Abstract
Background
Multiple idiopathic cervical root resorption (MICRR) is a rare entity distinct from pathological root resorption that occurs as a result of several local and systemic factors.
Methods
This report describes a familial pattern of MICRR, including a recently identified case and a 30-year follow-up on previously described cases.
Results
The previously reported father (95-years) and son (64-years), and the recently affected daughter (61-years), presented noncontributory medical history. The resorptive lesions were asymptomatic, unassociated with any predisposing factors, and were first identified during the fourth to sixth decades of life. All tooth types were affected, with posterior teeth being affected earlier and with greater frequency, however, distal root surfaces were never affected. The resorptive lesions were progressive in nature with additional teeth becoming involved as the condition was followed over time. In many instances, surrounding alveolar bone extended into the existing resorptive defects, but without clinical evidence of ankylosis. Gingival tissues, periodontal probing, and tooth mobility were within normal limits. Micro-computed tomography of extracted teeth demonstrated that the lesions were more extensive than clinically evident and rarely invaded the pulp chamber. Histologically, many resorptive lesions were noted along the cementum surface, with evidence of isolated cemental repair. Management of MICRR focused on restoring damaged root surfaces and extracting teeth with extensive root resorption.
Conclusions
MICRR is a challenging entity with unknown etiology, and a lack of well-established preventative and management strategies. The familial pattern noted in this report necessitates future studies to investigate the role of genetic components in MICRR development.
Keywords: Root resorption, idiopathic, cervical, familial, tooth, tooth resorption
Introduction
Root resorption, characterized by the progressive loss of dentin and cementum, is a normal physiologic process occurring in primary dentition that leads to exfoliation of deciduous teeth.1 However, root resorption in permanent teeth is largely pathological and can occur as a result of several local and systemic factors including trauma, inflammation, tumors, endocrine imbalances, and Paget’s disease.2–6 Root resorption can be classified into various categories based on the cause, location, type of resorptive process, and its clinical and histological appearance.7–10 Broadly, resorptive lesions are classified into physiologic or pathologic, and internal or external.2, 10, 11 In most instances, the resorptive process is mediated by odontoclasts/osteoclasts.2, 12
In contrast to the well-known etiological forms of root resorption2, 10, multiple idiopathic cervical root resorption (MICRR) is an aggressive form of external root resorption that occurs at the cemento-enamel junction (CEJ), often with an unknown etiology.13–16 It can affect multiple teeth within the same arch or can be widely distributed throughout the dentition. MICRR is usually detected as an incidental finding on radiographs or during routine dental examination.16–18 No correlation exists between MICRR and age, gender, ethnicity, or systemic conditions.10, 13, 19 Clinically, the lesions are often asymptomatic with no signs of overt inflammation. The resorption cavities are hard, non-carious and exhibit sharp knife-edge borders with a pinkish hue representing vascular granulation tissue.13, 16, 19–21 In a vital tooth with minimal destruction, there is rarely any pulpal involvement19, 22, which distinguishes MICRR from external inflammatory root resorption where pulpal necrosis or infection is prerequisite.7 MICRR lesions often progress rapidly and/or recur despite intervention, although, in some cases the resorptive process has been arrested for extended periods with appropriate treatment.20, 21 Management of MICRR lesions is challenging, as they are often located subgingivally and/or interproximally, making them difficult to restore. Moreover, the lesions are generally located on dentin and cementum surfaces that are difficult to bond with restorative materials.20, 21, 23 Attempts to manage early resorptive lesions with root canal therapy have largely failed.21, 23, 24
Since the first case of MICRR described by Mueller and Rony in 193025, approximately 30 cases of MICRR have been reported in the literature.13–16, 20, 21, 26, 27 However, to date, the etiologic and pathologic process, as well as the management of MICRR remains poorly understood. The purpose of this report is to present a familial pattern of MICRR with a 30-year follow-up. The clinical, radiographic, and histological evidence associated with these cases helps to improve our understanding of the etiology, pathology and management of MICRR.
