Abstract
STUDY QUESTION
Are female young cancer survivors (YCS) able to self-collect high-quality dried blood spots (DBSs) at home to provide biospecimens for studying ovarian reserve?
SUMMARY ANSWER
YCS can self-collect high-quality DBS specimens in non-clinical settings, and anti-Mullerian hormone (AMH) levels can be assayed in such specimens.
WHAT IS KNOWN ALREADY
Large-scale biosample collection is a barrier to studying ovarian reserve in YCS. DBS collected by research personnel has high acceptability. AMH levels measured in DBS are highly correlated with those measured by serum-based methods.
STUDY DESIGN, SIZE, DURATION
In a prospective cohort study, YCS were recruited to self-collect DBS samples. AMH levels were assayed in 112 samples.
PARTICIPANTS/MATERIALS, SETTING, METHODS
YCS participants, ages 18–44, were recruited from a nationwide longitudinal cohort and DBS collection materials were posted to them. AMH levels were assayed by the Ansh DBS AMH ELISA and compared according to participant characteristics.
MAIN RESULTS AND THE ROLE OF CHANCE
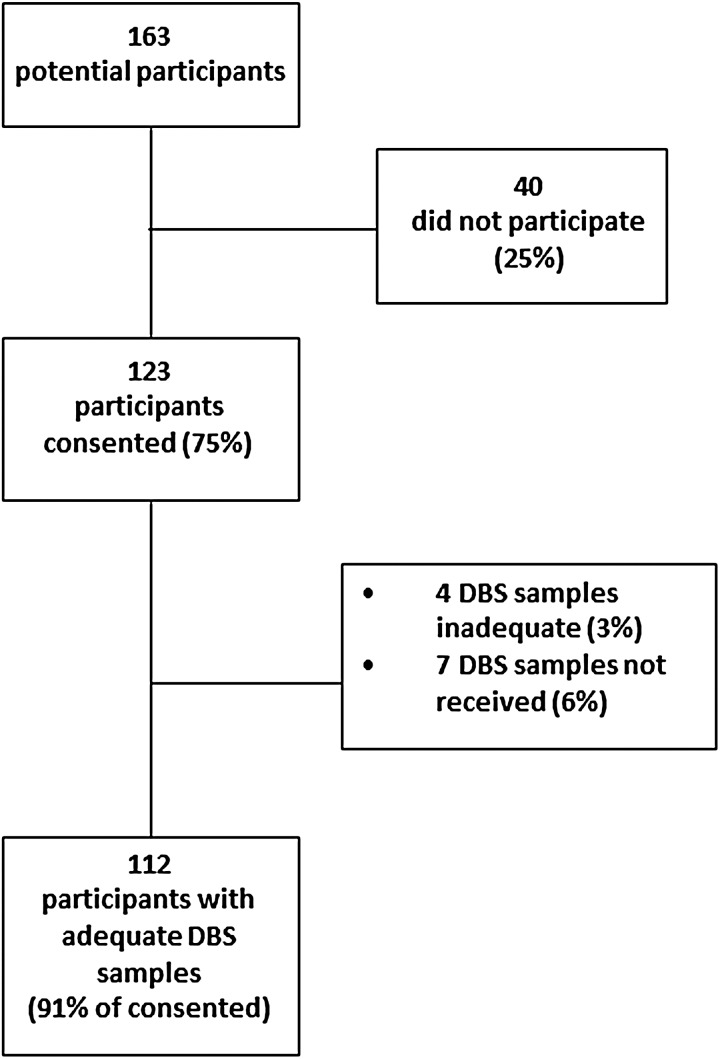
Among 163 potential participants, 123 (75%) were enrolled. Of those enrolled, 112 (91%) were able to complete DBS self-collection and submit mailed samples adequate for measuring AMH. Participants (mean age 31.6 [SD 5.5]) were 85% white, 87% college graduates and 46% reported higher income. Common cancer types were lymphoma and leukemia (34%), breast cancer (30%) and thyroid or skin cancer (8%). The geometric mean (95% confidence interval) AMH level in DBS samples was 0.24 ng/ml (0.16–0.36). In adjusted analysis, AMH levels for survivors of breast cancer (0.02 ng/ml [0.01–0.07]) or leukemia/lymphoma (0.03 ng/ml [0.01–0.08]) were lower than the levels in thyroid or skin cancer survivors (0.12 ng/ml [0.03–0.44]). Pelvic radiation remained associated with lower AMH levels (0.20 ng/ml [0.10–0.40] in unexposed versus 0.02 ng/ml [0.01–0.06] in exposed). Amenorrheic survivors had AMH levels (0.02 ng/ml [0.01–0.06]) that were lower than those of YCS with 7–9 (0.09 ng/ml [0.03–0.32]) or ≥10 (0.17 ng/ml [0.08–0.37]) menstrual periods in the past year.
LIMITATIONS, REASONS FOR CAUTION
The results are generalizable to a population of highly educated, higher income YCS. It is unclear how generalizable the results are to other populations.
WIDER IMPLICATIONS OF THE FINDINGS
Self-collected DBS is a patient-friendly and minimally invasive tool for studying ovarian reserve in geographically diverse populations.
STUDY FUNDING/COMPETING INTERESTS
Research related to the development of this paper was supported by the National Institutes of Health, grants UL1 RR024926 pilot and HD080952-02, and by the American Cancer Society MRSG-08-110-01-CCE. The authors report no competing interests.
Keywords: dried blood spot, AMH, ovarian reserve, cancer survivor, self-collection
Introduction
More than 80% of young girls and women who are diagnosed with cancer before age 40 will survive long-term (Howlader et al., 2013). As a result, there are nearly 400 000 reproductive-aged women with a history of cancer and cancer treatment in the USA. These young cancer survivors (YCS) are at risk of shortened reproductive lifespans, infertility and primary ovarian insufficiency, all of which result in poorer quality of life (Schover, 2008; Gorman et al., 2010, 2012; Levine et al., 2010; Canada and Schover, 2012). Because most YCS will have residual ovarian function after cancer treatment, estimating their remaining ovarian reserve is important to identify potential opportunities for biologic parenthood (Green et al., 2009).
