Abstract
Uncovering the mechanisms that underlie central noradrenergic neuron heterogeneity is essential to understanding selective subtype vulnerability to disease and environmental insult. Using recombinase-based intersectional genetic fate mapping we have previously demonstrated that molecularly distinct progenitor populations give rise to mature noradrenergic neurons differing in their anatomical location, axon morphology and efferent projection pattern. Here we review the findings from our previous study and extend our analysis of the noradrenergic subpopulation defined by transient developmental expression of Hoxb1. Using a combination of intersectional genetic fate mapping and analysis of a targeted loss of function mutation in Hoxb1, we have now uncovered additional heterogeneity based on the requirement of some noradrenergic neurons for Hoxb1 expression. By comparing the distribution of noradrenergic neurons derived from the Hoxb1 expression domain in wild-type and mutant mice, we demonstrate that Hoxb1 expression is required by a subset of neurons in the pons. Additional fate mapping, using a Hoxb1 enhancer element that drives Cre recombinase expression exclusively in rhombomere 4 of the hindbrain, reveals the existence of a subpopulation of noradrenergic neurons in the pons with more restricted axonal targets than the full Hoxb1-derived subpopulation. The unique projection profile of this newly defined subpopulation suggests that it may be functionally distinct. These analyses shed new light on the molecular determinants of noradrenergic identity in the pons and the overall complexity of the central noradrenergic system.
Keywords: norepinephrine, Hoxb1, fate map, recombinase-based, intersectional genetic fate mapping, efferents, rhombomere
1. Introduction
It is now well accepted that central noradrenergic neurons are heterogeneous. Collectively defined by their ability to synthesize the neuromodulator norepinephrine, these neurons differ in their connectivity, molecular and physiological properties, and function (Berridge and Waterhouse, 2003; Chandler et al., 2014; Grzanna and Fritschy, 1991; Rinaman, 2011). Little is known about the ways in which this complexity arises, but such knowledge is critical to understanding the basis of selective noradrenergic neuron subtype vulnerability to disease and environmental insult. Variation in developmental gene expression is one source of heterogeneity in the nervous system (Brust et al., 2014; Champagnat et al., 2009; Dasen and Jessell, 2009), and the focus of our research has been the relationship between this early gene expression and mature noradrenergic neuron subtype identity.
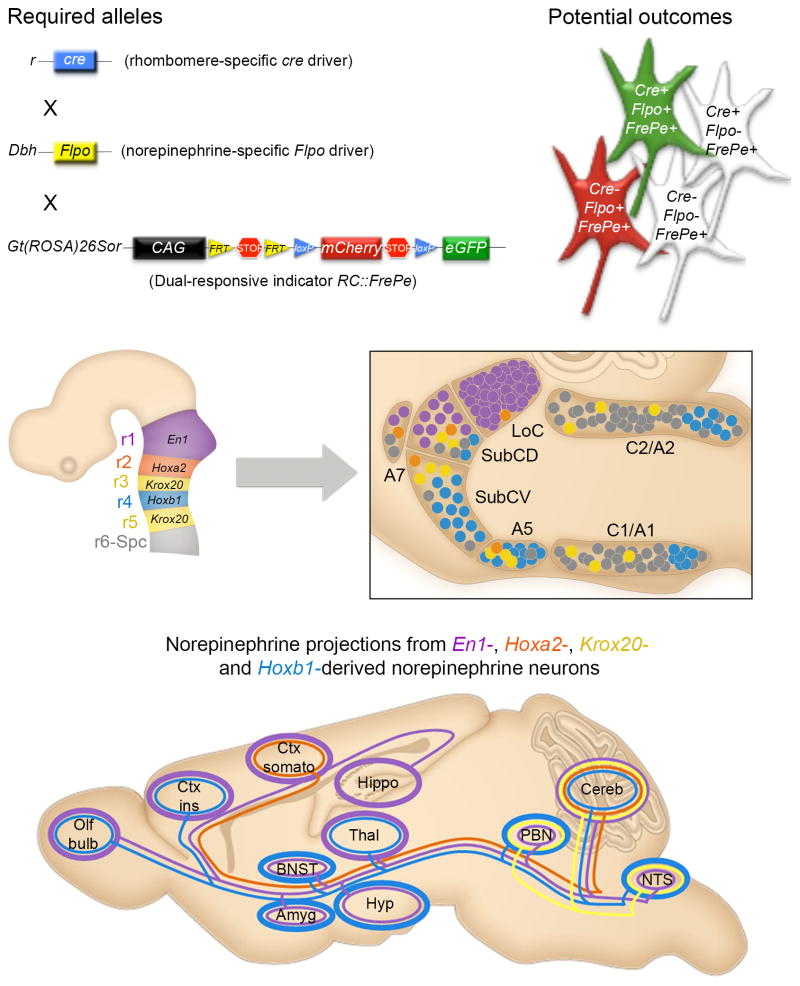
To investigate how developmental gene expression differences generate diversity in the noradrenergic system, we adopted a recombinase-based intersectional genetic fate mapping approach in mice (Awatramani et al., 2003) (Fig 1). Recombinase-based fate mapping requires two genetically engineered alleles: a recombinase driver which expresses a site-specific recombinase such as Cre or Flp expressed in the cell population of interest, and an indicator allele which expresses a heritable marker, such as enhanced green fluorescent protein (eGFP), in response to recombinase activity (Jensen and Dymecki, 2014). For intersectional labeling, the indicator allele expresses a marker after recombination by two recombinases controlled by different driver alleles, so labeled cells represent the “intersection” or overlap of two independent gene expression domains (Fig 1, top). Recombination of the indicator allele is irreversible, so cells remain permanently labeled after transient driver expression. The labeled cells and their descendants share developmental history and constitute a genetically defined lineage that can be traced throughout development and into the adult brain, even after extensive cell migration.
Figure 1. Intersectional labeling reveals genetically defined subpopulations of noradrenergic neurons with distinct efferent projection patterns.
