Despite increased human immunodeficiency virus (HIV) care policy efforts, and overall improvements in testing and care for HIV-infected persons, we found that disparities along the HIV care continuum remained for children, intravenous drug users, prisoners, and men who have sex with men.
Keywords: HIV care, disparity, HAART, continuum of care, China
Abstract
Background. The 90-90-90 targets recommended by the Joint United Nations Programme on HIV/AIDS require strengthening human immunodeficiency virus (HIV) care, which includes diagnosis, linkage to and retention in care, assessment for treatment suitability, and optimization of HIV treatment. We sought to quantify patient engagement along the continuum, 10 years after introduction of Chinese HIV care policies.
Methods. We included patients from Shandong, China, who were diagnosed with HIV from 1992 to 2013. Records were obtained from the HIV/AIDS Comprehensive Response Information Management System to populate a 7-step HIV care continuum. Pearson χ2 test and multivariate logistic regression were used for analysis.
Results. Of 6500 estimated HIV-infected persons, 60.1% were diagnosed, of whom 41.9% received highly active antiretroviral therapy (HAART). Only 59.6% of patients on HAART and 15% of all infected persons achieved viral suppression. Children infected by mother-to-child transmission (MTCT) and persons infected by intravenous drug use were less likely to be linked to and retained in care (odds ratio [OR], 0.33 [95% confidence interval {CI}, .14–.80] and OR, 0.58 [95% CI, .40–.90], respectively). Persons tested in custodial institutions were substantially less likely to be on HAART (OR, 0.22 [95% CI, .09–.59]) compared with those tested in medical facilities. Patients on HAART infected by homosexual or heterosexual transmission and those infected by MTCT were less likely to achieve viral suppression (OR, 0.18 [95% CI, .09–.34]; OR, 0.12 [95% CI, .06–.22]; OR, 0.07 [95% CI, .02–.20], respectively).
Conclusions. Our report suggests, at the current rate, Shandong Province has to accelerate HIV care efforts to close disparities in HIV care and achieve the 90-90-90 goals equitably.
Gardner and colleagues described the continuum of human immunodeficiency virus (HIV) care as starting with diagnosis and continuing to enrollment of patients in services; subsequently, HIV patients have follow-up maintenance visits, and, based on eligibility, patients receive highly active antiretroviral therapy (HAART) with the ultimate goal of viral suppression [1]. Along these lines, the Joint United Nations Programme on HIV/AIDS (UNAIDS) recently set objectives to end the AIDS epidemic by 2020. These include (1) increasing testing so that 90% of people living with HIV and AIDS (PLWHA) know their status; (2) ensuring 90% of diagnosed PLWHA receive sustained antiretroviral therapy; and (3) helping 90% of PLWHA on HAART achieve viral suppression [2]. However, few HIV-infected persons successfully navigate the HIV continuum. Only 6.3% of the general Chinese population has ever received an HIV test [3]. Researchers have found that substantial loss to follow-up (LTFU) occurs after testing. Pretreatment losses to care before HAART initiation range from 25% to 45%. Furthermore, only half of HIV-infected patients who know their infection status are regularly engaged in care [1]. Missed opportunities for PLWHA to link to care services and continue their engagement are associated with increased HAART resistance and greater mortality [4–6]. Patients on HAART should also have their treatment optimized, which means at least 70%–80% adherence for durable viral suppression to occur [7].Wang et al, however, reported that 20% of patients in central China missed at least 1 dose in the last week [8].
Shandong Province, a coastal province in eastern China of 97.3 million inhabitants, began to tackle gaps in HIV testing and treatment in 2004. Since 2004, policies such as Four Frees and One Care have provided no-cost HIV services to nearly 100 million people [9]. Testing, counseling, and psychological support became widely available at Chinese Center for Disease Control and Prevention (CDC) locations. In 2013, there were 457 voluntary counseling and testing (VCT) locations. Patients began review for HAART eligibility and received HAART, as well as regular monitoring of CD4 counts and viral load (VL). Furthermore, government finance provided reimbursement to patients for meals and transportation to improve retention in care.
Limited evidence suggests that disparities in HIV testing and care in China remain despite supportive national HIV policies [10]. We investigate the magnitude of disparities by stage of engagement in HIV care cascade, age at entry to care, sex, mode of transmission, education level, and testing venue. We hypothesized that inequalities in HIV testing and treatment would remain in Shandong Province despite a free, inclusive, nationwide HIV care policy.
MATERIALS AND METHODS
Study Population
We conducted a cross-sectional study of HIV patient records in 2013 from Shandong Province, China. Patients selected were aged ≥18 months. Both community-dwelling and patients under custodial care were included.
Database
We extracted cases from the Shandong Provincial HIV/AIDS Comprehensive Response Information Management System (CRIMS) at the end of 31 October 2014. CRIMS is a Web-based surveillance system used for public health reporting of HIV cases. Confirmed HIV cases are reported in CRIMS by the testing physician. Thereafter, the local health department (referred to as the CDC) contacts the newly diagnosed patient to gather additional epidemiological information. Upon enrollment, patients typically are interviewed face-to-face every 3–6 months. During the course of care, additional information entered into CRIMS may include age, sex, education level, patient address, likely transmission route, testing venue, CD4 count, VL, and HAART use. Interviewers collect data using a confidential standardized interview questionnaire and then record responses and clinical data in the system. CRIMS has been used extensively in HIV research in China [11, 12].