Anti-Mullerian hormone (AMH), a glycoprotein expressed by granulosa cells of small growing ovarian follicles, is a biomarker of ovarian reserve. Levels of AMH rise through adolescence, peak by the mid-20s and decline thereafter to undetectable levels in women in their 40s; AMH levels have been demonstrated to be associated with time to menopause (Hagen et al., 2010; Kelsey et al., 2011; Tehrani et al., 2011; Nair et al., 2015). With regard to fertility, AMH levels have been also associated with responses to fertility treatment, and there are conflicting data on its ability to predict time to pregnancy (Scott et al., 2008; Broer et al., 2009; Steiner et al., 2011; Hagen et al., 2012). In the context of cancer, serum AMH levels are lower in YCS who are exposed to more gonadotoxic treatments compared with those exposed to less gonadotoxic treatments, and YCS who are amenorrheic have lower AMH than YCS who are menstruating (Bath et al., 2003; Anderson et al., 2006; van Beek et al., 2007; Partridge et al., 2010; Su et al., 2010; Gracia et al., 2012). In addition, prechemotherapy AMH levels predict post-chemotherapy ovarian function (Anderson et al., 2013; Su et al., 2014). Hence, AMH is a promising marker for measuring the residual window of ovarian function after cancer. However, this area of investigation has been limited by small sample sizes due to the costs and logistics associated with venipuncture blood collection (Gorman et al., 2014; Su and Lin, 2014). A significant barrier to studying ovarian function after gonadotoxic treatment has been the lack of an efficient, valid tool for sampling ovarian reserve in the large, geographically diverse YCS population.
Dried blood spots (DBSs) are a novel tool for measuring AMH. DBS is an approach that collects drops of whole blood on filter paper following a simple finger prick (McDade et al., 2007). Validation work has demonstrated that assays for quantifying AMH in DBS samples are highly sensitive, specific, precise and reliable, with a high level of agreement with serum-based assays (Worthman and Stallings, 1994, 1997; Edelman et al., 2007; McDade et al., 2012). Sampling is safe and relatively less painful, invasive and costly than venipuncture. Following collection, samples are stable for 2 weeks at room temperature for AMH and may be mailed to the lab for analysis (Worthman and Stallings, 1997; McDade et al., 2012). DBS have been collected in large health surveys and in young adults with high acceptability (Costello et al., 1996; Frank et al., 1997; Worthman and Stallings, 1997).
While DBS have been collected in clinical settings, self-collection of these samples, which would facilitate accruing larger samples of YCS, has not been described. The objectives of this study were (i) to determine the feasibility of obtaining self-collected DBS to measure ovarian reserve in a large, geographically diverse population of female YCS and (ii) to characterize the association of self-collected DBS AMH with participant characteristics and cancer treatment exposures.
Materials and Methods
Study population
Participants for the DBS study were recruited from a pool of YCS followed in the Fertility Information Research Study (FIRST). FIRST is an ongoing prospective cohort study assessing the reproductive outcomes of reproductive-aged cancer survivors (Gorman et al., 2014). Eligibility requirements for FIRST are: female, ages 18–44 at study enrollment, and history of cancer or exposure to cancer treatment. FIRST participants were recruited from social media outreach through young adult cancer survivor advocacy groups, six university-based fertility preservation programs and additional sources including healthcare provider referrals. Participants were recruited from 44 states across the USA.
Among 295 FIRST participants who enrolled between May 2011 and February 2013, 286 (97%) agreed to be contacted for future studies. For recruitment to the DBS study, these women were emailed DBS study information. Up to three recruitment emails were sent to each potential participant over 6 months. Those who provided consent then underwent study procedures. DBS participants were compensated $20 for completing the study. The Institutional Review Board at the University of California, San Diego approved this study.
Sample collection
Each interested participant was mailed a study packet that included: a brochure on DBS collection, two lancets (BD Microtainer, Franklin Lakes, NJ, USA), a blood spot collection card with five DBS circles (Whatman Inc./GE Healthcare Bio-Science Corp., Westborough, MA, USA), a biohazard foil bag (Whatman Inc., Sanford, ME, USA), two alcohol pads, four pieces of gauze, two band aids, a desiccant, a brief questionnaire on current hormone use and last menstrual period, the study consent and a preaddressed/prepaid FedEx 2-day shipping envelope. DBS samples were collected, dried overnight at room temperature, placed in the biohazard foil bag with desiccant, and mailed back to investigators. Once received, DBS samples were frozen at −80°C. Telephone support before and/or during DBS collection was offered to all participants.
The DBS collection brochure was generated by the investigators and included instructions on sample collection and pictures of each procedure step (Supplemental data, Fig. S1). Ten participants local to San Diego initially evaluated the brochure for content and clarity and were observed by study staff as they underwent DBS self-collection guided by only the brochure.
AMH measurements
AMH was measured using a three-step, sandwich-type enzymatic microplate assay developed for blood spots (AL-129, Ansh Labs, Webster, TX, USA). The assay uses stabilized recombinant human AMH as calibrators. From the DBS collection card, two 7.9 mm discs were punched out and placed in extraction buffer. Samples were shaken at slow speed at room temperature for 60 min to elute the AMH. Following extraction, the liquid was separated from the blood spot and analyzed for AMH. Calibrators in the assay were mapped to two equivalent extracted DBS samples, and the AMH measurements are reported as such. The mapping experiment is a comparison between the serum and two equivalent DBSs from the same subject, performed on 32 subjects in duplicates. The slope from this comparison was used to define the serum equivalence of the DBS AMH measurements. Intra-assay and inter-assay coefficients of variation were 0.1–6.0% and 2.2–4.5%, respectively. The lower limit of detection was 0.014 ng/ml, comparable to the serum assay.