Case Description and Results
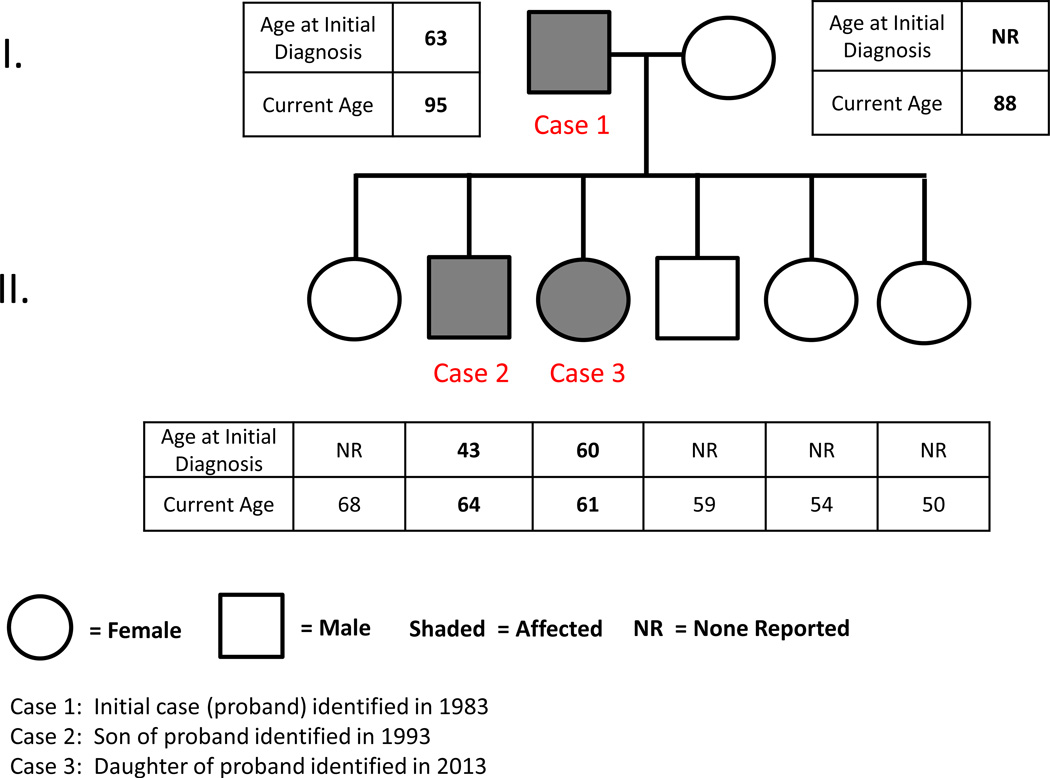
A 22-year follow-up of MICRR in a father and son was previously reported in 2007.21 This report describes the 30-year follow-up of the father (Case 1) and son (Case 2), and a newly identified case of MICRR in the daughter (Case 3). Figure 1 is a pedigree showing family members affected with MICRR. Written informed consent was obtained from all patients presented in this study.
Figure 1.
Pedigree of family members affected with multiple idiopathic cervical root resorption. Siblings are arranged in birth order. Adjacently listed are age at initial diagnosis of MICRR and current ages of family members.
Case 1 and Case 2
At the time of last clinical examination in 2013, the father (Case 1/proband) was a healthy 93-year-old white male who had been followed and treated over 30 years for repeated episodes of MICRR. Briefly, his medical history was significant for history of vitiligo on the skin, mild heart attack in 1993, polypectomy for colon cancer in 1992, and chemotherapy for recurrence of colon cancer in 2006. He reported being treated for hypothyroidism from age 16 to 21 years. His wife and four daughters were also treated for hypothyroidism with hormone replacement therapy, while the two sons were free of hypothyroidism.
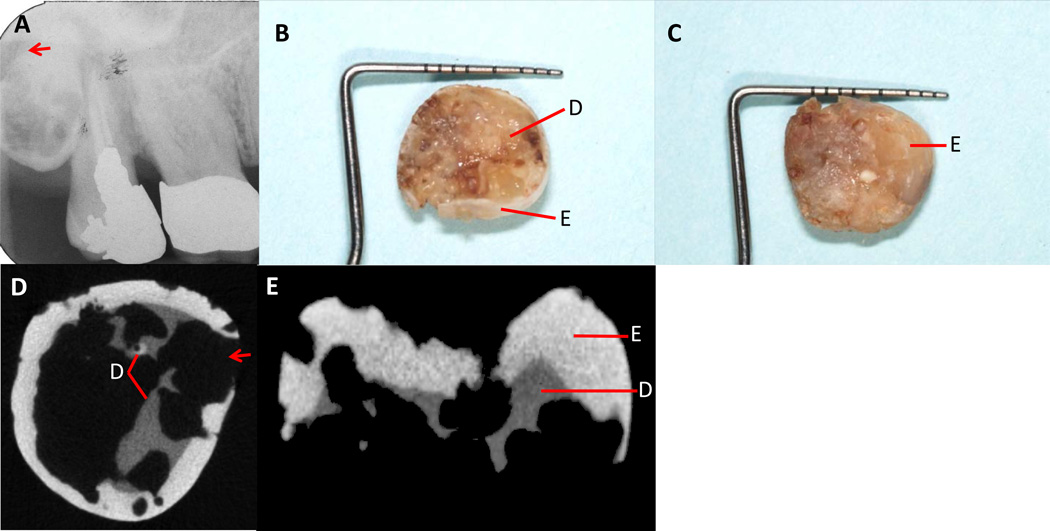
During the follow-up period between 2005 and 2013, two new resorptive lesions and six recurrent lesions were noted in the proband (see Table 1). Lesions in five of the affected teeth (#2, #6, #13, #21 and #22) were so extensive that they required extraction. Two lesions noted in tooth #6 have been previously reported21, however, the tooth was extracted in 2006 due to disease progression. Tooth #1, which was fully impacted on examination in 2005, exfoliated spontaneously in 2008 due to extensive root resorption involving all the root surfaces (Figure 2A). Figures 2B and 2C show roots of tooth #1 being completely resorbed. Micro-computed tomography (micro-CT) images (Figures 2D and 2E) show the extent of resorption within the crown structure. While some of the affected teeth were treated and retained, several others were maintained without treatment (#3, #20 and #27) as the patient was not in discomfort nor had infections. Moreover, the only treatment available for severely affected teeth would have been extractions and extensive prosthetic reconstruction. Slight progression of lesions was noted over time in untreated teeth #20 and #27, while no extractions or additional lesions occurred in any teeth between 2009 and 2013.
Table 1.