Data Management
Physicians are responsible for ordering HIV antibody testing and reporting results on the Uniform Infectious Disease Report Card. Local CDC staff personnel conducted field-level inquiries to confirm information as a means to reduce missing data. Shandong CDC staff verify entries from the provincial CRIMS database to identify errors and duplicates. All personal identifiers were removed from the database before analysis to protect participants' privacy.
Definition of Variables
Custodial institutions included reeducation centers/detention houses/intravenous drug user (IDU) prisons, temporary detention centers, and mandatory treatment programs. A child was defined as someone <15 years of age. A confirmed diagnosis of HIV was defined as a reactive Western blot (Genelabs Diagnostics Pet Ltd); linkage to care was defined as documentation of ≥1 CD4 or VL test result within 180 days of diagnosis. The following variables were defined as occurring within 12 months of censoring (October 2014): retention in HIV care (≥2 CD4 count or VL test results separated by ≥90 days); HAART eligibility as determined according to World Health Organization (WHO) criteria [13, 14]; HAART use (≥1 prescription refill); and viral suppression (defined as VL < 50 copies/mL).
Statistical Analysis
First, the prevalence of HIV-infected individuals in 2013, including diagnosed and undiagnosed cases, was estimated by workbook method [15–17]. Sex and transmission mode were also estimated by this method. The total number of infections by age, education, and testing venue could not be estimated by the workbook method. Second, we calculated the cumulative percentages for each stage of the continuum. For sex and transmission, the total number of estimated infected persons was used as the denominator for the stages from diagnosis to viral suppression; in contrast, for age at entry to care, education, and testing venue, the number of total patients diagnosed was used as the denominator for the stages from linkage to viral suppression. Pearson χ2 test was used to tabulate associations between sex, transmission route, age at entry to care, education level, and testing venue. Partition of χ2 test (ά) was used to determine whether differences in viral suppression were statistically significant between subpopulations when accounting for the number of comparisons. Third, we calculated the proportion of patients engaged in steps from linkage to viral suppression by using the number of patients who engaged in the preceding step—for example, the denominator for the step of linkage is the number of diagnosed patients, and the denominator for the step of retention is the number of patients who have been linked to HIV care. We modeled the association between steps in the care continuum and patient characteristics using multivariate logistic regression to calculate odd ratios (ORs) and 95% confidence intervals (CIs). SAS software, version 9.2 (SAS Institute, Cary, North Carolina) was used for analysis.
RESULTS
HIV Infection and Diagnosis
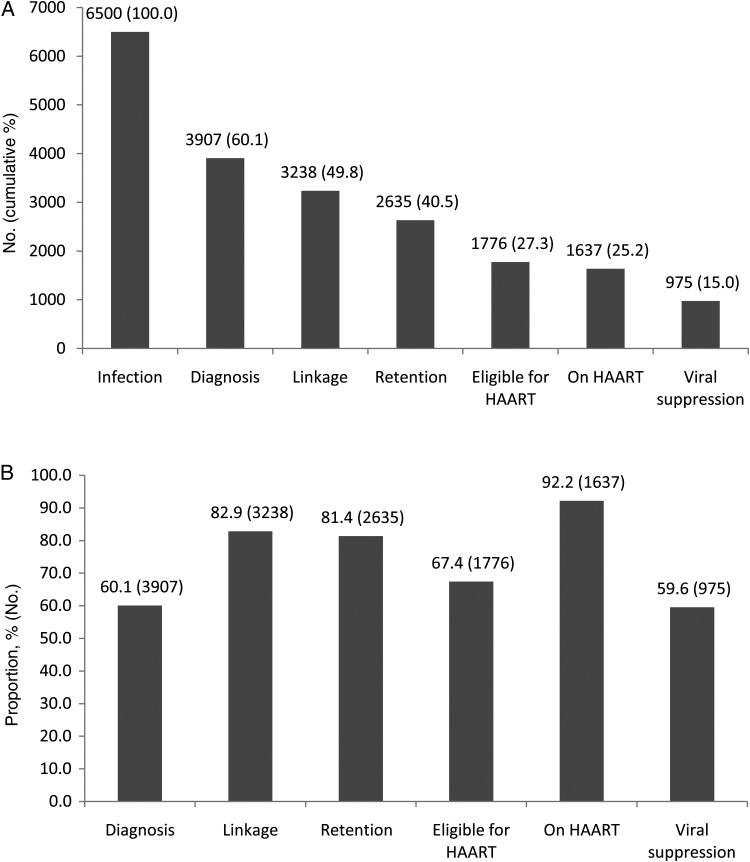
We estimated that there were 6500 (range, 4600–8400) PLWHA by the end of 2013. Table 1 shows patient characteristics by stage. Most infections occurred in men (n = 5550 [85.4%]) and were through homosexual transmission (n = 3810 [58.6%]); most diagnosed infections were among people aged 25–34 years (n = 1465 [37.5%]). VCT locations were the most utilized testing venue (n = 2444 [62.6%]). Among all infected persons, 60.1% were diagnosed (Figure 1A). Thus, 39.9% of PLWHA (n = 2593) were unaware of their HIV status. Among those unaware of their status, 1930 (74.4%) attributed their infection to homosexual transmission.