Statistical methods
Categorical variables were summarized by frequencies and proportions, while continuous variables were summarized by means, medians and range. Baseline characteristics were compared between DBS participants versus non-participants using Fisher's Exact or Student's t-test, as appropriate. AMH levels were log-transformed to approximate a normal distribution. Values below detection thresholds were given half of the threshold value in analyses. Linear regression models were used to compare log-transformed AMH levels by participant characteristics, while adjusting for confounding. Participant characteristics associated with log-transformed AMH at P < 0.05 in the bi-variable analysis were included in the final adjusted linear regression model. Resulting estimates were exponentiated to yield geometric means and 95% confidence intervals (CIs). Analyses were conducted using SAS statistical software version 9.4 (Cary, NC, USA), and two-tailed P-values ≤0.05 were considered significant.
Results
Feasibility of DBS self-collection
There were 163 YCS who expressed interest in the DBS study, of which 123 (75%) provided consent (Fig. 1). Table I summarizes the characteristics of DBS study participants and non-participants. There were no significant differences in baseline characteristics between survivors who completed the DBS study and those who did not. The mean age (standard deviation) of DBS participants at recruitment was 31.6 (5.5) years. DBS participants were mainly white (85%), college graduates (87%) and in a marriage or marriage-like relationship (55%). The most common cancer types among DBS participants were lymphoma/leukemia (34%), breast cancer (30%) and thyroid or skin cancer (8%). DBS participants were mainly 1–4 years post cancer diagnosis, and their mean age at diagnosis was 27.0 (6.7) years.
Figure 1.
Flow of the DBS study participants.
Table I.
Baseline characteristics by DBS study participation status in female YCS (n = 163).
| Participant characteristics | Overall (n= 163)a | Participant (n= 123) | Non-participant (n= 40) | P-valueb |
|---|---|---|---|---|
| Mean age (SD) at DBS recruitment | 31.8 (5.5) | 31.6 (5.5) | 32.5 (5.7) | 0.40 |
| Mean age (SD) at cancer diagnosis | 27.1 (6.8) | 27.0 (6.7) | 27.6 (7.2) | 0.60 |
| Race | 0.15 | |||
| White | 133 (82.0) | 104 (84.5) | 29 (74.4) | |
| Non-White | 29 (18.0) | 19 (15.5) | 10 (25.6) | |
| Ethnicity | 0.73 | |||
| Hispanic | 11 (6.7) | 8 (6.5) | 3 (7.5) | |
| Non-Hispanic | 152 (93.3) | 115 (93.5) | 37 (92.5) | |
| Education | 0.75 | |||
| <College graduate | 22 (13.5) | 16 (13.0) | 6 (15.0) | |
| ≥College graduate | 141 (86.5) | 107 (87.0) | 34 (85.0) | |
| Marital status | 0.56 | |||
| Single/widow/divorce | 75 (46.0) | 55 (44.7) | 20 (50.0) | |
| Marriage/marriage-like relationship | 88 (54.0) | 68 (55.3) | 20 (50.0) | |
| Income | 0.23 | |||
| ≤50 000 | 57 (35.0) | 40 (32.5) | 17 (42.5) | |
| >50 000 | 76 (46.6) | 57 (46.4) | 19 (47.5) | |
| Prefer not to answer | 30 (18.4) | 26 (21.1) | 4 (10.0) | |
| BMI | 0.38 | |||
| <25 | 95 (58.3) | 75 (61.0) | 20 (50.0) | |
| 25–29 | 37 (22.7) | 25 (20.3) | 12 (30.0) | |
| ≥30 | 31 (19.0) | 23 (18.7) | 8 (20.0) | |
| Cancer type | 0.67 | |||
| Thyroid/skin | 13 (8.0) | 10 (8.1) | 3 (7.5) | |
| Breast | 46 (28.2) | 37 (30.1) | 9 (22.5) | |
| Lymphoma/leukemia | 55 (33.7) | 42 (34.2) | 13 (32.5) | |
| Other | 49 (30.1) | 34 (27.6) | 15 (37.5) | |
| Surgery | 98 (60.1) | 72 (58.5) | 26 (65.0) | 0.47 |
| Chemotherapy | 131 (80.4) | 100 (81.3) | 31 (77.5) | 0.60 |
| Radiation | 89 (54.6) | 65 (52.9) | 24 (60.0) | 0.43 |
| Bone marrow transplant | 7 (4.3) | 5 (4.1) | 2 (5.0) | 0.68 |
| Years since cancer diagnosis | 0.61 | |||
| 1–4 | 112 (71.3) | 85 (72.0) | 27 (69.2) | |
| 5–9 | 31 (19.8) | 24 (20.4) | 7 (18.0) | |
| ≥10 | 14 (8.9) | 9 (7.6) | 5 (12.8) | |
| Ever been pregnant | 59 (35.8) | 43 (35.0) | 16 (40.0) | 0.56 |
aDue to missing values, not all numbers add to total.
bFisher's exact, χ2 or Student's t-test was used to compare characteristics between DBS participants and non-participants.
Returned DBS samples were inspected by study staff for suboptimal collections, including overlapping or unfilled blood spots. A total of 112 participants (91%) provided self-collected DBS that were adequate for AMH measurements, while seven participants (6%) reported sending back a completed DBS that was not received by study staff, and samples from four participants (3%) were excluded by study staff due to poor quality or inadequate quantity. No significant adverse effects of DBS self-collection were reported to the study team. To obtain self-collected DBS, only two participants used telephone-based coaching for DBS collection, while the remainder was able to collect following provided written instructions without requiring additional research staff coaching.