(Top) Visualization of noradrenergic neurons derived from a specific rhombomere in the embryonic hindbrain requires a rhombomere-specific Cre driver, noradrenergic specific Flp driver, and dual-recombinase responsive fluorescent indicator allele, RC::FrePe. In mice that inherit all three alleles, noradrenergic neurons derived from the Cre expression domain will be labeled with eGFP. All other noradrenergic neurons will be labeled with mCherry. (Middle) Four Cre driver lines expressed in the embryonic hindbrain (left, schematic sagittal view) define subpopulations of noradrenergic neurons that are distributed across the anatomically defined noradrenergic nuclei in the adult brainstem (right). The locus coeruleus (LoC), subcoeruleus dorsal (SubCD) and ventral (SubCV), A7, A5, C2/A2 and C1/A1 are shown with color-coded contribution from each genetically defined subpopulation represented as colored circles on a schematic sagittal view of the adult brainstem compressed along the mediolateral axis. (Bottom) Prominent axonal projections from the four genetically defined noradrenergic subopulations are illustrated as color-coded lines. The thickness of the open circle around each target region denotes the density of innervation. Olf bulb, olfactory bulb; Ctx ins, insular cortex; Ctx somato, somatosensory cortex; Hippo, hippocampus; BNST, bed nucleus of stria terminalis; Amyg, amygdala; Hippo, hippocampus; Thal, thalamus; Hyp, hypothalamus; PBN, parabranchial nucleus; Cereb, cerebellum; NTS, solitary nucleus. Adapted from Robertson et al., 2013.
In our initial analysis of the noradrenergic system, we exploited the unique gene expression profiles within rhombomeres (r), transient segmental units of the developing hindbrain. Four subpopulations of noradrenergic neurons were defined by intersectional expression of a noradrenergic specific Flp recombinase driver (DbhFlpo)(Robertson et al., 2013) and one of four rhombomere-specific Cre drivers (r1, En1cre (Li et al., 2002); r2, Hoxa2-cre (Awatramani et al., 2003); r3&5, Krox20cre (Voiculescu et al., 2000); and r4, Hoxb1cre (O’Gorman, 2005)). In mice heterozygous for the dual-recombinase responsive indicator allele RC::FrePe (Bang et al., 2012; Engleka et al., 2012), DbhFlpo, and a rhombomere-specific Cre driver, noradrenergic neurons derived from that rhombomere (expressing both Flp and Cre) are labeled with eGFP, while noradrenergic neurons derived from other rhombomeres (expressing Flp alone) are labeled with red fluorescent mCherry (Fig. 1, top).
When we mapped the fates of the four genetically defined noradrenergic subpopulations, we found that their distribution in the adult brain varies significantly from their traditional anatomical subdivisions (Fig. 1 middle row) and reveals previously unappreciated lineal relationships between noradrenergic neurons in disparate anatomical locations. In addition, these genetically defined subpopulations have distinct axon morphology (Robertson et al., 2013) and efferent projection patterns (Fig. 1 bottom). The smallest subpopulations, derived from the Hoxa2-cre and Krox20 expression domains in r2 and r3&5, respectively, provide input to relatively limited targets. In sharp contrast, the En1- and Hoxb1-derived noradrenergic subpopulations, which comprise the largest genetic lineages, have axonal projections distributed throughout the brain. En1-derived noradrenergic neurons provide the highest input to areas of higher cognitive function such as the hippocampus and cortex, while the Hoxb1-derived noradrenergic neurons preferentially provide input to key central autonomic sites such as the hypothalamus, solitary nucleus, parabrachial nucleus and bed nucleus of the stria terminalis (BNST).
Our ability to map the axonal projections of each rhombomere-derived subpopulation across the entire brain in complete isolation enabled us to visualize the architecture of the central noradrenergic efferent system at a resolution that is difficult to achieve with other approaches. Notably, we discovered an unexpected projection from non-LoC Hoxb1-derived neurons to the prefrontal cortex, challenging the long held notion that the LoC is the sole source of noradrenergic projections to the cortex (Robertson et al., 2013). The distinct projection profile of each genetically defined subpopulation suggests that this method of reclassifying the noradrenergic system is functionally relevant, and the experimental access provided by fluorescent labeling will facilitate additional investigation at all levels from individual cells to intact animals.
Although rhombomere-based labeling has demonstrated that noradrenergic neurons are heterogeneous with regard to their embryonic origins, it did not reveal any of the molecular determinants of noradrenergic subtype development. It was previously proposed that Hoxb1 is required for the development of medullary noradrenergic neurons (Gaufo et al., 2004). However, this conclusion was based on analysis of Hoxb1 mutant embryos and not long-term fate mapping. By combining intersectional labeling with a targeted loss of function mutation in Hoxb1, we can now determine which, if any, of the noradrenergic neurons in the adult brain require Hoxb1 expression. Here we present the results of further investigation into the Hoxb1-derived noradrenergic subpopulation. We demonstrate that Hoxb1 expression is required by a subset of pontine noradrenergic neurons. In addition, we uncover further genetically defined diversity in the Hoxb1 lineage.
2. Results
2.1. Hoxb1 is required for the development of a subset of pontine central noradrenergic neurons
Based on the absence of dopamine β-hydroxylase (Dbh) expression within rhombomere 4 of the embryonic day (E) 11.5 Hoxb1-null mouse hindbrain, it has been speculated that Hoxb1 expression is required for the differentiation of medullary A2 noradrenergic neurons (Gaufo et al., 2004). Given our previous finding that Hoxb1-derived noradrenergic neurons contribute to both the SubCV and A5 pontine nuclei and to caudal portions of the A1 and A2 medullary nuclei, we wanted to investigate whether Hoxb1 expression is required by all Hoxb1-derived noradrenergic neurons or just neurons destined for the A2 medullary nucleus. Toward this goal, we exploited the fact that the Hoxb1cre allele was generated by targeting cre to exon 1 of Hoxb1, resulting in a loss of function (Hoxb1-null) mutation (O’Gorman, 2005; Rottkamp et al., 2008). To visualize and map the fate of all noradrenergic neurons in the context of a Hoxb1-null mutation, we crossed Hoxb1cre mice with mice carrying DbhFlpo and RC::FrePe to generate triple transgenic Hoxb1cre;DbhFlpo;RC::FrePe animals that are either heterozygous or homozygous for the Hoxb1 mutation. In Hoxb1-null (Hoxb1cre/cre;DbhFlpo/+;RC::FrePe) and heterozygous (Hoxb1cre/+;DbhFlpo/+;RC::FrePe) mice, noradrenergic neurons with a history of Hoxb1cre expression will be labeled with eGFP, while all other noradrenergic neurons will be labeled with mCherry (Fig. 2 CTR panels).
