As bacteria become increasingly resistant to commonly used antibiotics, the development of alternative therapeutic approaches is critical. Here we describe novel strategies that are being developed for treatment of bacterial pathogens.
Keywords: antivirulence therapy, bacteria, phage therapy, secretion systems, microbiome
Abstract
The utility of conventional antibiotics for the treatment of bacterial infections has become increasingly strained due to increased rates of resistance coupled with reduced rates of development of new agents. As a result, multidrug-resistant, extensively drug-resistant, and pandrug-resistant bacterial strains are now frequently encountered. This has led to fears of a “postantibiotic era” in which many bacterial infections will be untreatable. Alternative nonantibiotic treatment strategies need to be explored to ensure that a robust pipeline of effective therapies is available to clinicians. In this review, we highlight some of the recent developments in this area, such as the targeting of bacterial virulence factors, utilization of bacteriophages to kill bacteria, and manipulation of the microbiome to combat infections.
The problem of antibiotic resistance in bacteria has reached the crisis stage. Coincident with ever-increasing rates of resistance to conventional antibiotics is the slowing in development of novel-acting antibiotics by the pharmaceutical industry. The convergence of these trends has led to the relatively common occurrence of multidrug- and extensively drug-resistant bacteria. The World Health Organization recently reported that “a postantibiotic era—in which common infections and minor injuries can kill—far from being an apocalyptic fantasy, is instead a very real possibility for the 21st century” [1]. To fathom such a future, one need only ponder the past. At the beginning of the 20th century, mortality rates were 100% for endocarditis [2], >95% for meningitis [3], 30% for pneumonia [4], and 10% for serious skin infections [5]. Indeed, the past success of antibiotics may be judged by the degree to which we have taken them for granted. As conventional antibiotics become less reliable, alternate strategies are receiving more attention. Here, we provide an overview of several “nonantibiotic” approaches that are being investigated for the treatment and prevention of bacterial infections. Vaccine development and modulation of host immunity have been covered in a recent review [6] and will not be discussed here.
ANTIVIRULENCE STRATEGIES
Successful bacterial pathogens produce virulence factors, molecules that allow them to resist clearance by the host, to invade and gain access to deeper tissues, and to damage host cells. A substantial amount of effort has been devoted to developing agents that block the activities of virulence factors and hence halt pathogenesis until the host immune response or adjunctive antibiotics kill the bacteria. Such an approach is not new—serum containing antibodies that bound and inactivated diphtheria toxin were used to treat individuals with diphtheria in the 1800s. A frequently touted rationale for antivirulence strategies is that resistance to such agents will be less likely because they do not directly kill or inhibit the growth of bacterial pathogens and therefore avoid the selective pressure that would allow resistant mutants to quickly overgrow the population. However, as recently discussed by Allen and colleagues [7], the situation is actually more complex. For example, an inhibitor that blocks an antiphagocytic virulence factor will result in killing of the bacterium by neutrophils and macrophages, which in turn will supply the selective pressure for emergence of resistance to the inhibitor. Nonetheless, some antivirulence strategies may indeed invoke a milder evolutionary pressure for the development of resistance than conventional antibiotics [7].
Targeting Toxins and Secretion Systems
Secreted toxins play a major role in the pathogenesis of many medically important bacteria, and several of these have been targeted with the aim of blocking infection (Table 1). (Here, we will use the term “toxin” to refer to factors secreted by bacteria that promote infection, including both traditional toxins and effector proteins directly injected into host cells by specialized secretion systems.) Agents developed to inhibit toxins predominantly fall into 1 of 2 categories: chemical inhibitors and antibodies.
Table 1.