AMH levels and participant characteristics
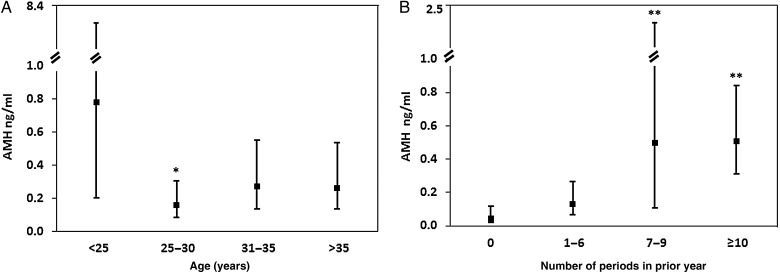
Among the 112 participants for whom AMH was measured, the geometric mean (95% CI) AMH level was 0.24 ng/ml (0.16–0.36). Table II depicts unadjusted and adjusted analyses comparing AMH levels by participant characteristics. In unadjusted analyses, lower AMH levels were associated with Hispanic ethnicity (P = 0.03), cancer type (P = 0.02), chemotherapy exposure (P = 0.03), pelvic radiation (P ≤ 0.01) and current hormone use (P ≤ 0.01). AMH levels were also associated with two reproductive characteristics: age and menstrual pattern. AMH levels were lower in participants older than age 25 than in the younger participants (Fig. 2A) and in participants who reported fewer menstrual cycles in the past year than in those with normal menstrual cycles (Fig. 2B). AMH levels were lower in women who were 25–29 years old, compared with levels in women who were younger than age 25 (P = 0.007). While AMH levels in women who were ages 30 and older appeared lower than in younger women, these comparisons were not statistically significantly different. Participants who were amenorrheic in the past year had significantly lower AMH levels than women who reported 7–9 or ≥10 menstrual periods in the past year (both P < 0.001). Because use of hormone therapy can impact menstrual pattern, we conducted a sensitivity analysis restricted to participants not on hormone therapy. Fewer periods were still significantly associated with lower AMH levels. The geometric mean AMH level (95% CI) for amenorrheic participants not on hormone therapy was 0.05 ng/ml (0.007–0.31), compared with 0.19 ng/ml (0.08–0.44) in participants reporting 1–6 menses, 1.3 ng/ml (0.29–5.7) in participants reporting 7–9 menses and 0.71 ng/ml (0.41–1.20) in participants reporting at least 10 menses in the past year (P = 0.008).
Table II.
Unadjusted and adjusted AMH levels by participant characteristics (n = 112).
| Participant characteristics | Overalla n (%) | Unadjusted AMH geometric mean (95% CI) | P-valueb | Adjusted AMH geometric mean (95% CI) | P-valueb |
|---|---|---|---|---|---|
| Age at DBS completion | 0.07 | 0.09 (0.03–0.26) | 0.32 | ||
| <25 | 15 (13.4) | 0.78 (0.20–8.35) | 0.04 (0.02–0.09) | ||
| 25–30 | 41 (36.6) | 0.16 (0.08–0.30) | 0.06 (0.02–0.16) | ||
| 31–35 | 27 (24.1) | 0.27 (0.13–0.55) | 0.07 (0.03–0.17) | ||
| >35 | 29 (25.9) | 0.26 (0.13–0.53) | |||
| Age at cancer diagnosis | 0.49 | — | |||
| <20 | 14 (12.6) | 0.50 (0.13–0.60) | |||
| 20–24 | 28 (25.3) | 0.28 (0.11–0.48) | — | ||
| 25–30 | 31 (27.9) | 0.23 (0.10–0.36) | |||
| >30 | 38 (34.2) | 0.19 (0.18–1.39) | |||
| Race | 0.57 | — | |||
| White | 93 (83.8) | 0.23 (0.15–5.81) | — | ||
| Non-white | 18 (16.2) | 0.31 (0.11–5.10) | |||
| Ethnicity | 0.03 | 0.11 | |||
| Hispanic | 8 (7.2) | 0.05 (0.01–3.03) | 0.04 (0.01–0.13) | ||
| Non-Hispanic | 103 (92.8) | 0.27 (0.19–5.90) | 0.10 (0.05–0.19) | ||
| BMI | 0.69 | — | — | ||
| <25 | 65 (58.6) | 0.26 (0.16–0.43) | |||
| 25–29 | 23 (20.7) | 0.18 (0.08–0.37) | |||
| ≥30 | 23 (20.7) | 0.28 (0.11–0.76) | |||
| Periods past year | <0.001 | <0.001 | |||
| 0 | 16 (14.6) | 0.05 (0.02–0.11) | 0.02 (0.01–0.06) | ||
| 1–6 | 29 (26.4) | 0.13 (0.07–0.26) | 0.05 (0.02–0.11) | ||
| 7–9 | 10 (9.0) | 0.50 (0.17–1.48) | 0.09 (0.03–0.32) | ||
| ≥10 | 55 (50.0) | 0.51 (0.31–0.83) | 0.17 (0.08–0.37) | ||
| Current hormone therapy | <0.001 | 0.06 | |||
| No | 61 (55.0) | 0.46 (0.28–5.00) | 0.08 (0.04–0.20) | ||
| Yes | 50 (45.0) | 0.12 (0.07–5.50) | 0.05 (0.02–0.10) | ||
| Cancer type | 0.02 | <0.001 | |||
| Thyroid/skin | 10 (9.0) | 1.43 (0.49–4.17) | 0.12 (0.03–0.44) | ||
| Breast | 35 (31.6) | 0.16 (0.09–0.28) | 0.02 (0.01–0.07) | ||
| Lymphoma/leukemia | 28 (25.2) | 0.21 (0.10–0.41) | 0.03 (0.01–0.08) | ||
| Other | 38 (34.2) | 0.29 (0.12–0.67) | 0.17 (0.07–0.38) | ||
| Chemotherapy | 0.03 | 0.83 | |||
| No | 22 (19.8) | 0.54 (0.20–5.60) | 0.06 (0.02–0.16) | ||
| Yes | 89 (80.2) | 0.20 (0.13–5.43) | 0.07 (0.03–0.15) | ||
| Pelvic radiation | 0.001 | <0.001 | |||
| No | 99 (89.2) | 0.30 (0.20–5.62) | 0.20 (0.10–0.40) | ||
| Yes | 12 (10. 8) | 0.04 (0.01–3.48) | 0.02 (0.01–0.06) |
aDue to missing values, not all numbers add to total.
bLinear regression was used to compare participant characteristics and AMH values.