Figure 2. Hoxb1 expression is required for the development of a subset of Hoxb1-derived noradrenergic neurons in the pontine SubC and A5 nuclei.
(TOP) Coronal sections from adult mouse brainstem reveal the significant absence of Hoxb1-derived noradrenergic neurons in the SubC and A5 nuclei of Hoxb1-null animals (Null-Hoxb1cre/cre;DbhFlpo;RC::FrePe) compared to control animals (CTR-Hoxb1cre/+;DbhFlpo;RC::FrePe). Hoxb1-derived noradrenergic neurons are labeled with eGFP (green) and all other noradrenergic neurons are labeled with mCherry (red) in representative sections corresponding to the labeled areas in the schematic (top right). (Bottom) Counts of labeled neurons confirm a significant decrease in the number of eGFP-labeled neurons (**P<0.01) and total noradrenergic neurons (*P<0.05) in A5 of Hoxb1-null mice (top right graph). No significant change in mCherry-labeled A5 neurons was observed. In the SubCD of Hoxb1-null animals, a significant decrease in eGFP-labeled neurons (*P<0.05), but no significant difference in mCherry or total noradrenergic neurons was observed (bottom left graph). In the SubCV of Hoxb1 null animals, significant decreases in eGFP-labeled and total noradrenergic neurons were observed(***P<0.001) (bottom right graph). Numbers are the sum of bilateral counts from 40-μm sections spaced 120 μm apart from the brainstems of seven mice for each fate map (n=7 with minimum of n=3 for each gender; error bars are mean ± s.e.m.). Scale bar represents 119 μm in all images.
In Hoxb1-null and heterozygous mice, we tracked all noradrenergic neurons into the adult brain and mapped them onto the anatomically defined nuclei in the pons (LoC, SubC, A5) and medulla (A2, A1) according to an adult mouse brain atlas (Paxinos and Franklin, 2004). For each anatomically defined nucleus, we counted mCherry- and eGFP-positive noradrenergic neurons in male and female mice (Fig. 2, 3, and Supplemental Table 1). The number of noradrenergic neurons in each nucleus did not vary significantly according to sex. It is important to note that our intersectional approach is incapable of distinguishing Dbh positive adrenergic neurons in C1 and C2, from noradrenergic neurons in A1 and A2.
Figure 3. Hoxb1 expression is not required for the development of medullary Hoxb1-derived noradrenergic neurons.
(Top) Coronal sections from adult mouse brainstem reveal an increase in Hoxb1-derived norepinephrine neurons in C1/A1 and C2/A2 nuclei of Hoxb1-null animals (Null-Hoxb1cre/cre;DbhFlpo;RC::FrePe) compared to control animals (CTR-Hoxb1cre/+;DbhFlpo;RC::FrePe). Hoxb1-derived noradrenergic neurons are labeled with eGFP and all other noradrenergic neurons are labeled with mCherry (red) in representative sections corresponding to the boxed areas in the schematic (left). Scale bar represents 100 μm (C2/A2) or 75 μm (C1/A1). (Bottom) Counts of labeled neurons confirm a significant increase of eGFP-labeled neurons (*P<0.05) but no significant change in numbers of mCherry-labeled or total noradrenergic neurons in C2/A2 (left graph). In C1/A1, a significant increase of eGFP-labeled neurons (***P<0.001) and total noradrenergic neurons was observed (right graph). Numbers are the sum of bilateral counts from 40-μm sections spaced 120 μm apart from the brainstems of six mice for each fate map (n=6 with n=3 for each gender; error bars are mean ± s.e.m.) For the total count of noradrenergic neurons (grey bars), the number of neurons co-expressing mCherry and eGFP was subtracted from the GFP population to prevent double counting.
In the pons of Hoxb1-null animals we observed a striking deficit in the number of Hoxb1-derived noradrenergic neurons (Fig. 2, eGFP-labeled neurons). In the SubCV, 90% of the Hoxb1-derived noradrenergic neurons are absent in Hoxb1 mutants. Similarly, 80% and 66% of the Hoxb1-derived noradrenergic neurons are missing in A5 and the SubCD of Hoxb1-null animals, respectively (Fig. 2). In the SubCV and A5, where Hoxb1-derived neurons constitute the majority of noradrenergic neurons (Fig 1.), the total number of neurons is also significantly decreased (Fig. 2, grey bars in graphs). The number of non-Hoxb1-derived (mCherry-positive) noradrenergic neurons was not significantly different in Hoxb1-null and heterozygous mice (Fig. 2, red neurons and bars).
In contrast, mapping of noradrenergic neurons onto the medullary A1 and A2 nuclei in Hoxb1-null mice revealed a significant increase in the number of Hoxb1-derived noradrenergic neurons (Fig. 3, eGFP-labeled neurons). In the C1/A1 nuclei, but not in C2/A2, an increase in the total number of labeled neurons was also observed (Fig 3, grey bars). We did not observe any significant change in the number of non-Hoxb1-derived (mCherry-positive) noradrenergic populations (Fig. 3, red neurons and bars), but we did detect co-expression of eGFP and mCherry in a few neurons. This co-labeling is never observed in heterozygous mice, because of the structure of the RC::FrePe allele (Fig 1) and the order in which the Hoxb1cre and DbhFlpo driver alleles are expressed. The loxP-flanked mCherry-stop cassette of RC::FrePe is excised by the early, transient expression of Hoxb1cre in progenitor cells (Freyer et al., 2011; Gaufo et al., 2000; Popperl et al., 1995). Later excision of the FRT-flanked stop cassette by DbhFlpo (Robertson et al., 2013) results in eGFP expression. Co-labeling can only be observed when Flp is expressed before Cre. In that situation, cells will express mCherry until Cre-mediated recombination deletes the mCherry-stop cassette and permits eGFP expression. The cells will transiently contain both fluorescent proteins until remaining mCherry mRNA and protein is turned over. Thus, co-expression of mCherry and eGFP in Hoxb1-null mice suggests that in at least a few cells, the cre-dependent excision of the mCherry-stop cassette was a recent event occurring in mature neurons.