Table 1.
Estimated Numbers of Patients With Human Immunodeficiency Virus (HIV)/AIDS in 2013 and Engagement in the HIV Care Continuum by Demographic Characteristics, Shandong Province, China
| Characteristic | Unaware | No. (Cumulative %) |
||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis | Linkage | Retention | Eligible for HAART | On HAART | Viral Suppression | P Value | ||
| Sex | ||||||||
| Male (n = 5550 [range, 3900–7200]) | 2332 | 3218* (58.0) | 2741 (49.4) | 2219* (40.0) | 1451* (26.1) | 1327* (23.9) | 760* (13.7) | ** |
| Female (n = 950 [range, 700–1200]) | 261 | 689 (72.5) | 497 (52.3) | 416 (43.8) | 325 (34.2) | 310 (32.6) | 215 (22.6) | a |
| Transmission mode | ||||||||
| Blood donation/transfusion (n = 260 [range, 180–340]) | 39 | 221* (85.0) | 143* (55.0) | 133* (51.2) | 125* (48.1) | 122* (46.9) | 97* (37.3) | a |
| Homosexual (n = 3810 [range, 2700–4920]) | 1930 | 1880 (49.3) | 1745 (45.8) | 1428 (37.5) | 846 (22.2) | 770 (20.2) | 432 (11.3) | ** |
| IDU (n = 190 [range, 130–250]) | 42 | 148 (77.9) | 85 (44.7) | 58 (50.5) | 48 (25.3) | 35 (18.4) | 20 (10.5) | ** |
| Heterosexual (n = 2050 [range, 1450–2650]) | 568 | 1482 (72.3) | 1173 (57.2) | 948 (46.2) | 712 (34.7) | 666 (32.5) | 406 (19.8) | ** |
| MTCT (n = 190 [range, 130–250]) | 14 | 176 (92.6) | 92 (48.4) | 68 (35.8) | 45 (23.7) | 44 (23.2) | 20 (10.5) | ** |
| Age at entry to care | ||||||||
| <15 y | … | 176 | 92* (52.3) | 68* (38.6) | 45* (25.6) | 44* (25.0) | 20* (11.4) | ** |
| 15–24 y | … | 846 | 784 (92.7) | 580 (68.6) | 330 (39.0) | 286 (33.8) | 166 (19.6) | ** |
| 25–34 y | … | 1465 | 1214 (82.9) | 984 (67.2) | 626 (42.7) | 581 (39.7) | 363 (24.8) | |
| 35–44 y | … | 960 | 770 (80.2) | 677 (70.5) | 514 (53.5) | 487 (50.7) | 284 (29.6) | |
| 45–54 y | … | 329 | 261 (79.3) | 227 (69.0) | 180 (54.7) | 165 (50.2) | 99 (30.1) | |
| ≥55 y | … | 131 | 117 (89.3) | 99 (75.6) | 81 (61.8) | 74 (56.5) | 43 (32.8) | a |
| Education | ||||||||
| Illiterate | … | 258 | 156* (60.5) | 124* (48.1) | 95* (36.8) | 88* (34.1) | 50* (19.4) | a |
| Primary | … | 505 | 353 (69.9) | 290 (57.4) | 236 (46.7) | 219 (43.4) | 139 (27.5) | |
| Junior middle | … | 1166 | 960 (82.3) | 783 (67.2) | 568 (48.7) | 533 (45.7) | 322 (27.6) | |
| Junior high | … | 948 | 834 (88.0) | 674 (71.1) | 428 (45.1) | 385 (40.6) | 237 (25.0) | |
| College or greater | … | 1030 | 935 (90.8) | 764 (74.2) | 449 (43.6) | 412 (40.0) | 227 (22.0) | |
| Testing venue | ||||||||
| Medical facility | … | 1166 | 1014* (87.0) | 849* (72.8) | 634* (54.4) | 595* (51.0) | 359* (30.8) | a |
| VCT | … | 2444 | 2041 (83.5) | 1656 (67.8) | 1045 (42.8) | 959 (39.2) | 563 (23.0) | ** |
| Custody institution | … | 177 | 94 (53.1) | 57 (32.2) | 44 (24.9) | 32 (18.1) | 16 (9.0) | ** |
| Other | … | 120 | 89 (74.2) | 73 (60.8) | 53 (44.2) | 51 (42.5) | 37 (30.8) | |
For sex and transmission, the total number of infected persons was used as the denominator when calculating cumulative percentages for each stage in the continuum. For age at entry to care, education, and testing venue, the total number diagnosed was used as the denominator for subsequent stages.