Figure 2.
AMH levels (geometric means and 95% CI) by age (A) and menstrual pattern (B). AMH, anti-Mullerian hormone. *P = 0.007 compared with <25 years. **P < 0.001 compared with zero periods in prior year.
In a multivariable model adjusting for age, ethnicity, menstrual pattern, cancer type, chemotherapy, pelvic radiation and current hormone therapy, several participant characteristics remained significantly associated with AMH levels (Table II). AMH levels (geometric mean [95% CI]) for survivors of breast cancer (0.02 ng/ml [0.01–0.07]) or leukemia and lymphoma (0.03 ng/ml [0.01–0.08]) were lower than levels in thyroid or skin cancer survivors (0.12 ng/ml [0.03–0.44]). Among cancer treatment exposures, only pelvic radiation remained significantly associated with lower AMH levels (0.20 [0.10–0.40] in unexposed versus 0.02 ng/ml [0.01–0.06] in exposed). Survivors who were amenorrheic had AMH levels (0.02 ng/ml [0.01–0.06]) that were lower than those of survivors with 7–9 (0.09 ng/ml [0.03–0.32]) or ≥10 (0.17 ng/ml [0.08–0.37]) menstrual periods in the past year. Chemotherapy, current hormone treatment, age and ethnicity were no longer significantly related to AMH levels.
Discussion
We describe the feasibility of obtaining self-collected DBSs for measuring ovarian reserve in young women with cancer. Ovarian function is a clinically important issue in female cancer survivors, and large-scale data are needed to elucidate whether biomarkers of ovarian reserve can estimate residual windows of ovarian function for this vulnerable population. As the number of YCS in single clinical sites is limited, the traditional approach of clinic-based recruitment and venipuncture blood collection is not efficient. In this proof-of-concept study, we demonstrated that YCS can collect high-quality DBS specimens by themselves at home, that AMH levels can be assayed in these specimens and that the levels appear to reflect ovarian reserve following exposure to gonadotoxic treatments. This new research tool appears to be feasible for studying ovarian reserve in YCS.
The logistics associated with DBS sample collection from YCS were relatively straightforward. Study recruitment was performed by sending standardized emails to a pool of potential participants, which required few study resources. With the exception of the DBS instructions brochure, all supplies were commercially available. Self-collection allowed participants to obtain the required biosamples without visits to a medical facility and venipuncture. This was particularly advantageous in this context, because many YCS experience limited venous access after cancer treatment. As a prior stability analysis showed that DBS samples can be stored at room temperature for up to 2 weeks without loss of AMH, biosamples could be returned via routine delivery by FedEx with shipment tracking (McDade et al., 2012). Inclusive of DBS materials and mailings, each sample cost ∼$24 to collect, far more cost-effective than in-clinic venipunctures and sample processing.
AMH levels measured in DBS were related to cancer type and cancer treatment characteristics in expected directions. In the post-treatment setting of this study, we used cancer type as a surrogate for cancer treatment exposures. Thyroid cancer and skin cancers were designated as reference groups as individuals with these conditions are generally not treated with gonadotoxic therapies. The mainstays of thyroid cancer treatment are thyroid surgery, thyroid hormone suppression, local radiation and/or radioiodine, none of which are known to be toxic to ovarian follicles (Haddad et al., 2015). Similarly, both melanoma and non-melanoma skin cancers are most frequently managed by surgery, possible radiation and possible immune therapy, which are not known to be gonadotoxic (NCCN ‘Basal Cell Skin Cancer’, 2016; NCCN ‘Melanoma’, 2016; NCCN ‘Squamous Cell Skin Cancer’, 2016). In contrast, the majority of young breast cancer survivors with invasive tumors will receive chemotherapy incorporating cyclophosphamide, an alkylating agent that is well studied in terms of its gonadotoxicity (Su et al., 2010). Likewise, leukemia and lymphoma patients are managed with multi-agent chemotherapy, most of which incorporate alkylating agents (Alvarnas et al., 2015; NCCN ‘Non-Hodgkins Lymphoma’, 2015). Consequently, our DBS results were in line with studies showing serum measures of ovarian reserve to be lower in survivors of these cancers compared with controls (Thomas-Teinturier et al., 2015). Whether cancer itself impairs ovarian reserve is more controversial, but such studies have primarily been performed in the pretreatment setting and are less generalizable to this study (Yu et al., 2010; Lawrenz et al., 2012; Su et al., 2013; van Dorp et al., 2014).
Pelvic radiation exposure was associated with decreased DBS AMH. This was anticipated because ionizing radiation to the ovary accelerates depletion of ovarian follicles (Wallace et al., 1989). Chemotherapy was no longer associated with AMH in the multivariable model, likely because of concomitant adjustment for cancer type. We postulate that self-reported cancer type is more accurate for gonadotoxic treatment exposure than self-reported specific cancer treatments. We recently compared medical record data with self-reported cancer type and stage, chemotherapy regimen and pelvic radiation in a subset of this cohort (Knight et al., 2015). Accuracy was highest in self-report of cancer type and stage, and less so with pelvic radiation and alkylating chemotherapy exposure. In addition, self-reported pelvic radiation was more accurate than self-reported alkylating chemotherapy exposure. The limitations of self-reported cancer information may have misclassified participants' cancer treatment exposure and contributed to a null association between alkylators and AMH. Alternatively, the effect size of alkylators on fertility was noted to be smaller than pelvic radiation, and this more modest association may not have been detected in our limited sample size (Bramswig et al., 2015).