Resorption lesions noted in the proband between 2005 and 2013 by tooth number, surface involved and treatment provided.
| Tooth Number | Surface(s) Involved | Initial Diagnosis |
Treatment Rendered |
|---|---|---|---|
| 6 | Mesial, Buccal | 2005 | Extracted (2006) |
| 3 | Palatal* | 2007 | No treatment |
| 13 | Palatal* | 2007 | Extracted (2008) |
| 21 | Buccal* | 2007 | Extracted (2008) |
| 22 | Lingual* | 2007 | Extracted (2008) |
| 27 | Lingual | 2007 | No treatment |
| 1 | Entire root (impacted) |
2008 | Exfoliated (2008) |
| 2 | Palatal* | 2008 | Extracted (2009) |
| 11 | Buccal* | 2008 | Composite |
| 20 | Lingual | 2008 | No treatment |
Text in bold highlights the final fate of the tooth.
Indicates recurrent lesions.
Note: No new lesions were identified between 2009 and 2013.
Figure 2.
A. Periapical radiograph showing fully impacted tooth #1 (2005). Note the evidence of resorption (arrow) on tooth #1. B. Inferior view of the exfoliated crown three years later (2008) showing complete resorption of the root with remnants of underlying enamel and dentin. C. Clinical view of the occlusal surface showing minimal alteration of the enamel. D. Micro-CT scan of crown remnant shown in B above. Note extensive resorption (arrow) with nearly complete loss of dentin. E. Micro-CT scan of the mesio-distal view of crown remnant shown in C above. Only a small amount of dentin remains intact while most of the enamel is untouched. (E=Enamel, D=dentin)
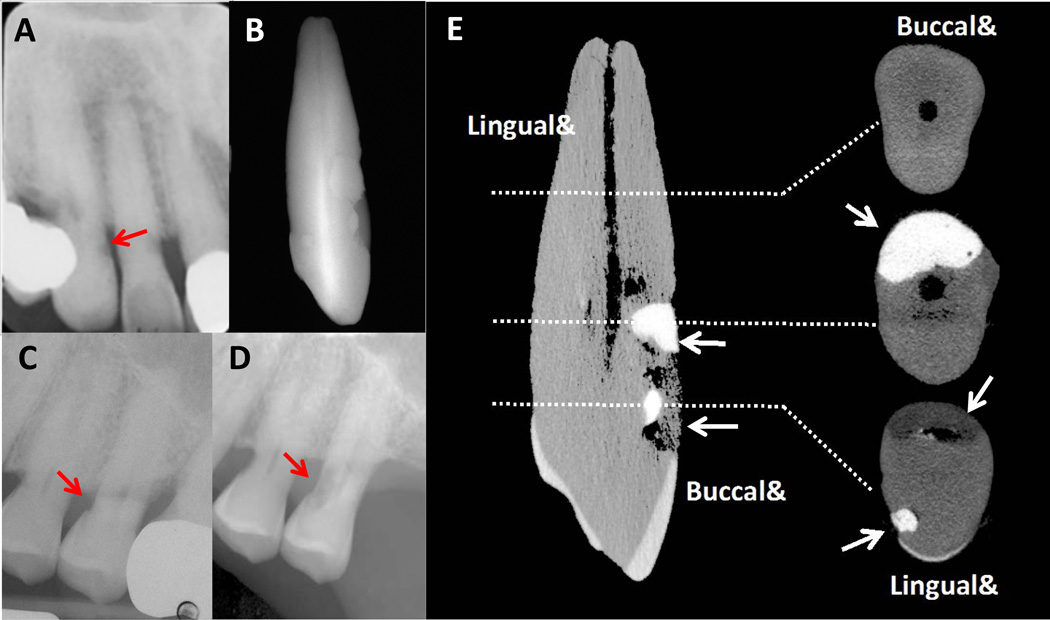
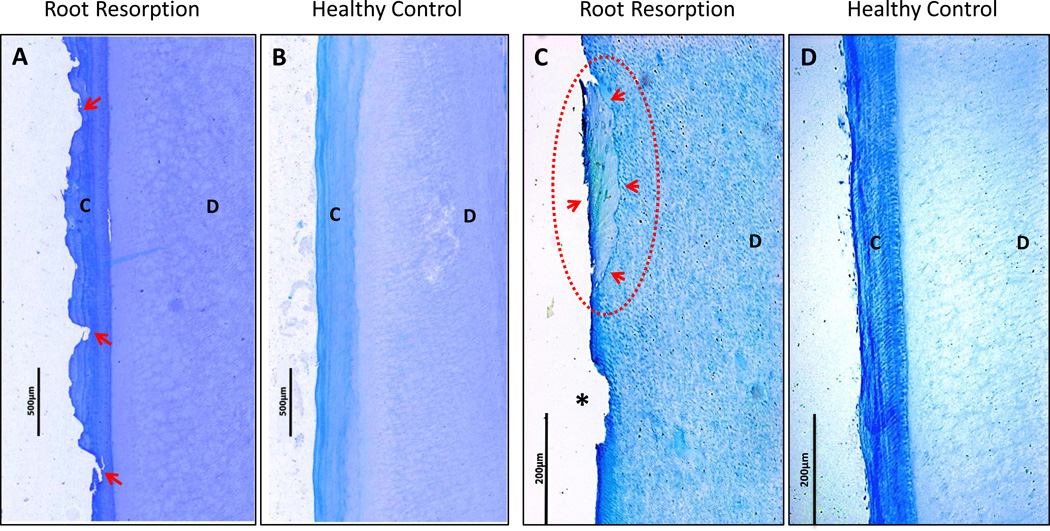
Radiographs and micro-CT images (Figure 3) of extracted teeth show the extent and progression of cervical root resorption. Figure 3C and 3D show radiographic evidence of progressive resorptive lesions on the mesial surface of tooth #13 from year 2005 to 2008. Micro-CT images of tooth #6 (Figure 3E) from the proband show expansive resorption without invasion of the pulp chamber. Histologic sections of representative root samples of tooth #6 and normal control roots obtained from healthy volunteers are shown in Figure 4. Figure 4A shows a section of the root with acellular cementum of relatively normal thickness, but with an uneven border and many small resorption pits. Another section of the same root (Figure 4C) shows complete resorption of cementum except for a single island of reparative cementum. Normal, intact cementum can be seen in control sections of teeth without root resorption (Figures 4B and 4D).