Next, we wanted to determine the specific targets of the noradrenergic neurons that are missing in the Hoxb1-null mice. We exploited the ability of the R/C::FrePe allele to label axons with eGFP (Bang et al., 2012; Brust et al., 2014; Robertson et al., 2013) in order to compare the efferent projection profiles of the Hoxb1-derived noradrenergic population in Hoxb1-null and heterozygous mice. We did not observe a decrease in fiber density at any target site in Hoxb1-null mice relative to heterozygous controls. To the contrary, there are significantly more labeled axons at several target sites in Hoxb1-null mice, likely reflecting the increased number of eGFP-labeled cells in C2/A2 and C1/A1 (Fig. 4). Interestingly, this increase is not observed in the paraventricular thalamic (PV) nucleus, suggesting that the additional eGFP-labeled cells in Hoxb1-null mice do not project to the PV region.
Figure 4. In Hoxb1-null mice, the number of noradrenergic axons is increased at target sites of the Hoxb1-derived noradrenergic subpopulation.
In Hoxb1-null (Null-Hoxb1cre/cre;DbhFlpo;RC::FrePe) and control (CTR-Hoxb1cre/+;DbhFlpo;RC::FrePe) mice, axons of Hoxb1-derived (eGFP-labeled) noradrenergic neurons are revealed by immunoperoxidase staining of representative sections from target sites of noradrenergic projections. Relative to controls, staining is heavier in Hoxb1-null sections indicating an increase in the number of labeled axons in the basolateral amygdala posterior part (BLP) and the bed nucleus of the stria terminalis (BNST) medial division ventral part (STMV) but not in the paraventricular (PV) thalamic nucleus. Grey boxes on coronal schematic diagrams indicate the approximate position of the imaged axons. Scale bar represents 136 μm (Thalamus and BNST) or 47 μm (Amygdala). The total projection pixel intensities (arbitrary intensity units) of eGFP-positive axon fibers from three Hoxb1-null and control mice are shown (BLP n=7, BNST STMV n=5, and PV n=6 images; error bars are mean ± s.e.m.). A two-tailed, unpaired t-test was used to determine significance. *P = 0.01 t = 2.896 df = 17 (BLP), ***P = 0.0002 t = 5.111 df = 13 (BNST STMV), Not significantly different P = 0.389 t = 0.8878 df = 15 (PV).
The increase in the number of eGFP-positive noradrenergic neurons observed in C2/A2 and C1/A1 nuclei of Hoxb1-null mice may be due to the ectopic expression of cre observed in the hindbrain caudal to rhombomere 4 in Hoxb1-null embryos (Yang et al., 2008). However, ectopic cre expression does not explain the absence of a corresponding decrease in the number of mCherry labeled neurons and the statistically significant increase in the total number of noradrenergic neurons in C1/A1. Other possible explanations include increased proliferation of noradrenergic progenitors in the absence of Hoxb1 expression or disrupted cell migration such that the noradrenergic neurons missing from the pons migrate to the medulla. However, the latter explanation is not supported by our cell counts: more cells are gained in the medulla than are lost in the pons. Also consistent with these data is the possibility that in the absence of Hoxb1 expression, other cells in the brainstem may undergo a fate switch—becoming noradrenergic neurons, as defined by expression of Dbh and tyrosine hydroxylase (TH) — similar to what has been reported for motor neurons in Hoxb1 mutants (Gavalas et al., 2003).
In summary, fate mapping on a Hoxb1-null genetic background demonstrates that Hoxb1 expression is required for the development of a major subset of pontine SubCV and A5 noradrenergic neurons. These data reveal genetic and developmental complexity within the Hoxb1-derived noradrenergic neuron subpopulation and suggest that at least a portion of the pontine neurons may have a developmental history that is distinct from those of the medullary neurons. In the hindbrain, Hoxb1cre expression is restricted to r4, but cre is also expressed in the spinal cord, caudal of the hindbrain (Freyer et al., 2011; Gaufo et al., 2000; Marshall et al., 1994; Popperl et al., 1995) and unpublished observations). Therefore, one plausible hypothesis is that the Hoxb1cre-derived noradrenergic neurons consist of two lineages with distinct anatomic origins.
2.2. b1r4-cre defines a subset of Hoxb1-derived pontine noradrenergic neurons
To further investigate the developmental origins of Hoxb1-derived noradrenergic neurons, we used a b1r4-cre driver transgene, in which Cre expression is under the control of the Hoxb1 r4 enhancer that restricts Cre expression exclusively to rhombomere 4 (Di Bonito et al., 2013; Studer et al., 1994). In b1r4-cre;DbhFlpo;RC::FrePe mice, noradrenergic neurons with a history of b1r4-cre expression (hereafter designated b1r4-cre-derived) express eGFP, while all non-b1r4-cre noradrenergic neurons express mCherry. We mapped the fate of b1r4-cre-derived noradrenergic neurons onto the mature anatomically defined noradrenergic nuclei and found that b1r4-cre-derived noradrenergic neurons contribute to the SubCD, SubCV, and A5 pontine nuclei, but not to the medullary C2/A2, C1/A1 nuclei (Fig. 5). To determine the percent contribution to each nucleus we counted mCherry and eGFP positive neurons in male and female mice. We found that b1r4-cre-derived noradrenergic neurons comprise 2.8%, 46.5%, and 51.5% of the SubCD, SubCV, and A5 nuclei, respectively (Fig. 5). We also observed a small but significant increase in the number of b1r4-cre-derived noradrenergic neurons in the A5 nucleus of female mice compared to males (Supplemental Table 1). Regardless of this interesting sex difference, b1r4-cre labels fewer neurons than Hoxb1cre in all three pontine nuclei.
Figure 5. b1r4-cre expression defines a subpopulation of noradrenergic neurons in the pons.