Abbreviations: HAART, highly active antiretroviral therapy; IDU, intravenous drug user; MTCT, mother-to-child transmission; VCT, voluntary counseling and testing.
a Reference category for partition of χ2 test in the step of achieving viral suppression.
*P < .05 for χ2 test in each step of HIV care.
**P < ά for partition of χ2 test, ά = .00625 for transmission mode and education, ά = .001 for age at entry, ά = .0083 for testing venue.
Figure 1.
A, Estimated number of patients with human immunodeficiency virus (HIV)/AIDS in 2013 and engagement in HIV care continuum in Shandong Province, China. The total number of persons living with HIV is the denominator for all stages. B, Proportion of patients with HIV/AIDS engaged in each step of the care continuum among those engaged in the preceding step in Shandong Province, China. The denominator for the first step (diagnosis) is the total number of persons living with HIV (n = 6500), and for the other steps it is the number of patients who engaged in the preceding step. Abbreviation: HAART, highly active antiretroviral therapy.
Care Linkage and Retention
Among all estimated infections, 3238 (49.8%) patients were linked to care and 2635 (40.5%) subsequently were retained in care (Figure 1A). Figure 1B shows the proportion of patients engaged in each step of the care among those engaged in the preceding step. For every 10 patients diagnosed, 8 were linked to care (82.9%). Significant differences were found between men and women for retention to but not for linkage in care (Table 1). Sex, however, was not related to these stages of care in multivariate analysis (Table 2). Compared to patients infected by blood donation/transfusion, children infected by mother-to-child transmission (MTCT) were approximately 40% less likely to be linked to care (OR, 0.58 [95% CI, .40–.90]), and persons infected by intravenous drug use were almost 70% less likely to be retained in care (OR, 0.33 [95% CI, .14–.80]). Infected persons aged 25–54 were significantly less likely to be linked to care compared with persons ≥55 years. Patients aged 15–24 were almost half as likely to attend follow appointments (OR, 0.48 [95% CI, .27–.84]). Patients tested by the custody institutions were less likely to be retained in care than were those tested by medical facilities (OR, 0.43 [95% CI, .25–.76]).
Table 2.
Multivariate Logistic Regression Analysis of Human Immunodeficiency Virus (HIV)/AIDS Patient Attributes on Stage of HIV Care, Shandong Province, China
| Variable at Enrollment | Linkage |
Retention |
HAART Eligibility |
On HAART |
Viral Suppression |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | OR (95% CI) | No. | OR (95% CI) | No. | OR (95% CI) | No. | OR (95% CI) | No. | OR (95% CI) | |
| Sex | ||||||||||
| Male | 3218 | 1.25 (.95–1.65) | 2741 | 0.73 (.53–1.01) | 2219 | 0.90 (.65–1.26) | 1451 | 0.41 (.18–.94) | 1327 | 0.98 (.70–1.38) |
| Female | 689 | 1.00 | 497 | 1.00 | 416 | 1.00 | 325 | 1.00 | 310 | 1.00 |
| Transmission mode | ||||||||||
| Blood donation/transfusion | 221 | 1.00 | 143 | 1.00 | 133 | 1.00 | 125 | 1.00 | 122 | 1.00 |
| Homosexual | 1880 | 6.21 (4.07–9.48) | 1745 | 0.52 (.26–1.04) | 1428 | 0.12 (.05–.28) | 846 | 0.41 (.12–1.42) | 770 | 0.18 (.09–.34) |
| IDU | 148 | 0.50 (.31–1.08) | 85 | 0.33 (.14–.80) | 58 | 0.31 (.10–.97) | 48 | 0.12 (.03–.50) | 35 | 0.61 (.19–1.95) |
| Heterosexual | 1482 | 2.30 (1.62–3.27) | 1173 | 0.38 (.20–.75) | 948 | 0.18 (.08–.42) | 712 | 0.51 (.15–1.76) | 666 | 0.12 (.06–.22) |
| MTCT | 176 | 0.58 (.40–.90) | 92 | 0.33 (.11–.99) | 68 | 0.45 (.10–2.04) | 45 | 1.77 (.06–37.3) | 44 | 0.07 (.02–.20) |
| Age at entry to care | ||||||||||
| <15 y | 176 | 0.76 (.23–2.58) | 92 | 0.73 (.23–2.36) | 68 | 0.13 (.03–.58) | 45 | 0.95 (.04–21.6) | 44 | 1.07 (.29–3.97) |
| 15–24 y | 846 | 1.51 (.82–2.79) | 784 | 0.48 (.27–.84) | 580 | 0.49 (.27–.87) | 330 | 0.72 (.27–1.97) | 286 | 0.93 (.51–1.67) |
| 25–34 y | 1465 | 0.47 (.27–.83) | 1214 | 0.77 (.46–1.30) | 984 | 0.61 (.35–1.07) | 626 | 1.52 (.57–4.06) | 581 | 1.19 (.70–2.03) |
| 35–44 y | 960 | 0.49 (.27–.71) | 770 | 1.28 (.73–2.25) | 677 | 0.89 (.50–1.55) | 514 | 2.38 (.87–6.50) | 487 | 1.01 (.62–1.78) |
| 45–54 y | 329 | 0.37 (.16–.88) | 261 | 1.20 (.64–2.26) | 227 | 0.85 (.47–1.88) | 180 | 1.11 (.39–3.21) | 165 | 1.04 (.62–1.88) |
| ≥55 y | 131 | 1.00 | 117 | 1.00 | 99 | 1.00 | 81 | 1.00 | 74 | 1.00 |
| Education level | ||||||||||
| Illiterate | 258 | 1.00 | 156 | 1.00 | 124 | 1.00 | 95 | 1.00 | 88 | 1.00 |