Figure 3.
A. Radiograph of tooth #6 from the proband showing cervical root resorption (arrow). B. Radiograph of tooth #6 after extraction (2006). Note the presence of multiple resin restorations on the buccal and palatal surfaces. C. Radiograph of tooth #13 from the proband (2005). Resorption (arrow) is present on the palatal and mesial aspect of the tooth. D. Radiograph of tooth #13 showing progression of resorptive lesion (arrow) on the mesial surface just prior to extraction (2008). E. Micro-CT image of tooth #6 from the proband. The image on the left shows the entire tooth. Images on the right represent cross-sectional slices of the tooth. Despite the invasive nature of the resorption, there is no perforation into the pulp chamber. Arrows point towards resin restorations. Note: 3A adapted from “A Familial Pattern of Multiple Idiopathic Cervical Root Resorption in a Father and Son: A 22-Year Follow-Up”.21 Adapted with permission from the American Academy of Periodontology.
Figure 4.
A. Histology section of affected tooth #6 from the proband (presented in Figure 3) shows numerous areas of cementum resorption (arrows) along the entire root surface, with normal acellular cementum thickness and intact dentin clearly visible. B. A control root section from a healthy individual shows normal acellular cementum and dentin with no areas of root resorption. C. Histology section of tooth #6 (presented in Fig 3) shows evidence of a resorption lesion (asterisk) and an island of reparative cementum (dotted circle with arrows). The remainder of the specimen shows complete absence of cementum with dentin clearly visible. D. A control root section from a healthy individual shows normal cementum and dentin with no areas of root resorption. (C=cementum, D=dentin. Tartrate-resistant acid phosphatase (TRAP)-stain, A & B original magnification × 50, C & D original magnification × 100).
During the last dental examination of the proband in 2013, the 64-year old son (Case 2) was reportedly healthy with no systemic concerns. No new lesions were reported in the son during the follow-up period between 2005 and 2013.
Case 3
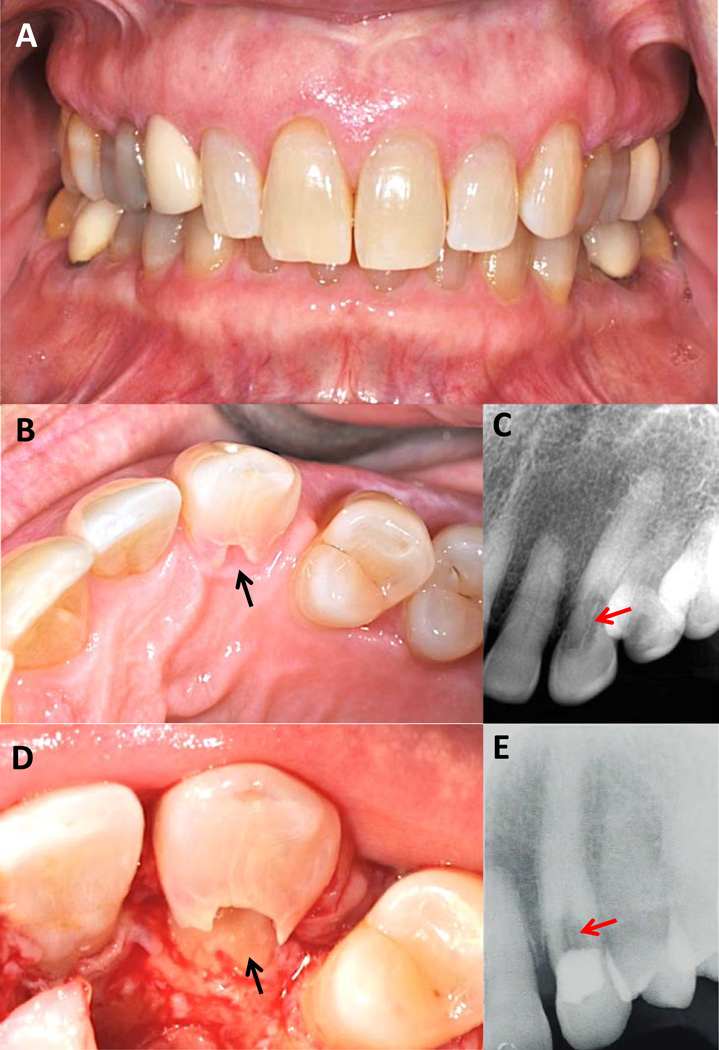
At initial diagnosis of MICRR by her dentist in 2013, the patient was a 60-year old healthy white female. She was referred to a periodontist for management of MICRR. Her medical history was unremarkable, except for hormone replacement therapy for hypothyroidism as a child. She is married and has two daughters who are reportedly healthy. On oral examination, the patient presented with no signs of overt gingival inflammation, bleeding on probing, increased pocket depth, or tooth mobility. Figure 5A shows a frontal view of her teeth on the day of MICRR treatment in 2014. Cervical resorption was noted on the palatal aspect of her maxillary left canine (Figure 5 B-D). The extent of the lesion is evident clinically on the palatal aspect of the tooth (Figure 5B) and radiographically (Figure 5C). Unlike most resorption lesions that are hidden subgingivally, this lesion was visible without manipulation of the marginal tissue (Figure 5B). The classic pinkish hue of the underlying soft tissue could be seen through the thin overlying enamel. Figure 5D shows an access flap being raised to evaluate the lesion and determine restorability. Although the lesion was extensive, there was no apparent invasion into the pulp. Note that the outline of the pulp chamber can still be seen on the radiograph (Figures 5C and 5E). After thorough lesion debridement, a resin restoration was placed and the flap closed (October of 2013). A radiograph taken five months later (March of 2014) (Figure 5E) showed that resorption continued to increase in size. Unfortunately, the tooth was given a hopeless prognosis and subsequently extracted by her dentist. According to the patient, the extraction and subsequent healing was uneventful and the site was treatment planned for an osseointegrated dental implant.