Among demographic and reproductive characteristics, menstrual pattern was related to DBS AMH. The fewer the number of menses reported in the past year, the lower the AMH levels. This finding is consistent with lower ovarian reserve with advancing stages of menopausal transition (van Rooij et al., 2004; Sowers et al., 2008). Of interest is that current age was no longer related to AMH levels in adjusted analysis, in contrast to known declines in AMH with chronologic age. We attribute this finding to the larger effect that cancer treatments had on ovarian reserve than the effect of chronologic age. One additional observation to note is the presence of detectable levels of AMH even in YCS with amenorrhea or very few periods in the past year. In amenorrheic YCS, 37% had AMH levels >0.01 ng/ml, while in YCS with 1–6 periods in the past year, 72% had AMH levels >0.01 ng/ml. The presence of AMH suggests the presence of early growing follicles and is inconsistent with menopause, the classic definition being 12 or more months of amenorrhea. In YCS, some very early longitudinal data show a drop in AMH with gonadotoxic therapy, often to undetectable levels. However, following cessation of treatment, there can be recovery of ovarian function as measured by both rising AMH and menstrual pattern (Dillon et al., 2013; Su et al., 2014). Theoretically, measurable AMH from growing follicles could precede recovery of ovulation and subsequent menses. But this chronology needs further longitudinal analyses for confirmation.
Several challenges in DBS self-collection were identified in the study. First, variability in the quality and quantity of specimens was observed. While only four participants had no usable blood spots, samples that did not fill the entire spot or consisted of overlapping blood spots were more common, despite explicit instructions in the brochure to avoid these suboptimal collection methods. Suboptimal collection limited the number of hormones that could be assayed for each participant. Based on this experience, we recommend video- or telephone-based coaching with the first DBS collection, and return of suboptimal specimens should trigger repeat collection.
Secondly, participants who ultimately completed DBS sample collection represented 56% of YCS who were willing to be contacted for future studies and 75% of YCS who expressed interest in the DBS study. None of the measured demographic, reproductive or cancer characteristics predicted participation. This high participation rate may be due in part to a participant pool that was highly educated and reported higher income, limiting the generalizability of our findings. This rate is higher than the 52% rate reported in 2350 participants with diabetes in the Health and Retirement Study, a nationally representative sample of adults over the age of 50 (Heisler et al., 2007). Adherence rates may be improved by the more resource-intensive approach of using interviewers to collect the samples, as the National Longitudinal Study of Adolescent Health (Wave IV, 2007–2008) and National Social Life, Health and Aging Project (2005–2006 survey) report 84–94% completion of DBS collection (Williams and McDade, 2009; McDade, 2011). An additional strategy may be to improve training for DBS collection (Spielberg et al., 2000). In a cohort study on DBS home collection for HIV testing, participants underwent semi-annual clinic visits, in-person training for DBS collection by watching video and practicing the technique until adequate samples were collected, and telephone-based reminders. This approach yields 84–90% specimen return rates. In the current study, several participants reported being unable to perform a finger prick, and some participants required more than one finger prick to complete the five DBSs. We found that sending supplies for two finger pricks was important. Future efforts to support self-collection by using training videos and/or real-time video- or telephone-based coaching should be considered.
Additional limitations should be noted. We did not measure FSH, LH and estradiol because specimens were not collected in a cycle day specific manner. Participants were recruited primarily from social media outreach by cancer advocacy groups and fertility preservation programs (Mersereau et al., 2013; Gorman et al., 2014). Therefore, results are generalizable only to this YCS population. On current hormone use, we did not solicit an indication, i.e. for contraception or menopausal hormone replacement, limiting our ability to determine if lower AMH on hormone therapy was due to hormone exposure or underlying ovarian insufficiency. Finally, the small number of post-treatment pregnancies (n = 16) and the significant time between the beginning of those pregnancies and DBS preclude assessment of AMH levels in relation to pregnancy.
DBS is a patient-friendly, minimally invasive approach to biosample collection. Self-collection, low costs and minimal sample processing of DBS renders this biosample collection approach a novel, feasible research tool for measuring ovarian reserve in geographically diverse populations.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Acknowledgements
We thank FIRST participants, Stupid Cancer!, Fertile Action and the Heart Beat Fertility Preservation Program, and Dr Ajay Kumar from Ansh Labs for their contributions to this study.
Authors' roles
H.I.S., T.W.M., J.R.G. contributed to the study design. H.I.S., S.C.R., S.A.D., T.W.M. contributed to study execution and data acquisition. All authors contributed to data analysis and interpretation. H.I.S., S.C.R. and S.M.S. drafted the manuscript. All authors critically reviewed and approved the final manuscript.
Funding
Research related to the development of this paper was supported by the National Institutes of Health, grants UL1 RR024926 pilot and HD080952-02, and by the American Cancer Society MRSG-08-110-01-CCE.
Conflict of interest
H.I.S., B.W.W., J.R.G., S.C.R. and S.A.D. all received funding from the above granting agencies for their work related to this project. Otherwise the authors report no competing interests.