| Primary | 505 | 1.03 (.67–1.57) | 353 | 1.22 (.71–2.10) | 290 | 1.32 (.73–2.37) | 236 | 1.46 (.46–4.65) | 219 | 0.80 (.44–1.46) |
| Junior middle | 1166 | 1.47 (.95–2.27) | 960 | 1.21 (.70–2.09) | 783 | 0.91 (.53–1.90) | 568 | 2.00 (.65–6.19) | 533 | 1.58 (. 88–2.86) |
| Junior high | 948 | 2.04 (1.26–3.34) | 834 | 1.24 (.70–2.16) | 674 | 0. 86 (.48–1.55) | 428 | 0.68 (.41–4.13) | 385 | 1.56 (.84–2.89) |
| College or greater | 1030 | 2.79 (1.64–4.74) | 935 | 1.43 (.80–2.55) | 764 | 0.84 (.46–1.54) | 449 | 0.99 (.60–6.66) | 412 | 1.68 (.89–3.17) |
| Testing venue | ||||||||||
| Medical facility | 1166 | 1.00 | 1014 | 1.00 | 849 | 1.00 | 634 | 1.00 | 595 | 1.00 |
| VCT | 2444 | 0.56 (.43–.73) | 2041 | 0.91 (.73–1.14) | 1656 | 0.74 (.60–.91) | 1045 | 0.82 (.51–1.33) | 959 | 0.82 (.64–1.03) |
| Custody institutions | 177 | 0.40 (.24–.68) | 94 | 0.43 (.25–.76) | 57 | 0.73 (.35–1.51) | 44 | 0.22 (.09–.59) | 32 | 0.86 (.45–1.67) |
| Other | 120 | 0.43 (.24–.75) | 89 | 1.04 (.56–1.92) | 73 | 0.98 (.56–1.85) | 53 | 1.36 (.30–6.09) | 51 | 2.25 (.87–5.82) |
ORs and 95% CIs were calculated based on the engagement in the preceding step of the continuum.
Abbreviations: CI, confidence interval; HAART, highly active antiretroviral therapy; IDU, intravenous drug user; MTCT, mother-to-child transmission; OR, odds ratio; VCT, voluntary counseling and testing.
Eligibility for HAART and Sustained HAART Treatment
Almost 1 in 4 persons infected with HIV eventually received HAART (Figure 1A). Among HAART-eligible patients, >90% had a record of use (Figure 1B). Compared with patients infected by blood donation/transfusion, those infected sexually and by intravenous drug use were less likely to be eligible for HAART. Children and persons aged 15–24 years were less likely to be eligible for HAART compared with those aged ≥55 years (OR, 0.13 [95% CI, .03–.58] and OR, 0.49 [95% CI, .27–.87], respectively) (Table 2). Patients infected by IDU were less likely to be prescribed HAART compared with those infected by blood donation/transfusion (OR, 0.12 [95% CI, .03–.50]). Patients tested by custodial institutions were less likely to be prescribed HAART compared with those tested by medical facilities (OR, 0.22 [95% CI, .09–.59]).
Viral Suppression
For every 7 HIV-infected persons, only 1 had documented viral suppression (Figure 1A). Stated differently, the gap between diagnosis and viral suppression was an absolute difference of 45.1%. For patients on HAART, 59.6% had documented viral suppression (Figure 1B). More men than women achieved viral suppression (Table 1; P < .001). This relationship, however, was insignificant in multivariate analysis (Table 2). Children on HAART infected by MTCT were almost 93% less likely to achieve viral suppression (OR, 0.07 [95% CI, .02–.20]) compared with those infected by blood donation/transfusion. Viral suppression was less likely to occur for patients on HAART when infections were acquired via homosexual sex (OR, 0.18 [95% CI, .09–.34]) or heterosexual sex (OR, 0.12 [95% CI, .06–.22]), in comparison to infections acquired by blood donation/transfusion.
DISCUSSION
We anticipated that more undiagnosed HIV infections would have been tested in Shandong Province. VCT and provider-initiated HIV testing and counseling in clinics are widely available at no cost in Shandong Province. Furthermore, free testing has been extended to outreach programs for high-risk populations, pregnant women, and prisoners to improve detection rates [9, 18–20]. In addition, considering the demographic characteristics of undiagnosed infections, we anticipated that a greater number of men who have sex with men (MSM) in our study would have been diagnosed given their high reported testing rates [21, 22]. Acceptance of HIV testing among the average-risk Chinese population, however, is low [23, 24]. As in other areas of China, stigma, not knowing where to access free testing, fear of testing HIV positive, privacy concerns, perception of low susceptibility to infection, and deficient referral mechanisms may have contributed to the low overall testing rates observed in our study [21, 25–27]. Factors facilitating testing such as hiring peer counselors, testing as part of routine care, and advertising the confidentiality of testing should be strengthened and incorporated into future testing programs [22, 28]. Rapid testing is a viable option that has been used successfully by community-based organizations in China to expand testing programs and decrease LTFU among the MSM population [29, 30].