Figure 5.
A. Frontal view of the affected daughter on the day of treatment (2014). B. Clinical presentation of the resorption lesion (arrow) on the palatal aspect of tooth #11. Note that the resorption lesion is large and extends coronally and apically past the free gingival margin. The pinkish hue of the underlying granulation tissue can be seen through the thin enamel. C. Radiograph of the lesion (arrow) taken at the time of lesion discovery (2013) (courtesy of Dr. Robert Nyberg, New Bern, NC). Note that the outline of the pulp chamber appears to be intact. D. View of the lesion (arrow) upon flap reflection, degranulation and removal of some of the unsupported enamel. E. Radiograph showing restoration of the lesion (arrow). (Photographs and radiographs in A, B, D and E are courtesy of Dr. Jeffery R. Thomas, New Bern, NC.).
All members of the family received routine dental care over the 30-year period and absence of MICCR in non-affected members was confirmed clinically and radiographically. In the three affected patients, resorptive lesions were first identified during the fourth to sixth decades of life. All tooth types were affected with posterior teeth being affected earlier and with greater frequency. Strangely, not all surfaces were equally affected as no lesions were noted on distal surfaces.
Discussion
This report describes three cases of MICRR, including a 30-year follow up on the previously reported father and son21, and a new case of an affected daughter in the same family. To the best of our knowledge, this is the first report of a familial pattern of MICRR with an extended follow-up period.
The described cases are of true idiopathic nature as none of the well-known predisposing factors for root resorption2–6 were noted. Consistent with the previously reported cases of MICRR13–16, the affected individuals were healthy with apparently non-contributory medical histories. The only unusual finding was that the proband and daughter reported being treated for hypothyroidism at a younger age, and the proband also reported a history of mild heart attack and colon cancer. Endocrine disorders such as hypothyroidism28 and hyperparathyroidism29 have been associated with apical root resorption induced by orthodontic treatment, although these findings have not been well established.30, 31 It is suggested that the low bone turnover rate noted in hypothyroidism decreases tooth movement while increasing the risk of root resorption.28 Based on this hypothesis, experimental clinical and animal studies have administered thyroxine to increase bone turnover rate and thereby reduce the risk of root resorption.32, 33 Nevertheless, to date, no studies have found an association between hypothyroidism and MICRR. The fact that the wife and three daughters with a history of hypothyroidism did not exhibit MICRR further questions the possible association between hypothyroidism and root resorption.
Other factors such as developmental defects, trauma, bruxism, environmental exposure, inflammatory reactions, and feline herpes virus 1 (FeHV-1) have been speculated to be associated with MICRR.2, 10, 22, 26, 34–36 In the present report none of the patients received orthodontic treatment nor displayed evidence of occlusal trauma or parafunctional habits. However, both proband21 and daughter (Figure 5A) exhibited deep overbites. The significance of deep overbite in MICRR development is currently unknown. All family members maintained good oral hygiene with no evidence of overt gingival inflammation or deeper pocket depths. Interestingly, resorptive lesions were noted in a fully impacted third molar clinically unexposed to the oral environment (Figure 2), which supports a non-bacterial origin of MICRR. However, histological analysis is needed to confirm the absence of microbes. In support of our findings, MICRR has been noted in impacted third molar unexposed to the oral environment.13 Previous association of herpes virus with pulpal and periapical pathologies in humans37 and the close similarity of MICRR to feline odontoclastic resorptive lesions (FORL) in cats26, 34, raises the possibility that FeHV-1 may be related to MICRR. von Arx et al., reported four cases of MICRR who had extended contact with cats and presented positive titers of neutralizing antibodies against FeHV-1 in their blood samples.26 Similarly, Wu et al., also reported a case of MICRR who had contact with cats.14 However, in the present study none of the patients had extended contacts with cats. Additionally, environmental exposure is an unlikely predisposing factor in this study, as the affected proband, son, and daughter had not resided in the same home or state for more than 20 years. Furthermore, cementoenamel disjunction, a histological variation seen in 10% of the teeth38, is thought to predispose root surfaces to resorption. It is suggested that the gap between cementum and enamel leaves the dentin without surface protection, allowing osteoclasts to bind to the dentin surface and initiate the resorption process.10 In the present study, there was no apparent evidence of cementoenamel disjunction, and histological analysis of two teeth extracted from the proband did not reveal cementum aplasia/hypoplasia that could predispose to cervical resorption.