Coronal sections of brain from b1r4-cre;DbhFlpo;RC::FrePe mice demonstrate that b1r4-cre-derived noradrenergic neurons (b1r4-cre and DbhFlpo expression; eGFP-labeled) populate the SubC dorsal, SubC ventral, and A5 noradrenergic nuclei in the pons. No b1r4-cre-derived noradrenergic neurons are observed in the pontine A7 nucleus and medullary C2/A2 and C1/A1 nuclei. Cell counts indicate that b1r4-cre-derived neurons constitute a smaller percentage of SubC and A5 neurons than those labeled by Hoxb1cre.The number of labeled neurons in each anatomically defined noradrenergic nucleus (mean ± s.e.m.) is the sum of bilateral counts from 40-μm sections spaced 120 μm apart in the brainstems of seven mice (3 males and 4 females). Scale bar represents 128 μm (A7), 119 μm (LoC, SubC, C2/A2, C1/A1), or 77 μm (A5)
These findings reveal the existence of at least two genetically distinct noradrenergic neuron subpopulations within the Hoxb1 lineage: neurons that express both b1r4-cre and Hoxb1cre, and those that express only Hoxb1cre. The precise nature of these two subpopulations remains unclear. One possibility is that the b1r4-cre transgene is not expressed uniformly throughout rhombomere 4, indicating heterogeneity in the molecular programs that control development within the rhombomere. Alternatively, the non-b1r4-derived neurons may originate from a Hoxb1 domain outside of rhombomere 4. Regardless of their origin, if these two genetically distinct subpopulations are also functionally distinct, they are likely to project to different target regions. Our genetic tools permit this hypothesis to be tested.
2.3. b1r4-cre defines a subpopulation of noradrenergic neurons with a distinct efferent projection pattern
To determine if the b1r4-cre- and Hoxb1-derived noradrenergic neurons project to the same or different target regions, we again exploited the ability of the R/C::FrePe allele to label axons with eGFP. We mapped the axonal projections of the b1r4-cre-derived noradrenergic subpopulation in b1r4-cre;DbhFlpo;RC::FrePe mice and those of the entire Hoxb1-derived noradrenergic subpopulation in Hoxb1cre;DbhFlpo;RC:FrePe mice.
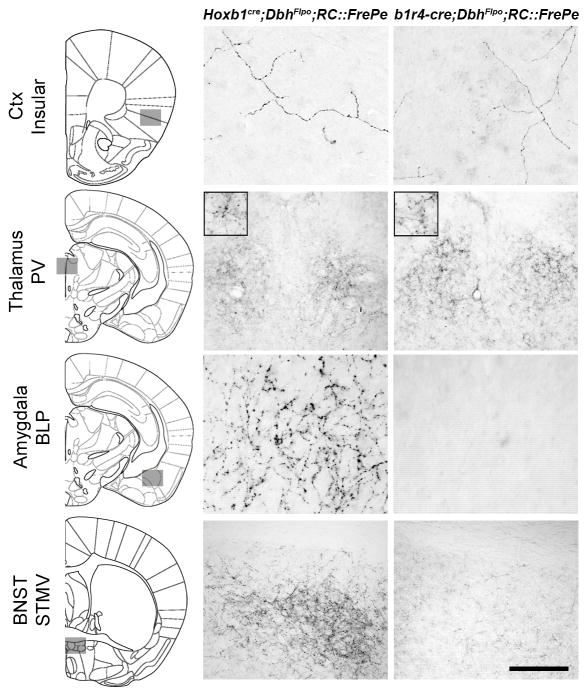
Consistent with our previous findings (Robertson et al., 2013), noradrenergic neurons labeled by Hoxb1cre provide input to key components of the central autonomic nervous system, including the insular cortex (Ctx), paraventricular thalamic (PV) nucleus, basolateral amygdala, bed nucleus of the stria terminalis (BNST), parabrachial nucleus, and solitary nucleus (Fig 6, left panels and Table 1). Upon mapping axonal projections from b1r4-cre-derived noradrenergic neurons, we found that these neurons project to many of the same targets, including the insular cortex (Fig 6, top right). As expected for a smaller subpopulation, the b1r4-cre-derived noradrenergic neurons appear to provide fewer projections at most target sites, although there are several exceptions (Table 1). In the PV thalamic nucleus, projections from the b1r4-cre and Hoxb1-derived subpopulations occur at approximately the same density (Fig 5), suggesting that the b1r4-cre subpopulation accounts for most, if not all, of the projections labeled by Hoxb1cre. In contrast, projections from the b1r4-cre-derived subpopulation are virtually absent in basolateral amygdala and BNST, two regions that are heavily targeted by the full Hoxb1-derived population (Fig 6, right panels and Table 1). Thus, the b1r4-cre-derived noradrenergic lineage projects more heavily to a specific subset of the Hoxb1-derived targets, rather than constituting the same proportion of the projections at all target sites. This unique projection pattern suggests that b1r4-cre expression may define a functionally distinct subpopulation of noradrenergic neurons within the Hoxb1 lineage. These results highlight the role of early molecular programs in generating subtle differences in the neuronal circuitry.
Figure 6. b1r4-cre defines a subpopulation of noradrenergic neurons with a distinct efferent projection pattern.
At representative target sites, axons of eGFP-labeled Hoxb1-derived and b1r4-cre-derived noradrenergic neurons are revealed by immunoperoxidase staining. Projections from Hoxb1-derived noradrenergic subpopulation can be observed in insular cortex (Ctx), paraventricular (PV) thalamic nucleus, basolateral amygdala posterior part (BLP), and the BNST medial division ventral part (STMV). In contrast, projections from b1r4-cre-derived neurons are almost completely absent from the Amygdala and very sparse in the BNST. The representative images reflect observations from a minimum of five animals of each genotype. Grey boxes on coronal schematic diagrams indicate the approximate position of the imaged axons. Scale bar represents 136 μm (Thalamus and BNST), 56 μm (Ctx), or 47 μm (Amygdala).
Table 1.