We found that >80% of those diagnosed established care and subsequently attended follow-up visits. The high level of transition to and through these stages is likely attributed to China's highly subsidized HIV care programs. Nevertheless, we confirmed our hypothesis that inequalities in HIV testing and treatment would remain in Shandong Province despite a free, inclusive, nationwide HIV care policy. We found inequalities in key risk groups such as IDUs and those in custodial institutions, which suggests that elimination of direct medical costs may be insufficient to increase linkage to and retention in care for some populations. CRIMS surveillance data show that in Shandong Province, patients infected by intravenous drug use are mostly minorities groups from Sichuan Province [31]. They are mostly tested while incarcerated. Because this population is highly mobile, many are lost to follow-up upon release. Language barriers also likely hinder this population from understanding follow-up appointment instructions. For all populations, delays in receiving a confirmatory test may have also contributed to LTFU. Research is needed to best develop postincarceration programs focused on improving care for those in custody.
Our research suggests that the gap between HAART eligibility and sustained treatment was lower than for any step in the care continuum. Furthermore, our findings suggest that coverage among PLWHA meeting treatment criteria in Shandong Province was higher than the national data reported in CRIMS in 2011 (76.1%) [32] and global ART coverage by the end of 2011 (54%) [33]. Thus, current health systems in Shandong Province are proving a high level of HIV treatment coverage to most patients enrolled in services. Although we did not segregate HAART coverage by year, it is likely that coverage increased in our sample starting in 2012. At that time, Shandong adopted the WHO guidelines that recommended HAART should be initiated for HIV-infected adults at higher CD4 counts than in previous years [34].
While the overall HAART coverage for Shandong Province was excellent, our report found that, although >90% of infections acquired by MTCT were diagnosed, few children infected via MTCT ultimately received therapy. Our findings are in contrast to the national average for HAART coverage, which stands at 85% for HIV-infected children [32]. Our study may suggest that significant challenges remain for treating children in areas of China where the national average is not the norm. Regional and population asymmetries may contribute to different HAART coverage among HIV-infected pregnant women and their newborns. A paucity of prevention of MTCT programs nationwide and the lack of knowledge may have also contributed to our finding of low HAART coverage among children in our sample [35]. In addition, IDUs and those tested by custodial institutions were less likely to receive HAART than blood donors and those tested by medical facilities. Likely, most prisoners are also IDUs and their time in custody is too short to allow assessment for and treatment with antiviral therapy.
Our study shows that only one-quarter of persons diagnosed with HIV eventually controlled their infection. Furthermore, we showed that the margin for virologic failure was substantial even for those on HAART. Few studies have reported on long-term (>5 years) trends in HAART virologic outcomes in China. A short-term study from Shandong Province found that approximately 80% of patients on HAART achieved viral suppression (defined as VL < 50 copies/mL) in 36 months [36]. Huang and colleagues studied the outcomes of antiretroviral therapy in PLWHA followed over 10 years and found that 6.7% and 31.5% of patients met the criteria for virologic failure (defined as VL < 1000 copies/mL) at 6 and 96 months, respectively [37]. Similar to Huang et al, we found that IDUs had an increased likelihood of treatment failure. Also similar to Huang et al, we found that age, sex, and transmission through homosexual sex were nonsignificant predictors of viral suppression. These observations may underscore the importance of additional factors in patients' ability to achieve viral suppression such as nonadherence and drug resistance. Nonadherence is common and of paramount importance in HAART virologic failure [6]. Wang et al, for example, found that patients who self-reported missing doses were up to 12.1 times more likely to fail than those with 100% self-reported adherence [38]. A study of 1 year's duration found that drug resistance among HAART-treated patients in Shandong Providence was 6.2% [39]. He et al demonstrated that drug resistance was the cause of 10.2% of Chinese patients switching to second-line therapy in their first year of treatment [40]. Although we did not measure adherence or drug resistance in our study, they may have played an important role in the low number of patients achieving viral suppression in our sample. Further research is needed to understand barriers to optimizing HAART effectiveness for PLWHA in Shandong Province.