The resorptive lesions were first identified in all the patients only during the fourth to sixth decades of life. The late onset of the disease suggests that genetic and environmental factors may play a substantial role in increasing susceptibility to MICRR. Several studies have noted MICRR during the fourth to sixth decades of life.15, 16, 20, 26, 39 Conversely, other studies have noted MICRR in younger populations.13, 14, 40 All tooth types were affected in this study, with posterior teeth being affected earlier and with greater frequency. Interestingly, not all surfaces were equally affected as no lesions were noted on distal surfaces. This finding is unusual and contrary to the other reports that have noted involvement of all root surfaces.13, 14, 16, 20, 26, 39
The pattern of root resorption in our cases is similar to other reported MICRR cases13–16, where larger areas of cervical root structure are affected on multiple teeth, rarely involving the pulp chamber. Micro-CT of the extracted teeth showed that the pulpal wall was intact and that the lesions were initiated and confined to the external surface of the root (Figure 3). Clinically, the lesions were not associated with signs of overt gingival inflammation, bleeding on probing, increased pocket depth, or tooth mobility, which is consistent with the findings noted by von Arx et al.26, Iwamatsu-Kobayashi et al.20, and others.13, 14 Similar to other reports, a pinkish hue representing vascular granulation tissue was noted within the resorption cavities.14, 20, 26 In many instances, the surrounding alveolar bone extended into the existing resorptive defects, but without clinical signs of ankylosis. Histologically, many resorptive lesions were noted along the cementum surface, with evidence of isolated cemental repair of lesions (Figure 4). Likewise, other studies have noted resorbed dentin to be partially covered with reparative cementum.20, 26 Numerous TRAP-positive osteoclasts/odontoclasts have also been noted in Howship's lacunae along the resorbed dentin border.20, 23, 26, 39
The resorptive lesions in MICRR are usually progressive, and often resistant to surgical and non-surgical interventions.13, 21 Of all the reported MICRR cases in the literature, progressive disease has been noted in 21 cases, while four cases presented no progression.14, 16, 26, 39 Following our previously published findings21, no new lesions were noted in the son, while nine new lesions were noted in the proband over the last 8 years despite regular dental care. The addition of these new lesions aggregates to 37 separate episodes of MICRR in the proband, resulting in a total of 11 extractions to date. Unfortunately, it was impossible to predict which teeth would become affected next and when. For example, the initial lesions were noted in the proband in 1983 without new occurrences until 1989–1990. New lesions were noted again at four to five-year intervals in 1996, 2001, and 2005. Clearly, there appears to be an active phase of resorption followed by an inert phase, making it extremely challenging for early identification or intervention. As the previous reports have noted13–16, 39, MICRR lesions tend to recur because of their aggressive nature. It is suggested that the osteoclasts create small infiltrative channels within the dentine that are interconnected with the periodontal ligament41, 42, and unless all osteoclasts within these channels are removed, the resorptive process will continue.9 Agents that modify osteoclast activity such as bisphosphonates and RANKL antibodies may prove valuable and warrant further investigation. An intriguing case report found root resorption ceased during a six-year period when treated with bisphosphonate, which was prescribed primarily for the management of osteoporosis and possible Paget’s disease.20
Management of MICRR is challenging due to the progressive nature of the disease and its widespread distribution throughout the dentition.13, 16, 19, 21, 43 The fundamental principals in management of MICRR involves arresting the disease progression, restoring damaged root surfaces, and preventing further root resorption.13, 16, 17, 19–21, 27, 43 The following are a range of case-dependent treatment options: (a) Observation, with early intervention involving surgical exposure, lesion curettage, restoration (e.g., glass ionomer, amalgam or resin composite), and root canal therapy if necessary; (b) Extraction of severely affected teeth and replacement with partial/complete denture or implant; and (c) crown resection with root submergence to preserve alveolar bone, which is useful for stabilization of the partial or complete denture. However, as noted in this study and several others13, 16, 19, 21, 43, restorative interventions do not prevent disease progression and tooth loss over time appears to be inevitable. Early restorative interventions may improve esthetics and delay tooth loss. Currently, there are no standard guidelines for managing MICRR, so individualized, patient-focused management should be followed. Regular clinical and radiographic examination is helpful in early detection and management of MICRR.
Conclusion
In summary, this report shows that MICRR lesions progress over time and often lead to tooth loss. Hence, frequent clinical examinations and early interventions are needed to manage this condition. The lack of definitive etiological factors makes it difficult to develop suitable preventative and therapeutic strategies. To date, no correlation exists between MICRR and age, gender, ethnicity or systemic conditions.2, 10, 13, 14, 20 The development of MICRR in another family member in this study further strengthens the familial nature of the condition. The inheritance pattern appears to be autosomal dominant and suggests a potential genetic component involved in the development of MICRR. Future studies need to investigate the role of genetic predisposition in the development of MICRR.
Acknowledgments
This work was supported in part by the Intramural Research Program of National Institute of Arthritis and Musculoskeletal and Skin Diseases (NAIMS) at the National Institutes of Health, Bethesda, MD (MJS). The authors thank Dr. Robert Nyberg, New Bern, NC and Dr. Jeffery R. Thomas, New Bern, NC for providing clinical photos and radiographs related to Case 3.
