2, ng/ml | 3.1±0.5 | 4.5±2.4 | 4.0±0.5 | 6.6±0.4*,#,$ | 4.2±0.4#,& | 0.209 | <0.0001 |
| PRA, ng/ml/h | 0.26 (0.23–0.27) | 0.23 (0.21–0.25) | 0.24 (0.16–0.27) | 0.25 (0.21–0.27) | 0.25 (0.23–0.26) | 0.001 | 0.083 |
| RBF, ml/min | 493.6±72.9 | 799.1±202.2* | 436.5±174.0# | 751.8±231.5*,$ | 455.1±106.4#,& | 0.090 | 0.002 |
| Cortical perfusion, ml/min/ml | 3.9 (3.1–4.4) | 4.3 (2.9–5.8) | 3.8 (3.5–4.9) | 3.9 (3.3–6.2) | 3.7 (3.1–5.2) | 0.048 | 0.958 |
| Medullary perfusion, ml/min/ml | 3.7 (2.4–4.4) | 2.0 (0.9–2.3) | 1.9 (1.4–4.0) | 1.5 (1.0–4.2) | 1.7 (0.6–6.3) | 0.040 | 0.130 |
| Cortical volume, ml | 105.7 (98.5–114.9) | 185.6 (123.2–210.8)* | 98.2 (72.8–130.3)#,& | 150.0 (114.4–168.2)*,$ | 89.1 (74.9–99.0)#,& | 0.041 | 0.0003 |
| Medullary volume, ml | 22.7±7.3 | 31.7±8.8 | 16.4±7.6# | 26.3±7.4 | 14.2±4.8#,& | 0.491 | 0.004 |
| GFR, ml/min | 69.7±7.3 | 114.6±20.6* | 68.8±24.3# | 108.4±24.5*,$ | 61.2±9.5#,& | 0.076 | <0.0001 |
Results are mean ± SD or median (range). n = 7 each group.
Abbreviations: ANOVA, analysis of variance; GFR, glomerular filtration rate; HT, hypertension; HTC, HT and hypercholesterolemia; LDL, low-density lipoprotein; MAP, mean arterial pressure; PG, prostaglandin; PRA, plasma renin activity; RBF, renal blood flow; RRHT, reversal of renovascular HT.
*P < 0.05 vs. normal; # P < 0.05 vs. HT; $ P < 0.05 vs. HT+RRHT; & P < 0.05 vs. HTC (Student’s t-test with Tukey’s post hoc or Kruskal–Wallis with Steel–Dwass post hoc).
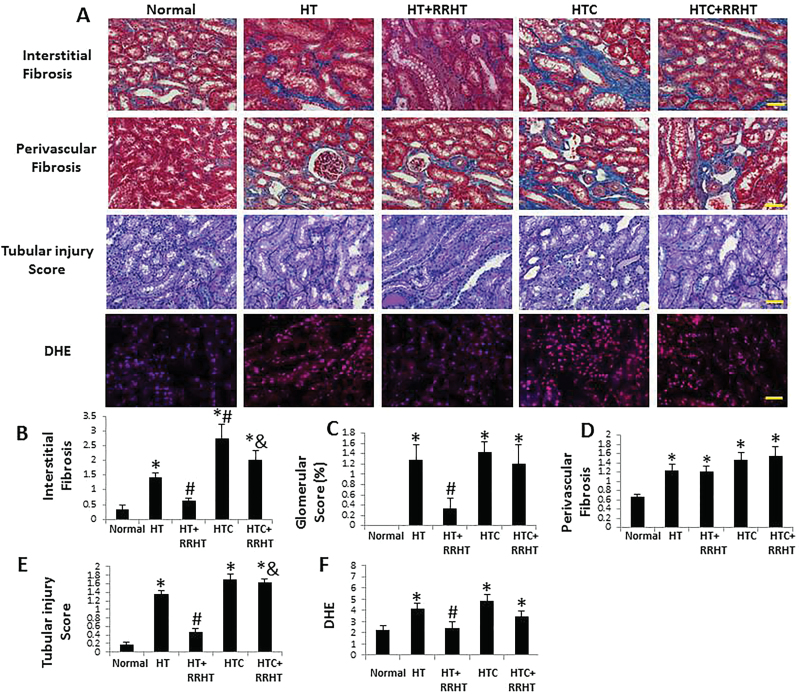
Figure 2.