Our study faced the following limitations. First, we were not able to evaluate the influence of national HIV care policies on the disparities observed in our study. Without a control group, we could not resolve the directionality of their impact. While national HIV care policies may have contributed to a decrease in testing and HIV care inequalities, it is also feasible that they may have increased inequalities for some groups. There are likely additional unexplored influences on our results (for example, changes in knowledge and attitudes about HIV). The purpose of our study, however, was merely to describe differences among subgroups rather than to attribute any individual factor as the cause of inequalities. Second, the quality of estimation done by the workbook method, which has been used since 2005 in Shandong and nationwide, depends on the quality of data used. Although data quality may vary from year to year in our sample, efforts have gradually improved data quality over the years. The process of data collection has become more systematic and involves multiple stakeholders in an iterative process [15, 17]. There are still, however, gaps in data on HIV prevalence such as on clients of female sex workers. Third, because our study was cross-sectional, we could not calculate the incidence of LTFU at each step or protective factors associated with progressing through the care continuum. Forth, we did not show how LTFU changed over time. However, we provide the absolute percentage progressing through each stage, which we believe is a fair representation of the population lost to follow-up over an approximate 10-year period. Fifth, we cannot exclude the possibility that overlapping experiences might have influenced the associations we observed. Finally, those who were prescribed HAART but did not meet the standard definition of retention in care may have been excluded; thus, we may have slightly underestimated the total HAART coverage.
CONCLUSIONS
Our study on HIV care in Shandong Province found that approximately 58.1% of persons diagnosed with HIV ultimately had no recorded HAART prescription. Furthermore, among all HAART-treated patients, 40.4% persons experienced virologic failure. Overall, only 15% of infected patients transitioned through the care continuum from initial diagnosis to viral suppression. Attrition occurred at each step of HIV care especially for HIV diagnosis, HAART eligibility for treatment, and achieving viral suppression. Our report suggests, at the current rate, Shandong Province has to accelerate its HIV care efforts to close disparities in HIV care and achieve the 90-90-90 goals equitably.
Notes
Acknowledgments. We acknowledge the staff of Comprehensive Response Information Management System in Shandong Province and all related individuals.
Financial support. This work was supported by the Department of Science and Technology of Shandong Province (grant number ZR2014HQ038) and the Health and Family Planning Commission of Shandong Province (originally the Health Department of Shandong Province, grant number 2013WS0166). S. B. contributed to this work during his fellowship, which was supported by the National Institutes of Health (NIH) (research training grant R25 TW009337), funded by the Fogarty International Center, the NIH Office of the Director, and the National Institute of Mental Health.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. The Joint UN Programme on HIV/AIDS, Geneva, Switzerland: UNAIDS, 2014. Available at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. Accessed 25 November 2015.
- 3.Wu D, Zhang H, Yao XL, Cui Y. Key quality indicators of the China Comprehensive AIDS response 2008–2011 program. China J Prev Med 2012; 46:1095–8. [PubMed] [Google Scholar]
- 4.Giordano TP, Gifford AL, White AC Jr et al. . Retention in care: a challenge to survival with HIV infection. Clin Infect Dis 2007; 44:1493–9. [DOI] [PubMed] [Google Scholar]
- 5.Bangsberg DR, Perry S, Charlebois ED et al. . Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS 2001; 15:1181–3. [DOI] [PubMed] [Google Scholar]
- 6.García de Olalla P, Knobel H, Carmona A, Guelar A, López-Colomés JL, Caylà JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr 2002; 30:105–10. [DOI] [PubMed] [Google Scholar]
- 7.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis 2006; 43:939–41. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, He G, Li X et al. . Self-reported adherence to antiretroviral treatment among HIV-infected people in central China. AIDS Patient Care STDS 2008; 22:71–80. [DOI] [PubMed] [Google Scholar]
- 9.Sun X, Lu F, Wu Z et al. . Evolution of information-driven HIV/AIDS policies in China. Int J Epidemiol 2010; 39:ii4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan HJ, Yang HT, Li JJ et al. . Emerging disparities in HIV/AIDS disease progression and mortality for men who have sex with men, Jiangsu Province, China. AIDS Behav 2014; 18(suppl 1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xing JN, Li YG, Tang WM et al. . HIV/AIDS epidemic among older adults in China during 2005–2012: results from trend and spatial analysis. Clin Infect Dis 2014; 59:e53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao Y, Wu Z, Katharine P et al. . Development of a unified Web-based national HIV/AIDS information system in China. Int J Epidemiol 2010; 39:ii79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach (2010 version). Geneva, Switzerland, 2010. Available at: http://www.who.int/hiv/pub/arv/adult2010/en/. Accessed 10 August 2015. [PubMed]
- 14.World Health Organization. Antiretroviral therapy for HIV infection in infants and children: towards universal access: recommendations for a public health approach (2010 version). Geneva, Switzerland, 2010. Available at: http://www.who.int/hiv/pub/paediatric/paed-prelim-summary.pdf. Accessed 10 August 2015. [PubMed]