Footnotes
Key Findings from the Study: This report presents a familial pattern of multiple idiopathic cervical root resorption (MICRR) with a 30-year follow-up period.
Conflicts of Interest: All authors report no conflicts of interest related to this study.
References
- 1.Harokopakis-Hajishengallis E. Physiologic root resorption in primary teeth: molecular and histological events. Journal of oral science. 2007;49:1–12. doi: 10.2334/josnusd.49.1. [DOI] [PubMed] [Google Scholar]
- 2.Fuss Z, Tsesis I, Lin S. Root resorption--diagnosis, classification and treatment choices based on stimulation factors. Dental traumatology : official publication of International Association for Dental Traumatology. 2003;19:175–182. doi: 10.1034/j.1600-9657.2003.00192.x. [DOI] [PubMed] [Google Scholar]
- 3.Gunraj MN. Dental root resorption. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 1999;88:647–653. doi: 10.1016/s1079-2104(99)70002-8. [DOI] [PubMed] [Google Scholar]
- 4.Ram D, Cohenca N. Therapeutic protocols for avulsed permanent teeth: review and clinical update. Pediatric dentistry. 2004;26:251–255. [PubMed] [Google Scholar]
- 5.Beertsen W, Piscaer M, Van Winkelhoff AJ, Everts V. Generalized cervical root resorption associated with periodontal disease. Journal of clinical periodontology. 2001;28:1067–1073. doi: 10.1034/j.1600-051x.2001.281112.x. [DOI] [PubMed] [Google Scholar]
- 6.Schatzle M, Tanner SD, Bosshardt DD. Progressive, generalized, apical idiopathic root resorption and hypercementosis. Journal of periodontology. 2005;76:2002–2011. doi: 10.1902/jop.2005.76.11.2002. [DOI] [PubMed] [Google Scholar]
- 7.Andreasen JO. External root resorption: its implication in dental traumatology, paedodontics, periodontics, orthodontics and endodontics. International endodontic journal. 1985;18:109–118. doi: 10.1111/j.1365-2591.1985.tb00427.x. [DOI] [PubMed] [Google Scholar]
- 8.Bakland LK. Root resorption. Dental clinics of North America. 1992;36:491–507. [PubMed] [Google Scholar]
- 9.Tronstad L. Root resorption--etiology, terminology and clinical manifestations. Endodontics & dental traumatology. 1988;4:241–252. doi: 10.1111/j.1600-9657.1988.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 10.Darcey J, Qualtrough A. Resorption: part 1. Pathology, classification and aetiology. British dental journal. 2013;214:439–451. doi: 10.1038/sj.bdj.2013.431. [DOI] [PubMed] [Google Scholar]
- 11.Andreasen JO. Luxation of permanent teeth due to trauma. A clinical and radiographic follow-up study of 189 injured teeth. Scandinavian journal of dental research. 1970;78:273–286. doi: 10.1111/j.1600-0722.1970.tb02074.x. [DOI] [PubMed] [Google Scholar]
- 12.Ne RF, Witherspoon DE, Gutmann JL. Tooth resorption. Quintessence international. 1999;30:9–25. [PubMed] [Google Scholar]
- 13.Yu VS, Messer HH, Tan KB. Multiple idiopathic cervical resorption: case report and discussion of management options. International endodontic journal. 2011;44:77–85. doi: 10.1111/j.1365-2591.2010.01820.x. [DOI] [PubMed] [Google Scholar]
- 14.Wu J, Lin LY, Yang J, et al. Multiple idiopathic cervical root resorption: a case report. International endodontic journal. 2015 doi: 10.1111/iej.12440. [DOI] [PubMed] [Google Scholar]
- 15.Beckett HA, Gilmour AG. Multiple idiopathic cervical root resorption in a male. British dental journal. 1993;175:33–34. doi: 10.1038/sj.bdj.4808213. [DOI] [PubMed] [Google Scholar]
- 16.Liang H, Burkes EJ, Frederiksen NL. Multiple idiopathic cervical root resorption: systematic review and report of four cases. Dento maxillo facial radiology. 2003;32:150–155. doi: 10.1259/dmfr/12925020. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald-Jankowski D. Multiple idiopathic cervical root resorption most frequently seen in younger females. Evidence-based dentistry. 2005;6:20. doi: 10.1038/sj.ebd.6400308. [DOI] [PubMed] [Google Scholar]
- 18.Jiang YH, Lin Y, Ge J, Zheng JW, Zhang L, Zhang CY. Multiple idiopathic cervical root resorptions: report of one case with 8 teeth involved successively. International journal of clinical and experimental medicine. 2014;7:1155–1159. [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Lin LY, Yang J, et al. Multiple idiopathic cervical root resorption: a case report. International endodontic journal. 2015 doi: 10.1111/iej.12440. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 20.Iwamatsu-Kobayashi Y, Satoh-Kuriwada S, Yamamoto T, et al. A case of multiple idiopathic external root resorption: a 6-year follow-up study. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2005;100:772–779. doi: 10.1016/j.tripleo.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 21.Neely AL, Gordon SC. A familial pattern of multiple idiopathic cervical root resorption in a father and son: a 22-year follow-up. Journal of periodontology. 2007;78:367–371. doi: 10.1902/jop.2007.060155. [DOI] [PubMed] [Google Scholar]