Renal tissue remodeling. (A) Representative renal trichrome, periodic acid Schiff (PAS), and dihydroethidium (DHE) staining (all ×20). Renal interstitial fibrosis, glomerulosclerosis, and tubular injury increased in renovascular hypertension (HT), and HT and hypercholesterolemia (HTC) kidneys. After reversal of renovascular HT (RRHT), interstitial fibrosis and tubular injury decreased in HT, but not in HTC+RRHT (B, C, and E). Perivascular fibrosis was similarly increased in the HT, HT+RRHT, HTC, and HTC+RRHT groups (D). DHE staining was elevated in HT, HTC, and HTC+RRHT, suggesting increased oxidative stress, which decreased in HT+RRHT (F). *P < 0.05 vs. Normal; # P < 0.05 vs. HT; & P < 0.05 vs. HT+RRHT. Scale bar = 50 µm.
Renal hemodynamics and function
The degree of renal artery stenosis in HT and HTC was comparable (Table 1), and CLK RBF and GFR were elevated in both (P ≤ 0.05 each). After successful revascularization of the stenotic renal artery, RBF and GFR decreased to normal levels in both HT+RRHT and HTC+RRHT (P ≤ 0.05 each). Cortical and medullary perfusion were not significantly different among the 5 groups. Renal cortical volume was elevated in HT and HTC (P ≤ 0.05 vs. Normal, both) and decreased in both groups after RRHT (P ≤ 0.05 each). Differences among and between the groups persisted after adjusting by body weight (see Supplementary Table 1).
Renal tissue injury
Trichrome staining showed increased interstitial fibrosis in HT compared with Normal pigs (Figure 2, P < 0.01), which was more severe in HTC kidneys (P = 0.01 vs. HT). After RRHT, interstitial fibrosis in HT+RRHT kidneys was no longer significantly different from Normal but remained elevated in HTC+RRHT kidneys (P = 0.002 vs. Normal; P = 0.001 vs. HT+RRHT). Contrarily, perivascular fibrosis was similarly elevated in HT, HT+RRHT, HTC, and HTC+RRHT (P < 0.05 vs. Normal, each). Glomerular score increased in HT and HTC kidneys (both P < 0.001 vs. Normal), and decreased after RRHT in HT+RRHT (P = 0.02 vs. HT), but not in HTC+RRHT (P = 0.57 vs. HTC). Tubular injury observed in HT and HTC (Figure 2, both P < 0.001 vs. Normal) decreased in HT+RRHT (P < 0.001 vs. HT), but not in HTC+RRHT, which was significantly higher than in HT+RRHT (P < 0.001). Similarly, dihydroethidium staining was elevated in HT, HTC, and HTC+RRHT (P ≤ 0.04 each) but was restored in HT+RRHT (P = 0.04 vs. HT).
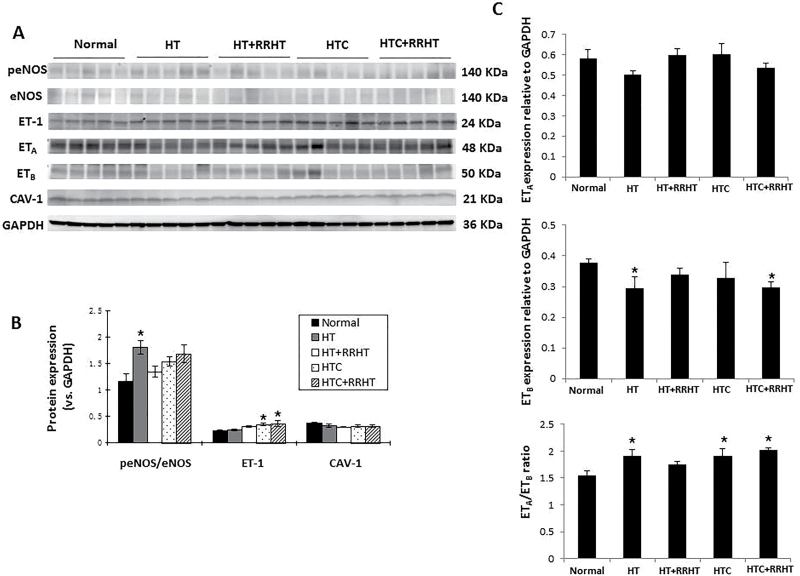
Compared with Normal, renal activation of eNOS (expression of peNOS/eNOS) increased in only HT kidneys (Figure 3A,B, P = 0.02 vs. Normal). Tissue expression of ET-1 increased in HTC and HTC+RRHT pigs (P = 0.05 and P = 0.03 vs. Normal, respectively). Expression of ETA was similar among the groups, whereas ETB expression decreased in HT and HTC+RRHT compared to Normal (Figure 2A,D, P ≤ 0.05 each). Consequently, ETA/ETB ratio increased in HT, HTC, and HTC+RRHT pigs (all P ≤ 0.05). Renal protein expression of CAV-1 showed no differences among the 5 groups.