- 15.Wang N, Wang L, Wu ZY et al. . Estimating the number of people living with HIV/AIDS in China: 2003–2009. Int J Epidemiol 2010; 39:ii21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker N, Stover J, Stanecki K et al. . The workbook approach to making estimates and projecting future scenarios of HIV/AIDS in countries with low level and concentrated epidemics. Sex Transm Infect 2013; 80(suppl 1):i10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang LY, Wang N, Wang L. The workbook approach to estimate HIV/AIDS prevalence. Chin J AIDS STD 2009; 2:180–2. [Google Scholar]
- 18.Zhao Y, Poundstone KE, Montaner J, Wu ZY. New policies and strategies to tackle HIV/AIDS in China. Chin Med J 2012; 125:1331–7. [PubMed] [Google Scholar]
- 19.Huang ZJ, He N, Nehl EJ et al. . Social network and other correlates of HIV testing: findings from male sex workers and other MSM in Shanghai, China. AIDS Behav 2012; 16:858–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han MJ, Chen QF, Hao Y et al. . Design and implementation of a China comprehensive AIDS response programme (China CARES), 2003–2008. Int J Epidemiol 2010; 39:ii47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao YJ, Zhang L, Zhang H et al. . HIV testing and preventive services accessibility among men who have sex with men at high risk of HIV infection in Beijing, China. Medicine (Baltimore) 2015; 94:e534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Xiao Y, Lu RR et al. . Predictors of HIV testing among men who have sex with men in a large Chinese city. Sex Transm Dis 2013; 40:235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma W, Detels R, Feng YJ et al. . Acceptance of and barriers to voluntary HIV counseling and testing among adults in Guizhou Province, China. AIDS 2007; 21:s129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang TJ, Zhang JL, Gao MY, He N, Detels R. Knowledge, attitudes and practices of voluntary HIV counseling and testing among rural migrants in central China: a cross-sectional study. Eur J Public Health 2012; 22:192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spielberg F, Branson BM, Goldbaum GM et al. . Overcoming barriers to HIV testing: preferences for new strategies among clients of a needle exchange, a sexually transmitted disease clinic, and sex venues for men who have sex with men. J Acquir Immune Defic Syndr 2003; 32:318–27. [DOI] [PubMed] [Google Scholar]
- 26.Rou KM, Guan JH, Wu ZY et al. . Demographic and behavioral factors associated with HIV testing in China. J Acquir Immune Defic Syndr 2009; 50:432–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Sun XY, Qian HZ et al. . Qualitative assessment of barriers and facilitators of access to HIV testing among men who have sex with men in China. AIDS Patient Care STDS 2015; 29:481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei CY, Yan HJ, Yang CK et al. . Accessing HIV testing and treatment among men who have sex with men in China: a qualitative study. AIDS Care 2014; 26:372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan HJ, Zhang RJ, Wei CY et al. . A peer-led, community-based rapid HIV testing intervention among untested men who have sex with men in China: an operational model for expansion of HIV testing and linkage to care. Sex Transm Infect 2014; 90:388–93. [DOI] [PubMed] [Google Scholar]
- 30.Zhang DP, Qi JL, Fu XJ, Meng SN, Li CM, Sun JP. Case finding advantage of HIV rapid tests in community settings: men who have sex with men in 12 programme areas in China, 2011. Int J STD AIDS 2015; 26:402–13. [DOI] [PubMed] [Google Scholar]
- 31.Zhang N, Wang GY, Su SL et al. . An analysis of sources of reported HIV/AIDS cases in Shandong Province in 2011. China J AIDS STD 2012; 18:591–3. [Google Scholar]
- 32.Ministry of Health of the People's Republic of China. 2012 China AIDS response progress report. Available at: http://www.unaids.org.cn/en/index/Document_view.asp?id=770. Accessed 9 December 2015.
- 33.World Health Organization. Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. Available at: http://apps.who.int/iris/bitstream/10665/44787/1/9789241502986_eng.pdf. Accessed 9 December 2015.
- 34.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: WHO, 2015. Available at: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/. Accessed 9 December 2015. [PubMed] [Google Scholar]
- 35.Kalembo FW, Maggie Z, Du YK. Progress of mother-to -child transmission of HIV programs in China: successes, challenges and way forward. Chin Med J (Engl) 2013; 126:3172–6. [PubMed] [Google Scholar]
- 36.Chen XJ, Tang XP, Cai WP et al. . The therapeutic effects of highly active anti-retroviral therapy in 74 treatment-naive patients with AIDS in China. Zhonghua Nei Ke Za Zhi 2011; 50:59–62. [PubMed] [Google Scholar]
- 37.Huang P, Tan JG, Ma WZ et al. . Outcomes of antiretroviral treatment in HIV-infected adults: a dynamic and observational cohort study in Shenzhen, China, 2003–2014. BMJ Open 2015; 5:e007508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Yang LT, Li HQ et al. . Factors associated with HIV virologic failure among patients on HAART for one year at three sentinel surveillance sites in China. Curr HIV Res 2011; 9:103–11. [DOI] [PubMed] [Google Scholar]
- 39.Lin B, Sun XG, Su SL et al. . The prevalence and evolution of HIV drug-resistant strains in people who live with HIV/AIDS during HIV antiretroviral therapy in Shandong Province. Zhonghua Yu Fang Yi Xue Za Zhi 2011; 45:995–8. [PubMed] [Google Scholar]
- 40.He M, Zheng YH, Zhou HY et al. . Prospective observation for seven-year's highly active antiretroviral therapy in Chinese HIV-1 infected patients. Curr HIV Res 2011; 9:160–5. [DOI] [PubMed] [Google Scholar]