Examples of Agents That Inhibit Toxins and Secretion Systems
| Name | Type | Target | Bacterium | Development Phase | Reference(s) |
|---|---|---|---|---|---|
| Shigamab | Monoclonal antibodies | Stx-1, Stx-2 | Escherichia coli | Phase 2 clinical trial | [8] |
| Bezlotoxumab | Monoclonal antibody | Toxin B | Clostridium difficile | Completed phase 3 clinical trial | [9, 10] |
| MEDI4893 | Monoclonal antibody | α-hemolysin | Staphylococcus aureus | Phase 2 clinical trial | [11] |
| Compounds 1–9 | Small molecule | Type 2 secretion | Pseudomonas aeruginosa, Burkholderia pseudomallei | Preclinical (inhibition of bacterial secretion | [12] |
| Compounds 7086, 7832, 7812 | Small molecules | Type 3 secretion | Yersinia pestis | Preclinical (efficacy in cell culture) | [13] |
| Salicylidene acylhydrazides | Small molecules | Type 3 secretion | Salmonella, Shigella, Chlamydia, Yersinia, Pseudomonas spp | Preclinical (efficacy in mice) | [14] |
| CHIR-1 | Small molecule | Type 4 secretion | Helicobacter pylori | Preclinical (efficacy in mice) | [15] |
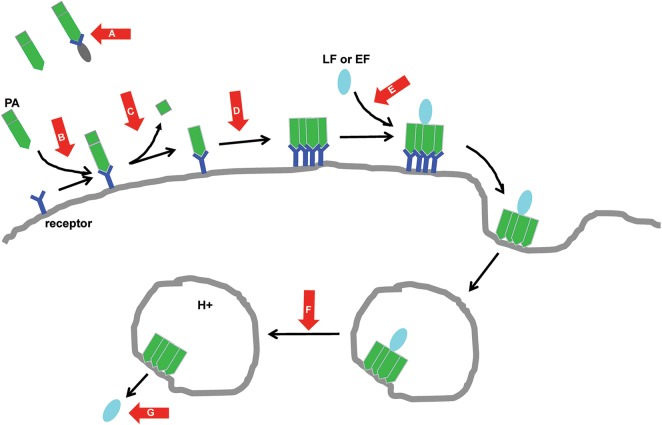
Work done on anthrax toxin, a major virulence determinant of Bacillus anthracis, illustrates the many vulnerabilities that have been exploited by toxin-directed therapeutics (Figure 1). The most clinically advanced of these agents is raxibacumab (GlaxoSmithKline), a fully humanized immunoglobulin G1 (IgG1) monoclonal antibody that prevents anthrax toxin binding to its host cell receptor. Raxibacumab was approved by the US Food and Drug Administration (FDA) in 2012 for prophylaxis and treatment of inhalational anthrax and is now recommended for the adjunctive (ie, along with conventional antibiotics) treatment of inhalational anthrax [16]. However, raxibacumab was approved on the basis of efficacy in animals, so human data are not available to inform decisions regarding the optimal timing of raxibacumab administration for treatment of anthrax.
Figure 1.
Strategies to block the cellular effects of anthrax toxin. Red arrows indicate critical steps in the intoxication process blocked by inhibitory compounds. Anthrax toxin is comprised of 3 proteins: protective antigen (PA), lethal factor (LF), and edema factor (EF). In the first step of the cell entry process, PA binds to receptors such as CMG2 on the surface of host cells. This interaction may be prevented by CMG2-Fc, a fusion protein consisting of the extracellular domain of CMG2 and human IgG Fc. Exogenously administered CMG2-Fc acts as a decoy receptor that sequesters PA before it binds to host cells (A). The interaction of PA with host cells can also be prevented by antibodies such as raxibacumab (GlaxoSmithKline) and obiltoxaximab (Elusys Therapeutics) that recognize and block host cell receptors (B). After binding its receptor, PA is cleaved by the host cell protease furin, a process that is blocked by the protease inhibitor IαIp (C). This cleavage event allows PA to oligomerize and form a prepore complex capable of binding the other 2 components of anthrax toxin, LF and EF. The monoclonal antibodies AVP-21D9 (Avanir Pharmaceuticals) and MDX1303 (PharmAthene/Medarex) block or disrupt the formation of the oligomer (D), and a heptavalent inhibitory peptide compound [17] blocks binding of LF to the oligomerized PA (E). The prepore complex bound to LF/EF then triggers endocytosis, and subsequent acidification of the endosome causes a structural change in the prepore complex that makes it competent to translocate LF/EF into the cytosol. The monoclonal antibody cAB29 and dominant-negative variants of PA (eg, V377E or F427A) prevent the formation of a functioning pore, blocking translocation of LF/EF into the cytosol (F). Likewise, the cationic compound AMBnTβCD effectively obstructs the pore (F). Once in the cytosol, LF and EF use their enzymatic activities to disrupt cellular processes. The monoclonal antibodies p6CO1/p6FO1 and the small-molecule inhibitor PT-8541 neutralize the activity of LF, whereas the monoclonal antibody EF13D and the small-molecule inhibitor DC-5 neutralize the activity of EF (G). A complete list of compounds targeting anthrax toxin as well as references for the preceding discussion can be found in [18, 19].