Figure 3.
Western blotting and ELISA results. (A) Western bands in the experimental groups (5 bands per group) normalized to GAPDH or to total endothelial nitric oxide synthase (eNOS) (phosphorylated eNOS, peNOS). (B) Renal activation of eNOS increased only in HT kidneys. Endothelin-1 (ET-1) expression increased in HTC and HTC+RRHT pigs, while caveolin-1 (CAV-1) protein expression showed no differences among the 5 groups. (C) Expression of ETA was similar among the groups, whereas ETB expression decreased in HT and HTC+RRHT compared to Normal. Consequently, ETA/ETB ratio increased in HT, HTC, and HTC+RRHT pigs. *P < 0.05 vs. Normal. Abbreviations: HT, hypertension; HTC, HT and hypercholesterolemia; RRHT, reversal of renovascular HT.
DISCUSSION
This study demonstrates that concurrent HC attenuates the regression of nonstenotic kidney injury following revascularization and reversal of swine renovascular HT. Although CLK RBF and GFR were not different from normal in the HTC+RRHT kidney 1 month after RRHT, oxidative stress, ET-1 expression, and ETA/ETB expression ratio remained upregulated, and persistence of tissue damage may contribute to progression of renal injury.
Unilateral renal artery stenosis leads to ischemia and excretory dysfunction in the poststenotic kidney.20 In the nonstenotic kidney, long-standing HT induces vascular and glomerular damage, including arteriolosclerosis and glomerulosclerosis,6 vascular injury, and tubular dysfunction,19,21 with a progressive decline in renal function. Indeed, comorbid cardiovascular risk factors like diabetes and HC increase CLK injury.22
HT and HTC are both characterized by increased oxidative stress, and their coexistence augments ROS production and renal dysfunction.14 Renal failure is less common in HT than in HTC, suggesting that atherogenic factors contribute to renal injury in HTC.23 In our HT and HTC models, both systemic and nonstenotic kidney oxidative stress increased (PGF2α isoprostane and dihydroethidium). However, after RRHT, oxidative stress decreased in HT+RRHT pigs but remained elevated in HTC+RRHT. Many forms of kidney diseases are aggravated by increased oxidative stress,2,24,25 and its persistence in the HTC+RRHT kidney likely reflects ongoing tissue injury. In addition, HT and HTC kidneys showed pronounced interstitial and perivascular fibrosis, as well as glomerulosclerosis and tubular injury. After successful RRHT, kidney injury declined in HT, but was again unresolved in HTC+RRHT pigs, possibly due to sustained HC, oxidative stress, and vasoconstriction.
HC is considered a surrogate for early atherosclerosis, and with coexisting HT magnifies tissue injury and fibrosis. Interestingly, eNOS was activated in the HT kidney, possibly due to increased shear stress, but failed to increase in HTC, possibly because HC reduces eNOS activation.26 Consequent deficiency in the protective effects of NO may lead to inflammation, thrombogenesis, vasoconstriction, and elevated prevalence of ROS. Increased generation of ROS can in turn result in predominance of renal vasoconstrictors, enhanced renal vascular tone, and premature senescence.27 Furthermore, HC upregulates the expression of ET-1,28,29 a potent vasoconstrictor and mitogenic peptide that contributes to the pathogenesis and maintenance of HT, oxidative stress, and inflammation. In this study, systemic and nonstenotic kidney ET-1 increased in HTC and remained elevated in HTC+RRHT, which might have aggravated kidney fibrosis and tubular injury. The physiological actions of the ET peptides may be mediated through changes in the ratio of the ETA and ETB receptor subtypes.30 While the expression of ETA was unchanged, ETB expression decreased in HT and HTC+RRHT compared to Normal. Consequently, ETA/ETB ratio increased in HT, HTC, and HTC+RRHT pigs, suggesting that ETA remained the dominating receptor subtype.31 These data implicate ET-1 signaling in kidney injury after RRHT. On the other hand, thromboxane-A2 enhances ROS, ET-1, microvascular remodeling, and contractility in mice with chronic kidney disease,32 but its systemic levels that were elevated in HTC fell after revascularization, linking it primarily to the HT that was resolved.