Comparison of the efferent projection patterns from Hoxb1- and b1r4-cre-derived noradrenergic neurons
| Brain region | Hoxb1cre | b1r4-cre |
|---|---|---|
| Cerebral Cortex | ||
| Insular Ctx | ++ | + |
| Orbital Ctx | + | +/− |
| Olfactory Bulb | + | +/− |
| Septum | ||
| Lateral Septal Nucleus | + | + |
| Medial Septal Nucleus | + | + |
| Bed nucleus of the stria terminalis | ||
| BNST, dorsal medial | ++ | +/− |
| BNST, dorsal lateral | +++ | +/− |
| BNST, ventral medial | ++++ | + |
| BNST, ventral lateral | ++++ | + |
| Hypothalamus | ||
| Paraventricular hypothalamic nucleus | ++++ | + |
| Dorsomedial hypothalamic nucleus | ++++ | ++ |
| Arcuate nucleus | + | + |
| Lateral hypothalamic area | +++ | + |
| Amygdala | ||
| Central Amygdaloid nucleus | +++ | +/− |
| Basolateral amygdaloid nucleus | ++++ | +/− |
| Thalamus | ||
| Central medial thalamic nucleus | +++ | ++ |
| Paraventricular thalamic nucleus | +++ | +++ |
| Brainstem | ||
| Dorsal raphe nucleus | ++++ | ++ |
| Median raphe nucleus | + | +/− |
| Locus Coeruleus | ++++ | +++ |
| Parabrachial Nucleus | ++++ | +++ |
| Parvicellular reticular nucleus | +++ | ++ |
| Intermediate reticular nucleus | ++++ | ++ |
| Solitary nucleus | ++++ | ++ |
| Cerebellum | + | + |
The relative density of noradrenergic innervation from Hoxb1-derived and b1r4-cre-derived neurons across various regions of the brain is shown from coronal sections immunostained for eGFP. The density of eGFP immunolabeling is graded as one of the following: undetectable in all animals (−), presence of rare fiber and not seen in all animals (+/−), sparse but consistent fibers in all animals (+), moderate presence with consistent fibers observed in all animals in multiple sections (++), strong innervation (+++), dense and heavy innervation (++++).
3. Discussion
Our results underline the complex molecular and developmental heterogeneity of central noradrenergic neurons, and the value of intersectional labeling for identifying the different genetic lineages that make up broadly defined cell types. In addition to the four genetically defined noradrenergic subpopulations described in our recent study (Robertson et al., 2013), we have now demonstrated that the noradrenergic neuron population defined by transient developmental expression of Hoxb1 consists of several distinct subpopulations. These new genetically defined subpopulations are correlated with, but do not precisely match, the anatomic division of the Hoxb1-derived population between the pons and medulla.
By combining intersectional labeling with a loss-of-function mutation of Hoxb1, we were able to assess whether Hoxb1 is a molecular determinant of noradrenergic development for those neurons originating within the Hoxb1 expression domain. Previous analysis of Hoxb1-null mice indicated that Hoxb1 is required for the specification of medullary noradrenergic neurons in the solitary nucleus (Gaufo et al., 2004). This conclusion was based on observations in embryonic hindbrains, where ongoing developmental changes can make identification of cellular populations challenging. Our analysis, in which neurons in the adult brain are unambiguously labeled on the basis of developmental gene expression, demonstrates that Hoxb1 is required for a subset of noradrenergic neurons in the pons. In the medulla of Hoxb1-null mice, the surprising increase in the number of labeled neurons hampers any attempt to determine whether Hoxb1 expression is required for specification of a subset of neurons in A1 or A2.
Intersectional labeling of noradrenergic neurons in b1r4-cre;DbhFlpo;RC::FrePe mice has revealed the existence of another genetically defined subpopulation of noradrenergic neurons restricted to the pons. This population—defined by activity of a rhombomere 4-specific enhancer element in the context of a transgenic construct—has a unique axonal projection pattern, which hints that it may be functionally distinct. We have previously shown by retrograde labeling that axons from Hoxb1-derived noradrenergic neurons in the pons and medulla share some target sites (Robertson et al., 2013), but the near absence of b1r4-cre-derived axons in BNST and basolateral amygdala suggest that those regions receive many of their noradrenergic inputs from Hoxb1-derived neurons in the medulla.
Current genetic tools do not permit us to determine the degree of overlap between the Hoxb1-dependent noradrenergic subpopulation and b1r4-cre-derived subpopulation, but it may be significant that both of these newly defined noradrenergic subpopulations are restricted to the pons. Our lab is currently developing new indicator alleles that incorporate a third recombinase system, Dre/rox (Sauer and McDermott, 2004), and offer the ability to label axonal projections from both cre+ and cre-negative cells (unpublished observations). These new tools may make it possible in the future to label the b1r4-cre-derived subpopulation on a Hoxb1-null background and to specifically label fibers from the subpopulation of Hoxb1-derived neurons that are not labeled by b1r4-cre. Future studies should also apply these new intersectional tools to explore noradrenergic efferents in regions beyond the forebrain such as the brainstem and spinal cord. Together, these analyses will shed new light on the Hoxb1-dependent noradrenergic neurons in the pons and the overall complexity of the noradrenergic system.
4. Experimental Procedure
4.1 Animal Care and Breeding
All animal procedures and breeding were carried out in accordance with the NIEHS Institutional Animal Care and Use Committee. Mice were maintained on a 12-hour light/dark cycle and given water and food ad libitum. Hoxb1cre (O’Gorman, 2005; Rottkamp et al., 2008), DbhFlpo (Robertson et al., 2013), RC::FrePe (Bang et al., 2012; Engleka et al., 2012), and b1r4-cre (Di Bonito et al., 2013) colonies were maintained on a C57BL/6J background. Triple transgenic Hoxb1cre;DbhFlpo;RC::FrePe animals were generated by crossing Hoxb1cre/+ to DbhFlpo/+;RC::FrePe. Offspring were intercrossed to generate Hoxb1-null (Hoxb1cre/cre;DbhFlpo/+;RC::FrePe), and heterozygous (Hoxb1cre/+;DbhFlpo/+;RC::FrePe) mice. Triple transgenic b1r4-cre;DbhFlpo;RC::FrePe animals were generated by crossing b1r4-cre to DbhFlpo/+;RC::FrePe. Hoxb1cre/cre and Hoxb1cre/+ mice were genotyped using primers 5′ ATTGGCCTGGGAGAGATCA (Hoxb1, forward), 5′-CCCGGTTACAAAGTGGGTACT (Hoxb1, reverse), and 5′-ATGTTTAGCTGGCCCAAATG (Cre, reverse) (http://jaxmice.jax.org/strain/012373.html). All other genotyping was performed as previously described (Robertson et al., 2013).
4.2 Immunohistochemistry
Adult Hoxb1-null (Hoxb1cre/cre;DbhFlpo/+;RC::FrePe), heterozygous (Hoxb1cre/+;DbhFlpo/+;RC::FrePe), b1r4-cre;DbhFlpo;RC::FrePe and control mice were anesthetized with phenobarbital and transcardially perfused with 0.1M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS. Brains were postfixed overnight in 4% PFA at 4 °C and then rinsed in PBS. Following equilibration in 30% sucrose in PBS brains were embedded and frozen in tissue-freezing medium (Triangle Biomedical Sciences). Brains were stored at −80°C until sectioned.