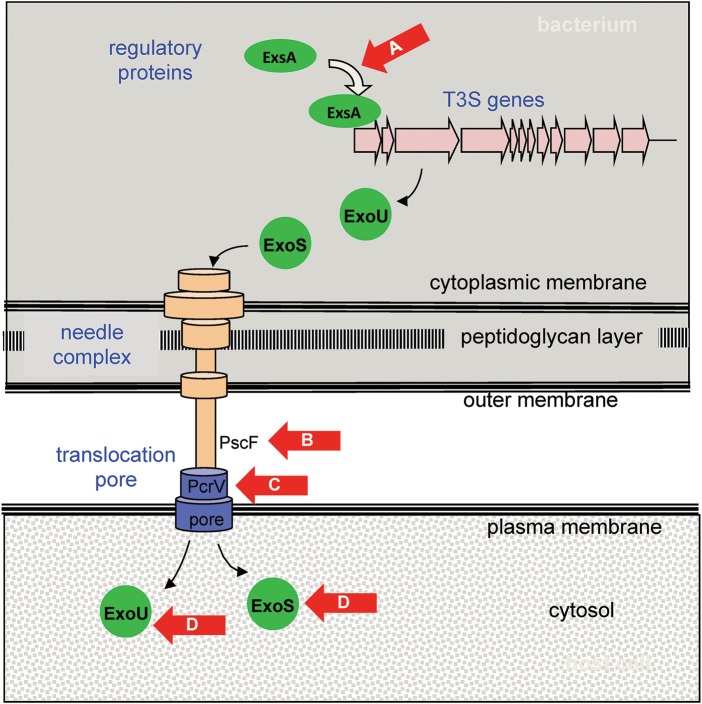
Because toxins are produced in the bacterial cytosol, they must first be transported across the bacterial cell envelope prior to gaining access to human cells. To accomplish this, bacteria have evolved a number of complex secretion systems, which themselves are attractive drug targets (Table 1). The agents furthest along the development pipeline are inhibitors of type 3 secretion (T3S) (reviewed in [14]). T3S systems are complex multiprotein, needle-like apparatuses used by many gram-negative bacteria to inject toxins directly into human cells (Figure 2). Although the secreted toxins are typically quite distinct, the components of the secretion apparatuses are somewhat conserved between the T3S systems of different bacterial species. Thus inhibitors of the T3S apparatuses may be active against multiple different bacteria [13]. Pseudomonas aeruginosa illustrates how agents have been developed that block a number of steps critical for the functioning of T3S (Figure 2). Although most of these compounds are still in the preclinical stage of development, inhibitors that target PcrV, a protein on the exposed tip of the T3S needle that is essential for appropriate insertion of a translocation pore into the plasma membrane of host cells, have entered clinical trials (reviewed in [20]). KB001 (KaloBios), a pegylated, humanized anti-PcrV antibody Fab′ fragment, was studied in two phase 2 clinical trials. In 35 mechanically ventilated patients colonized with P. aeruginosa, a single prophylactic dose of KB001 or placebo was given intravenously. KB001 was safe and well tolerated and showed a trend toward decreasing the development of ventilator-associated pneumonia [21]. In a second study, 27 P. aeruginosa–infected patients with cystic fibrosis were randomized to receive a single intravenous dose of KB001 or placebo. At enrollment, patients receiving antibiotics were excluded except for maintenance azithromycin or inhaled tobramycin. KB001 was again found to be safe. Although KB001-treated patients had several findings suggesting modest reductions in inflammation, no differences were noted in P. aeruginosa density in sputum, symptoms, or pulmonary spirometry [29]. Due to concerns about efficacy, there are currently no plans to further pursue this agent. An intriguing variation in the targeting of PcrV is MEDI3902 (AstraZeneca), a chimeric bispecific monoclonal antibody that recognizes both PcrV and the polysaccharide Psl located on the surface of P. aeruginosa [25]. The presence of both antigen-binding sites confers synergistic protection against P. aeruginosa in animal models of infection [25]. A phase 2 clinical trial for MEDI3902 is currently in the planning stages.
Figure 2.
Overview of antivirulence agents active against the type 3 secretion (T3S) system of Pseudomonas aeruginosa. Red arrows indicate the molecular targets of agents in development to prevent intoxication by the T3S system. ExsA is a transcriptional activator that induces expression of all genes in the T3S regulon. This induction is blocked by N-hydroxybenzimidazoles [22] (A). T3S genes encode proteins such as the PscF monomer, which polymerizes to form the needle complex. PscF is thought to be targeted by phenoxyacetamide compounds [23, 24] (B). PcrV is located at the tip of the needle complex and is essential for insertion of the translocation pore into the plasma membrane of the host cell. This protein is targeted by antibodies KB001 (KaloBios) [21] and MEDI3902 (AstraZeneca) [25] (C). Effector proteins ExoU and ExoS are injected into the host cell, where they manifest enzymatic activities that damage or subvert these cells. The activity of ExoU is inhibited by pseudolipasin A [26] and that of ExoS is inhibited by exosin and STO1101 [27, 28] (D).
Several interesting approaches are being tried to maximize the efficacy of antivirulence compounds. Because bacteria often utilize multiple and redundant pathogenic mechanisms, simultaneous targeting of several virulence determinants may improve the probability of success. In an extreme application of this principle, Rouha and colleagues identified a single human monoclonal IgG1 antibody that neutralized α-hemolysin and 4 leukocidin toxins of Staphylococcus aureus [30]. Combining antivirulence compounds with conventional antibiotics may provide synergistic enhancement of efficacy. For example, addition of MEDI3902 to tobramycin yielded improved survival rates in a mouse model of pneumonia compared to either agent used alone. Interestingly, the improved survival rates persisted even when the mice were infected with a tobramycin-resistant strain of P. aeruginosa [25]. Thus, antivirulence compounds may provide a way to extend the usefulness of current antibiotics in an era of multidrug-resistant (MDR) bacterial infections.