- 22.Bergmans L, Van Cleynenbreugel J, Verbeken E, Wevers M, Van Meerbeek B, Lambrechts P. Cervical external root resorption in vital teeth. Journal of clinical periodontology. 2002;29:580–585. doi: 10.1034/j.1600-051x.2002.290615.x. [DOI] [PubMed] [Google Scholar]
- 23.Moody AB, Speculand B, Smith AJ, Basu MK. Multiple idiopathic external resorption of teeth. International journal of oral and maxillofacial surgery. 1990;19:200–202. doi: 10.1016/s0901-5027(05)80389-3. [DOI] [PubMed] [Google Scholar]
- 24.Chowdhury S, Bashar A. Progressive idiopathic external root resorption in multiple teeth: A 6- year follow-up study. Updat Dent Coll. 2014;4:14–19. [Google Scholar]
- 25.Mueller E, Rony HR. Laboratory Studies of an Unusual Case of Resorption. Journal of the American Dental Association. 1930;17:326–334. [Google Scholar]
- 26.von Arx T, Schawalder P, Ackermann M, Bosshardt DD. Human and feline invasive cervical resorptions: the missing link?--Presentation of four cases. Journal of endodontics. 2009;35:904–913. doi: 10.1016/j.joen.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 27.Arora A, Acharya SR, Muliya VS, Sharma P. Multiple idiopathic cervical resorption: a diagnostic dilemma. Quintessence international. 2012;43:187–190. [PubMed] [Google Scholar]
- 28.Becks H, Cowden RC. Root resorptions and their relation to pathologic bone formation: Part II. Classification, degrees, prognosis and frequency. American Journal of Orthodontics and Oral Surgery. 1942;28:513–526. [Google Scholar]
- 29.Goultschin J, Nitzan D, Azaz B. ROOT RESORPTION - REVIEW AND DISCUSSION. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1982;54:586–590. doi: 10.1016/0030-4220(82)90199-2. [DOI] [PubMed] [Google Scholar]
- 30.Newman WG. Possible etiologic factors in external root resorption. American journal of orthodontics. 1975;67:522–539. doi: 10.1016/0002-9416(75)90298-5. [DOI] [PubMed] [Google Scholar]
- 31.Verna C, Dalstra M, Melsen B. Bone turnover rate in rats does not influence root resorption induced by orthodontic treatment. European journal of orthodontics. 2003;25:359–363. doi: 10.1093/ejo/25.4.359. [DOI] [PubMed] [Google Scholar]
- 32.Loberg EL, Engstrom C. Thyroid administration to reduce root resorption. The Angle orthodontist. 1994;64:395–399. doi: 10.1043/0003-3219(1994)064<0395:TATRRR>2.0.CO;2. discussion 399–400. [DOI] [PubMed] [Google Scholar]
- 33.Poumpros E, Loberg E, Engstrom C. Thyroid function and root resorption. The Angle orthodontist. 1994;64:389–393. doi: 10.1043/0003-3219(1994)064<0389:TFARR>2.0.CO;2. discussion 394. [DOI] [PubMed] [Google Scholar]
- 34.DeLaurier A, Boyde A, Jackson B, Horton MA, Price JS. Identifying early osteoclastic resorptive lesions in feline teeth: a model for understanding the origin of multiple idiopathic root resorption. Journal of periodontal research. 2009;44:248–257. doi: 10.1111/j.1600-0765.2008.01123.x. [DOI] [PubMed] [Google Scholar]
- 35.Dahl JE, Pallesen U. Tooth bleaching--a critical review of the biological aspects. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 2003;14:292–304. doi: 10.1177/154411130301400406. [DOI] [PubMed] [Google Scholar]
- 36.Southam JC. Clinical and histological aspects of peripheral cervical resorption. Journal of periodontology. 1967;38:534–538. doi: 10.1902/jop.1967.38.6_part1.534. [DOI] [PubMed] [Google Scholar]
- 37.Sabeti M, Slots J. Herpesviral-bacterial coinfection in periapical pathosis. Journal of endodontics. 2004;30:69–72. doi: 10.1097/00004770-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Carranza F, Bernard G. The tooth supporting structures. In: Newman MG, Takei HH, Carranza FA, editors. Carranza's Clinical Periodontology. Philadelphia: W.B. Saunders Company; 2003. pp. 36–57. [Google Scholar]
- 39.Kerr DA, Courtney RM, Burkes EJ. Multiple idiopathic root resorption. Oral surgery, oral medicine, and oral pathology. 1970;29:552–565. doi: 10.1016/0030-4220(70)90467-6. [DOI] [PubMed] [Google Scholar]
- 40.Hopkins R, Adams D. Multiple idiopathic resorption of the teeth. British dental journal. 1979;146:309–312. doi: 10.1038/sj.bdj.4804245. [DOI] [PubMed] [Google Scholar]
- 41.Heithersay GS. Treatment of invasive cervical resorption: an analysis of results using topical application of trichloracetic acid, curettage, and restoration. Quintessence international. 1999;30:96–110. [PubMed] [Google Scholar]
- 42.Heithersay GS. Invasive cervical resorption: an analysis of potential predisposing factors. Quintessence international. 1999;30:83–95. [PubMed] [Google Scholar]
- 43.Darcey J, Qualtrough A. Resorption: part 2. Diagnosis and management. British dental journal. 2013;214:493–509. doi: 10.1038/sj.bdj.2013.482. [DOI] [PubMed] [Google Scholar]