To label noradrenergic cell bodies, free-floating 40 μm coronal cryosections were incubated with chicken anti-GFP (1:10,000; ab13970 Abcam) and Rabbit anti-dsRed (1:1000; 632496 Clontech), or chicken anti-GFP, Rabbit anti-dsRed, and Mouse anti-tyrosine hydroxylase (1:500; GTX10372 GeneTex), followed by detection with Alexa 488 conjugated goat anti-chicken, Alexa 568 conjugated anti-rabbit, and Alexa 647 anti-mouse secondary antibodies (1:500; A11039, A11036, A21236 Life Technologies) as previously described (Robertson et al., 2013).
Immunoperoxidase labeling was used to achieve the highest-sensitivity detection of eGFP in axons. 40-μm free-floating coronal sections were incubation with chicken anti-GFP (1:10,000; ab13970 Abcam) followed by incubaton with a biotinylated goat anti-chicken secondary (1:500, BA9010), Vectastain Elite ABC kit and DAB (all Vector Laboratories). We identified eGFP positive projections throughout various brain regions according to an adult mouse brain atlas (Paxinos and Franklin, 2004) and collected representative images on a light microscope (Zeiss Axio Imager.Z2).
4.3 Cell Counts and Statistical Analysis
To quantify eGFP+ and mCherry+ noradrenergic neuron number in adult Hoxb1-null (Hoxb1cre/cre;DbhFlpo/+;RC::FrePe), heterozygous (Hoxb1cre/+;DbhFlpo/+;RC::FrePe), and b1r4-cre;DbhFlpo;RC::FrePe mice, the anatomically defined nuclei (SubCD, SubCV, A5, A2 and A1) were counted as previously described (Jin et al., 2004; Robertson et al., 2013). Cell numbers are the sum of bilateral counts from 40 μm sections spaced 120 μm apart from the brainstem of a minimum of six mice for each strain of animals and a minimum of n=3 for each gender. We manually counted cells for each animal and anatomically matched the sections to an adult mouse brain atlas (Paxinos and Franklin, 2004). Data are expressed as the mean ± standard error (s.e.m.) and for each experiment all genotypes were represented. We compared noradrenergic neuron cell counts for each nucleus between Hoxb1-null (Hoxb1cre/cre;DbhFlpo/+;RC::FrePe) and heterozygous (Hoxb1cre/+;DbhFlpo/+;RC::FrePe) controls using unpaired, two-tailed t tests. Statistical tests were performed using GraphPad Prism (GraphPad Software). The exact animal numbers, gender distributions and the statistical analyses are also indicated in the figure legends. Data collection and analysis were not preformed blind to experimental conditions, because the noradrenergic neuron phenotype in Hoxb1-null mice is distinct enough for investigators to tell the genotype of the animals by observation of the tissue section.
4.4 Total projection pixel intensity quantification
We performed analysis of projection pixel intensity on sections labeled for eGFP by the immunoperoxidase methods described above. We photographed multiple fields from the region of interest (PV thalamus, BNST STMV, and BLP amygdala) at 20× magnification. For quantification, we visualized the region of interest as a maximal projection of a z-stack using a Zeiss Axio Imager.Z2 light microscope (7 images for BLP amygdala, 5 images for BNST STMV, and 6 images for PV thalamus from three Hoxb1-null and control animals). Using NIH ImageJ software a manual threshold function was applied to identify and isolate projections with the eGFP label. Following isolation of the projections, the total area above threshold and the average pixel intensity was measured. The total projection pixel intensity was calculated by multiplying total area times the average pixel intensity. We statistically analyzed the total projection pixel intensity by an unpaired t-test using GraphPad Prism.
Supplementary Material
The number of mCherry-positive, eGFP-positive, and all noradrenergic neurons in each anatomically defined nucleus of male and female mice are shown for each genotype (CTR-Hoxb1cre/+;DbhFlpo;RC::FrePe, Null-Hoxb1cre/cre;DbhFlpo;RC::FrePe, and b1r4-cre;DbhFlpo;RC::FrePe). Numbers are the sum of bilateral counts from 40-μm sections spaced 120 μm apart (mean ± s.e.m.). The average cell counts for male and female mice within a single genotype were compared using unpaired, two-tailed t tests for each nucleus. No significant differences were observed in the counts from male and female mice of the same genotype, with the exception of the A5 nucleus counts in the b1r4-cre;DbhFlpo;RC::FrePe strain. In the A5 nucleus of b1r4-cre;DbhFlpo;RC::FrePe animals, a small but significant difference in the number of noradrenergic neurons between male and female mice is observed (*p=0.0496).
Highlights.