Targeting Biofilms and Adherence
Biofilms growing on inert surfaces, such as catheters or prosthetic joints, and biofilms growing on body structures, such as heart valves and teeth, are major sources of infections [31]. Their eradication can be difficult in part because bacteria growing in biofilms are in a physiological state that allows them to persist in the presence of antibiotics that typically kill planktonic-growing bacteria [31, 32]. In addition, the extracellular matrix of the biofilm itself can prevent antibiotic penetration into the biofilm [31, 32]. With our increased understanding of the factors required for biofilm formation and stability, novel methods are being developed that are designed to prevent biofilm formation and to disaggregate biofilms once formed; however, to date these newer strategies outlined below have not reached the clinical testing stage (reviewed in [33]), although previous modifications of inert substances have been described.
Because many biofilms form on abiotic surfaces such as catheters or plastic implants, work has progressed on coatings for these devices that prevent bacterial adherence, the first step in biofilm formation. Presumably, by preventing colonization, these modifications would reduce infections. Indeed, in one study, catheters coated with the zwitterionic polymeric sulfobetaine had reduced amounts of both S. aureus and Escherichia coli adhesion, and animals treated with these catheters experienced fewer infections [34]. In a rat model of infection, implants coated with an antiadhesive glycocalyx-like compound, methylcellulose, were resistant to the formation of biofilms and infected thrombi [35]. Quorum sensing (QS) also plays an important role in biofilm formation and has been a target of novel therapeutics (discussed below). In addition, the small signaling molecule c-di-GMP has also been a recent target to prevent infections by biofilm-forming pathogens because it regulates the switch that allows planktonically grown bacteria to form biofilms. Likewise, targeting of the factors that allow bacteria in biofilms to form persister cells that resist antibiotics is being explored with the goal of rendering biofilms sensitive to antibiotics, and a variety of strategies to disperse biofilms once they have developed are also being pursued [36]. Targeting adhesins required for colonization is another strategy that has been used to prevent infections by microbes in both the biofilm and planktonic phase of growth (reviewed in [37]). Notably, uropathogenic E. coli (UPEC), which causes urinary tract infections, uses a lectin-type fimbriae adhesin to attach to epithelial cells. Small molecules have been developed that interfere with the binding of the fimbriae to sugar moieties on epithelial cell surfaces. For example, the small molecule ZFH-04269 caused a 1000-fold reduction in the number of UPEC bacteria in the bladders of chronically infected mice [38].
Targeting Signaling and Regulation
QS is a cell density–dependent communication system that utilizes low-molecular-weight signaling molecules (autoinducers) to regulate virulence in many bacterial pathogens. In general, gram-negative species use N-acylhomoserine lactones (AHLs) or related compounds, and gram-positive species use ribosomally produced autoinducing peptides for QS. Two basic drug-discovery strategies are to identify inhibitors of the synthesis of the QS signaling molecules or inhibitors of the interactions between these molecules and their receptors. The latter strategy is employed by the naturally occurring halogenated furanones and by several AHL analogues [39]. A synthetic analogue of the furanone C-30 reduced P. aeruginosa PAO1 colony-forming units in a murine lung infection model in a dose-dependent manner [40]. M64, a phenoxy derivative of a substituted benzamide moiety with endocyclic aromatic amines, follows the former strategy by inhibiting MvfR, a transcriptional regulator of the 4-hydroxy-2-alkylquinoline QS system of P. aeruginosa [26]. MvfR is required for full virulence in several animal infection models, and no clinical isolates carrying mvfR mutations have been found to date [41]. M64 exhibited efficacy in murine burn and acute pneumonia infection models and demonstrated additive protective effects when combined with an antibiotic (ciprofloxacin) at a subtherapeutic dose in the burn infection model [41].
A common theme throughout bacterial species is the use of 2-component systems to sense and respond to environmental signals of either host or bacterial origin. For example, the enterohemorrhagic E. coli (EHEC) membrane-bound 2-component sensor QseC responds to the human hormones epinephrine and norepinephrine and to the bacterial signal AI-3 [42]. Upon sensing any of those signals, QseC indirectly regulates expression of several sets of EHEC genes. QseC homologues are found in many gram-negative species, indicating that an inhibitor may have a relatively broad spectrum. Indeed, a small-molecular-weight inhibitor of QseC, LED209 [N-phenyl-4-(3-phenylthioureido) benzenesulfonamide], reduced the expression of virulence factors by EHEC, Salmonella enterica serovar Typhimurium, and Francisella tularensis in a QseC-dependent manner in vitro. Furthermore, administration of LED209 suppressed the pathogenicity of Salmonella Typhimurium and F. tularensis in a murine infection model [43].