Supplementary Material
References
- Alvarnas JC, Brown PA, Aoun P, Ballen KK, Barta SK, Borate U, Boyer MW, Burke PW, Cassaday R, Castro JE et al. Acute lymphoblastic leukemia, Version 2.2015. J Natl Compr Canc Netw 2015;10:1240–1279. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod 2006;21:2583–2592. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Rosendahl M, Kelsey TW, Cameron DA. Pretreatment anti-Mullerian hormone predicts for loss of ovarian function after chemotherapy for early breast cancer. Eur J Cancer 2013;49:3404–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath LE, Wallace WH, Shaw MP, Fitzpatrick C, Anderson RA. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum Reprod 2003;18:2368–2374. [DOI] [PubMed] [Google Scholar]
- Bramswig JH, Riepenhausen M, Schellong G. Parenthood in adult female survivors treated for Hodgkin's lymphoma during childhood and adolescence: a prospective, longitudinal study. Lancet Oncol 2015;16:667–675. [DOI] [PubMed] [Google Scholar]
- Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of anti-Mullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril 2009;91:705–714. [DOI] [PubMed] [Google Scholar]
- Canada AL, Schover LR. The psychosocial impact of interrupted childbearing in long-term female cancer survivors. Psychooncology 2012;21:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Angold A, Burns BJ, Stangl DK, Tweed DL, Erkanli A, Worthman CM. The great smoky mountains study of youth. Goals, design, methods, and the prevalence of DSM-III-R disorders. Arch Gen Psychiatry 1996;53:1129–1136. [DOI] [PubMed] [Google Scholar]
- Dillon KE, Sammel MD, Prewitt M, Ginsberg JP, Walker D, Mersereau JE, Gosiengfiao Y, Gracia CR. Pretreatment anti-Mullerian hormone levels determine rate of post-therapy ovarian reserve recovery: acute changes in ovarian reserve during and after chemotherapy. Fertil Steril 2013;99:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman A, Stouffer R, Zava DT, Jensen JT. A comparison of blood spot vs. plasma analysis of gonadotropin and ovarian steroid hormone levels in reproductive-age women. Fertil Steril 2007;88:1404–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank AP, Wandell MG, Headings MD, Conant MA, Woody GE, Michel C. Anonymous HIV testing using home collection and telemedicine counseling: a multicenter evaluation. Arch Intern Med 1997;157:309–314. [PubMed] [Google Scholar]
- Gorman JR, Malcarne VL, Roesch SC, Madlensky L, Pierce JP. Depressive symptoms among young breast cancer survivors: the importance of reproductive concerns. Breast Cancer Res Treat 2010;123:477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JR, Bailey S, Pierce JP, Su HI. How do you feel about fertility and parenthood? The voices of young female cancer survivors. J Cancer Surviv 2012;6:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JR, Roberts SC, Dominick SA, Malcarne VL, Dietz AC, Su HI. A diversified recruitment approach incorporating social media leads to research participation among young adult-aged female cancer survivors. J Adolesc Young Adult Oncol 2014;3:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, Vance A, Ginsberg JP. Impact of cancer therapies on ovarian reserve. Fertil Steril 2012;97:134–140 e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Sklar CA, Boice JD Jr, Mulvihill JJ, Whitton JA, Stovall M, Yasui Y. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol 2009;27:2374–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad RI, Lydiatt WM, Ball DW, Busaidy NL, Byrd D, Callender G, Dickson P, Duh QY, Ehya H, Haymart M et al. Anaplastic thyroid carcinoma, Version 2.2015. J Natl Compr Canc Netw 2015;13:1140–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen CP, Aksglaede L, Sorensen K, Main KM, Boas M, Cleemann L, Holm K, Gravholt CH, Andersson AM, Pedersen AT et al. Serum levels of anti-Mullerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab 2010;95:5003–5010. [DOI] [PubMed] [Google Scholar]
- Hagen CP, Vestergaard S, Juul A, Skakkebaek NE, Andersson AM, Main KM, Hjollund NH, Ernst E, Bonde JP, Anderson RA et al. Low concentration of circulating anti-Mullerian hormone is not predictive of reduced fecundability in young healthy women: a prospective cohort study. Fertil Steril 2012;98:1602–1608 e1602. [DOI] [PubMed] [Google Scholar]
- Heisler M, Faul JD, Hayward RA, Langa KM, Blaum C, Weir D. Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older Americans in the health and retirement study. Arch Intern Med 2007;167:1853–1860. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone A, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z et al. (eds). SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute, 2013. [Google Scholar]
- Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-Mullerian hormone from conception to menopause. PLoS One 2011;6:e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AN, Whitcomb BW, Gorman J, Su I. Validity of self-reported cancer diagnosis and gonadotoxic treatments among female young adult cancer survivors. Fertil Steril. 2015;3:e266 (Elsevier). [Google Scholar]
- Lawrenz B, Fehm T, von Wolff M, Soekler M, Huebner S, Henes J, Henes M. Reduced pretreatment ovarian reserve in premenopausal female patients with Hodgkin lymphoma or non-Hodgkin-lymphoma—evaluation by using anti-Mullerian hormone and retrieved oocytes. Fertil Steril 2012;98:141–144. [DOI] [PubMed] [Google Scholar]
- Levine J, Canada A, Stern CJ. Fertility preservation in adolescents and young adults with cancer. J Clin Oncol 2010;28:4831–4841. [DOI] [PubMed] [Google Scholar]
- McDade TW. The state and future of blood-based biomarkers in the health and retirement study. Forum Health Econ Policy 2011;14:5. [Google Scholar]
- McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography 2007;44:899–925. [DOI] [PubMed] [Google Scholar]
- McDade TW, Woodruff TK, Huang YY, Funk WE, Prewitt M, Kondapalli L, Gracia CR. Quantification of anti-Mullerian hormone (AMH) in dried blood spots: validation of a minimally invasive method for assessing ovarian reserve. Hum Reprod 2012;27:2503–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersereau JE, Goodman LR, Deal AM, Gorman JR, Whitcomb BW, Su HI. To preserve or not to preserve: how difficult is the decision about fertility preservation? Cancer 2013;119:4044–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Slaughter JC, Terry JG, Appiah D, Ebong I, Wang E, Siscovick DS, Sternfeld B, Schreiner PJ, Lewis CE et al. Anti-Mullerian hormone (AMH) is associated with natural menopause in a population-based sample: the CARDIA Women's Study. Maturitas 2015;81:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCN, National Comprehensive Cancer Network. Basal Cell Skin Cancer (Version 1.2016) http://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf (2 November 2015, date last accessed).