Limitations
We used young animals with short duration of disease and no additional comorbidities. This might limit the clinical translation power of our observations. Nevertheless, this study has a number of strengths. Our swine model reproduces the effects of early HT and HTC, and allows studying single-kidney function and structure using clinically applicable tools, offering the opportunity to assess the difference of RRHT to HT and HTC. Our tomographic imaging allowed quantification of individual kidney hemodynamics and function and demonstrated the blunted efficacy of RRHT in HTC.
In conclusion, this study demonstrates that HTC induces systemic and renal oxidative stress and elicits renal injury and dysfunction. RRHT alone can attenuate the nonstenotic kidney injury in experimental renovascular HT, but fails to reverse it in HTC, possibly due to enduring HC and persistent oxidative stress and activation of ET-1 signaling. RRHT leads to a decrease in compensatory hypertrophy and hyperfiltration in the nonstenotic HTC kidney but fails to achieve complete regression of tissue injury. Possibly, drugs like HMG CO-A inhibitors might not only lower cholesterol levels but also improve kidney outcomes by their pleiotropic effects.33 Taken together, these observations suggest a need for multipronged approach to blunt underlying mechanisms in order to halt progressive loss of renal function in atherosclerotic renovascular disease.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
This work was partly supported by National Institutes of Health grant numbers DK73608, HL12160, DK106427, DK104273, DK100081, and DK102325. We thank the Jiangsu Overseas & Training Program for University Prominent Young & Middle-aged Teachers and Presidents, the project of key medical personnel of Jiangsu Province (RC2011116), and the project of National Natural Science Foundation of China (81270769).
REFERENCES
- 1. Appel LJ, Wright JT, Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 2002; 106:1165–1171. [DOI] [PubMed] [Google Scholar]
- 3. Li Y, Xie D, Qin X, Tang G, Xing H, Li Z, Xu X, Xu X, Hou F. Metabolic syndrome, but not insulin resistance, is associated with an increased risk of renal function decline. Clin Nutr 2015; 34:269–275. [DOI] [PubMed] [Google Scholar]
- 4. Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA. Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens 2013; 22:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ofstad J, Iversen BM. Glomerular and tubular damage in normotensive and hypertensive rats. Am J Physiol Renal Physiol 2005; 288:F665–F672. [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez-Porcel M, Krier JD, Lerman A, Sheedy PF, II, Romero JC, Napoli C, Lerman LO. Combination of hypercholesterolemia and hypertension augments renal function abnormalities. Hypertension 2001; 37:774–780. [DOI] [PubMed] [Google Scholar]
- 7. Tonelli M, Wiebe N, Guthrie B, James MT, Quan H, Fortin M, Klarenbach SW, Sargious P, Straus S, Lewanczuk R, Ronksley PE, Manns BJ, Hemmelgarn BR. Comorbidity as a driver of adverse outcomes in people with chronic kidney disease. Kidney Int 2015; 88:859–866. [DOI] [PubMed] [Google Scholar]
- 8. Lavi R, Zhu XY, Chade AR, Lin J, Lerman A, Lerman LO. Simvastatin decreases endothelial progenitor cell apoptosis in the kidney of hypertensive hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol 2010; 30:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB, Sr, Dworkin LD. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eirin A, Zhu XY, Urbieta-Caceres VH, Grande JP, Lerman A, Textor SC, Lerman LO. Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am J Physiol Renal Physiol 2011; 300:F1394–F1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Favreau F, Zhu XY, Krier JD, Lin J, Warner L, Textor SC, Lerman LO. Revascularization of swine renal artery stenosis improves renal function but not the changes in vascular structure. Kidney Int 2010; 78:1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saad A, Herrmann SM, Crane J, Glockner JF, McKusick MA, Misra S, Eirin A, Ebrahimi B, Lerman LO, Textor SC. Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ Cardiovasc Interv 2013; 6:428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu XY, Rodriguez-Porcel M, Bentley MD, Chade AR, Sica V, Napoli C, Caplice N, Ritman EL, Lerman A, Lerman LO. Antioxidant intervention attenuates myocardial neovascularization in hypercholesterolemia. Circulation 2004; 109:2109–2115. [DOI] [PubMed] [Google Scholar]