Once environmental conditions conducive to infection are sensed by a bacterium, this information must be relayed via a system of regulators to cause appropriate transcription of virulence determinants. In Vibrio cholerae, ToxT is the master regulator that controls expression of the genes encoding cholera toxin and the toxin-coregulated pilus [44]. Two inhibitors, the small molecule toxtazin B, which blocked production of an upstream regulator necessary for expression of the toxT gene [44], and virstatin, which inhibited ToxT dimerization [45], each reduced colonization of V. cholerae in a mouse model of infection [45, 46].
PHAGE THERAPY
Bacteriophages were used for antibacterial therapy in Russia and Eastern Europe before the advent of antibiotics, and recent dramatic increases in infections with MDR bacterial strains are driving new interest in this approach [47]. Phages offer several important advantages over traditional antibiotics. They are specific for bacteria and even particular strains and species of bacteria, they do not infect human cells, and they have little or no effect on normal microbial flora. Limitations include the development of bacterial resistance and immune responses, difficulties in purification from bacterial endo- and exotoxins, and formulation and stability issues in systemic delivery [48]. For these reasons, most studies have been done with topical, gastrointestinal, or pulmonary deliveries.
As mentioned, phages have the advantage of being exquisitely specific, but this is also a disadvantage, as cocktails of multiple phages are required to target multiple species and even most strains within a species. Nevertheless, several phage cocktails have exhibited efficacy in animal infection models (reviewed in [47]). Strikingly, a phage cocktail has been tested successfully in a human phase 1/2 clinical trial for efficacy against P. aeruginosa–mediated chronic otitis [49]. A cocktail of 6 anti–P. aeruginosa phages called Biophage-PA applied as a suspension directly into the ear was more effective than placebo in decreasing bacterial counts in the ears of 24 patients (12 in each group) with chronic P. aeruginosa otitis. Of note, the patients did not receive antibiotics during the trial. No serious adverse events were reported, and the mild to moderate adverse events noted were not considered to be treatment related. In summary, Biophage-PA appeared to be safe and effective in this small clinical trial.
Strategies to improve phage therapy have involved engineering phages to increase their infectivity and host range and purifying individual phage components to target bacteria (reviewed in [50]). In one study, investigators repeatedly passaged a phage on a mucoid MDR P. aeruginosa strain obtained from a patient with cystic fibrosis to select for phage variants with enhanced infectivity [51]. Intranasal administration of one of these phage variants (P3-CHA) either prior to or along with inoculation with the same P. aeruginosa strain in a mouse pneumonia model resulted in improved survival [51]. In a second study, researchers described in vivo proof-of-principle for a relatively broad-acting purified phage lysin—a phage-encoded peptidoglycan hydrolase that normally lyses bacteria to allow progeny phage escape [52]. A single intraperitoneal dose of phage lysin PlySs2 rescued mice with methicillin-resistant S. aureus and Streptococcus pyogenes peritonitis and bacteremia. Concerns about the relatively short in vivo half-life (20 minutes) and the potential for the development of neutralizing antibodies suggest that the initial use of purified lysins may be for the treatment of infections of mucosal surfaces. In that regard, purified lysins have shown efficacy in an oral colonization model for S. pyogenes, a nasal model for pneumococci, and a vaginal model for group B streptococci (reviewed in [53]).
MODULATING THE MICROBIOME
In the past decade, our understanding of the importance of the microbiome to human health and disease has grown exponentially, and a number of striking associations have been revealed between an individual's microbiota and his or her disease states, including diabetes, cardiovascular disease, and even mental health. To date, the most successful use of the microbiome as a therapy is in the treatment of recalcitrant or recurrent Clostridium difficile infection (CDI) using a strategy called fecal microbiota transplant (FMT) (reviewed in [54]). Cure rates after FMT are very high for recalcitrant CDI, and the introduced microbiota appears to be stable for several months (reviewed in [54]). Recently, human stool for use in FMT has been classified as a biological agent by the FDA and should be regulated, but exactly how remains to be determined, and many questions remain regarding how to optimize FMT treatment. For instance, how does the donor's microbiota influence the health of the recipient after recovery from CDI? Is there one “best” mixture of microbiota to use for everyone, or would different components be better for some people based on what microbiota they carry or their other medical treatments? Excitingly, microbiome therapy may also be useful for treating drug-resistant bacterial infections other than CDI. For example, in mice and in people undergoing allogeneic hematopoietic stem cell transplant, the commensal bacterium Barnesiella protected against gastrointestinal colonization and subsequent bloodstream infection with vancomycin-resistant enterococci [55]. Similarly, FMT successfully displaced an MDR strain of Klebsiella pneumoniae in another patient who had undergone hematopoietic cell transplant [56].