Intersectional genetic fate mapping reveals noradrenergic neuron diversity
Hoxb1 is required for a subset of pontine noradrenergic neurons
A subset of pontine Hoxb1-derived noradrenergic neurons are defined by b1r4-cre
b1r4-cre+ pontine noradrenergic neurons have a distinct efferent projection pattern
Acknowledgments
We thank Dr. S. Dymecki for RC::FrePe mice. We thank T. Wolfgang, G. Keeley and NIEHS Fluorescence Microscopy, Vivarium, Knockout Mice, and Statistics services for assistance. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (ZIA-ES-102805).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awatramani R, Soriano P, Rodriguez C, Mai JJ, Dymecki SM. Cryptic boundaries in roof plate and choroid plexus identified by intersectional gene activation. Nature genetics. 2003;35:70–5. doi: 10.1038/ng1228. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM, Commons KG. Projections and interconnections of genetically defined serotonin neurons in mice. The European journal of neuroscience. 2012;35:85–96. doi: 10.1111/j.1460-9568.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain research. Brain research reviews. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Brust RD, Corcoran AE, Richerson GB, Nattie E, Dymecki SM. Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell Rep. 2014;9:2152–65. doi: 10.1016/j.celrep.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagnat J, Morin-Surun MP, Fortin G, Thoby-Brisson M. Developmental basis of the rostro-caudal organization of the brainstem respiratory rhythm generator. Philos Trans R Soc Lond B Biol Sci. 2009;364:2469–76. doi: 10.1098/rstb.2009.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DJ, Gao WJ, Waterhouse BD. Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc Natl Acad Sci U S A. 2014;111:6816–21. doi: 10.1073/pnas.1320827111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS, Jessell TM. Hox networks and the origins of motor neuron diversity. Curr Top Dev Biol. 2009;88:169–200. doi: 10.1016/S0070-2153(09)88006-X. [DOI] [PubMed] [Google Scholar]
- Di Bonito M, Narita Y, Avallone B, Sequino L, Mancuso M, Andolfi G, Franze AM, Puelles L, Rijli FM, Studer M. Assembly of the auditory circuitry by a Hox genetic network in the mouse brainstem. PLoS Genet. 2013;9:e1003249. doi: 10.1371/journal.pgen.1003249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleka KA, Manderfield LJ, Brust RD, Li L, Cohen A, Dymecki SM, Epstein JA. Islet1 derivatives in the heart are of both neural crest and second heart field origin. Circulation research. 2012;110:922–6. doi: 10.1161/CIRCRESAHA.112.266510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer L, Aggarwal V, Morrow BE. Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development. 2011;138:5403–14. doi: 10.1242/dev.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaufo GO, Flodby P, Capecchi MR. Hoxb1 controls effectors of sonic hedgehog and Mash1 signaling pathways. Development. 2000;127:5343–54. doi: 10.1242/dev.127.24.5343. [DOI] [PubMed] [Google Scholar]
- Gaufo GO, Wu S, Capecchi MR. Contribution of Hox genes to the diversity of the hindbrain sensory system. Development. 2004;131:1259–66. doi: 10.1242/dev.01029. [DOI] [PubMed] [Google Scholar]
- Gavalas A, Ruhrberg C, Livet J, Henderson CE, Krumlauf R. Neuronal defects in the hindbrain of Hoxa1, Hoxb1 and Hoxb2 mutants reflect regulatory interactions among these Hox genes. Development. 2003;130:5663–79. doi: 10.1242/dev.00802. [DOI] [PubMed] [Google Scholar]
- Grzanna R, Fritschy JM. Efferent projections of different subpopulations of central noradrenaline neurons. Progress in brain research. 1991;88:89–101. doi: 10.1016/s0079-6123(08)63801-7. [DOI] [PubMed] [Google Scholar]
- Jensen P, Dymecki SM. Essentials of recombinase-based genetic fate mapping in mice. Methods Mol Biol. 2014;1092:437–54. doi: 10.1007/978-1-60327-292-6_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SH, Kim HJ, Harris DC, Thomas SA. Postnatal development of the cerebellum and the CNS adrenergic system is independent of norepinephrine and epinephrine. The Journal of comparative neurology. 2004;477:300–9. doi: 10.1002/cne.20263. [DOI] [PubMed] [Google Scholar]
- Li JY, Lao Z, Joyner AL. Changing requirements for Gbx2 in development of the cerebellum and maintenance of the mid/hindbrain organizer. Neuron. 2002;36:31–43. doi: 10.1016/s0896-6273(02)00935-2. [DOI] [PubMed] [Google Scholar]
- Marshall H, Studer M, Popperl H, Aparicio S, Kuroiwa A, Brenner S, Krumlauf R. A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature. 1994;370:567–71. doi: 10.1038/370567a0. [DOI] [PubMed] [Google Scholar]
- O’Gorman S. Second branchial arch lineages of the middle ear of wild-type and Hoxa2 mutant mice. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;234:124–31. doi: 10.1002/dvdy.20402. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Elsevier Academic Press; Amsterdam ; Boston: 2004. [Google Scholar]
- Popperl H, Bienz M, Studer M, Chan SK, Aparicio S, Brenner S, Mann RS, Krumlauf R. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell. 1995;81:1031–42. doi: 10.1016/s0092-8674(05)80008-x. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;300:R222–35. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SD, Plummer NW, de Marchena J, Jensen P. Developmental origins of central norepinephrine neuron diversity. Nat Neurosci. 2013;16:1016–23. doi: 10.1038/nn.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottkamp CA, Lobur KJ, Wladyka CL, Lucky AK, O’Gorman S. Pbx3 is required for normal locomotion and dorsal horn development. Dev Biol. 2008;314:23–39. doi: 10.1016/j.ydbio.2007.10.046. [DOI] [PubMed] [Google Scholar]
- Sauer B, McDermott J. DNA recombination with a heterospecific Cre homolog identified from comparison of the pac-c1 regions of P1-related phages. Nucleic Acids Res. 2004;32:6086–95. doi: 10.1093/nar/gkh941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer M, Popperl H, Marshall H, Kuroiwa A, Krumlauf R. Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science. 1994;265:1728–32. doi: 10.1126/science.7916164. [DOI] [PubMed] [Google Scholar]
- Voiculescu O, Charnay P, Schneider-Maunoury S. Expression pattern of a Krox-20/Cre knock-in allele in the developing hindbrain, bones, and peripheral nervous system. Genesis. 2000;26:123–6. doi: 10.1002/(sici)1526-968x(200002)26:2<123::aid-gene7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhou Y, Barcarse EA, O’Gorman S. Altered neuronal lineages in the facial ganglia of Hoxa2 mutant mice. Dev Biol. 2008;314:171–88. doi: 10.1016/j.ydbio.2007.11.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The number of mCherry-positive, eGFP-positive, and all noradrenergic neurons in each anatomically defined nucleus of male and female mice are shown for each genotype (CTR-Hoxb1cre/+;DbhFlpo;RC::FrePe, Null-Hoxb1cre/cre;DbhFlpo;RC::FrePe, and b1r4-cre;DbhFlpo;RC::FrePe). Numbers are the sum of bilateral counts from 40-μm sections spaced 120 μm apart (mean ± s.e.m.). The average cell counts for male and female mice within a single genotype were compared using unpaired, two-tailed t tests for each nucleus. No significant differences were observed in the counts from male and female mice of the same genotype, with the exception of the A5 nucleus counts in the b1r4-cre;DbhFlpo;RC::FrePe strain. In the A5 nucleus of b1r4-cre;DbhFlpo;RC::FrePe animals, a small but significant difference in the number of noradrenergic neurons between male and female mice is observed (*p=0.0496).