- NCCN, National Comprehensive Cancer Network. Melanoma (Version 1.2016) http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf (2 November 2015, date last accessed).
- NCCN, National Comprehensive Cancer Network. Non-Hodgkin's Lymphoma (Version 2.2015) http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf (2 November 2015, date last accessed). [DOI] [PMC free article] [PubMed]
- NCCN, National Comprehensive Cancer Network. Squamous Cell Skin Cancer (Version 1.2016) http://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf (2 November 2015, date last accessed).
- Partridge AH, Ruddy KJ, Gelber S, Schapira L, Abusief M, Meyer M, Ginsburg E. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril 2010;94:638–644. [DOI] [PubMed] [Google Scholar]
- Schover LR. Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility. J Clin Oncol 2008;26:753–758. [DOI] [PubMed] [Google Scholar]
- Scott RT Jr, Elkind-Hirsch KE, Styne-Gross A, Miller KA, Frattarelli JL. The predictive value for in vitro fertility delivery rates is greatly impacted by the method used to select the threshold between normal and elevated basal follicle-stimulating hormone. Fertil Steril 2008;89:868–878. [DOI] [PubMed] [Google Scholar]
- Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, Harlow S, Randolph JF Jr. Anti-Mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab 2008;93:3478–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg F, Critchlow C, Vittinghoff E, Coletti AS, Sheppard H, Mayer KH, Metzgerg D, Judson FN, Buchbinder S, Chesney M et al. Home collection for frequent HIV testing: acceptability of oral fluids, dried blood spots and telephone results. HIV Early Detection Study Group. AIDS 2000;14:1819–1828. [DOI] [PubMed] [Google Scholar]
- Steiner AZ, Herring AH, Kesner JS, Meadows JW, Stanczyk FZ, Hoberman S, Baird DD. Anti-Mullerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet Gynecol 2011;117:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HI, Lin K. Research recruitment and dissemination in young adults with cancer. In: Woodruff TK, Clayman M, Waimey KE (eds). Oncofertility Communication: Sharing Information and Building Relationships Across Disciplines. Berlin: Springer, 2014. [Google Scholar]
- Su HI, Sammel MD, Green J, Velders L, Stankiewicz C, Matro J, Freeman EW, Gracia CR, DeMichele A. Anti-Mullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer 2010;116:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HI, Flatt SW, Natarajan L, DeMichele A, Steiner AZ. Impact of breast cancer on anti-Mullerian hormone levels in young women. Breast Cancer Res Treat 2013;137:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HI, Haunschild C, Chung K, Komrokian S, Boles S, Sammel MD, DeMichele A. Prechemotherapy anti-Mullerian hormone, age, and body size predict timing of return of ovarian function in young breast cancer patients. Cancer 2014;120:3691–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani FR, Shakeri N, Solaymani-Dodaran M, Azizi F. Predicting age at menopause from serum anti-Mullerian hormone concentration. Menopause 2011;18:766–770. [DOI] [PubMed] [Google Scholar]
- Thomas-Teinturier C, Allodji RS, Svetlova E, Frey MA, Oberlin O, Millischer AE, Epelboin S, Decanter C, Pacquement H, Tabone MD et al. Ovarian reserve after treatment with alkylating agents during childhood. Hum Reprod 2015;30:1437–1446. [DOI] [PubMed] [Google Scholar]
- van Beek RD, van den Heuvel-Eibrink MM, Laven JS, de Jong FH, Themmen AP, Hakvoort-Cammel FG, van den Bos C, van den Berg H, Pieters R, de Muinck Keizer-Schrama SM. Anti-Mullerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin's lymphoma during childhood. J Clin Endocrinol Metab 2007;92:3869–3874. [DOI] [PubMed] [Google Scholar]
- van Dorp W, van den Heuvel-Eibrink MM, de Vries AC, Pluijm SM, Visser JA, Pieters R, Laven JS. Decreased serum anti-Mullerian hormone levels in girls with newly diagnosed cancer. Hum Reprod 2014;29:337–342. [DOI] [PubMed] [Google Scholar]
- van Rooij IA, Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, Themmen AP, te Velde ER. Anti-Mullerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause 2004;11:601–606. [DOI] [PubMed] [Google Scholar]
- Wallace WH, Shalet SM, Crowne EC, Morris-Jones PH, Gattamaneni HR. Ovarian failure following abdominal irradiation in childhood: natural history and prognosis. Clin Oncol (R Coll Radiol) 1989;1:75–79. [DOI] [PubMed] [Google Scholar]
- Williams SR, McDade TW. The use of dried blood spot sampling in the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci 2009;64(Suppl 1):i131–i136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthman CM, Stallings JF. Measurement of gonadotropins in dried blood spots. Clin Chem 1994;40:448–453. [PubMed] [Google Scholar]
- Worthman CM, Stallings JF. Hormone measures in finger-prick blood spot samples: new field methods for reproductive endocrinology. Am J Phys Anthropol 1997;104:1–21. [DOI] [PubMed] [Google Scholar]
- Yu B, Douglas N, Ferin MJ, Nakhuda GS, Crew K, Lobo RA, Hershman DL. Changes in markers of ovarian reserve and endocrine function in young women with breast cancer undergoing adjuvant chemotherapy. Cancer 2010;116:2099–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.