In contrast with FMT, the use of probiotics to alter the microbiome and prevent or cure CDI has been less well validated, although several studies indicate that Saccharomyces boulardii and Lactobacillus species may reduce the risk of CDI and antibiotic-associated diarrhea (reviewed in [54]). Another infection that may be preventable or treatable with probiotics is peridontal disease. This approach involves applying Streptococcus salivarius and Lactobacillus species to compete for sites with disease-causing bacteria in the oral cavity (reviewed in [57]).
FUTURE CHALLENGES
The extraordinary success of conventional antibiotics led to a focus on development of these agents to the exclusion of other antibacterial strategies [58]. A silver lining in the current dark cloud of antibiotic resistance is that these alternative strategies are again being pursued, although significant challenges remain before they can be widely adopted into clinical practice. First, since many of these approaches are species or even strain specific rather than broad spectrum, rapid diagnostic technologies will be necessary to identify candidate patients in a timely manner. Second, the narrow spectrum of many of these compounds and the corresponding smaller sales markets make them less attractive for development by pharmaceutical companies. Third, it remains unclear whether these alternate antibacterial therapies will fall prey to the same rapid emergence of resistance that has plagued conventional antibiotics. Fourth, it is likely that many of these agents will require the simultaneous use of an active antibiotic, which may be problematic for some MDR bacteria. Finally, many of these compounds are still in the preclinical phase of development, and a substantial investment in time and resources will be necessary before they significantly impact the ability of physicians to treat patients infected with MDR bacteria. For a detailed discussion of the time and funding required to advance nonantibiotic therapeutics to the marketplace, and of the obstacles that must be overcome for this to occur, the reader is referred to a recent review [59]. If these difficulties can be surmounted, alternatives to antibiotics may become important therapeutic options for bacterial infections.
Notes
Financial support. This work was supported by the National Institutes of Health (NIH) (grant numbers AI099269 to D. T. M., J. M., and A. R. H.; AI053674, AI04831, and AI118257 to A. R. H.; and AI113166 to J. M.).
Potential conflicts of interest. All 3 authors currently hold a NIH research grant to characterize and optimize inhibitors of type III secretion systems. A. R. H. has served as a consultant for AstraZeneca and Microbiotix, Inc. D. T. M. is an employee of Microbiotix, Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Antimicrobial resistance: global report on surveillance. 2014. Available at: http://www.who.int/drugresistance/documents/surveillancereport/en/ Accessed 14 January 2016.
- 2.Bishara J, Leibovici L, Gartman-Israel D et al. Long-term outcome of infective endocarditis: the impact of early surgical intervention. Clin Infect Dis 2001; 33:1636–43. [DOI] [PubMed] [Google Scholar]
- 3.Shulman ST. Type 19 pneumococcal meningitis: a patient with 75-year clinical and serologic follow up, with a review of the evolution of therapy from antiserum to sulfonamides to penicillin. J Pediatric Infect Dis Soc 2015; 4:349–55. [DOI] [PubMed] [Google Scholar]
- 4.Podolsky SH. Pneumonia before antibiotics: therapeutic evolution and evaluation in twentieth-century America. Baltimore, MD: Johns Hopkins University Press, 2006. [Google Scholar]
- 5.Spellberg B, Talbot GH, Boucher HW et al. Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Antimicrobial agents for complicated skin and skin-structure infections: justification of noninferiority margins in the absence of placebo-controlled trials. Clin Infect Dis 2009; 49:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hancock RE, Nijnik A, Philpott DJ. Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol 2012; 10:243–54. [DOI] [PubMed] [Google Scholar]
- 7.Allen RC, Popat R, Diggle SP, Brown SP. Targeting virulence: can we make evolution-proof drugs? Nat Rev Microbiol 2014; 12:300–8. [DOI] [PubMed] [Google Scholar]
- 8.López EL, Contrini MM, Falaschi A et al. A phase II study of chimeric monoclonal antibodies to Shiga toxins 1 and 2 (Shigamabs) administered concomitantly to children with Shiga toxin–producing Escherichia coli infection and bloody diarrhea (SHIGATEC trial). In: VTEC Meeting, Boston, MA, 13–16 September 2015.