- 14. Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 2009; 119:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Urbieta-Caceres VH, Lavi R, Zhu XY, Crane JA, Textor SC, Lerman A, Lerman LO. Early atherosclerosis aggravates the effect of renal artery stenosis on the swine kidney. Am J Physiol Renal Physiol 2010; 299:F135–F140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun D, Eirin A, Zhu XY, Zhang X, Crane JA, Woollard JR, Lerman A, Lerman LO. Experimental coronary artery stenosis accelerates kidney damage in renovascular hypertensive swine. Kidney Int 2015; 87:719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eirin A, Ebrahimi B, Zhang X, Zhu XY, Tang H, Crane JA, Lerman A, Textor SC, Lerman LO. Changes in glomerular filtration rate after renal revascularization correlate with microvascular hemodynamics and inflammation in Swine renal artery stenosis. Circ Cardiovasc Interv 2012; 5:720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ebrahimi B, Eirin A, Li Z, Zhu XY, Zhang X, Lerman A, Textor SC, Lerman LO. Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS One 2013; 8:e67474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higashi Y, Oshima T, Ozono R, Matsuura H, Kajiyama G. Aging and severity of hypertension attenuate endothelium-dependent renal vascular relaxation in humans. Hypertension 1997; 30:252–258. [DOI] [PubMed] [Google Scholar]
- 20. Dean RH, Tribble RW, Hansen KJ, O’Neil E, Craven TE, Redding JF., II Evolution of renal insufficiency in ischemic nephropathy. Ann Surg 1991; 213:446–455; discussion 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ploth DW, Roy RN, Huang WC, Navar LG. Impaired renal blood flow and cortical pressure autoregulation in contralateral kidneys of Goldblatt hypertensive rats. Hypertension 1981; 3:67–74. [DOI] [PubMed] [Google Scholar]
- 22. Hartono SP, Knudsen BE, Lerman LO, Textor SC, Grande JP. Combined effect of hyperfiltration and renin angiotensin system activation on development of chronic kidney disease in diabetic db/db mice. BMC Nephrol 2014; 15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med 2001; 344:431–442. [DOI] [PubMed] [Google Scholar]
- 24. Chade AR, Rodriguez-Porcel M, Herrmann J, Zhu X, Grande JP, Napoli C, Lerman A, Lerman LO. Antioxidant intervention blunts renal injury in experimental renovascular disease. J Am Soc Nephrol 2004; 15:958–966. [DOI] [PubMed] [Google Scholar]
- 25. Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol 2004; 24:1854–1859. [DOI] [PubMed] [Google Scholar]
- 26. Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem 1999; 274:32512–32519. [DOI] [PubMed] [Google Scholar]
- 27. Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009; 52:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Catar RA, Muller G, Brandt A, Langbein H, Brunssen C, Goettsch C, Frenzel A, Hofmann A, Goettsch W, Steinbronn N, Strasser RH, Schubert U, Ludwig B, Bornstein SR, Morawietz H. Increased gene expression of the cardiac endothelin system in obese mice. Horm Metab Res 2015; 47:509–515. [DOI] [PubMed] [Google Scholar]
- 29. Chade AR, Krier JD, Textor SC, Lerman A, Lerman LO. Endothelin-a receptor blockade improves renal microvascular architecture and function in experimental hypercholesterolemia. J Am Soc Nephrol 2006; 17:3394–3403. [DOI] [PubMed] [Google Scholar]
- 30. O’Reilly G, Charnock-Jones DS, Davenport AP, Cameron IT, Smith SK. Presence of messenger ribonucleic acid for endothelin-1, endothelin-2, and endothelin-3 in human endometrium and a change in the ratio of ETA and ETB receptor subtype across the menstrual cycle. J Clin Endocrinol Metab 1992; 75:1545–1549. [DOI] [PubMed] [Google Scholar]
- 31. Möller S, Uddman R, Granström B, Edvinsson L. Altered ratio of endothelin ET(A)- and ET(B) receptor mRNA in bronchial biopsies from patients with asthma and chronic airway obstruction. Eur J Pharmacol 1999; 365:R1–R3. [DOI] [PubMed] [Google Scholar]
- 32. Wang C, Luo Z, Kohan D, Wellstein A, Jose PA, Welch WJ, Wilcox CS, Wang D. Thromboxane prostanoid receptors enhance contractions, endothelin-1, and oxidative stress in microvessels from mice with chronic kidney disease. Hypertension 2015; 65:1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Juncos LI, Juncos LA, García NH. The antihypertensive actions of statins: modulation by salt intake. Am J Hypertens 2012; 25:1140–1148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.