- 9.Wilcox M, Gerding D, Poxton I et al. Bezlotoxumab (BEZ) alone and with actoxumab (ACT) for prevention of recurrent C. difficile infection (rCDI) in patients on standard of care (SoC) antibiotics: integrated results of 2 phase 3 studies (MODIFY I and MODIFY II). In: IDWeek, San Diego, CA, 7–11 October 2015. [Google Scholar]
- 10.Lowy I, Molrine DC, Leav BA et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010; 362:197–205. [DOI] [PubMed] [Google Scholar]
- 11.Hua L, Cohen TS, Shi Y et al. MEDI4893* promotes survival and extends the antibiotic treatment window in a Staphylococcus aureus immunocompromised pneumonia model. Antimicrob Agents Chemother 2015; 59:4526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moir DT, Di M, Wong E et al. Development and application of a cellular, gain-of-signal, bioluminescent reporter screen for inhibitors of type II secretion in Pseudomonas aeruginosa and Burkholderia pseudomallei. J Biomol Screen 2011; 16:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swietnicki W, Carmany D, Retford M et al. Identification of small-molecule inhibitors of Yersinia pestis type III secretion system YscN ATPase. PLoS One 2011; 6:e19716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall NC, Finlay BB. Targeting the type III secretion system to treat bacterial infections. Expert Opin Ther Targets 2014; 18:137–52. [DOI] [PubMed] [Google Scholar]
- 15.Hilleringmann M, Pansegrau W, Doyle M et al. Inhibitors of Helicobacter pylori ATPase Cag-alpha block CagA transport and cag virulence. Microbiology 2006; 152:2919–30. [DOI] [PubMed] [Google Scholar]
- 16.Hendricks KA, Wright ME, Shadomy SV et al. Centers for Disease Control and Prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis 2014; 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi A, Kate S, Poon V et al. Structure-based design of a heptavalent anthrax toxin inhibitor. Biomacromolecules 2011; 12:791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Moayeri M, Purcell R. Monoclonal antibody therapies against anthrax. Toxins (Basel) 2011; 3:1004–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nestorovich EM, Bezrukov SM. Designing inhibitors of anthrax toxin. Expert Opin Drug Discov 2014; 9:299–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawa T, Ito E, Nguyen VH, Haight M. Anti-PcrV antibody strategies against virulent Pseudomonas aeruginosa. Hum Vaccin Immunother 2014; 10:2843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francois B, Luyt CE, Dugard A et al. Safety and pharmacokinetics of an anti-PcrV pegylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized,double-blind, placebo-controlled trial. Crit Care Med 2012; 40:2320–6. [DOI] [PubMed] [Google Scholar]
- 22.Marsden AE, King JM, Spies MA, Kim OK, Yahr TL. Inhibition of Pseudomonas aeruginosa ExsA DNA-binding activity by N-hydroxybenzimidazoles. Antimicrob Agents Chemother 2015; 60:766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aiello D, Williams JD, Majgier-Baranowska H et al. Discovery and characterization of inhibitors of Pseudomonas aeruginosa type III secretion. Antimicrob Agents Chemother 2010; 54:1988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowlin NO, Williams JD, Knoten CA et al. Mutations in the Pseudomonas aeruginosa needle protein gene pscF confer resistance to phenoxyacetamide inhibitors of the type III secretion system. Antimicrob Agents Chemother 2014; 58:2211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiGiandomenico A, Keller AE, Gao C et al. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci Transl Med 2014; 6:262ra155. [DOI] [PubMed] [Google Scholar]
- 26.Lee VT, Pukatzki S, Sato H et al. Pseudolipasin A is a specific inhibitor for phospholipase A2 activity of Pseudomonas aeruginosa cytotoxin ExoU. Infect Immun 2007; 75:1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnoldo A, Curak J, Kittanakom S et al. Identification of small molecule inhibitors of Pseudomonas aeruginosa exoenzyme S using a yeast phenotypic screen. PLoS Genet 2008; 4:e1000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto AF, Ebrahimi M, Saleeb M, Forsberg A, Elofsson M, Schuler H. Identification of inhibitors of Pseudomonas aeruginosa exotoxin-S ADP-ribosyltransferase activity. J Biomol Screen 2016; doi:10.1177/1087057116629923. [DOI] [PubMed] [Google Scholar]
- 29.Milla CE, Chmiel JF, Accurso FJ et al. Anti-PcrV antibody in cystic fibrosis: a novel approach targeting Pseudomonas aeruginosa airway infection. Pediatr Pulmonol 2014; 49:650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rouha H, Badarau A, Visram ZC et al. Five birds, one stone: neutralization of alpha-hemolysin and 4 bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody. MAbs 2015; 7:243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribeiro SM, Felicio MR, Boas EV et al. New frontiers for anti-biofilm drug development. Pharmacol Ther 2016; 160:133–44. [DOI] [PubMed] [Google Scholar]
- 32.Kester JC, Fortune SM. Persisters and beyond: mechanisms of phenotypic drug resistance and drug tolerance in bacteria. Crit Rev Biochem Mol Biol 2014; 49:91–101. [DOI] [PubMed] [Google Scholar]
- 33.Beloin C, Renard S, Ghigo JM, Lebeaux D. Novel approaches to combat bacterial biofilms. Curr Opin Pharmacol 2014; 18:61–8. [DOI] [PubMed] [Google Scholar]
- 34.Smith RS, Zhang Z, Bouchard M et al. Vascular catheters with a nonleaching poly-sulfobetaine surface modification reduce thrombus formation and microbial attachment. Sci Transl Med 2012; 4:153ra132. [DOI] [PubMed] [Google Scholar]
- 35.Chauhan A, Bernardin A, Mussard W et al. Preventing biofilm formation and associated occlusion by biomimetic glycocalyxlike polymer in central venous catheters. J Infect Dis 2014; 210:1347–56. [DOI] [PubMed] [Google Scholar]
- 36.Conlon BP, Nakayasu ES, Fleck LE et al. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 2013; 503:365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krachler AM, Orth K. Targeting the bacteria-host interface: strategies in anti-adhesion therapy. Virulence 2013; 4:284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Totsika M, Kostakioti M, Hannan TJ et al. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J Infect Dis 2013; 208:921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rampioni G, Leoni L, Williams P. The art of antibacterial warfare: deception through interference with quorum sensing-mediated communication. Bioorg Chem 2014; 55:60–8. [DOI] [PubMed] [Google Scholar]
- 40.Hentzer M, Wu H, Andersen JB et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 2003; 22:3803–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Starkey M, Lepine F, Maura D et al. Identification of anti-virulence compounds that disrupt quorum-sensing regulated acute and persistent pathogenicity. PLoS Pathog 2014; 10:e1004321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clarke MB, Hughes DT, Zhu C, Boedeker EC, Sperandio V. The QseC sensor kinase: a bacterial adrenergic receptor. Proc Natl Acad Sci U S A 2006; 103:10420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curtis MM, Russell R, Moreira CG et al. QseC inhibitors as an antivirulence approach for gram-negative pathogens. MBio 2014; 5:e02165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anthouard R, DiRita VJ. Small-molecule inhibitors of toxT expression in Vibrio cholerae. MBio 2013; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shakhnovich EA, Hung DT, Pierson E, Lee K, Mekalanos JJ. Virstatin inhibits dimerization of the transcriptional activator ToxT. Proc Natl Acad Sci U S A 2007; 104:2372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science 2005; 310:670–4. [DOI] [PubMed] [Google Scholar]
- 47.Knoll BM, Mylonakis E. Antibacterial bioagents based on principles of bacteriophage biology: an overview. Clin Infect Dis 2014; 58:528–34. [DOI] [PubMed] [Google Scholar]
- 48.Pires DP, Vilas Boas D, Sillankorva S, Azeredo J. Phage therapy: a step forward in the treatment of Pseudomonas aeruginosa infections. J Virol 2015; 89:7449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright A, Hawkins CH, Anggard EE, Harper DR. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa: a preliminary report of efficacy. Clin Otolaryngol 2009; 34:349–57. [DOI] [PubMed] [Google Scholar]
- 50.Nobrega FL, Costa AR, Kluskens LD, Azeredo J. Revisiting phage therapy: new applications for old resources. Trends Microbiol 2015; 23:185–91. [DOI] [PubMed] [Google Scholar]
- 51.Morello E, Saussereau E, Maura D, Huerre M, Touqui L, Debarbieux L. Pulmonary bacteriophage therapy on Pseudomonas aeruginosa cystic fibrosis strains: first steps towards treatment and prevention. PLoS One 2011; 6:e16963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilmer DB, Schmitz JE, Euler CW, Fischetti VA. Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2013; 57:2743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischetti VA. Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol 2008; 11:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crow JR, Davis SL, Chaykosky DM, Smith TT, Smith JM. Probiotics and fecal microbiota transplant for primary and secondary prevention of Clostridium difficile infection. Pharmacotherapy 2015; 35:1016–25. [DOI] [PubMed] [Google Scholar]
- 55.Ubeda C, Bucci V, Caballero S et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect Immun 2013; 81:965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bilinski J, Grzesiowski P, Muszynski J et al. Fecal microbiota transplantation inhibits multidrug-resistant gut pathogens: preliminary report performed in an immunocompromised host. Arch Immunol Ther Exp (Warsz) 2016; doi:10.1007/s00005-016-0387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saha S, Tomaro-Duchesneau C, Tabrizian M, Prakash S. Probiotics as oral health biotherapeutics. Expert Opin Biol Ther 2012; 12:1207–20. [DOI] [PubMed] [Google Scholar]
- 58.Wei H. Bacteriophages, revitalized after 100 years in the shadow of antibiotics. Virol Sin 2015; 30:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Czaplewski L, Bax R, Clokie M et al. Alternatives to antibiotics—a pipeline portfolio review. Lancet Infect Dis 2016; 16:239–51. [DOI] [PubMed] [Google Scholar]